Furfural Influences Hydrogen Evolution and Energy Conversion in Photo-Fermentation by Rhodobacter capsulatus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Furfural on Photo-Fermentative Hydrogen Production from Glucose
2.2. Glucose Consumption during Photo-Fermentation
2.3. Soluble Metabolic Residues Formed during Photo-Fermentation
2.4. Energy Conversion Efficiency Analysis
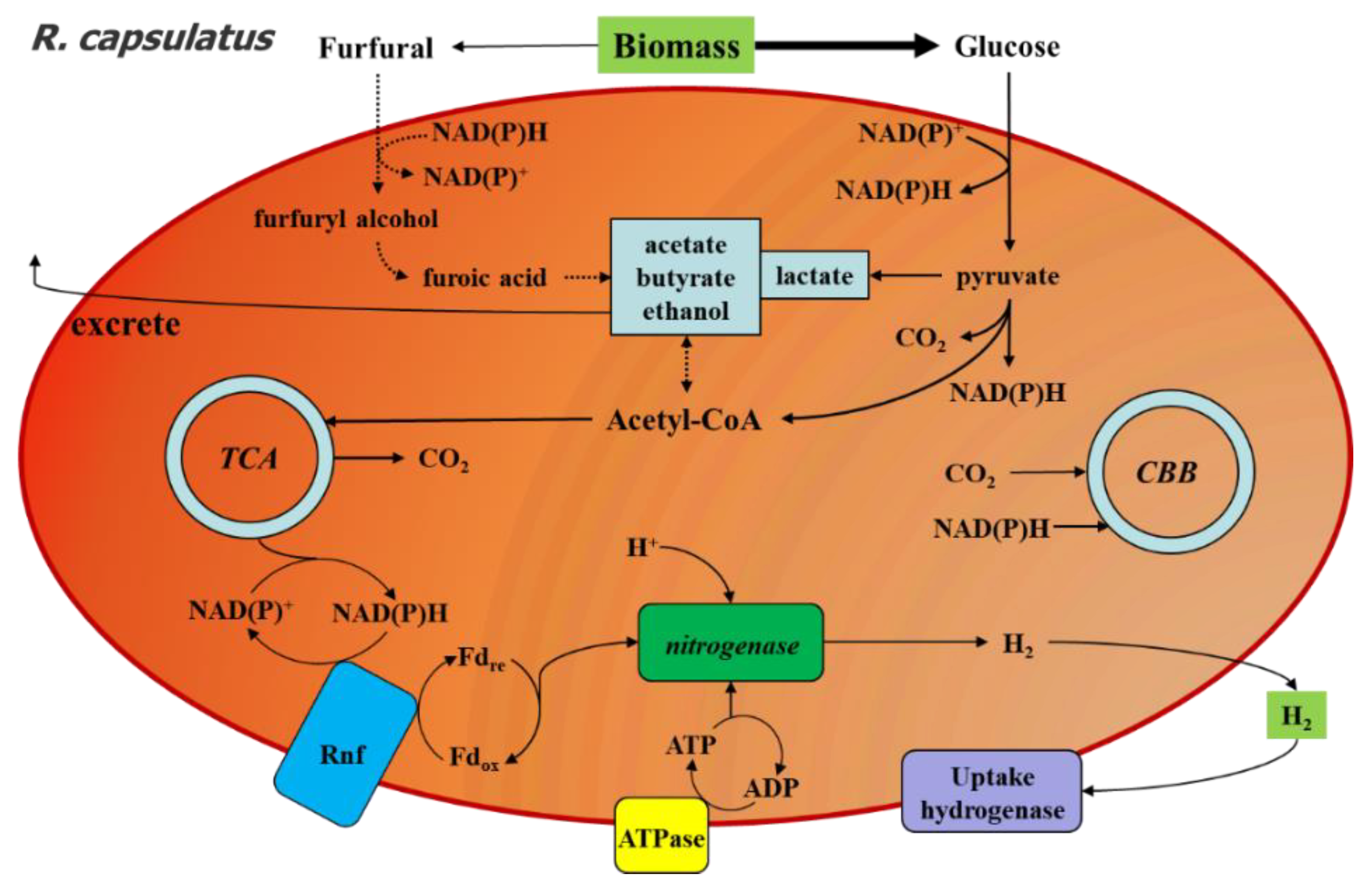
2.5. Proposed Response Mechanism of R. capsulatus to Furfural during Photo-Fermentation
3. Materials and Methods
3.1. Bacterial Strains and Pre-Culture
3.2. Batch Photo-Fermentative Hydrogen Production Process
3.3. Analytical Methods
3.4. Calculations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fixen, K.R.; Chowdhury, N.P.; Martinez-Perez, M.; Poudel, S.; Boyd, E.S.; Harwood, C.S. The path of electron transfer to nitrogenase in a phototrophic alpha-proteobacterium. Environ. Microbiol. 2018, 20, 2500–2508. [Google Scholar] [CrossRef]
- Seefeldt, L.C.; Peters, J.W.; Beratan, D.N.; Bothner, B.; Minteer, S.D.; Raugei, S.; Hoffman, B.M. Control of electron transfer in nitrogenase. Curr. Opin. Chem. Biol. 2018, 47, 54–59. [Google Scholar] [CrossRef]
- Rutledge, H.L.; Tezcan, F.A. Electron Transfer in Nitrogenase. Chem. Rev. 2020, 120, 5158–5193. [Google Scholar] [CrossRef]
- Ghosh, S.; Dairkee, U.K.; Chowdhury, R.; Bhattacharya, P. Hydrogen from food processing wastes via photofermentation using Purple Non-sulfur Bacteria (PNSB)–A review. Energy Convers. Manag. 2017, 141, 299–314. [Google Scholar] [CrossRef]
- Akroum-Amrouche, D.; Akroum, H.; Lounici, H. Green hydrogen production by Rhodobacter sphaeroides. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 1–19. [Google Scholar] [CrossRef]
- Tiang, M.F.; Hanipa, M.A.F.; Abdul, P.M.; Jahim, J.M.; Mahmod, S.; Takriff, M.S.; Lay, C.-H.; Reungsang, A.; Wu, S.-Y. Recent advanced biotechnological strategies to enhance photo-fermentative biohydrogen production by purple non-sulphur bacteria: An overview. Int. J. Hydrogen Energy 2020, 45, 13211–13230. [Google Scholar] [CrossRef]
- Chica, B.; Ruzicka, J.; Kallas, H.; Mulder, D.W.; Brown, K.A.; Peters, J.W.; Seefeldt, L.C.; Dukovic, G.; King, P.W. Defining Intermediates of Nitrogenase MoFe Protein during N2 Reduction under Photochemical Electron Delivery from CdS Quantum Dots. J. Am. Chem. Soc. 2020, 142, 14324–14330. [Google Scholar] [CrossRef]
- Mao, L.; Lu, B.; Shi, J.; Zhang, Y.; Kang, X.; Chen, Y.; Jin, H.; Guo, L. Rapid high-temperature hydrothermal post treatment on graphitic carbon nitride for enhanced photocatalytic H2 evolution. Catal. Today 2022. [Google Scholar] [CrossRef]
- Irfan, R.M.; Tahir, M.H.; Nadeem, M.; Maqsood, M.; Bashir, T.; Iqbal, S.; Zhao, J.; Gao, L. Fe3C/CdS as noble-metal-free composite photocatalyst for highly enhanced photocatalytic H2 production under visible light. Appl. Catal. A Gen. 2020, 603, 117768. [Google Scholar] [CrossRef]
- Irfan, R.M.; Tahir, M.H.; Iqbal, S.; Nadeem, M.; Bashir, T.; Maqsood, M.; Zhao, J.; Gao, L. Co3C as a promising cocatalyst for superior photocatalytic H2 production based on swift electron transfer processes. J. Mater. Chem. C 2021, 9, 3145–3154. [Google Scholar] [CrossRef]
- Divya, D.; Gopinath, L.; Christy, P.M. A review on current aspects and diverse prospects for enhancing biogas production in sustainable means. Renew. Sustain. Energy Rev. 2015, 42, 690–699. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef]
- Akhtar, N.; Gupta, K.; Goyal, D.; Goyal, A. Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ. Prog. Sustain. Energy 2015, 35, 489–511. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Hosseini Koupaie, E.; Dahadha, S.; Bazyar Lakeh, A.A.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production-A review. J. Environ. Manag. 2019, 233, 774–784. [Google Scholar] [CrossRef]
- Jilani, S.B.; Prasad, R.; Yazdani, S.S. Overexpression of Oxidoreductase YghA Confers Tolerance of Furfural in Ethanologenic Escherichia coli Strain SSK42. Appl. Environ. Microbiol. 2021, 87, e01855-21. [Google Scholar] [CrossRef]
- Tan, Z.; Li, X.; Yang, C.; Liu, H.; Cheng, J.J. Inhibition and disinhibition of 5-hydroxymethylfurfural in anaerobic fermentation: A review. Chem. Eng. J. 2021, 424, 130560. [Google Scholar] [CrossRef]
- Liu, Z.L.; Ma, M.; Song, M. Evolutionarily engineered ethanologenic yeast detoxifies lignocellulosic biomass conversion inhibitors by reprogrammed pathways. Mol. Genet. Genom. 2009, 282, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.A.; Clark, W.; McCaffery, J.M.; Cai, Z.; Lanctot, A.; Slininger, P.J.; Liu, Z.L.; Gorsich, S.W. Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol. Biofuels 2010, 3, 2. [Google Scholar] [CrossRef]
- Zhang, Y.; Ujor, V.; Wick, M.; Ezeji, T.C. Identification, purification and characterization of furfural transforming enzymes from Clostridium beijerinckii NCIMB 8052. Anaerobe 2015, 33, 124–131. [Google Scholar] [CrossRef]
- Cho, H.Y.; Nam, M.S.; Hong, H.J.; Song, W.S.; Yoon, S.-I. Structural and Biochemical Analysis of the Furan Aldehyde Reductase YugJ from Bacillus subtilis. Int. J. Mol. Sci. 2022, 23, 1882. [Google Scholar] [CrossRef]
- Quemeneur, M.; Hamelin, J.; Barakat, A.; Steyer, J.-P.; Carrere, H.; Trably, E. Inhibition of fermentative hydrogen production by lignocellulose-derived compounds in mixed cultures. Int. J. Hydrogen Energy 2012, 37, 3150–3159. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, D.; Shui, X.; Lei, T.; Zhang, H.; Zhang, Z.; Zhang, X.; Zhu, S.; Zhang, Q. Effect of 5-HMF and furfural additives on bio-hydrogen production by photo-fermentation from giant reed. Bioresour. Technol. 2022, 347, 126743. [Google Scholar] [CrossRef]
- Hu, J.; Wei, W.; Li, Q.; Cao, W.; Zhang, A.; Wang, X.; Ni, Y.; Guo, L. Metabolically versatile Rhodobacter sphaeroides as a robust biocatalyst for H2 production from lignocellulose-derived mix substrates. Fuel 2021, 302, 121108. [Google Scholar] [CrossRef]
- Kumka, J.E.; Schindel, H.; Fang, M.; Zappa, S.; Bauer, C.E. Transcriptomic analysis of aerobic respiratory and anaerobic photosynthetic states in Rhodobacter capsulatus and their modulation by global redox regulators RegA, FnrL and CrtJ. Microb. Genom. 2017, 3, e000125. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.-P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Khan, Q.A.; Hadi, S.M. Inactivation and repair of bacteriophage lambda by furfural. Biochem. Mol. Biol. Int. 1994, 32, 379–385. [Google Scholar]
- De Vrije, T.; Bakker, R.R.; Budde, M.A.; Lai, M.H.; Mars, A.E.; Claassen, P.A. Efficient hydrogen production from the lignocellulosic energy crop Miscanthus by the extreme thermophilic bacteria Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Biotechnol. Biofuels 2009, 2, 12–15. [Google Scholar] [CrossRef]
- Siqueira, M.R.; Reginatto, V. Inhibition of fermentative H2 production by hydrolysis byproducts of lignocellulosic substrates. Renew. Energy 2015, 80, 109–116. [Google Scholar] [CrossRef]
- Akobi, C.; Hafez, H.; Nakhla, G. Impact of furfural on biological hydrogen production kinetics from synthetic lignocellulosic hydrolysate using mesophilic and thermophilic mixed cultures. Int. J. Hydrogen Energy 2017, 42, 12159–12172. [Google Scholar] [CrossRef]
- Lane, N.; Martin, W.F. The Origin of Membrane Bioenergetics. Cell 2012, 151, 1406–1416. [Google Scholar] [CrossRef]
- Calisto, F.; Sousa, F.M.; Sena, F.V.; Refojo, P.N.; Pereira, M.M. Mechanisms of Energy Transduction by Charge Translocating Membrane Proteins. Chem. Rev. 2021, 121, 1804–1844. [Google Scholar] [CrossRef]
- Bartacek, J.; Zabranska, J.; Lens, P.N.L. Developments and constraints in fermentative hydrogen production. Biofuels Bioprod. Biorefin. 2007, 1, 201–214. [Google Scholar] [CrossRef]
- Davila-Vazquez, G.; Alatriste-Mondragón, F.; De León-Rodríguez, A.; Razo-Flores, E. Fermentative hydrogen production in batch experiments using lactose, cheese whey and glucose: Influence of initial substrate concentration and pH. Int. J. Hydrogen Energy 2008, 33, 4989–4997. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Ghosh, D. Advances in fermentative biohydrogen production: The way forward? Trends Biotechnol. 2009, 27, 287–297. [Google Scholar] [CrossRef]
- Saraphirom, P.; Reungsang, A. Optimization of biohydrogen production from sweet sorghum syrup using statistical methods. Int. J. Hydrogen Energy 2010, 35, 13435–13444. [Google Scholar] [CrossRef]
- Lin, R.; Deng, C.; Cheng, J.; Murphy, J.D. Low concentrations of furfural facilitate biohydrogen production in dark fermentation using Enterobacter aerogenes. Renew. Energy 2019, 150, 23–30. [Google Scholar] [CrossRef]
- Sun, C.; Liao, Q.; Xia, A.; Fu, Q.; Huang, Y.; Zhu, X.; Zhu, X.; Wang, Z. Degradation and transformation of furfural derivatives from hydrothermal pre-treated algae and lignocellulosic biomass during hydrogen fermentation. Renew. Sustain. Energy Rev. 2020, 131, 109983. [Google Scholar] [CrossRef]
- Hu, B.-B.; Wang, J.-L.; Wang, Y.-T.; Zhu, M.-J. Specify the individual and synergistic effects of lignocellulose-derived inhibitors on biohydrogen production and inhibitory mechanism research. Renew. Energy 2019, 140, 397–406. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Ding, L.; Song, W.; Zhou, J.; Cen, K. Inhibitory effects of furan derivatives and phenolic compounds on dark hydrogen fermentation. Bioresour. Technol. 2015, 196, 250–255. [Google Scholar] [CrossRef]
- Carlson, S.J.; Fleig, A.; Baron, M.K.; Berg, I.A.; Alber, B.E. Barriers to 3-Hydroxypropionate-Dependent Growth of Rhodobacter sphaeroides by Distinct Disruptions of the Ethylmalonyl Coenzyme A Pathway. J. Bacteriol. 2019, 201, e00556-18. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Zhou, X.; Hu, J.; Ge, X.; Xia, C.; Zhao, J.; Wang, Y.; Jing, Y.; Li, Y.; et al. Effect of substrate concentration on hydrogen production by photo-fermentation in the pilot-scale baffled bioreactor. Bioresour. Technol. 2017, 247, 1173–1176. [Google Scholar] [CrossRef]
- Xia, A.; Cheng, J.; Song, W.; Su, H.; Ding, L.; Lin, R.; Lu, H.; Liu, J.; Zhou, J.; Cen, K. Fermentative hydrogen production using algal biomass as feedstock. Renew. Sustain. Energy Rev. 2015, 51, 209–230. [Google Scholar] [CrossRef]
- Wang, S.; He, Z.; Yuan, Q. Xylose enhances furfural tolerance in Candida tropicalis by improving NADH recycle. Chem. Eng. Sci. 2017, 158, 37–40. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Yen, H.C.; Marrs, B. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata. J. Bacteriol. 1976, 126, 619–629. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Zhang, L.; Guo, L. Enhanced hydrogen production performance of Rubrivivax gelatinosus M002 using mixed carbon sources. Int. J. Hydrogen Energy 2012, 37, 13296–13303. [Google Scholar] [CrossRef]
- Wei, X.; Feng, J.; Cao, W.; Guo, L. Enhanced biohydrogen production by an ammonium-tolerant Rhodobacter capsulatus from sugarcane bagasse. Fuel 2021, 300, 121009. [Google Scholar] [CrossRef]
- Oh, Y.-K.; Seol, E.-H.; Kim, M.-S.; Park, S. Photoproduction of hydrogen from acetate by a chemoheterotrophic bacterium Rhodopseudomonas palustris P4. Int. J. Hydrogen Energy 2004, 29, 1115–1121. [Google Scholar] [CrossRef]
- Feng, J.; Li, Q.; Zhang, Y.; Yang, H.; Guo, L. High NH3N tolerance of a cheR2-deletion Rhodobacter capsulatus mutant for photo-fermentative hydrogen production using cornstalk. Int. J. Hydrogen Energy 2018, 44, 15833–15841. [Google Scholar] [CrossRef]
- Han, K.; Levenspiel, O. Extended monod kinetics for substrate, product, and cell inhibition. Biotechnol. Bioeng. 1988, 32, 430–447. [Google Scholar] [CrossRef]
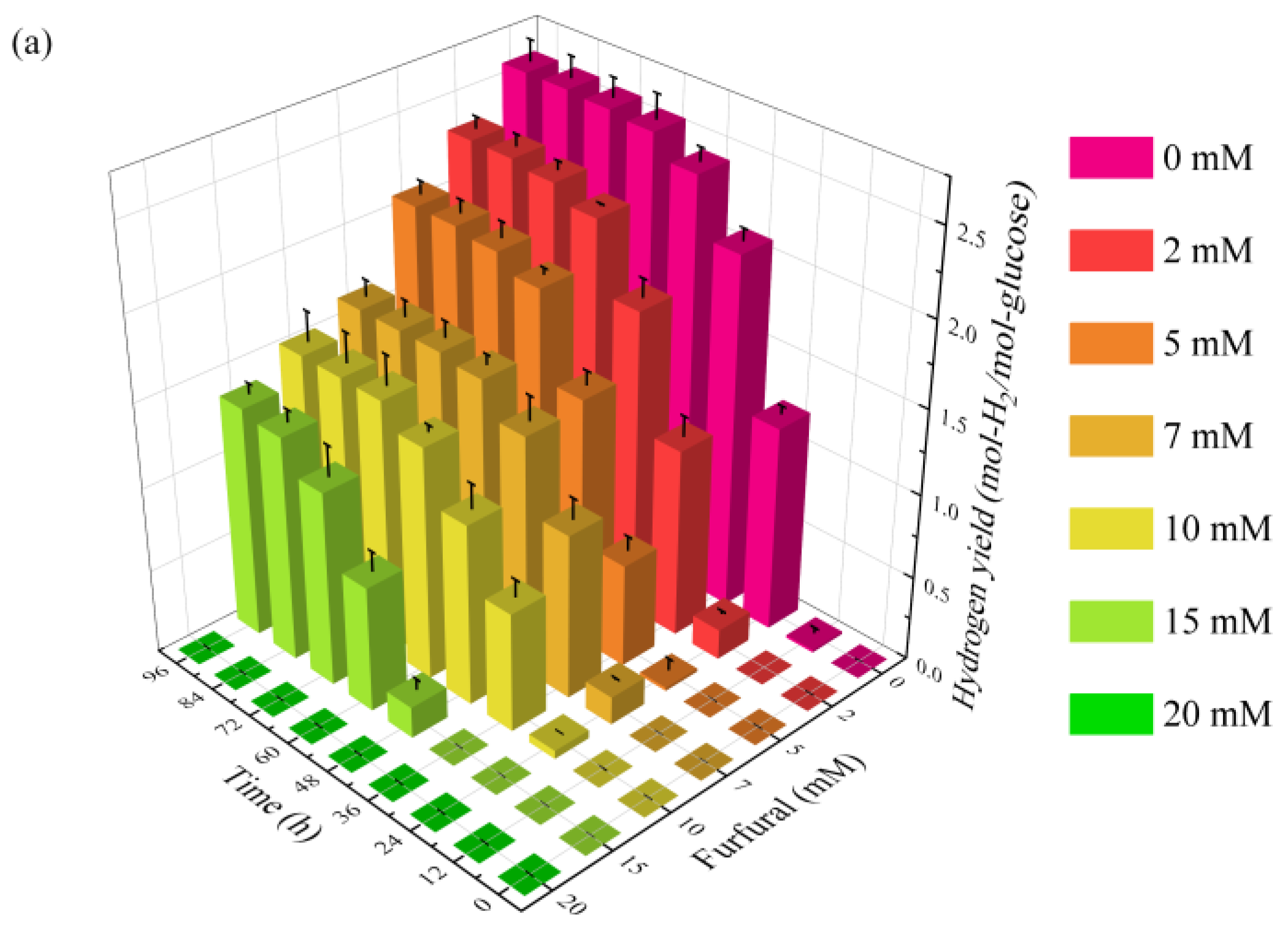
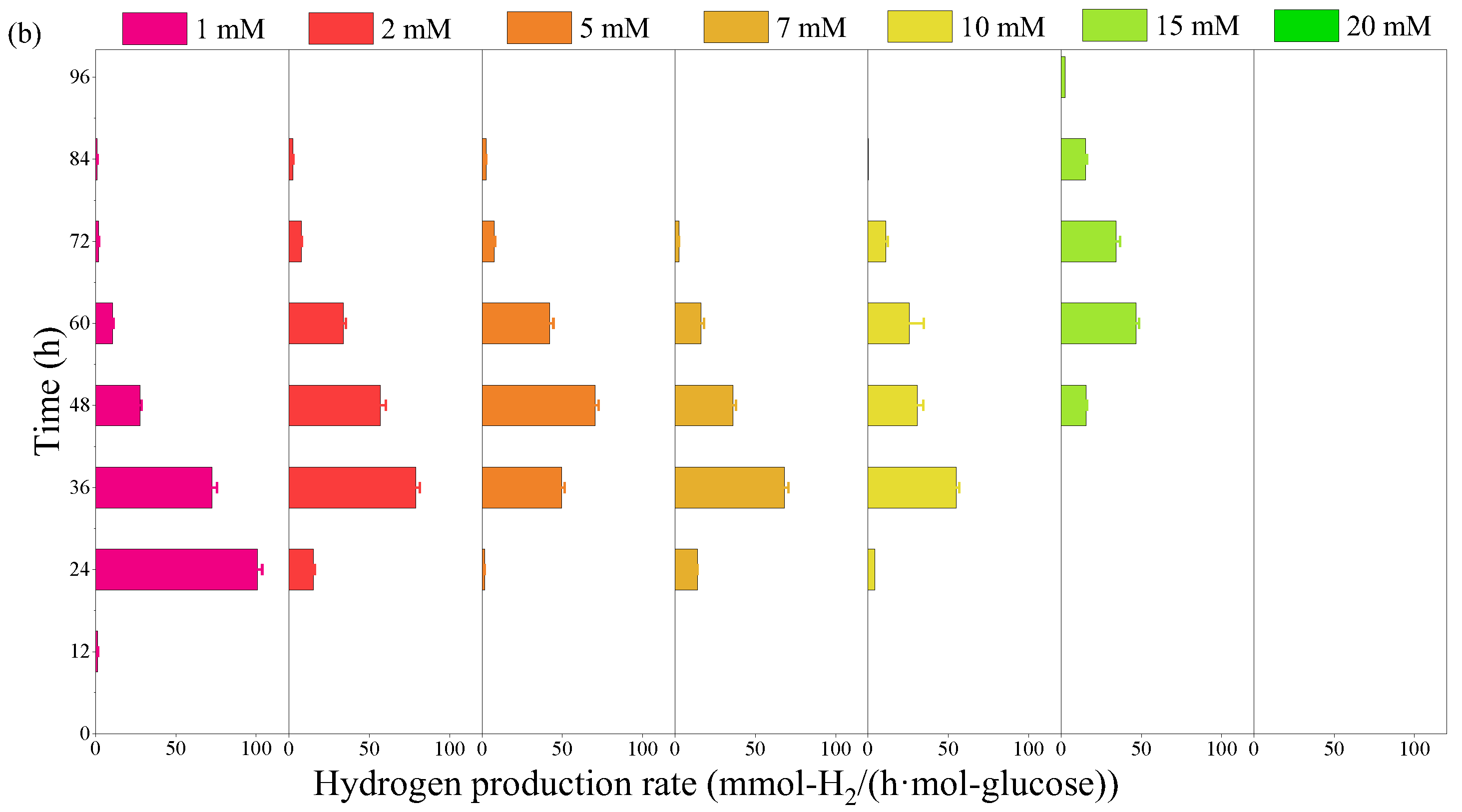
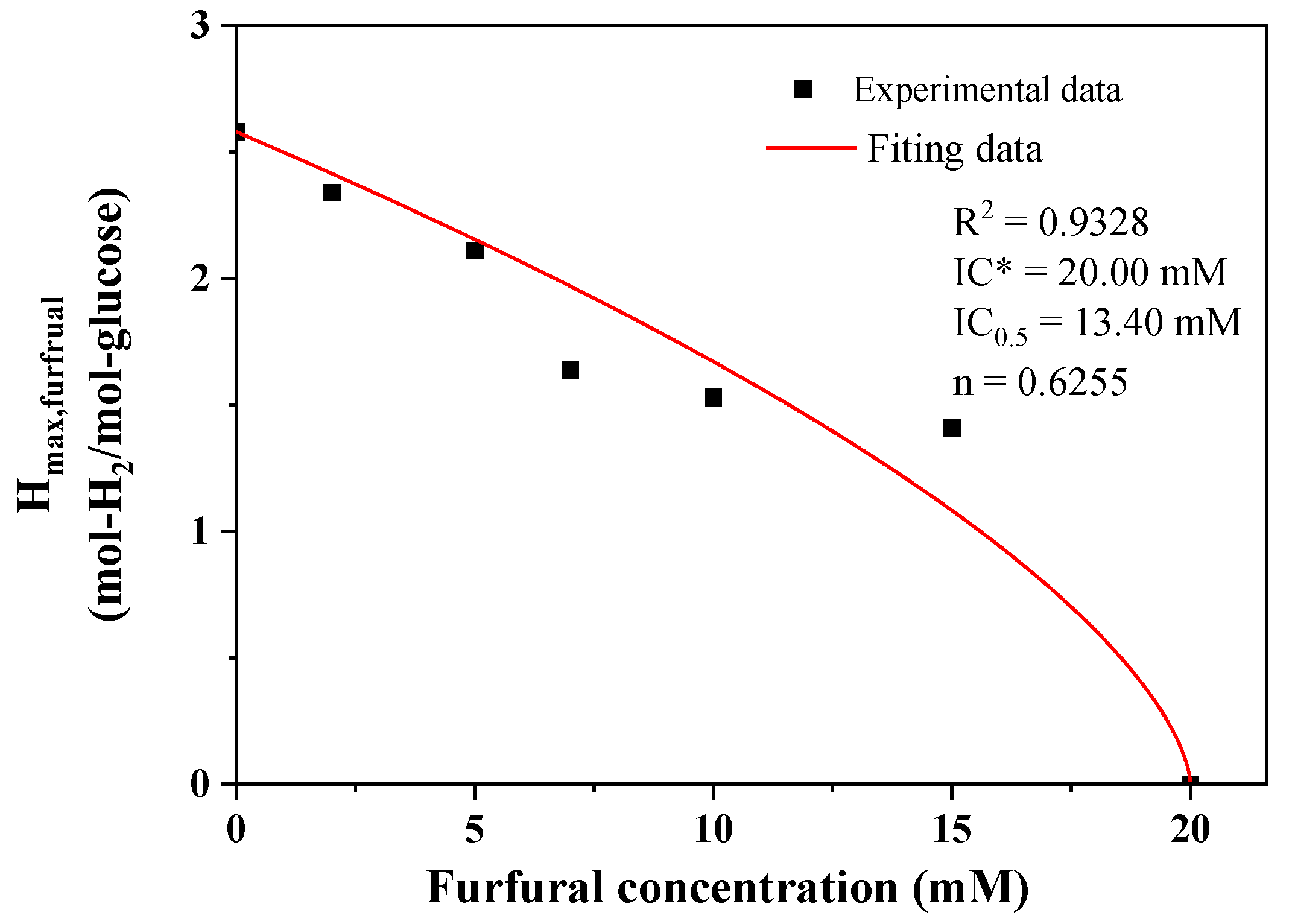
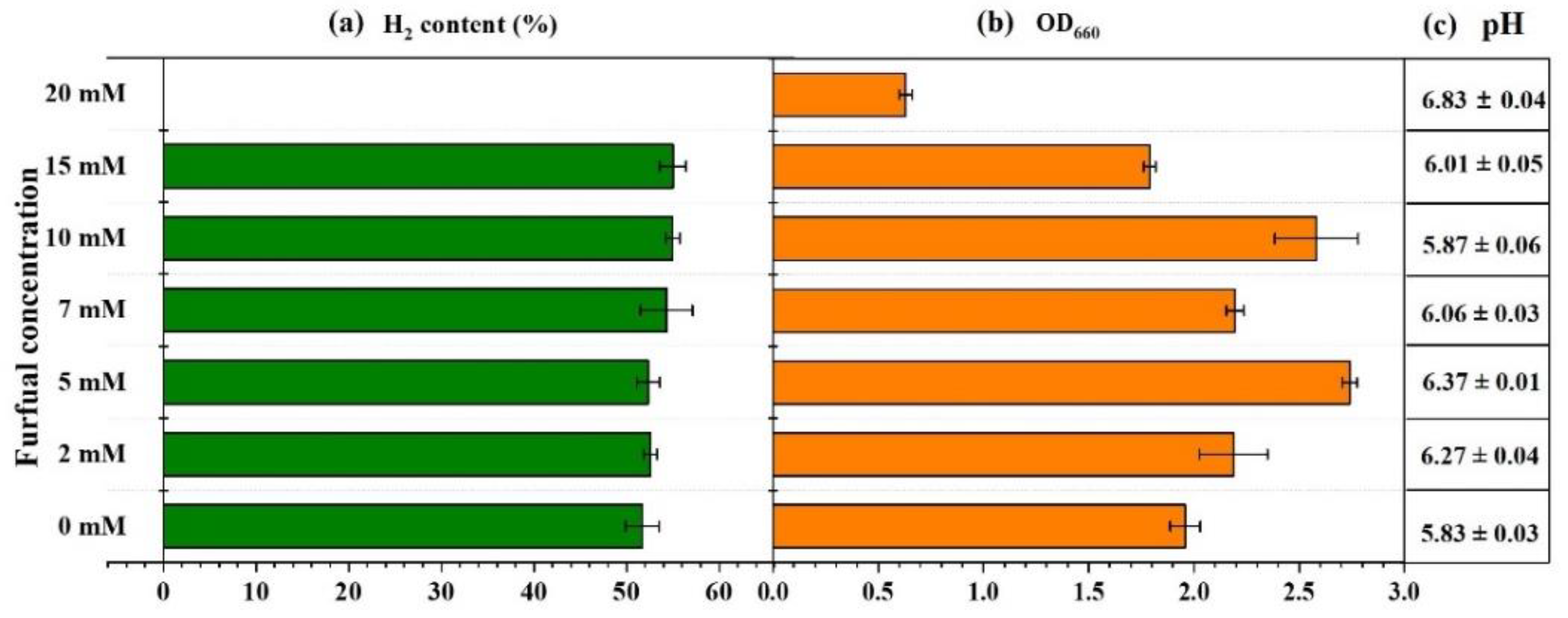
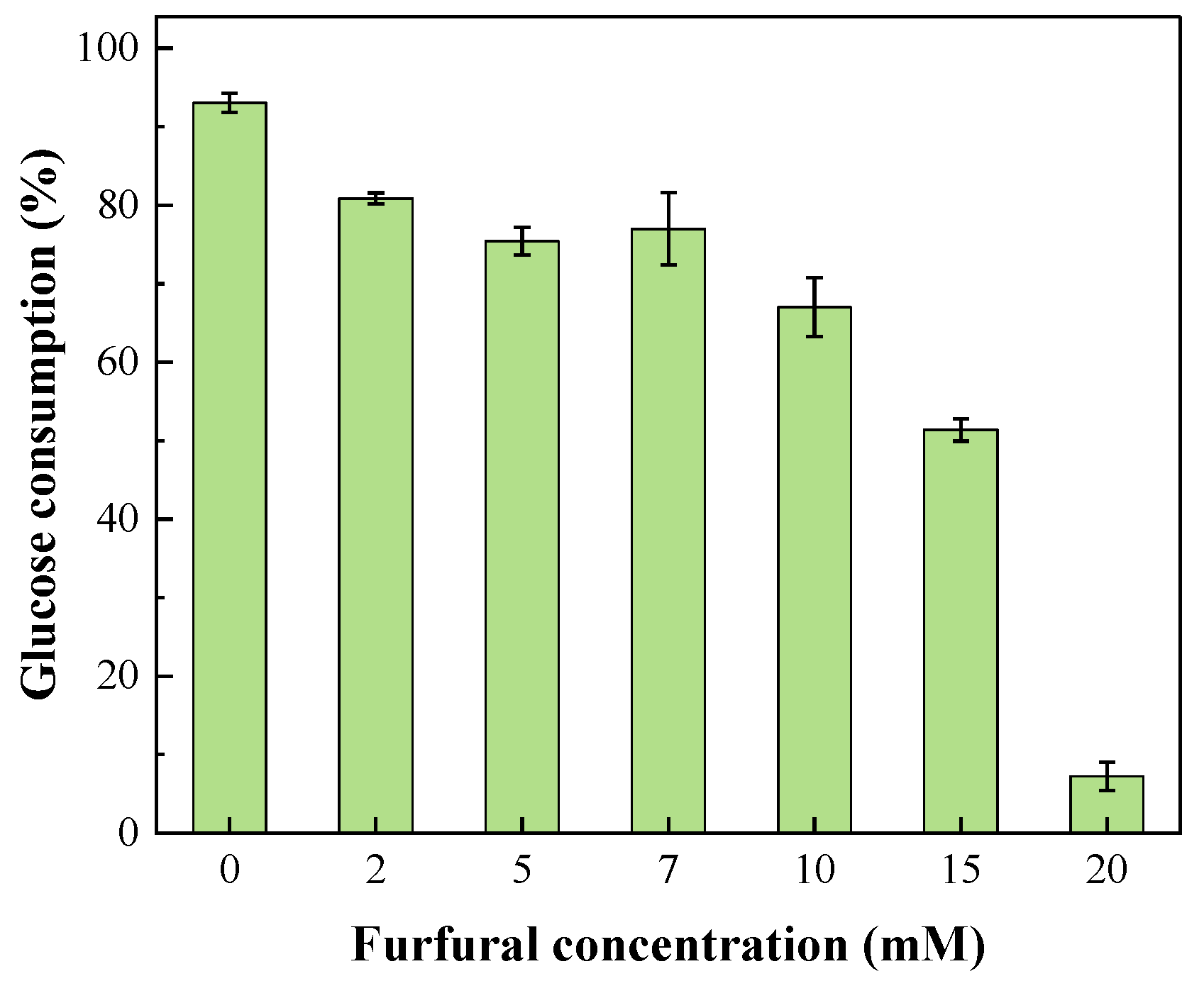
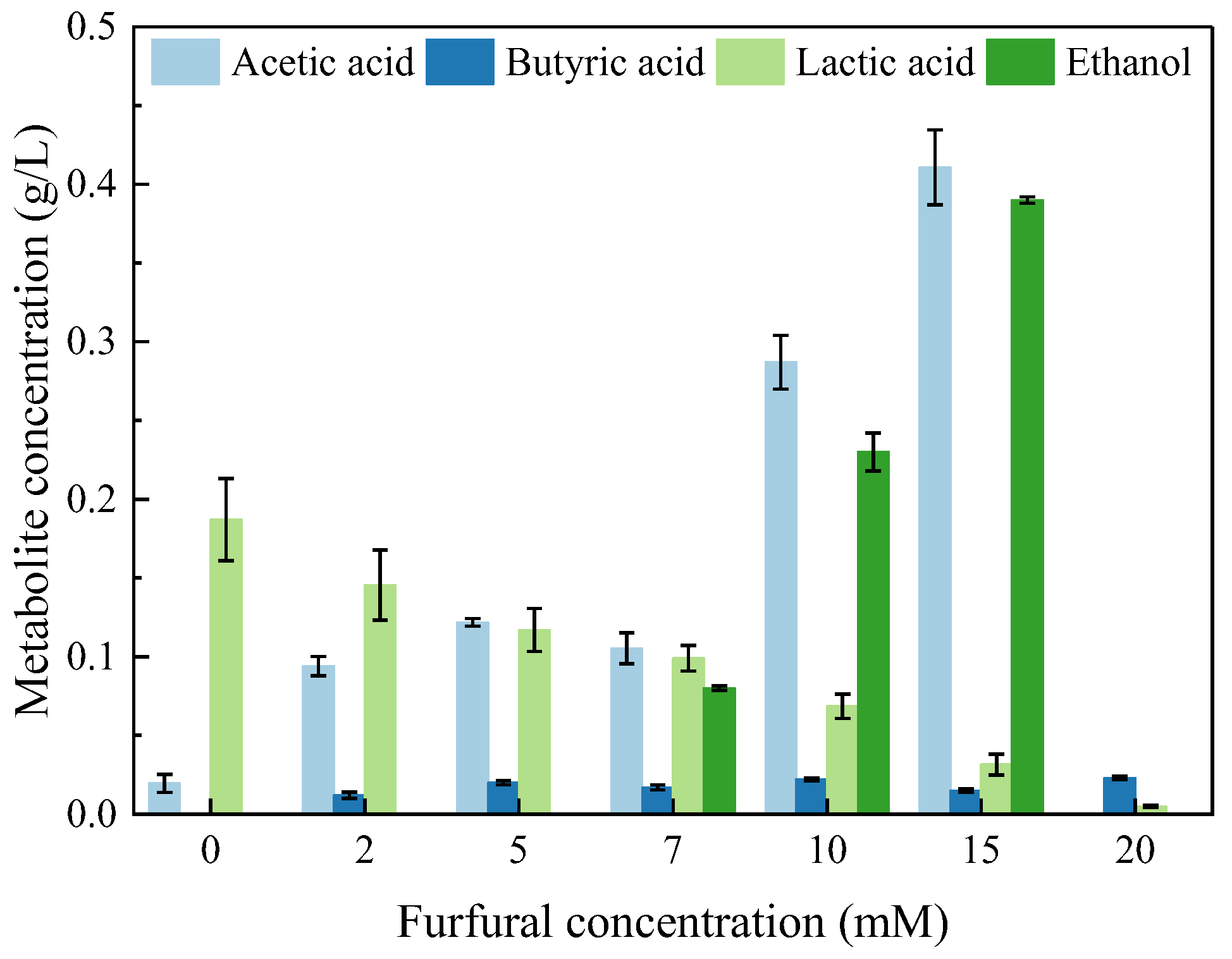
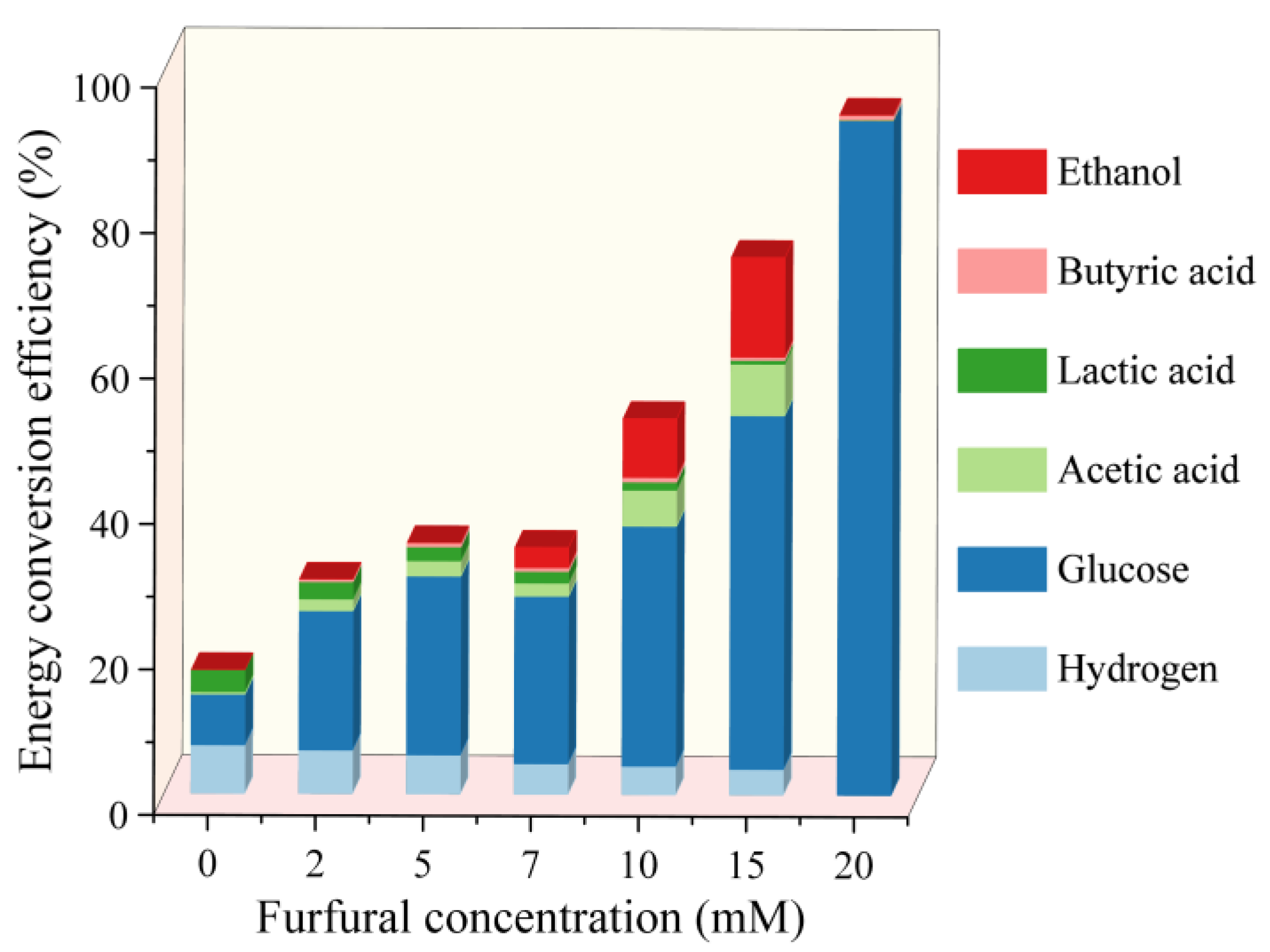
| Furfural Conc. (mM) | Hmax (mol-H2/mol-glucose) | Vmax (mmol-H2/(h·mol-glucose)) | L (h) | R2 |
|---|---|---|---|---|
| 0 | 2.58 ± 0.02 | 113.69 ± 7.02 | 13.64 ± 0.77 | 0.9983 |
| 2 | 2.34 ± 0.02 | 84.34 ± 2.94 | 22.99 ± 0.51 | 0.9993 |
| 5 | 2.11 ± 0.02 | 83.16 ± 3.32 | 28.78 ± 0.51 | 0.9993 |
| 7 | 1.64 ± 0.01 | 72.19 ± 2.18 | 22.35 ± 0.37 | 0.9995 |
| 10 | 1.53 ± 0.03 | 51.39 ± 4.83 | 23.56 ± 1.46 | 0.9949 |
| 15 | 1.41 ± 0.01 | 50.78 ± 0.83 | 45.09 ± 0.23 | 0.9998 |
| 20 | 0 | 0 | ∞ | 1.0000 |
| Strains | Substrate | Furfural | Hydrogen Yield | References |
|---|---|---|---|---|
| Photosynthetic consortium | giant reed | 0.2 g/L | 2.99 mol/mol | [25] |
| E. aerogenes ATCC13408 | glucose | 5 mM | 1.56 mol/mol | [39] |
| Rhodobacter sphaeroides HY01 | glucose and xylose | 5 mM | 1.79 mol/mol | [26] |
| Hydrogen producing bacteria | glucose | 1 g/L | 2 mol/mol | [40] |
| T. thermosaccharolyticum MJ1 | cellobiose | 2 g/L | 2.69 mol/mol | [41] |
| Rhodobacter capsulatus SB1003 | glucose | 2 mM | 2.34 mol/mol | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Wei, X.; Jiang, Y.; Feng, J.; Gao, Z.; Tang, C. Furfural Influences Hydrogen Evolution and Energy Conversion in Photo-Fermentation by Rhodobacter capsulatus. Catalysts 2022, 12, 979. https://doi.org/10.3390/catal12090979
Cao W, Wei X, Jiang Y, Feng J, Gao Z, Tang C. Furfural Influences Hydrogen Evolution and Energy Conversion in Photo-Fermentation by Rhodobacter capsulatus. Catalysts. 2022; 12(9):979. https://doi.org/10.3390/catal12090979
Chicago/Turabian StyleCao, Wen, Xuan Wei, Youmin Jiang, Jiali Feng, Zixuan Gao, and Canfang Tang. 2022. "Furfural Influences Hydrogen Evolution and Energy Conversion in Photo-Fermentation by Rhodobacter capsulatus" Catalysts 12, no. 9: 979. https://doi.org/10.3390/catal12090979
APA StyleCao, W., Wei, X., Jiang, Y., Feng, J., Gao, Z., & Tang, C. (2022). Furfural Influences Hydrogen Evolution and Energy Conversion in Photo-Fermentation by Rhodobacter capsulatus. Catalysts, 12(9), 979. https://doi.org/10.3390/catal12090979