Abstract
The global development of the bioeconomy is impossible without technologies for comprehensive processing of plant renewable resources. The use of proven pretreatment technologies raises the possibility of the industrial implementation of the enzymatic conversion of polysaccharides from lignocellulose considering the process’s complexity. For instance, a well-tuned kraft pulping produces a substrate easily degraded by cellulases and hemicelulases. Enzymatic hydrolysis of bleached hardwood kraft pulp was carried out using an enzyme complex of endoglucanases, cellobiohydrolases, β-glucosidases, and xylanases produced by recombinant strains of Penicillium verruculosum at a 10 FPU/g mixture rate and a 10% substrate concentration. As a result of biocatalysis, the following products were obtained: sugar solution, mainly glucose, xylobiose, xylose, as well as other minor reducing sugars; a modified complex based on cellulose and xylan. The composition of the biomodified kraft pulp was determined by HPLC. The method for determining the crystallinity on an X-ray diffractometer was used to characterize the properties. The article shows the possibility of producing biomodified cellulose cryogels by amorphization with concentrated 85% H3PO4 followed by precipitation with water and supercritical drying. The analysis of the enzymatic hydrolysate composition revealed the predominance of glucose (55–67%) among the reducing sugars with a maximum content in the solution up to 6% after 72 h. The properties and structure of the modified kraft pulp were shown to change during biocatalysis; in particular, the crystallinity increased by 5% after 3 h of enzymatic hydrolysis. We obtained cryogels based on the initial and biomodified kraft pulp with conversion rates of 35, 50, and 70%. The properties of these cryogels are not inferior to those of cryogels based on industrial microcrystalline cellulose, as confirmed by the specific surface area, degree of swelling, porosity, and SEM images. Thus, kraft pulp enzymatic hydrolysis offers prospects not only for producing sugar-rich hydrolysates for microbiological synthesis, but also cellulose powders and cryogels with specified properties.
1. Introduction
Cellulose-based wood resources are the most-widespread renewable resources in the world. The necessity of including them in the circular bioeconomy model has been growing over the last few years [1,2,3]. The crucial biotechnological process of this model is enzymatic hydrolysis, which involves deep polysaccharide degradation: cellulose and hemicelluloses to simple sugars [4,5,6]. It is possible to use the hydrolysate in further microbiological conversion with such biosynthesis products as bioethanol or organic or amino acids.
The issue of lignin-containing wood raw materials’ pretreatment to enzymatic conversion is still thrown into sharp relief in terms of all the possibilities of their full use [7,8]. Raw materials’ pretreatment aims at increasing the fiber accessibility for the enzyme action [9]. The indispensable stages are the raw materials’ grinding, pulp crystalline structure destruction, and complete or partial delignification [4,10]. There are several technologies of wood chemical pretreatment. Among these, for example, are acid treatment, alkali cooking, and kraft pulping. Kraft pulping is a cost-efficient and effective method of preparing wood raw materials for the production of pulp having good reactivity for enzymatic conversion [11,12,13,14,15,16]. Currently, the high level of technology allows the integration of biorefineries into existing pulp and paper industrial enterprises [5,6,17,18,19,20].
The choice of effective enzymes acting on carbohydrates, which include a complex of glycosidases with various degradation pathways of macromolecules, is a decisive factor for deep and controlled bioconversion [8,21,22]. Enzymatic technologies based on the use of isolated hydrolytic enzymes (endoxylanases and endoglucanases) have been extensively introduced in the pulp and paper industry starting from the late 1990s [23]. Currently, there is a wide range of multienzyme commercial complexes for polysaccharide degradation of plant raw materials. Novozymes (Denmark), Genencor, and Danisco Division (USA) are the international companies producing cellulolytic enzymes of the fungus Trichoderma. About 80% of multienzyme complexes for the production of biofuels and organic acids from lignin-containing raw materials are produced when using the fungus [4,24]. Their market prevalence is due to intense development of microbial platforms in recent decades. The enzyme complex based on the producer strains of Penicillium verruculosum has been proposed as their alternative [25]. Their effectiveness in the saccharification of kraft pulp has been confirmed by a number of studies [26,27].
The implementation of a comprehensive strategy in renewable resources pretreatment presumes a propensity to maximize the use of all resource components. Kraft pulp production has an advantage for the utilization of lignin, the most part of which is used in the chemical and energy recovery cycle. However, studying the processes of further enzymatic conversion of kraft pulp polysaccharides has usually been carried out for soluble products (glucose and reducing sugars). There are a limited number of papers devoted to the insoluble residue after hydrolysis [28]. Along with this, it is known that the structure of plant fibers is modified during substrate enzymatic conversion; kraft pulp acquires new characteristics of crystallinity, the degree of polymerization, etc. [29]. Thus, we can speak about the possibility of producing a new product, biomodified kraft pulp, the properties of which are not fully disclosed. According to the waste-free bioeconomy concept, it is necessary to carry out follow-up studies of this product in the economic dimension in order to use it in nanotechnology, pharmaceutics, energy, and other industries.
The production of porous, lightweight materials with a high specific surface area known as cryogels is a possible way to use biomodified kraft pulp. These polysaccharide gels are produced by amorphization in a solvent, freezing, and subsequent supercritical drying, which makes their production simple and cost-efficient [30]. Polysaccharide cryogels have the potential to be used in regenerative biomedicine as cell scaffolds and tissue engineering, with high fluid absorption parameters [31,32].
The present work is a continuation of the previous works [9,11,30], which aimed at obtaining hydrolysates with a high content of cellulosic sugars after the kraft method of wood raw materials’ pretreatment by means of conversion with enzyme complexes based on the P. verruculosum producer strains. This study deals with modified kraft pulp produced by such biocatalysis. Its composition, properties, and structure can be regulated during deep hydrolysis to meet various purposes, including high-quality cryogel production.
2. Results
2.1. Yield and Component Composition of Hydrolysate and Biomodified Kraft Pulp during Biocatalysis
The process of the biocatalysis of kraft pulp by P. verruculosum enzymes resulted in the formation of a mixture of monosaccharides, disaccharides, and oligosaccharides of wood origin in the liquid phase. The predominant products after 72 h of enzymatic hydrolysis were glucose with a concentration of 6.0% and the xylan hydrolysis products, such as xylose (1.0%) and xylobiose (0.7%); in addition, there were minor amounts of mannose (0.2%), galactose (0.2%), and arabinose (less than 0.1%) (Table S1).
The simultaneously flowing enzymatic modification of the kraft pulp significantly reduced its yield, from 75% after 1 h of biocatalysis to 25% after 72 h of hydrolysis (Figure 1). There were changes in the amounts of the main components of the biomodified kraft pulp: cellulose and xylan. Their initial amounts were 72% and 22%, respectively. The maximum amount of cellulose in the biomodified kraft pulp ranged from the initial value to 82.7%; the maximum value was achieved during treatment for 24 h with the P. verruculosum enzyme complex. The xylan content decreased sharply as a result of the action of xylanases after the first hour of the process to 15.3% and reached a minimum of 9% after 24 h of enzymatic modification.
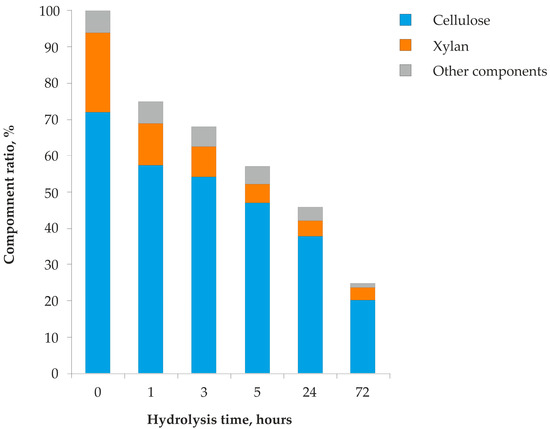
Figure 1.
Changes in the yield and component composition of biomodified kraft pulp during enzymatic hydrolysis by the P. verruculosum complex.
2.2. Degree of Polymerization of Biomodified Kraft Pulp
After hardwood pulping and several bleaching stages, the degree of polymerization (DP) of the kraft pulp used in the experiments was within 1000. As a result of the action of P. verruculosum cellulases, a sharp change in the DP of the biomodified kraft pulp was observed: up to 761 units in the first hour, then up to 400 units in 3 h, gradually decreasing to 240–250 units by 72 h, which corresponds to the level of commercial microcrystalline cellulose (MCC) (Figure 2).
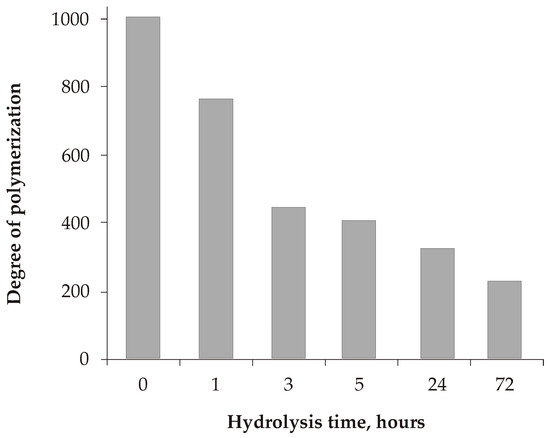
Figure 2.
Changes in the degree of polymerization of biomodified kraft pulp during biocatalysis.
2.3. Characteristics of Cryogels Produced from Original and Biomodified Kraft Pulps
The following samples were chosen as test samples to obtain the polysaccharide cryogels: initial bleached hardwood pulp (P) and biomodified pulp (BP) with different conversion rates: 5 h (BP1), 24 h (BP2), and 72 h (BP3). The BP1 sample after amorphization in phosphoric acid, precipitation, freezing, and freeze-drying had the highest yield: 70.4%. Samples of initial P, BP2, and BP3 had a lower yield of 2.0–6.8% (Table 1). The lowest cryogel volume is typical for BP2, and this may be due to the high shrinkage (70%) and the lowest porosity (91.6%). The remaining samples had a volume of about 3.0 cm3, a shrinkage rate of 34–40%, and an average porosity of 96%. All cryogels from the initial and biomodified kraft pulp had a low density of 0.049–0.115.

Table 1.
Properties of cryogel samples from biomodified kraft pulp with different conversion rates.
Figure 3 shows the SEM images of the cryogel samples from the initial bleached and biomodified kraft pulps.
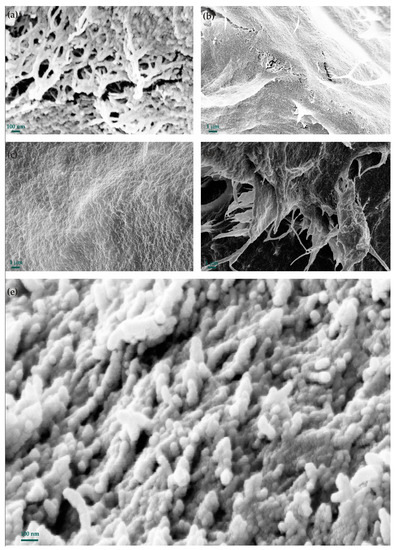
Figure 3.
SEM images of cryogel samples from initial and biomodified kraft pulps. (a,b)—cryogel samples from initial kraft pulp; (c–e)—cryogel samples from biomodified kraft pulp.
3. Discussion
3.1. Composition of Biomodified Kraft Pulp
Previous studies on kraft pulp enzymatic hydrolysis with specially designed enzymes focused mostly on the determination of the monosaccharide composition and content [33,34,35], as well as the cellulose hydrolysis efficiency [36,37]. A well-selected enzyme complex and the absence of inhibitors are crucial for the production of glucose-rich hydrolysates [9,33]. In this work, we used bleached kraft pulps to eliminate the limiting factors of hydrolysis, such as high lignin content [9,38]. Furthermore, the enzyme complex of P. verruculosum has been shown previously to be highly efficient with respect to kraft pulps [9]. We monitored the compositional and structural changes of the kraft pulp during the enzymatic digestion. The substrate contained more than 25% of hemicelluloses, which were also hydrolyzed by the hemicelulases of P. verruculosum into monosaccharides, disaccharides and oligosaccharides (Table S1). The pulp composition changed continuously as the hydrolysis proceeded (Figure 1). The composition at particular time points depended on the hydrolysis rates of individual P. verruculosum enzymes, the accessibility of pulp polysaccharides, as well as the synergistic action of cellulases and xylanases. The initial cellulose: xylan ratio of 3.2:1 changed to 5:1 during the first hour, then to 6:1 during the second hour, and by 24 h, it reached the maximum value of 9:1. Further hydrolysis up to 72 h resulted in the final ratio of 6.5:1 in the biomodified kraft pulp. Hemicelluloses other than xylan were less affected by P. verruculosum enzymes; however, with increasing hydrolysis time, more mannose, galactose, and arabinose were found in the solution (Table S1). The maximum amount of hemicelluloses dissolved after 5–24 h of hydrolysis, and the total amount of non-cellulosic components decreased from 30 to 17–19%.
Thus, this work demonstrated for the first time that biomodified kraft pulp of different compositions could be obtained together with glucose-rich hydrolysates using the enzyme complex of P. verruculosum. The resulting biomodified kraft pulp together with the hydrolysates become essential for lignocellulose biorefineries. Similar technologies have been recommended for the implementation at pulp and paper mills for several years [16,39], and the implementation results can be integrated well as new elements in the forest industry. For instance, 100 tons (dry weight) of bleached kraft pulp after 24 h of hydrolysis with an enzyme dosage of 10 FPU/g can give 60 tons of sugars (approximately 40 tons of glucose) and 46 tons of biomodified kraft pulp (cellulose content greater than 80%).
3.2. Structure and Properties of Biomodified Kraft Pulp
The degree of polymerization of commercial kraft pulps is essential for their application. Biocatalytic treatment of lignocelluloses with cellulolytic complexes is known to decrease the DP depending on the substrate type and the cooperative action of cellulases and xylanases [40,41]. Kaffle et al. reported a decrease in the DP of bleached softwood kraft pulp from 1700 to 250 after 96 h of hydrolysis by Trichoderma cellulases [28]. The DP of 250 corresponds to Avicel celluloses intensively applied in various industries. We observed a decrease in the DP of the hardwood kraft pulp during hydrolysis with P. verruculosum enzymes. The DP decreased from 1000 to 322 during the first 24 h; further hydrolysis resulted in a further smooth decrease of the DP up to 240 after 72 h. Thus, to obtain pulp samples with a higher DP (1.5–2-times higher than commercial Avicell), hydrolysis should be stopped at no longer than 24 h. However, the further microbiological treatment of the hydrolysates, glucose, and reducing sugar concentration should be considered as well.
Crystallinity is another important characteristic of lignocelluloses [28,40]. We used a new original method for calculating the crystallinity of the kraft pulp and biomodified cellulosic product based on XRD data and NMR calibration [42]. The destruction of the cellulose and hemicelluloses of the kraft pulp by P. verruculosum enzymes led to wave-like changes in pulp crystallinity (Figure 4). At the first stage of hydrolysis up to 30% conversion, the crystallinity increased by almost 2.5% compared to the initial sample. Further conversion to 70% resulted in a decrease in the crystallinity to the initial value of the bleached kraft pulp. Interestingly, the crystallinity of 5–65% of converted kraft pulp was higher than that for commercial MCC.
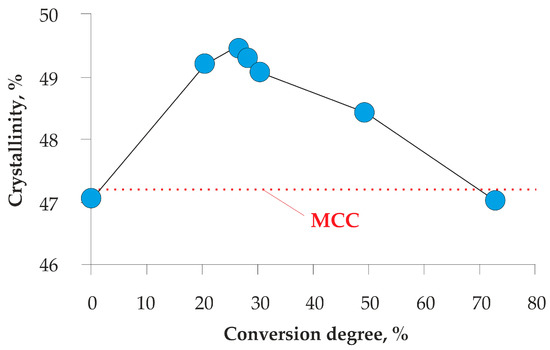
Figure 4.
Changes in crystallinity of biomodified kraft pulp during enzymatic hydrolysis by P. verruculosum cellulases.
The observed composition, DP, and crystallinity of the pulps together with the high sugar concentration of hydrolysates confirmed the high catalytic performance of P. verruculosum enzymes. After achieving the required quality of hydrolysate, one can vary the composition and properties of the new product, the biomodified kraft pulp: high purity, a DP of 250–500, and a crystallinity higher than for commercial MCC.
3.3. Application of Biomodified Kraft Pulp as Polysaccharide Matrix for Cryogel Formation
All cryogels obtained from the kraft pulps before and after enzymatic hydrolysis were stable in water. Stability was measured at room temperature (20 ± 3 °C); the samples were weighed after soaking in water for 24 h. The swelling ratios (SRs) are collected in Table 2. The less-dense samples (P, BP1, BP3) gave the biggest SRs. The dense structure of the BP2 sample hindered efficient water penetration; therefore, BP2 had the smallest SR. Similar values of 10–15 g/g were obtained earlier for cryogels from MCC [32].

Table 2.
The properties of cryogels obtained from biomodified pulp of different conversion levels.
Cellulosic gel can shrink in its volume by 40% during cellulose regeneration [43]. The replacement of a solvent by a non-solvent results in a closer arrangement of cellulose macromolecules. The ΔV values above 0 correspond to cellulose swelling after regeneration (Table 1). The sample with the highest density (BP2) had the lowest volume shrinkage (36%). The degree of swelling of the hydrogel (Qmax) corresponds to the volume shrinkage of the cryogel. The greater the density of the cryogel is, the less swelling the corresponding hydrogel has (Table 1).
Specific surface area is one of the most-important parameters of porous materials [44]. The cryogel with the highest density and the cryogel from the initial kraft pulp had the highest values of the specific surface area (7.94 and 8.97 m2/g, respectively). The results were similar to those obtained previously for cryogels from MCC [45]. Other samples showed a specific surface area similar to cryogels from pharmaceutical-grade MCC [32].
Mechanical tests showed that the BP3 sample had the lowest strength (Table 2). It corresponded to the lowest DS of the biomodified kraft pulp. The BP2 sample showed the highest compressive modulus (E) value. The irregular shape of the sample could contribute to the results, as well as its high volume shrinkage and low porosity and SR. Other samples had a regular cylindrical shape. Therefore, our cryogels demonstrated similar mechanical strength to previously obtained cryogels from MCC (126–3675 kPa) [32,45].
The morphologies of the cryogel samples were similar to each other; it was represented by networks, flat (layered) or spherical structures, and clusters of spherical structures (Figure 3). It was previously reported that regenerated cellulose can form 10–100 nm nanospheres and lamellar structures [46,47,48]. In our case, the diameter of the spheres was 20–100 nm. A similar morphology was previously observed for gels obtained after supercritical CO2 drying [49,50] and aerogels obtained by the direct regeneration of dissolved cellulose [51]. This morphology of cellulose cryogels is suitable for biomedical applications, sorption, filtration, and others.
Thus, we investigated the ability of the commercial hardwood bleached kraft pulp before and after profound enzymatic hydrolysis to form the cryogels. The bleached pulps had suitable purity, and theirs non-hydrolyzed residues were similar to commercial MCC. The cryogel properties and morphology were found to be similar to those observed previously for cryogels from microcrystalline cellulose [32,45]. Thus, biomodified kraft pulps can be promising materials for producing scaffolds in tissue engineering.
4. Materials and Methods
4.1. Kraft Pulp
In the experiments, we used samples of bleached hardwood pulp obtained from a mixture of aspen and birch at the Arkhangelsk Pulp and Paper Mill (Arkhangelsk region, Russia; N 64.424251°, E 40.825241°). Bleaching was carried out under industrial conditions using chlorine dioxide and sodium hydroxide in several stages. The polysaccharide composition of the freeze-dried pulp was determined using acid hydrolysis (72% H2SO4, 4%, 1 h, 121 °C) [52], and further analysis of the monosaccharides was performed by high-performance liquid chromatography using the LC-20 system.
Before use, the substrate was additionally washed with distilled warm water for three cycles in order to remove residual chemical content and moisture to a humidity of 70–80%. After that, the pulp was refrigerated (+4 °C) for 24 h till a homogeneous humidity of all the substrate.
4.2. Enzymes
The research used enzyme complexes (B1-221-151, 562 FPA U/g, and F10, 134 FPA U/g) produced by the recombinant strains of P. verruculosum. The enzyme complex B1 contained endoglucanases, cellobiohydrolases, and xylanases, whereas the enzyme complex F10 predominantly contained β-glucosidase. A balanced composition, comprising cellobiohydrolases 1 and 2 (40%), β-glucosidase (25%), endoglucanases 1, 2, and 3 (10%), and endoxylanase (2%), determined the effective use of the enzyme complex for the hydrolysis of the industrial samples of the cellulose-based raw materials. Approximately 23% of the enzyme complex consisted of ballast proteins [53]. Enzyme activity towards various substrates was determined using common methods [54]. The enzyme dosage was adjusted so that the total activity was 10 FPU/g of dry pulp, as in previous studies [9,55].
4.3. Enzymatic Hydrolysis
Enzymatic hydrolysis involved the use of the Biostat A Plus laboratory bioreactor. The pulp concentration was 10%, with an enzyme dosage of 10 FPU/g; the hydrolysis temperature was kept at about 50 °C with constant stirring (200–300 rpm) for 72 h, and the pH was kept at 5.0 with 0.05 M sodium acetate buffer. The mixture was centrifuged at 4200 rpm for 15 min to separate the biomodified kraft pulp and hydrolysate (Eppendorf 5804R). The supernatant was sampled and analyzed for sugars. Samples were taken every hour during the first 6 h, as well as after 24, 48, and 72 h.
4.4. Biomodified Kraft Pulp Analysis
After hydrolysis, the biomodified kraft pulp was washed thoroughly with distilled water to remove the soluble hydrolysis products. The washing was performed by successive repeated cycles of mixing the biomodified kraft pulp with water and centrifugation at 4200 rpm for 15 min (Eppendorf 5804R). After each cycle, the supernatant was carefully drained and tested for sugars, and a fresh batch of distilled water was added. This procedure was carried out before zero glucose values. The conversion rate and the biomodified kraft pulp yield were calculated as described in the paper by Aksenov et al. [9].
The crystallinity of the initial and biomodified kraft pulp was analyzed relative to microcrystalline bacterial cellulose in accordance with [42,56] using the XRD-7000S diffractometer (Shimadzu, Japan). For this purpose, samples were taken after 1, 3, 4, 5, 24, and 72 h of hydrolysis.
The viscometric method was used to determine the degree of polymerization, and cadmium ethylenediamine with a pulp concentration of 0.1% (state standard GOST 25438-82) was used as a solvent. The average degree of polymerization was determined based on the obtained results of intrinsic viscosity. For this purpose, samples were taken at 2–5, 24, 48, and 72 h.
We used the inversion method to study the biomodified kraft pulp composition. The method involved adding H2SO4 to the hydrolysates to a concentration of 4% by weight and incubating them for 20 min at 100 °C. The resulting mixture was cooled and neutralized using 25% NaOH. The concentrations of sugars (glucose, xylose, mannose, arabinose, galactose) and disaccharides (cellobiose, xylobiose) in the hydrolysate before and after acidic inversion were determined using the LC-20 Prominence system (Shim-pack ISA-07/S2504 column, Shimadzu, Kyoto, Japan) with post-column derivatization and fluorometric detection. Then, the calculation of the polysaccharide composition of the biomodified kraft pulp was determined for each point by the difference between the initial pulp and the amount of monosaccharides after inversion in a particular enzymatic hydrolysate [52].
4.5. Preparation and Characterization of Biomodified Kraft Pulp Cryogels
Cryogels based on the kraft pulp before and after 5, 24, and 72 h of enzymatic hydrolysis were obtained using phosphoric acid according to the method presented in [32]. For this, solutions containing 5% cellulose were prepared. The dissolution time was 25 h at a temperature of 22–24 °C. Distilled water was used as a cellulose precipitant from phosphoric acid solutions. The regenerated cellulose was washed by distilled water until neutral with phenolphthalein, frozen at −18 °C, and freeze-dried. The parameters (yield of cryogel, volume variation, density, porosity, swelling, and swelling ratio), specific surface area, mechanical characteristics of cryogels, and morphology were evaluated according to the study [32].
Scanning electron microscopy (SEM) of the cryogels was used to evaluate the morphology. The images were obtained using the SIGMA VP instrument (Zeiss, Germany) operated at a 10 kV accelerated voltage. Prior to SEM, the cryogel samples were sputter-coated with a 5 nm Pt/Pd mixture using the Q150TES spattering system (Quorum, Laughton, UK).
5. Conclusions
The application of biocatalytic technologies based on the conversion of polysaccharides to sugars in plant raw materials’ processing requires procedures considering the degree of modification of cellulose-containing matrices preserved as a result of incomplete hydrolysis. Bleached kraft pulp can serve as a commercially available model substrate for hydrolysis by cellulases and hemicellulases. We showed that the monitoring of the composition and characteristics of the non-hydrolyzed residue can be useful for the biocatalytic performance assessment using the interaction between P. verruculosum enzymes and kraft pulp as a case study. The targeted modification of a complex consisting of cellulose, xylan, and other hemicelluloses changes the characteristics of kraft pulp for its use both as a finished product and as a raw material for cryogel production. These processes are highly scalable to existing pulp and paper mills and lay the groundwork for the bioeconomy development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13010103/s1, Table S1: The concentration of sugars in the hydrolysate after enzymatic hydrolysis at various stages of conversion.
Author Contributions
Conceptualization, A.R.S. and A.S.A.; methodology, A.M.R., D.G.C., I.V.G. and A.S.A.; resources, E.A.T. and V.D.T.; data curation, A.V.M.; writing—original draft preparation, I.V.T., A.R.S. and A.S.A.; writing—review and editing, O.A.S.; visualization, D.G.C. and A.S.A.; cryogel production and characterization I.V.T.; project administration, I.V.T. and A.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
The Russian Science Foundation supported all biocatalysis studies and biomodified kraft pulp analysis (Project 22-24-20136), and the Russian Foundation for Basic Research supported the cryogel production and characterization (Project 19-33-60014).
Data Availability Statement
Not applicable.
Acknowledgments
In this work, we used the instrumentation of the Core Facility Center “Arktika” of the Northern (Arctic) Federal University named after M.V. Lomonosov and the Centre for Diagnostics of Functional Materials for Medicine, Pharmacology, and Nanoelectronics at the Research Park of Saint Petersburg State University. The authors are grateful to Alexander S. Sakhatsky for specific surface area measurements and Danil I. Falev from NArFU for sugar analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hernández-Chaverri, R.A.; Buenrostro-Figueroa, J.J. Biomass: Biorefinery as a model to boost the bioeconomy in Costa Rica, a review. Agron. Mesoam. 2021, 32, 1047–1070. [Google Scholar] [CrossRef]
- Novy, V.; Nielsen, F.; Seiboth, B.; Nidetzky, B. The influence of feedstock characteristics on enzyme production in Trichoderma reesei: A review on productivity, gene regulation and secretion profiles. Biotechnol. Biofuels 2019, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Nagy, M.; Kim, D.H.; Eckert, C.A.; Hallett, J.P.; Liotta, C.L. From wood to fuels: Integrating biofuels and pulp production. Ind. Biotechnol. 2006, 2, 55–65. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Parreiras, L.S.; Murakami, M.T. Rational engineering of the Trichoderma reesei RUT-C30 strain into an industrially relevant platform for cellulase production. Biotechnol. Biofuels 2020, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.B.; Jameel, H.; Chang, H.M. Integration of pulp and paper technology with bioethanol production. Biotechnol. Biofuels. 2013, 6, 13. [Google Scholar] [CrossRef]
- Johnson, M.A.; Hart, P.W. Integrating a biorefinery into an operating kraft mill. BioResources 2016, 11, 10677–10710. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Brunecky, R.; Donohoe, B.S.; Yarbrough, J.M.; Mittal, A.; Scott, B.R.; Ding, H.; Bomble, Y.J. The Multi Domain Caldicellulosiruptor bescii CelA Cellulase Excels at the Hydrolysis of Crystalline Cellulose. Sci. Rep. 2017, 7, 9622. [Google Scholar] [CrossRef]
- Aksenov, A.S.; Tyshkunova, I.V.; Poshina, D.N.; Guryanova, A.A.; Chukhchin, D.G.; Sinelnikov, I.G.; Terentyev, K.Y.; Skorik, Y.A.; Novozhilov, E.V.; Synitsyn, A.P. Biocatalysis of industrial kraft pulps: Similarities and differences between hardwood and softwood pulps in hydrolysis by enzyme complex of Penicillium verruculosum. Catalysts 2020, 10, 536. [Google Scholar] [CrossRef]
- Zheng, Y.; Shi, J.; Tu, M.; Cheng, Y.S. Principles and development of lignocellulosic biomass pretreatment for biofuels. Adv. Bioenergy 2017, 2, 1–68. [Google Scholar] [CrossRef]
- Novozhilov, E.V.; Sinel’nikov, I.G.; Aksenov, A.S.; Chukhchin, D.G.; Tyshkunova, I.V.; Rozhkova, A.M.; Osipov, D.O.; Zorov, I.N.; Sinitsyn, A.P. Biocatalytic conversion of kraft pulp using cellulase complex of Penicillium verruculosum. Catal. Ind. 2016, 8, 95–100. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Xu, H.; Che, X.; Ding, Y.; Kong, Y.; Li, B.; Tian, W. Effect of crystallinity on pretreatment and enzymatic hydrolysis of lignocellulosic biomass based on multivariate analysis. Bioresour. Technol. 2019, 279, 271–280. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, S.J.; Zhong, C.; Li, B.Z.; Yuan, Y.J. Alkali-based pretreatment-facilitated lignin valorization: A review. Ind. Eng. Chem. Res. 2020, 59, 16923–16938. [Google Scholar] [CrossRef]
- Shewale, J.G.; Sadana, J.C. Enzymatic hydrolysis of cellulosic materials by Sclerotium rolfsii culture filtrate for sugar production. Can. J. Microbiol. 1979, 25, 773–783. [Google Scholar] [CrossRef]
- Płaza, G.A.; Wandzich, W. Biorefineries-new green strategy for development of smart and innovative industry. Manag. Syst. Prod. Eng. 2016, 3, 150–155. [Google Scholar] [CrossRef][Green Version]
- Branco, R.H.; Serafim, L.S.; Xavier, A.M. Second generation bioethanol production: On the use of pulp and paper industry wastes as feedstock. Fermentation 2018, 5, 4. [Google Scholar] [CrossRef]
- Ajao, O.; Marinova, M.; Savadogo, O.; Paris, J. Hemicellulose based integrated forest biorefineries: Implementation strategies. Ind. Crops Prod. 2018, 126, 250–260. [Google Scholar] [CrossRef]
- Ahmadvand, S.; Khadivi, M.; Arora, R.; Sowlati, T. Bi-objective optimization of forest-based biomass supply chains for minimization of costs and deviations from safety stock. Energy Convers. Manag. X 2021, 11, 100101. [Google Scholar] [CrossRef]
- Martin-Sampedro, R.; Eugenio, M.E.; Moreno, J.A.; Revilla, E.; Villar, J.C. Integration of a kraft pulping mill into a forest biorefinery: Pre-extraction of hemicellulose by steam explosion versus steam treatment. Bioresour. Technol. 2014, 153, 236–244. [Google Scholar] [CrossRef]
- Bajpai, P. Application of enzymes in the pulp and paper industry. Biotechnol. Prog. 1999, 15, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Christopher, L.P. Recent trends and developments in dissolving pulp production and application. Cellulose 2017, 24, 2347–2365. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Esteghlaglian, A.R. Application of biotechnology in the forest products industry. In Applications of Enzymes to Lignocellulosics; Mansfield, S.D., Saddler, J.N., Eds.; American Chemical Society: Washington, DC, USA, 2003; Volume ACS Symposium Series 855, pp. 2–29. [Google Scholar]
- Bischof, R.H.; Ramoni, J.; Seiboth, B. Cellulases and beyond: The first 70 years of the enzyme producer Trichoderma reesei. Microb. Cell Factories 2016, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Rozhkova, A.M.; Gusakov, A.V.; Dotsenko, A.S.; Sinitsyna, O.A.; Sinitsyn, A.P. Capabilities of the ascomycete fungus Penicillium verruculosum and its enzymes for conversion of cellulosic feedstock. In Liquid Biofuels: Bioethanol. Biofuel and Biorefinery Technologies; Soccol, C.R., Amarante Guimarães Pereira, G., Dussap, C.G., Porto de Souza Vandenberghe, L., Eds.; Springer: Cham, Switzerland, 2022; Volume 12. [Google Scholar] [CrossRef]
- Sinitsyn, A.P.; Sinitsyna, O.A.; Rozhkova, A.M. Production of Industrial Enzymes Based on the Expression System of the Fungus Penicillium verruculosum. Appl. Biochem. Microbiol. 2021, 57, 851–865. [Google Scholar] [CrossRef]
- Kislitsin, V.Y.; Chulkin, A.M.; Zorov, I.N.; Denisenko, Y.A.; Sinitsyn, A.P.; Rozhkova, A.M. The effect of cellobiohydrolase 1 gene knockout for composition and hydrolytic activity of the enzyme complex secreted by filamentous fungus Penicillium verruculosum. Bioresour. Technol. Rep. 2022, 18, 101023. [Google Scholar] [CrossRef]
- Kafle, K.; Shin, H.; Lee, C.M.; Park, S.; Kim, S.H. Progressive structural changes of Avicel, bleached softwood and bacterial cellulose during enzymatic hydrolysis. Sci. Rep. 2015, 5, 15102. [Google Scholar] [CrossRef]
- Carrillo-Varela, I.; Vidal, C.; Vidaurre, S.; Parra, C.; Machuca, Á.; Briones, R.; Mendonça, R.T. Alkalization of kraft pulps from pine and eucalyptus and its effect on enzymatic saccharification and viscosity control of cellulose. Polymers 2022, 14, 3127. [Google Scholar] [CrossRef]
- Shevchenko, A.; Aksenov, A.; Tyshkunova, I.; Falev, D.; Toptunov, E. Products of hydrolysis of bleached hardwood kraft pulp by carbohydrate-active enzymes. In The International Conference on Advances in Emerging Trends and Technologies; Springer: Cham, Switzerland, 2021; pp. 114–123. [Google Scholar] [CrossRef]
- Budtova, T.; Aguilera, D.A.; Beluns, S.; Berglund, L.; Chartier, C.; Espinosa, E.; Buwalda, S.J. Biorefinery approach for aerogels. Polymers 2020, 12, 2779. [Google Scholar] [CrossRef]
- Tyshkunova, I.V.; Chukhchin, D.G.; Gofman, I.V.; Poshina, D.N.; Skorik, Y.A. Cellulose cryogels prepared by regeneration from phosphoric acid solutions. Cellulose 2021, 28, 4975–4989. [Google Scholar] [CrossRef]
- Buzała, K.; Przybysz, P.; Rosicka-Kaczmarek, J.; Kalinowska, H. Production of glucose-rich enzymatic hydrolysates from cellulosic pulps. Cellulose 2015, 22, 663–674. [Google Scholar] [CrossRef]
- Buzała, K.P.; Przybysz, P.; Kalinowska, H.; Przybysz, K.; Kucner, M.; Dubowik, M. Evaluation of pine kraft cellulosic pulps and fines from papermaking as potential feedstocks for biofuel production. Cellulose 2016, 23, 649–659. [Google Scholar] [CrossRef]
- Branco, R.H.; Amândio, M.S.; Serafim, L.S.; Xavier, A.M. Ethanol Production from Hydrolyzed Kraft Pulp by Mono-and Co-Cultures of Yeasts: The Challenge of C6 and C5 Sugars Consumption. Energies 2020, 13, 744. [Google Scholar] [CrossRef]
- Sinitsyn, A.P.; Sinitsyna, O.A. Bioconversion of renewable plant biomass. Second-generation biofuels: Raw materials, biomass pretreatment, enzymes, processes, and cost analysis. Biochemistry 2021, 86, S166–S195. [Google Scholar] [CrossRef]
- Proskurina, O.; Korotkova, O.; Rozhkova, A.; Matys, V.Y.; Koshelev, A.; Okunev, O.; Nemashkalov, V.; Sinitsyna, O.; Sinitsyn, A. Application of the “fusion” approach for the production of highly efficient biocatalysts based on recombinant strains of the fungus Penicillium verruculosum for the conversion of cellulose-containing biomass. Catal. Ind. 2013, 5, 327–334. [Google Scholar] [CrossRef]
- Przybysz Buzała, K.; Kalinowska, H.; Małachowska, E.; Boruszewski, P.; Krajewski, K.; Przybysz, P. The Effect of lignin content in birch and beech kraft cellulosic pulps on simple sugar yields from the enzymatic hydrolysis of cellulose. Energies 2019, 12, 2952. [Google Scholar] [CrossRef]
- Van Heiningen, A. Converting a kraft pulp mill into an integrated forest biorefinery. Pulp Pap. Can. 2006, 107, 38–43. [Google Scholar]
- Sinitsyn, A.P.; Gusakov, A.V.; Vlasenko, E.Y. Effect of structural and physico-chemical features of cellulosic substrates on the efficiency of enzymatic hydrolysis. Appl. Biochem. Biotechnol. 1991, 30, 43–59. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Mooney, C.; Saddler, J.N. Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol. Prog. 1999, 15, 804–816. [Google Scholar] [CrossRef]
- Vydrina, I.; Malkov, A.; Vashukova, K.; Tyshkunova, I.; Mayer, L.; Faleva, A.; Shestakov, S.; Novozhilov, E.; Chukhchin, D. A new method for determination of lignocellulose crystallinity from XRD data using NMR calibration. Carbohydr. Polym. 2023; in press. [Google Scholar]
- Sescousse, R.; Budtova, T. Influence of processing parameters on regeneration kinetics and morphology of porous cellulose from cellulose–NaOH–water solutions. Cellulose 2009, 16, 417–426. [Google Scholar] [CrossRef]
- Korhonen, O.; Budtova, T. All-cellulose composite aerogels and cryogels. Compos. Part A Appl. Sci. Manuf. 2020, 137, 106027. [Google Scholar] [CrossRef]
- Tyshkunova, I.V.; Gofman, I.V.; Chukhchin, D.G.; Malkov, A.V.; Mishanin, A.I.; Golovkin, A.S.; Pavlova, E.N.; Poshina, D.N.; Skorik, Y.A. Biophysical characterization and cytocompatibility of cellulose cryogels reinforced with chitin nanowhiskers. Polymers 2022, 14, 2694. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent strategies in preparation of cellulose nanocrystals and cellulose nanofibrils derived from raw cellulose materials. Int. J. Polym. Sci. 2018, 2018, 7923068. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Deng, X.-Y.; Shen, W.-H.; Jia, M.-Y. Preparation and characterization of the spherical nanosized cellulose by the enzymatic hydrolysis of pulp fibers. Carbohydr. Polym. 2018, 181, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Ram, B.; Chauhan, G.S. New spherical nanocellulose and thiol-based adsorbent for rapid and selective removal of mercuric ions. Chem. Eng. J. 2018, 331, 587–596. [Google Scholar] [CrossRef]
- Buchtova, N.; Budtova, T. Cellulose aero-, cryo-and xerogels: Towards understanding of morphology control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- Demilecamps, A.; Beauger, C.; Hildenbrand, C.; Rigacci, A.; Budtova, T. Cellulose–silica aerogels. Carbohydr. Polym. 2015, 122, 293–300. [Google Scholar] [CrossRef]
- Willför, S.; Pranovich, A.; Tamminen, T.; Puls, J.; Laine, C.; Suurnäkki, A.; Saake, B.; Uotila, K.; Simolin, H.; Hemming, J.; et al. Carbohydrate analysis of plant materials with uronic acid-containing polysaccharides—A comparison between different hydrolysis and subsequent chromatographic analytical techniques. Ind. Crops Prod. 2009, 29, 571–580. [Google Scholar] [CrossRef]
- Chekushina, A.V.; Dotsenko, G.S.; Sinitsyn, A.P. Comparing the efficiency of plant material bioconversion processes using biocatalysts based on Trichoderma and Penicillium verruculosum enzyme preparations. Catal. Ind. 2013, 5, 98–104. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Tenkanen, M.; Tamminen, T.; Hortling, B. Investigation of lignin-carbohydrate complexes in kraft pulps by selective enzymatic treatments. Appl. Microbiol. Biotechnol. 1999, 51, 241–248. [Google Scholar] [CrossRef]
- Chukhchin, D.G.; Malkov, A.V.; Tyshkunova, I.V.; Mayer, L.V.; Novozhilov, E.V. Diffractometric method for determining the degree of crystallinity of materials. Crystallogr. Rep. 2016, 61, 371–375. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).