Production of Propanediols through In Situ Glycerol Hydrogenolysis via Aqueous Phase Reforming: A Review
Abstract
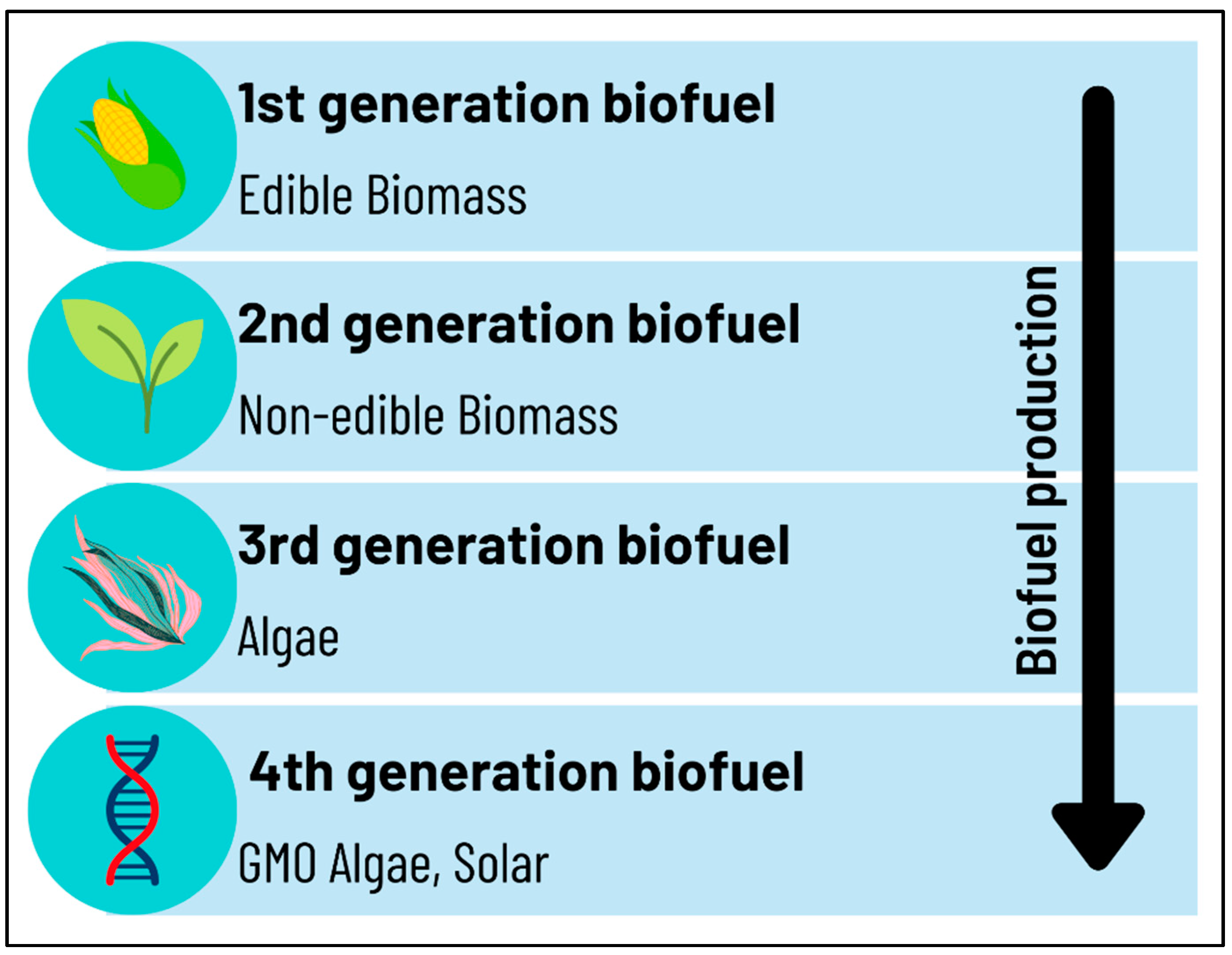
1. Introduction
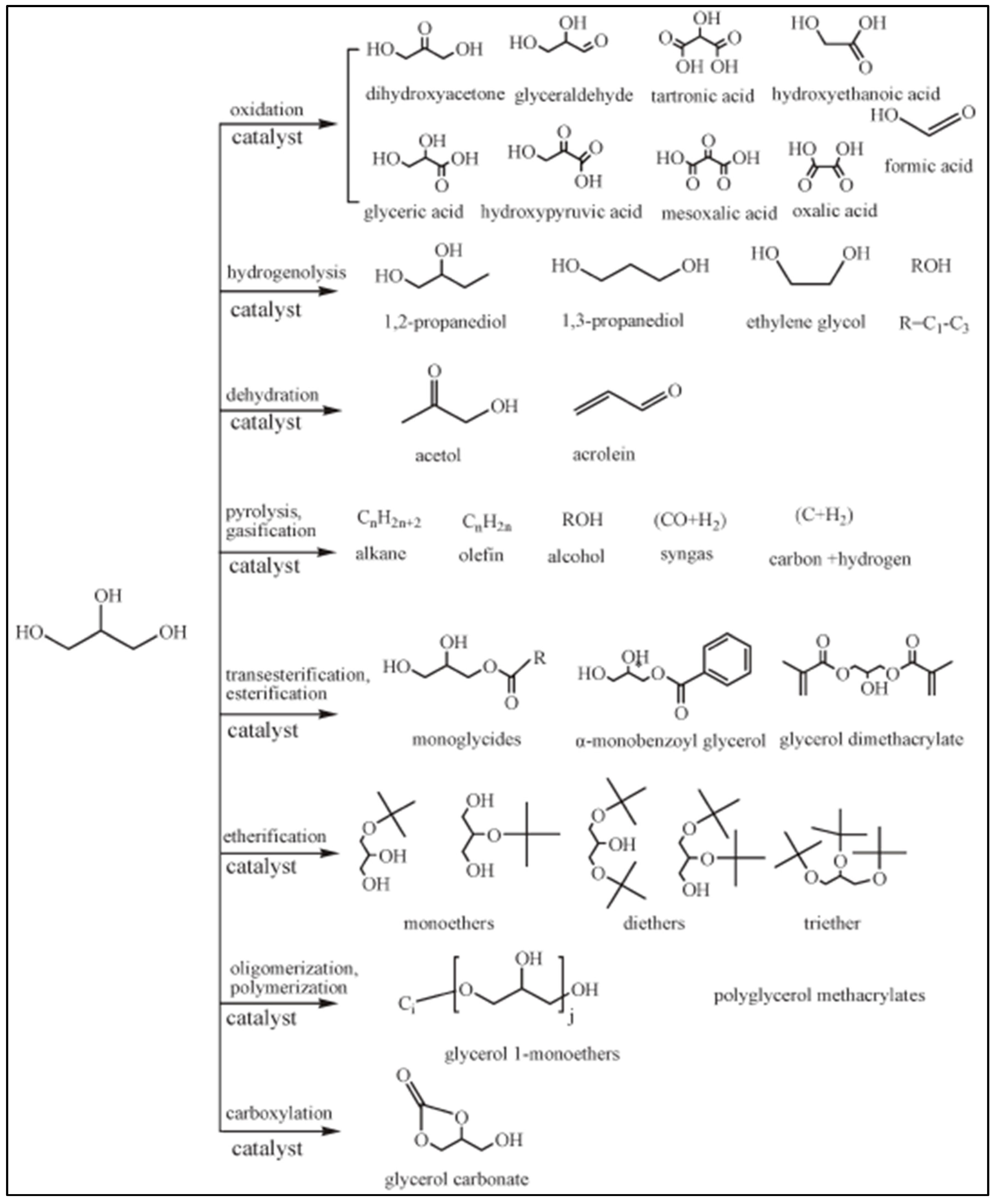
2. Glycerol as the Feedstock for Production of 1,2-Propanediol and 1,3-Propanediol
2.1. Glycerol
2.2. 1,2-Propanediol and 1,3-Propanediol
3. Aqueous Phase Reforming as the Technology of Choice for In Situ Glycerol Hydrogenolysis
Aqueous Phase Reforming of Glycerol: Hydrogen Gas vs. Liquid Products
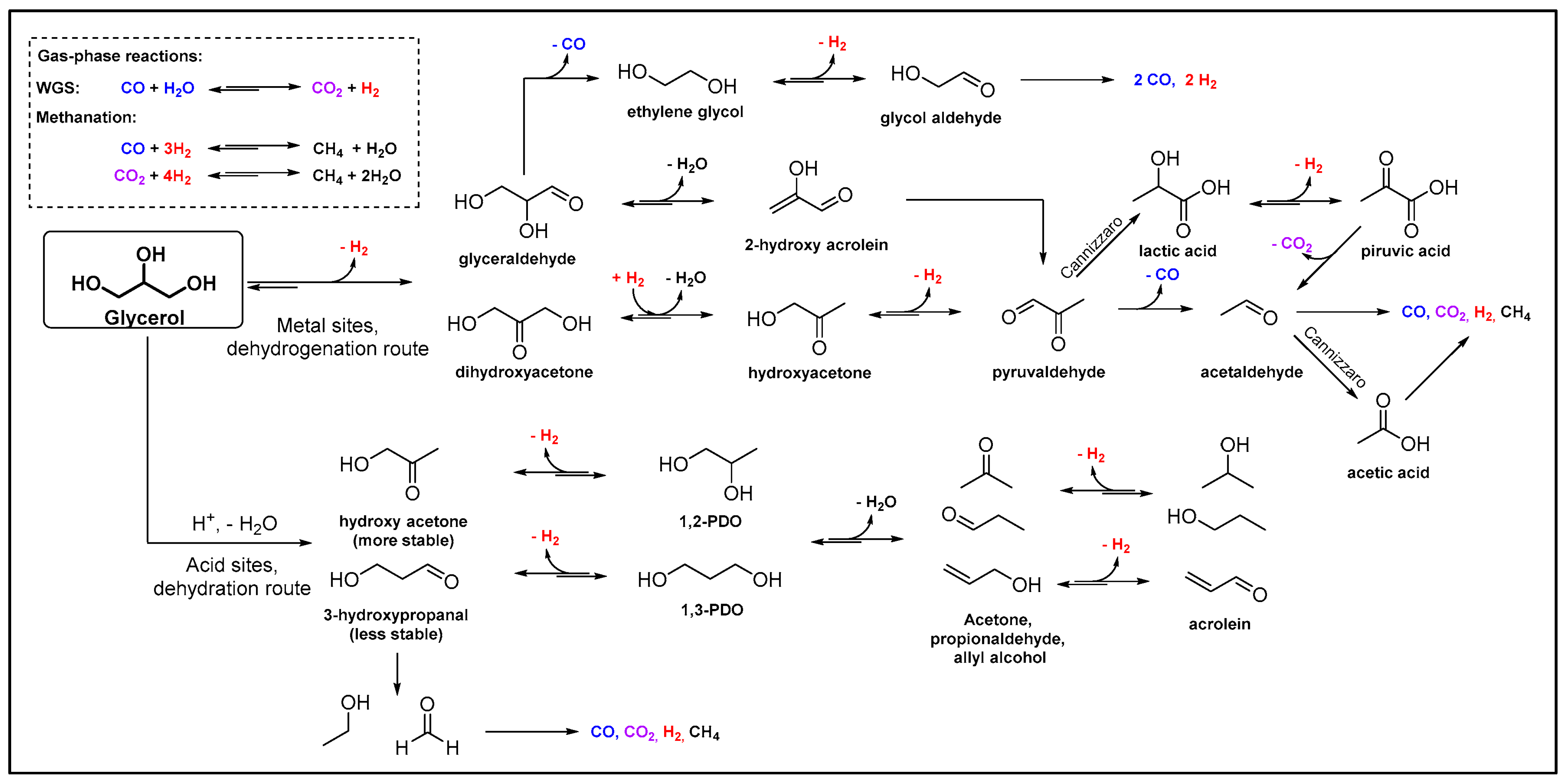
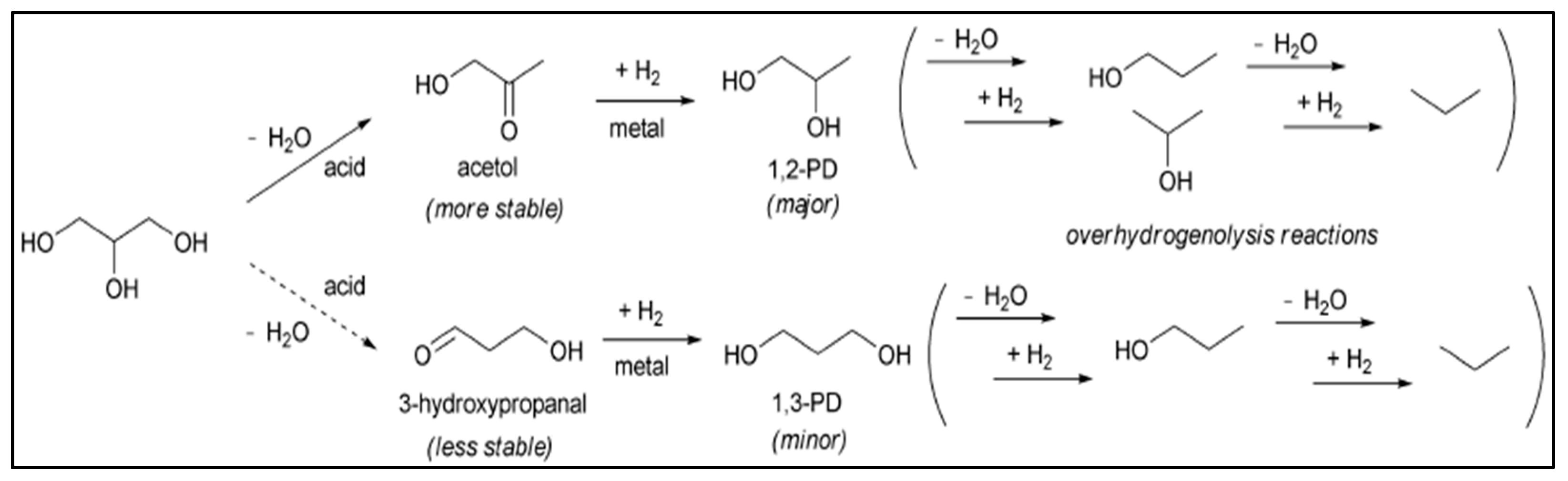
4. Production of Propanediols via Glycerol Hydrogenolysis
| Catalyst | Temp. (°C) | Reactor | X (%) | S a (%) | Y (%) | Ref. |
|---|---|---|---|---|---|---|
| Cu/Dolomite | 200 | Batch | 78.5 | 79 | Not reported | Azri, et al. [111] |
| Cu/Al2O3 | 28 | 66.8 | Not reported | |||
| Cu/Bentonite | 68.8 | 14.6 | Not reported | |||
| Cu/Al2O3 | 220 | Batch | 55.5 | 91.7 | 66.1 | Kunthakudee, et al. [109] |
| Cu/SBA-15 | 230 | Batch | 90.3 | 97.3 | Not reported | Shan, et al. [108] |
| CuMgFe-xLDO | 180 | Batch | 47.8 | 97.5 | Not reported | Yu, et al. [112] |
| Co-Ca/Al2O3 | 210 | Batch | 95 | 90 | Not reported | Gong, et al. [113] |
| Ce-Ni/SBA-15 | 200 | Batch | 51 | 29 | 24 | Jiménez-Morales, et al. [114] |
| Ru/Al2O3 | 200 | Batch | 32.8 | 41.7 | Not reported | Soares, et al. [115] |
| Ru/ZrO2 | 30.1 | 69.8 | Not reported | |||
| Ru-Cu/ZrO2 | 45 | 94 | Not reported | |||
| Raney Cu | 225 | Fixed-bed | 100 | 96.3 | Not reported | Tanielyan, et al. [110] |
| Ni/SiO2-C | 260 | Batch | 56 | 84.7 b | 43.3 | Gatti, et al. [116] |
| Pd/m-ZrO2 + ZnO | 220 | Batch | Not reported | 94.1 c | Not reported | Sun, et al. [117] |
| PtSn | 200 | Batch | 16 | 84 | 13 | Barbelli, et al. [118] |
5. Production of Propanediols via In Situ Glycerol Hydrogenolysis on Aqueous Phase Reforming of Glycerol
6. Discussions, Recommendations on Production of Propanediols via In-Situ Glycerol Hydrogenolysis via Aqueous Phase Reforming
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, F.; Hanna, M.A. Biodiesel Production: A Review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Shay, E.G. Diesel Fuel from Vegetable Oils: Status and Opportunities. Biomass Bioenergy 1993, 4, 227–242. [Google Scholar] [CrossRef]
- Albers, S.C.; Berklund, A.M.; Graff, G. The rise and fall of innovation in biofuels. Nat. Biotechnol. 2016, 34, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowska, J.R. Chapter 1—Biofuels Technologies: An Overview of Feedstocks, Processes, and Technologies. In Biofuels for a More Sustainable Future; Ren, J., Scipioni, A., Manzardo, A., Liang, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–19. [Google Scholar]
- Ziolkowska, J.R.; Simon, L. Recent Developments and Prospects for Algae-Based Fuels in the US. Renew. Sustain. Energy Rev. 2014, 29, 847–853. [Google Scholar] [CrossRef]
- Abdullah, B.; Muhammad, S.A.F.S.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.A. Fourth generation biofuel: A review on risks and mitigation strategies. Renew. Sustain. Energy Rev. 2019, 107, 37–50. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Bellière-Baca, V.; Rey, P.; Dumeignil, F. Glycerol dehydration to acrolein in the context of new uses of glycerol. Green Chem. 2010, 12, 2079–2098. [Google Scholar] [CrossRef]
- Grand View Research. Biodiesel Market Analysis by Feedstock [Vegetable Oils (Canola Oil, Soybean Oil, Palm Oil, Corn Oil), Animal Fats (Poultry, Tallow, White Grease)], by Application (Fuel, Power Generation), and Segment Forecasts, 2018–2025. Available online: https://www.grandviewresearch.com/Industry-Analysis/Biodiesel-Market (accessed on 28 July 2022).
- Inkwood Research. North America Biofuels & Biodiesel Market Forecast 2018–2026. Available online: https://www.inkwoodresearch.com/Reports/North-America-Biofuels-Biodiesel-Market/ (accessed on 2 October 2021).
- Inkwood Research. Europe Biofuels & Biodiesel Market Forecast 2018–2026. Available online: https://www.inkwoodresearch.com/Reports/Europe-Biofuels-Biodiesel-Market/ (accessed on 2 October 2021).
- Gholami, Z.; Abdullah, A.Z.; Lee, K.-T. Dealing with the surplus of glycerol production from biodiesel industry through catalytic upgrading to polyglycerols and other value-added products. Renew. Sustain. Energy Rev. 2014, 39, 327–341. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. Production of glycerol carbonate using a novel Ti-SBA-15 catalyst. Chem. Eng. J. 2018, 346, 477–488. [Google Scholar] [CrossRef]
- Chong, C.C.; Aqsha, A.; Ayoub, M.; Sajid, M.; Abdullah, A.Z.; Yusup, S.; Abdullah, B. A review over the role of catalysts for selective short-chain polyglycerol production from biodiesel derived waste glycerol. Environ. Technol. Innov. 2020, 19, 100859. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-Valerolactone, A Sustainable Platform Molecule Derived from Lignocellulosic Biomass. Green Chem. 2013, 15, 584–595. [Google Scholar] [CrossRef]
- Mordor Intelligents. 1,3-Propanediol Market Growths, Trends, And Forecast 2020–2025. Available online: https://www.mordorintelligence.com/Industry-Reports/1-3-Propanediol-Pdo-Market (accessed on 21 July 2022).
- Wang, S.; Wang, J.; Okoye, P.U.; Chen, S.; Li, X.; Duan, L.; Zhou, H.; Li, S.; Tang, T.; Zhang, L.; et al. Application of corncob residue-derived catalyst in the transesterification of glycerol with dimethyl carbonate to synthesize glycerol carbonate. BioResources 2019, 15, 17. [Google Scholar] [CrossRef]
- Tabah, B.; Varvak, A.; Pulidindi, I.N.; Foran, E.; Banin, E.; Gedanken, A. Production of 1,3-propanediol from glycerol via fermentation by Saccharomyces cerevisiae. Green Chem. 2016, 18, 4657–4666. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pina, C.D.; Rossi, M.; Pagliaro, M. Understanding the Glycerol Market. Eur. J. Lipid Sci. 2014, 116, 1432–1439. [Google Scholar] [CrossRef]
- Beroe Inc. Glycerin Market Intelligence. Available online: https://www.beroeinc.com/Category-Intelligence/Glycerin-Market/ (accessed on 27 July 2022).
- Abdurahman, M.H.; Yaacob, M.H.; Basir, N.I.; Abdullah, A.Z. Selective Transformation of Glycerol to Lactic Acid by Porous Multifunctional Mixed Oxide Catalysts under Alkaline Environment. In Applications of Nanotechnology for Green Synthesis; Inamuddin, Abdullah, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 223–245. [Google Scholar]
- Wang, F.; Chu, X.; Zhao, P.; Zhu, F.; Li, Q.; Wu, F.; Xiao, G. Shape selectivity conversion of biomass derived glycerol to aromatics over hierarchical HZSM-5 zeolites prepared by successive steaming and alkaline leaching: Impact of acid properties and pore constraint. Fuel 2020, 262, 116538. [Google Scholar] [CrossRef]
- Din, N.S.M.N.M.; Idris, Z.; Kian, Y.S.; Hassan, H. Preparation of Polyglycerol from Palm-Biodiesel Crude Glycerin. J. Oil Palm Res. 2013, 25, 289–297. [Google Scholar]
- Choi, H.-J.; Yu, S.-W. Influence of Crude Glycerol on the Biomass and Lipid Content of Microalgae. Biotechnol. Biotechnol. Equip. 2015, 29, 506–513. [Google Scholar] [CrossRef]
- Pyle, D.J.; Garcia, R.A.; Wen, Z. Producing Docosahexaenoic Acid (Dha)-Rich Algae from Biodiesel-Derived Crude Glycerol: Effects of Impurities on Dha Production and Algal Biomass Composition. J. Agric. Food Chem. 2008, 56, 3933–3939. [Google Scholar] [CrossRef]
- Kong, W.B.; Yang, H.; Cao, Y.T.; Song, H.; Hua, S.F.; Xia, C.G. Effect of Glycerol and Glucose on the Enhancement of Biomass, Lipid and Soluble Carbohydrate Production by Chlorella Vulgaris in Mixotrophic Culture. Food Technol. Biotechnol. 2013, 51, 62. [Google Scholar]
- Sandesh, K.; Ujwal, P. Trends and Perspectives of Liquid Biofuel—Process and Industrial Viability. Energy Convers. Manag. X 2021, 10, 100075. [Google Scholar] [CrossRef]
- Keita, V.M.; Gonzalez-Villanueva, M.; Wong, T.S.; Tee, K.L. Microbial Utilization of Glycerol for Biomanufacturing. In Engineering of Microbial Biosynthetic Pathways; Singh, V., Singh, A.K., Bhargava, P., Joshi, M., Joshi, C.G., Eds.; Springer: Singapore, 2020; pp. 245–302. [Google Scholar]
- Thompson, J.C.; He, B.B. Characterization of Crude Glycerol from Biodiesel Production from Multiple Feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Beltramini, J.N.; Fan, Y.-X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef]
- Liu, C.; Hirohara, M.; Maekawa, T.; Chang, R.; Hayashi, T.; Chiang, C.-Y. Selective electro-oxidation of glycerol to dihydroxyacetone by a non-precious electrocatalyst—CuO. Appl. Catal. B Environ. 2019, 265, 118543. [Google Scholar] [CrossRef]
- Choi, Y.-B.; Nunotani, N.; Imanaka, N. Glyceraldehyde Production from Glycerol over Pt/CeO2-Zro2-Fe2O3/Sba-16 Catalysts Around Room Temperature in Open Air System. Mater. Lett. 2020, 278, 128392. [Google Scholar] [CrossRef]
- Cai, J.; Ma, H.; Zhang, J.; Du, Z.; Huang, Y.; Gao, J.; Xu, J. Catalytic oxidation of glycerol to tartronic acid over Au/HY catalyst under mild conditions. Chin. J. Catal. 2014, 35, 1653–1660. [Google Scholar] [CrossRef]
- Yan, H.; Yao, S.; Liang, W.; Zhao, S.; Jin, X.; Feng, X.; Liu, Y.; Chen, X.; Yang, C. Ni–Co oxide catalysts with lattice distortions for enhanced oxidation of glycerol to glyceric acid. J. Catal. 2020, 381, 248–260. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Wang, A. Selective Hydrogenolysis of Glycerol to 1, 3-Propanediol over Pt-W Based Catalysts. Chin. J. Catal. 2020, 41, 1311–1319. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, G.; Wei, M. Ptin Alloy Catalysts Towards Selective Hydrogenolysis Of Glycerol To 1, 2-Propanediol. Ind. Eng. Chem. Res. 2020, 59, 12999–13006. [Google Scholar] [CrossRef]
- Kinage, A.K.; Upare, P.P.; Kasinathan, P.; Hwang, Y.K.; Chang, J.-S. Selective conversion of glycerol to acetol over sodium-doped metal oxide catalysts. Catal. Commun. 2010, 11, 620–623. [Google Scholar] [CrossRef]
- Ma, T.; Ding, J.; Liu, X.; Chen, G.; Zheng, J. Gas-phase dehydration of glycerol to acrolein over different metal phosphate catalysts. Korean J. Chem. Eng. 2020, 37, 955–960. [Google Scholar] [CrossRef]
- Shahirah, M.N.N.; Gimbun, J.; Lam, S.S.; Ng, Y.H.; Cheng, C.K. Synthesis and Characterization of a Lani/A-Al2O3 Catalyst and Its Use in Pyrolysis of Glycerol to Syngas. Renew. Energy 2019, 132, 1389–1401. [Google Scholar] [CrossRef]
- Almeida, A.; Pilão, R.; Ramalho, E.; Ribeiro, A.; Pinho, C. Co-gasification of glycerol/fat mixtures in a downflow fixed bed reactor: Preliminary results. Energy Rep. 2020, 6, 460–465. [Google Scholar] [CrossRef]
- Riascos, L.D.R.; Sanabria, A.E.R.; Rodríguez, G.A.T.; Sachse, A.; Muñoz, C.D.M. Sulfonated Reduced Graphene Oxide: An Acid Catalyst that Efficiently Promotes the Esterification of Glycerol. Top. Catal. 2022, 65, 957–965. [Google Scholar] [CrossRef]
- Khumho, R.; Tocuweang, K.; Sangkhum, P.; Kuchonthara, P.; Ashokkumar, V.; Ngamcharussrivichai, C. Etherification of glycerol into short-chain polyglycerols over MgAl LDH/CaCO3 nanocomposites as heterogeneous catalysts to promote circular bioeconomy. Chemosphere 2022, 291, 133091. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.; Altino, H.; Soares, R. Oxidative Dehydration of Glycerol over Molybdenum-and Vanadium-Based Catalysts. J. Braz. Chem. Soc. 2019, 30, 1025–1033. [Google Scholar] [CrossRef]
- Crosse, A.J.; Brady, D.; Zhou, N.; Rumbold, K. Biodiesel’s Trash is a Biorefineries’ Treasure: The Use of “Dirty” Glycerol as an Industrial Fermentation Substrate. World J. Microbiol. Biotechnol. 2020, 36, 2. [Google Scholar] [CrossRef] [PubMed]
- Dasari, M.A.; Kiatsimkul, P.-P.; Sutterlin, W.R.; Suppes, G.J. Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl. Catal. A Gen. 2005, 281, 225–231. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H. Selective Hydrogenolysis of Glycerol to Propylene Glycol on Cu–Zno Catalysts. Catal. Lett. 2007, 117, 62–67. [Google Scholar] [CrossRef]
- Bertrand, P.; Bonnarme, V.; Piccirilli, A.; Ayrault, P.; Lemée, L.; Frapper, G.; Pourchez, J. Physical and Chemical Assessment of 1,3 Propanediol as a Potential Substitute of Propylene Glycol in Refill Liquid for Electronic Cigarettes. Sci. Rep. 2018, 8, 10702. [Google Scholar] [CrossRef]
- Vivek, N.; Pandey, A.; Binod, P. An Efficient Aqueous Two Phase Systems Using Dual Inorganic Electrolytes to Separate 1,3-Propanediol from the Fermented Broth. Bioresour. Technol. 2018, 254, 239–246. [Google Scholar] [CrossRef]
- Hong, Y.K. Purification of 1,3-Propanediol for Production of Polytrimethylene Terephthalate (PTT) from Biomass. Adv. Mater. Res. 2011, 320, 191–195. [Google Scholar] [CrossRef]
- Khatib, S.; Oyama, S. Direct Oxidation of Propylene to Propylene Oxide with Molecular Oxygen: A Review. Catal. Rev. 2015, 57, 306–344. [Google Scholar] [CrossRef]
- Magdouli, S.; Rouissi, T.; Brar, K.; Blais, J.-F. Propylene Glycol: An Industrially Important C3 Platform Chemical. In Platform Chemical Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 77–100. [Google Scholar]
- Tjahjasari, D.; Kaeding, T.; Zeng, A.P. 3.22-1,3-Propanediol and Polytrimethyleneterephthalate. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, MA, USA, 2011; pp. 229–242. [Google Scholar]
- He, L.; Yang, J.; Chen, D. Chapter 6—Hydrogen from Biomass: Advances in Thermochemical Processes. In Renewable Hydrogen Technologies; Gandía, L.M., Arzamendi, G., Diéguez, P.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 111–133. [Google Scholar]
- Vilcocq, L.; Cabiac, A.; Especel, C.; Guillon, E.; Duprez, D. Transformation of Sorbitol to Biofuels by Heterogeneous Catalysis: Chemical and Industrial Considerations. Oil Gas Sci. Technol. Rev. d’IFP Energy Nouv. 2013, 68, 841–860. [Google Scholar] [CrossRef]
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from Catalytic Reforming of Biomass-Derived Hydrocarbons in Liquid Water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, T.; Li, B.; Jiang, T.; Ma, L.; Zhang, X.; Liu, Q. Aqueous phase reforming of sorbitol to bio-gasoline over Ni/HZSM-5 catalysts. Appl. Energy 2012, 97, 509–513. [Google Scholar] [CrossRef]
- Aiouache, F.; McAleer, L.; Gan, Q.; Al-Muhtaseb, A.H.; Ahmad, M.N. Path lumping kinetic model for aqueous phase reforming of sorbitol. Appl. Catal. A Gen. 2013, 466, 240–255. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Chen, F.; Wu, C.; Dong, L.; Vassallo, A.; Williams, P.T.; Huang, J. Characteristics and Catalytic Properties of Ni/Caalox Catalyst for Hydrogen-Enriched Syngas Production from Pyrolysis-Steam Reforming of Biomass Sawdust. Appl. Catal. B Environ. 2016, 183, 168–175. [Google Scholar] [CrossRef]
- Wei, Y.; Lei, H.; Liu, Y.; Wang, L.; Zhu, L.; Zhang, X.; Yadavalli, G.; Ahring, B.; Chen, S. Renewable Hydrogen Produced from Different Renewable Feedstock by Aqueous-Phase Reforming Process. J. Sustain. Bioenergy Syst. 2014, 4, 113–127. [Google Scholar] [CrossRef][Green Version]
- El Doukkali, M.; Iriondo, A.; Arias, P.; Requies, J.; Gandarías, I.; Jalowiecki-Duhamel, L.; Dumeignil, F. A comparison of sol–gel and impregnated Pt or/and Ni based γ-alumina catalysts for bioglycerol aqueous phase reforming. Appl. Catal. B Environ. 2012, 125, 516–529. [Google Scholar] [CrossRef]
- Martin, A.; Armbruster, U.; Gandarias, I.; Arias, P.L. Glycerol hydrogenolysis into propanediols using in situ generated hydrogen—A critical review. Eur. J. Lipid Sci. Technol. 2012, 115, 9–27. [Google Scholar] [CrossRef]
- Bindwal, A.B.; Vaidya, P.D. Toward Hydrogen Production from Aqueous Phase Reforming of Polyols on Pt/Al2O3 Catalyst. Int. J. Hydrogen Energy 2015, 41, 6085–6093. [Google Scholar] [CrossRef]
- d’Angelo, M.N.; Ordomsky, V.; Van Der Schaaf, J.; Schouten, J.C.; Nijhuis, T.A. Continuous Hydrogen Stripping during Aqueous Phase Reforming of Sorbitol in a Washcoated Microchannel Reactor with a Pt–Ru Bimetallic Catalyst. Int. J. Hydrogen Energy 2014, 39, 18069–18076. [Google Scholar]
- Kirilin, A.V.; Tokarev, A.V.; Kustov, L.; Salmi, T.; Mikkola, J.-P.; Murzin, D.Y. Aqueous phase reforming of xylitol and sorbitol: Comparison and influence of substrate structure. Appl. Catal. A Gen. 2012, 435–436, 172–180. [Google Scholar] [CrossRef]
- Sepúlveda, J.; Manuale, D.; Santiago, L.; Carrara, N.; Torres, G.; Vera, C.; Goncalves, M.; Carvalho, W.; Mandelli, D. Selective Hydrogenolysis of Glycerol to Propylene Glycol in a Continuous Flow Trickle Bed Reactor Using Copper Chromite and Cu/Al2O3 Catalysts. Química Nova 2017, 40, 371–377. [Google Scholar]
- Lehnert, K.; Claus, P. Influence of Pt Particle Size and Support Type on The Aqueous-Phase Reforming of Glycerol. Catal. Commun. 2008, 9, 2543–2546. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a Support for Catalysts: A Review of Fundamental Aspects. Chemistry 2005, 36, 3393–3403. [Google Scholar]
- Chen, Z.; Kukushkin, R.G.; Yeletsky, P.M.; Saraev, A.A.; Bulavchenko, O.A.; Millan, M. Coupling Hydrogenation of Guaiacol with in situ Hydrogen Production by Glycerol Aqueous Reforming over Ni/Al2O3 and Ni-X/Al2O3 (X= Cu, Mo, P) Catalysts. Nanomaterials 2020, 10, 1420. [Google Scholar] [CrossRef]
- King, D.L.; Zhang, L.; Xia, G.; Karim, A.M.; Heldebrant, D.J.; Wang, X.; Peterson, T.; Wang, Y. Aqueous phase reforming of glycerol for hydrogen production over Pt–Re supported on carbon. Appl. Catal. B Environ. 2010, 99, 206–213. [Google Scholar] [CrossRef]
- Buffoni, I.N.; Pompeo, F.; Santori, G.F.; Nichio, N.N. Nickel catalysts applied in steam reforming of glycerol for hydrogen production. Catal. Commun. 2009, 10, 1656–1660. [Google Scholar] [CrossRef]
- IIriondo, A.; Cambra, J.F.; Güemez, M.B.; Barrio, V.L.; Requies, J.; Sánchez-Sánchez, M.C.; Navarro, R.M. Effect of Zro2 Addition on Ni/Al2O3 Catalyst to Produce H2 from Glycerol. Int. J. Hydrogen Energy 2012, 37, 7084–7093. [Google Scholar] [CrossRef]
- Chakinala, A.G.; van Swaaij, W.P.; Kersten, S.R.; de Vlieger, D.; Seshan, K.; Brilman, D.W.F. Catalytic Reforming of Glycerol in Supercritical Water over Bimetallic Pt–Ni Catalyst. Ind. Eng. Chem. Res. 2013, 52, 5302–5312. [Google Scholar] [CrossRef]
- Reynoso, A.; Iriarte-Velasco, U.; Gutiérrez-Ortiz, M.; Ayastuy, J. Highly Stable Pt/Coal2O4 Catalysts in Aqueous-Phase Reforming of Glycerol. Catal. Today 2020, 367, 278–289. [Google Scholar] [CrossRef]
- Gogoi, P.; Kanna, N.; Begum, P.; Deka, R.C.; Satyanarayana, C.V.V.; Raja, T. Controlling and Stabilization of Ru Nanoparticles by Tuning the Nitrogen Content of the Support for Enhanced H2 Production through Aqueous-Phase Reforming of Glycerol. ACS Catal. 2019, 10, 2489–2507. [Google Scholar] [CrossRef]
- Rahman, M.M. Aqueous-Phase Reforming of Glycerol over Carbon-Nanotube-Supported Catalysts. Catal. Lett. 2020, 150, 2674–2687. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Wang, Y. Catalytic and Drifts Studies of Pt-Based Bimetallic Alloy Catalysts in Aqueous-Phase Reforming of Glycerol. Ind. Eng. Chem. Res. 2019, 58, 2749–2758. [Google Scholar] [CrossRef]
- Hemings, E.B.; Cavallotti, C.; Cuoci, A.; Faravelli, T.; Ranzi, E. A Detailed Kinetic Study of Pyrolysis and Oxidation of Glycerol (Propane-1,2,3-triol). Combust. Sci. Technol. 2012, 184, 1164–1178. [Google Scholar] [CrossRef]
- Shahirah, M.N.N.; Gimbun, J.; Ideris, A.; Khan, M.R.; Cheng, C.K. Catalytic pyrolysis of glycerol into syngas over ceria-promoted Ni/α-Al2O3 catalyst. Renew. Energy 2017, 107, 223–234. [Google Scholar] [CrossRef]
- May, A.; Salvadó, J.; Torras, C.; Montané, D. Catalytic gasification of glycerol in supercritical water. Chem. Eng. J. 2010, 160, 751–759. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; Zhang, X.; Jin, H.; Lu, Y. Hydrogen production from coal gasification in supercritical water with a continuous flowing system. Int. J. Hydrogen Energy 2009, 35, 3036–3045. [Google Scholar] [CrossRef]
- Siew, K.W.; Lee, H.C.; Gimbun, J.; Cheng, C.K. Production of CO-rich hydrogen gas from glycerol dry reforming over La-promoted Ni/Al2O3 catalyst. Int. J. Hydrogen Energy 2014, 39, 6927–6936. [Google Scholar] [CrossRef]
- Mohd Arif, N.N.; Abidin, Z.N.; Osazuwa, O.U.; Vo, D.-V.N.; Azizan, M.T.; Taufiq-Yap, Y.H. Hydrogen Production via Co2 Dry Reforming of Glycerol over Reni/Cao Catalysts. Int. J. Hydrogen Energy 2019, 44, 20857–20871. [Google Scholar] [CrossRef]
- Rennard, D.C.; Kruger, J.S.; Schmidt, L.D. Autothermal Catalytic Partial Oxidation of Glycerol to Syngas and to Non-Equilibrium Products. ChemSusChem 2009, 2, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Skrzyńska, E.; El Roz, A.; Paul, S.; Capron, M.; Dumeignil, F. Glycerol Partial Oxidation over Pt/Al2O3 Catalysts under Basic and Base-Free Conditions—Effect of the Particle Size. J. Am. Oil Chem. Soc. 2019, 96, 63–74. [Google Scholar] [CrossRef]
- Dauenhauer, P.J.; Salge, J.R.; Schmidt, L.D. Renewable Hydrogen by Autothermal Steam Reforming of Volatile Carbohydrates. J. Catal. 2006, 244, 238–247. [Google Scholar] [CrossRef]
- Swami, S.M.; Abraham, M.A. Integrated Catalytic Process for Conversion of Biomass To Hydrogen. Energy Fuels 2006, 20, 2616–2622. [Google Scholar] [CrossRef]
- Pairojpiriyakul, T.; Croiset, E.; Kiatkittipong, K.; Kiatkittipong, W.; Arpornwichanop, A.; Assabumrungrat, S. Catalytic reforming of glycerol in supercritical water with nickel-based catalysts. Int. J. Hydrogen Energy 2014, 39, 14739–14750. [Google Scholar] [CrossRef]
- Fasolini, A.; Cespi, D.; Tabanelli, T.; Cucciniello, R.; Cavani, F. Hydrogen from Renewables: A Case Study of Glycerol Reforming. Catalysts 2019, 9, 722. [Google Scholar] [CrossRef]
- Ouyang, K.; Huang, Y.; Chen, H.; Li, T.; Cao, F.; Fang, D. Thermodynamic Analysis of Liquid Phase in situ Hydrogenation of Glycerol for 1,3-Propanediol Synthesis. Front. Chem. Sci. Eng. 2011, 5, 67–73. [Google Scholar] [CrossRef]
- Samudrala, S.P. Glycerol Transformation to Value-Added 1, 3-Propanediol Production: A Paradigm for a Sustainable Biorefinery Process. In Glycerine Production and Transformation—An Innovative Platform for Sustainable Biorefinery and Energy; Frediani, M., Bartoli, M., Rosi, L., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Nakagawa, Y.; Tomishige, K. Heterogeneous Catalysis of The Glycerol Hydrogenolysis. Catal. Sci. Technol. 2011, 1, 179–190. [Google Scholar] [CrossRef]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol Hydrogenolysis into Useful C3 Chemicals. Appl. Catal. B Environ. 2016, 193, 75–92. [Google Scholar] [CrossRef]
- Zhou, W.; Luo, J.; Wang, Y.; Liu, J.; Zhao, Y.; Wang, S.; Ma, X. Wox Domain Size, Acid Properties and Mechanistic Aspects of Glycerol Hydrogenolysis over Pt/Wox/ZrO2. Appl. Catal. B Environ. 2019, 242, 410–421. [Google Scholar] [CrossRef]
- Liu, L.; Kawakami, S.; Nakagawa, Y.; Tamura, M.; Tomishige, K. Highly Active Iridium–Rhenium Catalyst Condensed on Silica Support for Hydrogenolysis of Glycerol to 1,3-Propanediol. Appl. Catal. B Environ. 2019, 256, 117775. [Google Scholar] [CrossRef]
- Ruy, A.D.D.S.; Alves, R.M.D.B.; Hewer, T.L.R.; Pontes, D.D.A.; Teixeira, L.S.G.; Pontes, L.A.M. Catalysts for glycerol hydrogenolysis to 1,3-propanediol: A review of chemical routes and market. Catal. Today 2020, 381, 243–253. [Google Scholar]
- Wang, S.; Liu, H. Selective Hydrogenolysis of Glycerol to Propylene Glycol on Hydroxycarbonate-Derived Cu-Zno-Al2O3 Catalysts. Chin. J. Catal. 2014, 35, 631–643. [Google Scholar] [CrossRef]
- Wen, G.; Xu, Y.; Ma, H.; Xu, Z.; Tian, Z. Production of hydrogen by aqueous-phase reforming of glycerol. Int. J. Hydrogen Energy 2008, 33, 6657–6666. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Grand View Research. Green Hydrogen Market Size, Share & Trends Analysis Report by Technology (Pem Electrolyzer, Alkaline Electrolyzer), by Region (North America, Europe, Apac), and Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/Industry-Analysis/Green-Hydrogen-Market (accessed on 25 August 2021).
- Markets And Markets. Propylene Glycol Market by Source (Petroleum-Based, Bio-Based), Grade (Industrial, Pharmaceutical), End-Use Industry (Transportation, Building & Construction, Food & Beverage, Pharmaceuticals, Cosmetics & Personal Care), Region—Global Forecast to 2024. Available online: https://www.marketsandmarkets.com/Market-Reports/Propylene-Glycol-Market-264488864.Html (accessed on 25 August 2021).
- Grand View Research. 1,3 Propanediol (Pdo) Market Size, Share & Trends Analysis Report by Application (Polytrimethylene Terephthalate (Ptt), Polyurethane, Personal Care & Detergents) and Segment Forecasts to 2022. Available online: https://www.grandviewresearch.com/Industry-Analysis/1-3-Propanediol-Pdo-Market (accessed on 4 August 2022).
- Market Watch. Acrolein Market Size, Share 2020, Cagr of 2%, Globally Industry Demand, Trends, Regional Overview, Top Manufacture, Business Growth and Forecast to 2026, Says Industry Research Biz. Available online: https://www.marketwatch.com/Press-Release/Acrolein-Market-Size-Share-2020-Cagr-Of-2-Globally-Industry-Demand-Trends-Regional-Overview-Top-Manufacture-Business-Growth-And-Forecast-To-2026-Says-Industry-Research-Biz-2020-07-12 (accessed on 27 August 2021).
- Grand View Research. Propanol Market Size, Share & Trends Analysis Report by Product (N-Propanol, Isopropyl Alcohol), by Region (North America, Europe, Apac, Csa, Mea), and Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/Industry-Analysis/Propanol-Market (accessed on 27 August 2021).
- Zheng, L.; Xia, S.; Hou, Z. Hydrogenolysis of glycerol over Cu-substituted hydrocalumite mediated catalysts. Appl. Clay Sci. 2015, 118, 68–73. [Google Scholar] [CrossRef]
- Mane, R.B.; Rode, C.V. Simultaneous glycerol dehydration and in situ hydrogenolysis over Cu–Al oxide under an inert atmosphere. Green Chem. 2012, 14, 2780–2789. [Google Scholar] [CrossRef]
- Azri, N.; Ramli, I.; Nda-Umar, U.I.; Shamsuddin, M.R.; Saiman, M.I.; Taufiq-Yap, Y.H. Copper-Dolomite as Effective Catalyst for Glycerol Hydrogenolysis to 1,2-Propanediol. J. Taiwan Inst. Chem. Eng. 2020, 112, 34–51. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, L.; Li, X.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol to 1,2-propanediol over Cu-based catalysts: A short review. Catal. Today 2019, 355, 84–95. [Google Scholar] [CrossRef]
- Shan, J.; Liu, H.; Lu, K.; Zhu, S.; Li, J.; Wang, J.; Fan, W. Identification of the Dehydration Active Sites in Glycerol Hydrogenolysis to 1,2-Propanediol over Cu/SiO2 Catalysts. J. Catal. 2020, 383, 13–23. [Google Scholar] [CrossRef]
- Kunthakudee, N.; Khemthong, P.; Luadthong, C.; Panpranot, J.; Mekasuwandumrong, O.; Witoon, T.; Faungnawakij, K. CuAl2O4–CuO–Al2O3 Catalysts Prepared by Flame-Spray Pyrolysis for Glycerol Hydrogenolysis. Mol. Catal. 2021, 523, 111426. [Google Scholar] [CrossRef]
- Tanielyan, S.K.; Marin, N.; Alvez, G.; Bhagat, R.; Miryala, B.; Augustine, R.L.; Schmidt, S.R. An Efficient, Selective Process for the Conversion of Glycerol to Propylene Glycol Using Fixed Bed Raney Copper Catalysts. Org. Process Res. Dev. 2013, 18, 1419–1426. [Google Scholar] [CrossRef]
- Azri, N.; Irmawati, R.; Nda-Umar, U.I.; Saiman, M.I.; Taufiq-Yap, Y.H. Effect of Different Supports for Copper as Catalysts on Glycerol Hydrogenolysis to 1,2-Propanediol. J. King Saud Univ. Sci. 2021, 33, 101417. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, F.; Wang, Y.; Cheng, D. Easily Recycled CuMgFe Catalysts Derived from Layered Double Hydroxides for Hydrogenolysis of Glycerol. Catalysts 2021, 11, 232. [Google Scholar] [CrossRef]
- Gong, H.; Zhao, X.; Li, X.; Chen, M.; Ma, Y.; Fang, J.; Wei, X.; Peng, Q.; Hou, Z. Boosting The Long-Term Stability of Hydrotalcite-Derived Catalysts in Hydrogenolysis of Glycerol by Incorporation of Ca(Ii). ACS Sustain. Chem. Eng. 2021, 9, 2246–2259. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Vila, F.; Mariscal, R.; Jiménez-López, A. Hydrogenolysis of Glycerol to Obtain 1,2-Propanediol on Ce-Promoted Ni/Sba-15 Catalysts. Appl. Catal. B Environ. 2012, 117–118, 253–259. [Google Scholar] [CrossRef]
- Soares, A.V.H.; Salazar, J.B.; Falcone, D.D.; Vasconcellos, F.A.; Davis, R.J.; Passos, F.B. A Study of Glycerol Hydrogenolysis over Ru–Cu/Al2O3 and Ru–Cu/Zro2 Catalysts. J. Mol. Catal. A Chem. 2016, 415, 27–36. [Google Scholar] [CrossRef]
- Gatti, M.N.; Pompeo, F.; Santori, G.F.; Nichio, N.N. Bio-Propylene Glycol by Liquid Phase Hydrogenolysis of Glycerol with Ni/SiO2-C Catalysts. Catal. Today 2017, 296, 26–34. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, S.; Liu, H. Selective Hydrogenolysis of Glycerol to Propylene Glycol on Supported Pd Catalysts: Promoting Effects of Zno and Mechanistic Assessment of Active Pdzn Alloy Surfaces. ACS Catal. 2017, 7, 4265–4275. [Google Scholar] [CrossRef]
- Barbelli, M.L.; Santori, G.F.; Nichio, N.N. Aqueous phase hydrogenolysis of glycerol to bio-propylene glycol over Pt–Sn catalysts. Bioresour. Technol. 2012, 111, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.-Y.; Wang, A.-Q.; Ji, N.; Pang, J.F.; Wang, X.D.; Zhang, T. Transition Metal–Tungsten Bimetallic Catalysts for the Conversion of Cellulose into Ethylene Glycol. ChemSusChem 2010, 3, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Asano, T.; Nakagawa, Y.; Tamura, M.; Tomishige, K. One-pot synthesis of 1,3-butanediol by 1,4-anhydroerythritol hydrogenolysis over a tungsten-modified platinum on silica catalyst. Green Chem. 2020, 22, 2375–2380. [Google Scholar] [CrossRef]
- Zhu, S.; Zhu, Y.; Hao, S.; Chen, L.; Zhang, B.; Li, Y. Aqueous-Phase Hydrogenolysis of Glycerol to 1,3-Propanediol over Pt-H4SiW12O40/SiO2. Catal. Lett. 2012, 142, 267–274. [Google Scholar] [CrossRef]
- Numpilai, T.; Cheng, C.K.; Seubsai, A.; Faungnawakij, K.; Limtrakul, J.; Witoon, T. Sustainable utilization of waste glycerol for 1,3-propanediol production over Pt/WOx/Al2O3 catalysts: Effects of catalyst pore sizes and optimization of synthesis conditions. Environ. Pollut. 2021, 272, 116029. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Asano, T.; Nakagawa, Y.; Tamura, M.; Okumura, K.; Tomishige, K. Selective Hydrogenolysis of Glycerol to 1,3-Propanediol over Rhenium-Oxide-Modified Iridium Nanoparticles Coating Rutile Titania Support. ACS Catal. 2019, 9, 10913–10930. [Google Scholar] [CrossRef]
- Amada, Y.; Shinmi, Y.; Koso, S.; Kubota, T.; Nakagawa, Y.; Tomishige, K. Reaction Mechanism of the Glycerol Hydrogenolysis To 1,3-Propanediol over Ir–Reox/SiO2 Catalyst. Appl. Catal. B Environ. 2011, 105, 117–127. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Yang, M.; Lei, N.; Li, L.; Hou, B.; Miao, S.; Pan, X.; Wang, A.; Zhang, T. Selective Hydrogenolysis of Glycerol to 1,3-Propanediol: Manipulating the Frustrated Lewis Pairs by Introducing Gold to Pt/Wox. ChemSusChem 2017, 10, 819–824. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Y.; Zheng, H.; Ding, G.; Li, Y. Direct Conversion of Glycerol into 1,3-Propanediol over Cu-H4SiW12O40/SiO2 in Vapor Phase. Catal. Lett. 2009, 131, 312–320. [Google Scholar] [CrossRef]
- Li, D.; Zhou, Z.; Qin, J.; Li, Y.; Liu, Z.; Wu, W. Cu-Wox-TiO2 Catalysts by Modified Evaporation-Induced Self-Assembly Method for Glycerol Hydrogenolysis to 1, 3-Propanediol. Chemistryselect 2018, 3, 2479–2486. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, X.; Ren, Y.; Wang, J.; Lei, N.; Wang, A.; Zhang, T. Pt/Nb-Wox for the Chemoselective Hydrogenolysis of Glycerol to 1,3-Propanediol: Nb Dopant Pacifying the over-Reduction of Wox Supports. Chin. J. Catal. 2018, 39, 1027–1037. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.; Wang, S.; Ma, X. The effect of metal properties on the reaction routes of glycerol hydrogenolysis over platinum and ruthenium catalysts. Catal. Today 2017, 298, 2–8. [Google Scholar] [CrossRef]
- Chaminand, J.; Djakovitch, L.A.; Gallezot, P.; Marion, P.; Pinel, C.; Rosier, C. Glycerol Hydrogenolysis on Heterogeneous Catalysts. Green Chem. 2004, 6, 359–361. [Google Scholar] [CrossRef]
- Tamura, M.; Amada, Y.; Liu, S.; Yuan, Z.; Nakagawa, Y.; Tomishige, K. Promoting Effect of Ru on Ir-Reox/SiO2 Catalyst in Hydrogenolysis of Glycerol. J. Mol. Catal. A Chem. 2014, 388–389, 177–187. [Google Scholar] [CrossRef]
- De Andrade, T.S.; Souza, M.M.V.M.; Manfro, R.L. Hydrogenolysis of Glycerol to 1,2-Propanediol without External H2 Addition in Alkaline Medium Using Ni-Cu Catalysts Supported on Y Zeolite. Renew. Energy 2020, 160, 919–930. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, D.S.; Yi, J. Effect of nickel on catalytic behaviour of bimetallic Cu–Ni catalyst supported on mesoporous alumina for the hydrogenolysis of glycerol to 1,2-propanediol. Catal. Sci. Technol. 2014, 4, 3191–3202. [Google Scholar] [CrossRef]
- Mishra, N.K.; Kumar, P.; Srivastava, V.C.; Štangar, U.L. Synthesis of Cu-Based Catalysts for Hydrogenolysis of Glycerol to 1,2-Propanediol with in-situ Generated Hydrogen. J. Environ. Chem. Eng. 2021, 9, 105263. [Google Scholar] [CrossRef]
- Seretis, A.; Tsiakaras, P. Hydrogenolysis of Glycerol to Propylene Glycol by in situ Produced Hydrogen from Aqueous Phase Reforming of Glycerol over SiO2–Al2O3 Supported Nickel Catalyst. Fuel Process. Technol. 2016, 142, 135–146. [Google Scholar] [CrossRef]
- Yfanti, V.-L.; Yfanti, V.-L.; Yfanti, V.-L.; Vasiliadou, E.S.; Vasiliadou, E.S.; Vasiliadou, E.S.; Sklari, S.; Sklari, S.; Sklari, S.; Lemonidou, A.A.; et al. Hydrodeoxygenation of glycerol with in situ H2 formation over Pt catalysts supported on Fe modified Al2O3: Effect of Fe loading. J. Chem. Technol. Biotechnol. 2017, 92, 2236–2245. [Google Scholar] [CrossRef]
- D’Hondt, E.; Van de Vyver, S.; Sels, B.F.; Jacobs, P.A. Catalytic glycerol conversion into 1,2-propanediol in absence of added hydrogen. Chem. Commun. 2008, 45, 6011–6012. [Google Scholar] [CrossRef]
- Pendem, C.; Gupta, P.; Chaudhary, N.; Singh, S.; Kumar, J.; Sasaki, T.; Datta, A.; Bal, R. Aqueous Phase Reforming of Glycerol to 1,2-Propanediol over Pt-Nanoparticles Supported on Hydrotalcite in the Absence of Hydrogen. Green Chem. 2012, 14, 3107–3113. [Google Scholar] [CrossRef]
- Liu, S.; Tamura, M.; Shen, Z.; Zhang, Y.; Nakagawa, Y.; Tomishige, K. Hydrogenolysis of Glycerol with in-situ Produced H2 by Aqueous-Phase Reforming of Glycerol Using Pt-Modified Ir-Reox/SiO2 Catalyst. Catal. Today 2018, 303, 106–116. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Y.; Jiang, T.; Xiao, T.; Edwards, P.P.; Cao, F. Glycerol hydrogenolysis over a Pt–Ni bimetallic catalyst with hydrogen generated in situ. RSC Adv. 2017, 7, 38251–38256. [Google Scholar] [CrossRef]
- Roy, D.; Subramaniam, B.; Chaudhari, R.V. Aqueous phase hydrogenolysis of glycerol to 1,2-propanediol without external hydrogen addition. Catal. Today 2010, 156, 31–37. [Google Scholar] [CrossRef]
- Yin, A.-Y.; Guo, X.-Y.; Dai, W.-L.; Fan, K.-N. The synthesis of propylene glycol and ethylene glycol from glycerol using Raney Ni as a versatile catalyst. Green Chem. 2009, 11, 1514–1516. [Google Scholar] [CrossRef]
- Syuhada, A.; Ameen, M.; Sher, F.; Azizan, M.T.; Aqsha, A.; Yusoff, M.H.M.; Ruslan, M.S.H. Effect of Calcium Doping Using Aqueous Phase Reforming of Glycerol over Sonochemically Synthesized Nickel-Based Supported ZrO2 Catalyst. Catalysts 2021, 11, 977. [Google Scholar] [CrossRef]
- Syuhada, A.; Ameen, M.; Azizan, M.T.; Aqsha, A.; Yusoff, M.H.M.; Ramli, A.; Alnarabiji, M.S.; Sher, F. In-situ hydrogenolysis of glycerol using hydrogen produced via aqueous phase reforming of glycerol over sonochemically synthesized nickel-based nano-catalyst. Mol. Catal. 2021, 514, 111860. [Google Scholar] [CrossRef]
- Soares, A.V.-H.; Perez, G.; Passos, F.B. Alumina supported bimetallic Pt–Fe catalysts applied to glycerol hydrogenolysis and aqueous phase reforming. Appl. Catal. B Environ. 2016, 185, 77–87. [Google Scholar] [CrossRef]
- Callison, J.; Subramanian, N.; Rogers, S.; Chutia, A.; Gianolio, D.; Catlow, C.; Wells, P.; Dimitratos, N. Directed aqueous-phase reforming of glycerol through tailored platinum nanoparticles. Appl. Catal. B Environ. 2018, 238, 618–628. [Google Scholar] [CrossRef]
- Dietrich, P.J.; Sollberger, F.G.; Akatay, M.C.; Stach, E.A.; Delgass, W.N.; Miller, J.T.; Ribeiro, F.H. Structural and catalytic differences in the effect of Co and Mo as promoters for Pt-based aqueous phase reforming catalysts. Appl. Catal. B Environ. 2014, 156–157, 236–248. [Google Scholar] [CrossRef]
- Foo, G.S.; Wei, D.; Sholl, D.S.; Sievers, C. Role of Lewis and Brønsted Acid Sites in the Dehydration of Glycerol over Niobia. ACS Catal. 2014, 4, 3180–3192. [Google Scholar] [CrossRef]
- Oloye, F.F.; Ololade, I.A. Influence of Molybdenum Loadings on the Properties of MoO3/Zirconia Catalysts. Chem. Afr. 2018, 1, 119–126. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.; Ramli, A.; Yusup, S.; Alnarabiji, M.S. Catalytic Hydrodeoxygenation of Rubber Seed Oil over Sonochemically Synthesized Ni-Mo/Γ-Al2O3 Catalyst for Green Diesel Production. Ultrason. Sonochem. 2019, 51, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, L.; Xu, G.; Jia, W.; Ma, Y.; Zhang, Y. Selective Hydrodeoxygenation of Lignin-Derived Phenols to Cyclohexanols or Cyclohexanes over Magnetic Conx@ Nc Catalysts under Mild Conditions. ACS Catal. 2016, 6, 7611–7620. [Google Scholar] [CrossRef]
- Liu, X.; Jia, W.; Xu, G.; Zhang, Y.; Fu, Y. Selective Hydrodeoxygenation of Lignin-Derived Phenols to Cyclohexanols over Co-Based Catalysts. ACS Sustain. Chem. Eng. 2017, 5, 8594–8601. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Zhang, L.; Chi, Z.; Yang, Y.; Zhang, Z.; Zhang, B.; Lin, J.; Wan, S. Hydrogenolysis of Aryl Ether Bond over Heterogeneous Cobalt-Based Catalyst. Ind. Eng. Chem. Res. 2020, 59, 17357–17364. [Google Scholar] [CrossRef]
- Pflügl, S.; Marx, H.; Mattanovich, D.; Sauer, M. 1,3-Propanediol production from glycerol with Lactobacillus diolivorans. Bioresour. Technol. 2012, 119, 133–140. [Google Scholar] [CrossRef]
- Vafaeian, Y.; Haghighi, M.; Aghamohammadi, S. Ultrasound assisted dispersion of different amount of Ni over ZSM-5 used as nanostructured catalyst for hydrogen production via CO2 reforming of methane. Energy Convers. Manag. 2013, 76, 1093–1103. [Google Scholar] [CrossRef]

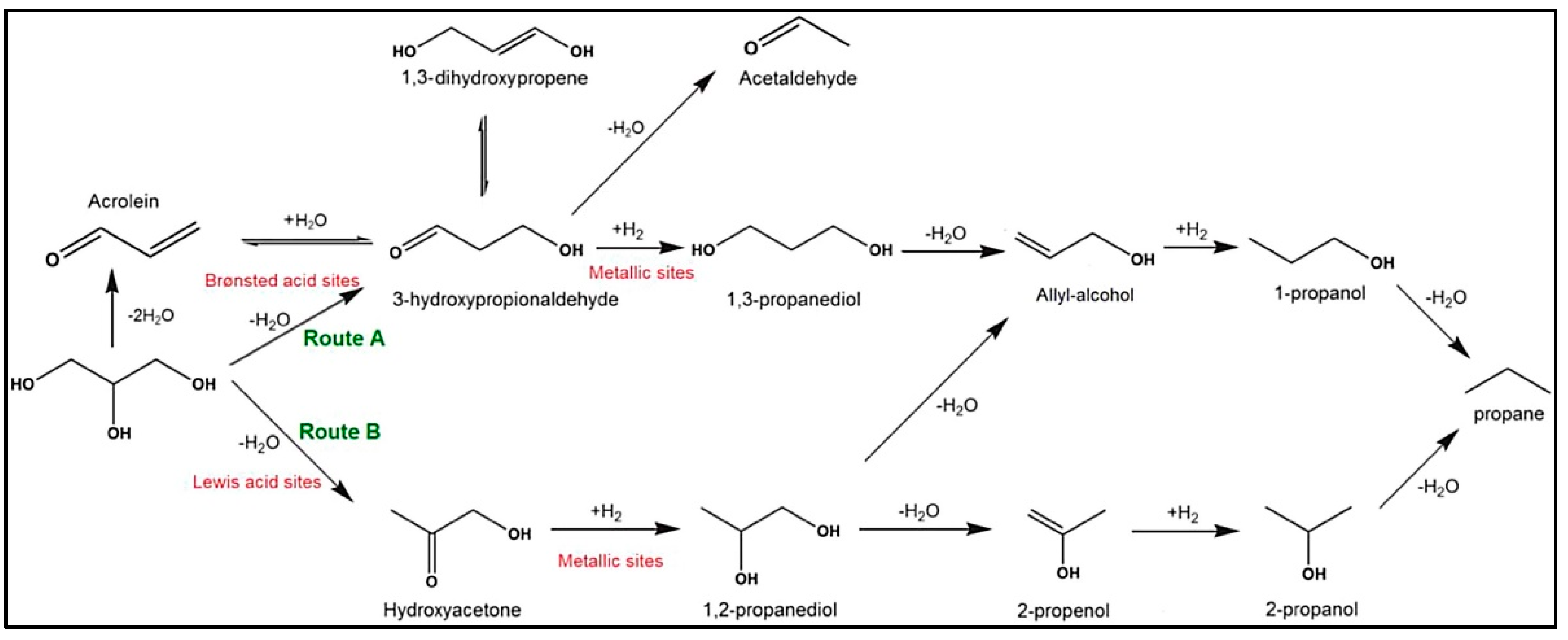
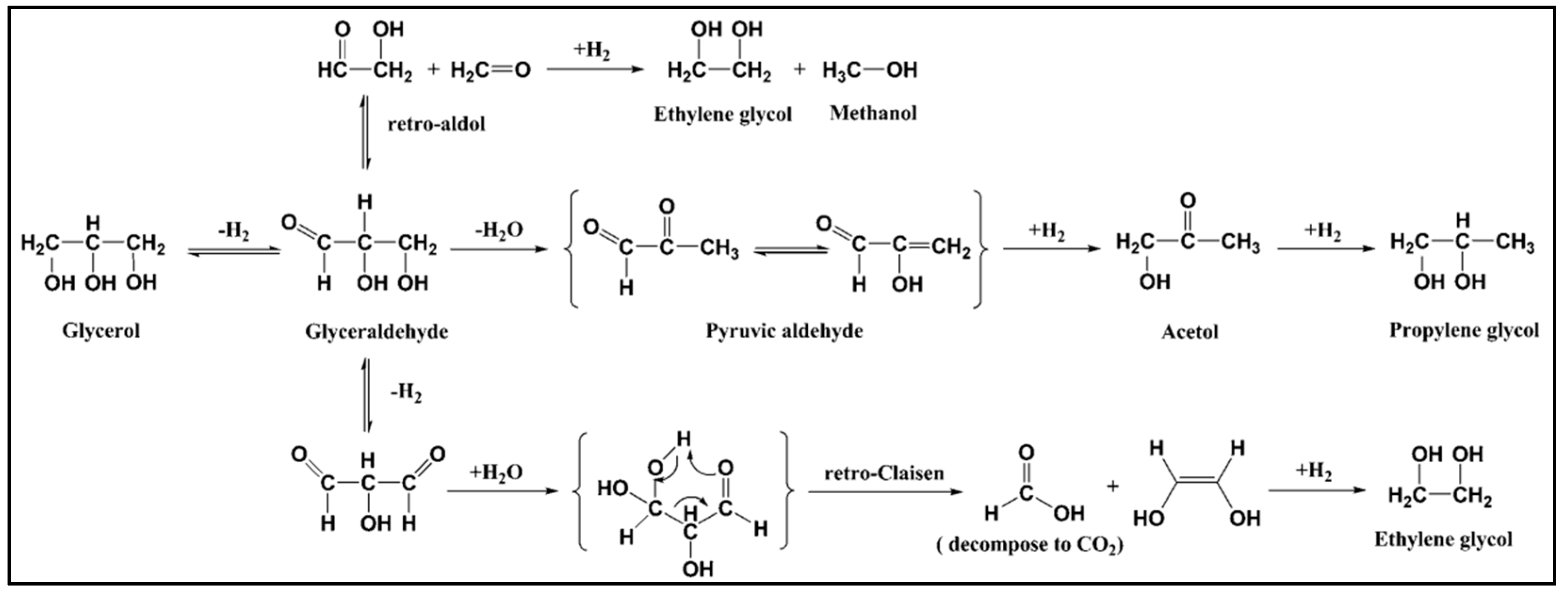
| Catalyst | Technique | Temperature (°C)/Pressure (Bar) | H2 Selectivity (%) | Alkane Selectivity (%) | Liquid Products | Ref. |
|---|---|---|---|---|---|---|
| Pt/CoAl2O3 | APR | 260/50 | 32 | 40 | Ethanol, acetone, propanol, propionic acid | Reynoso, et al. [73] |
| Ru-Pt-NMC3 | APR | 250/40 | 82.2 | Not reported | Not reported | Gogoi, et al. [74] |
| Pt-Ni/MWNT | APR | 240/40 | 90.9 | Not reported | Acetol, lactic acid | Rahman [75] |
| Cu-Ni/MWNT | 85.6 | |||||
| Pt/Al2O3 | APR | 225/27.6 | 40.4 | 6.8 | Not reported | Guo, et al. [76] |
| Pt-Ni/Al2O3 | 52.1 | 13.6 | ||||
| Pt-Co/Al2O3 | 50.9 | 8.4 | ||||
| Pt-Cu/Al2O3 | 129.9 | 1.3 | ||||
| Pt-Fe/Al2O3 | 54.6 | 9.8 | ||||
| Ni/Al2O3 | APR | 250/75 | 39.6 | Not reported | Not reported | Chen, et al. [68] |
| Ni-P/Al2O3 | 60 | |||||
| Ni-Cu/Al2O3 | 36.5 | |||||
| Ni-Mo/Al2O3 | 68.9 | |||||
| Pt-Re/C | APR | 225/29 | 24.5 | Not reported | Ethylene glycol, propylene glycol, 1-propanol, 2-propanol, ethanol, methanol, acids | King, et al. [69] |
| Ni/α-Al2O3 | Steam reforming | 450–600/1 | Not reported | Not reported | 1-hydroxy-2-propanone, acetic acid, 1-2 propanediol, propanol, 2-methyl-2-cyclopentenone | Buffoni, et al. [70] |
| Ni/Al2O3 | Steam reforming | 500–600/1 | Not reported | Not reported | Acetaldehyde, acrolein, propanal, acetone, acetic acid, methanol, ethanol, 1,2-propanediol | Iriondo, et al. [71] |
| Not reported | Pyrolysis | 650–700/1 | Not reported | Not reported | Acrolein, acetaldehyde, formaldehyde, acetol, 3-hydroxypropanal | Hemings, et al. [77] |
| Ni/α-Al2O3 | Pyrolysis | 700–900/1 | Not reported | Not reported | Not reported | Shahirah, et al. [78] |
| Ru/ZrO2 | Gasification | 510–550/350 | Not reported | Not reported | Acetic acid, acetaldehyde, hydroxyacetone | May, et al. [79] |
| Raney Ni/K2CO3 | Gasification | 650–800/230–270 | Not reported | Not reported | Acetaldehyde, propionaldehyde, acrolein, allyl alcohol, hydroxyacetone, propionic acid, | Li, et al. [80] |
| La-Ni/Al2O3 | Dry reforming | 750/1 | Not reported | Not reported | Not reported | Siew, et al. [81] |
| Re-Ni/CaO | Dry reforming | 600–900/1 | Not reported | Not reported | Not reported | Mohd Arif, et al. [82] |
| Rh/Ce | Partial oxidation | 600/2–6 | Not reported | Not reported | Methylglyoxal, acetaldehyde, hydroxyacetone, ethylene, acetic acid, glycols, benzene, acetone | Rennard, et al. [83] |
| Pt/Al2O3 | Partial oxidation | 100/5 | Not reported | Not reported | Glyceric acid, glycolic acid, lactic acid, oxalic acid, tartronic acid, formic acid, glyceraldehyde, dihydroxyacetone | Skrzyńska, et al. [84] |
| Rh-Ce/γ-Al2O3 | Autothermal reforming | 500–1050/1 | Not reported | Not reported | Not reported | Dauenhauer, et al. [85] |
| Pd/Ni/Cu/K supported on γ-Al2O3 | Autothermal reforming | 550–850/1 | Not reported | Not reported | Not reported | Swami and Abraham [86] |
| Pt-Ni/Al2O3 | Catalytic reforming | 380–500/250 | Not reported | Not reported | Glycerol, 1,2-propanediol, acetaldehyde, ethanol | Chakinala, et al. [72] |
| Ni supported on La2O3/α-Al2O3/γ-Al2O3/ZrO2/YSZ | Catalytic reforming | 450–580/250 | Not reported | Not reported | Acetaldehyde, acetic acid, methanol, acetol | Pairojpiriyakul, et al. [87] |
| Catalyst | Temp. (°C) | Reactor | X (%) | S a (%) | Y (%) | Ref. |
|---|---|---|---|---|---|---|
| AuPt/WOx | 140 | Batch | 81.4 | 57.1 b | 29.3 | Zhao et al. [125] |
| Cu-HSiW/SiO2 | 210 | Batch | 83.4 | 32.1 | Not reported | Huang et al. [126] |
| Cu-WOx-TiO2 | 180 | Batch | 12.7 | 32.3 | Not reported | Li et al. [127] |
| Ir–ReOx/SiO2 | 120 | Batch | 22.6 | 64.6 | Not reported | Amada et al. [124] |
| Ir-ReOx/rutile TiO2 | 120 | Batch | 80 | 69 | 36 | Liu et al. [123] |
| Pt/Nb-WOx | 160 | Batch | 40 | 29.7 | 11.9 | Yang et al. [128] |
| Pt/ZrW | 180 | Batch | 10.4 | 30.6 | ~5 | Zhou et al. [129] |
| Pt-HSiW/SiO2 | 200 | Batch | 81.2 | 38.7 | 31.4 | Zhu et al. [121] |
| Pt/WOx/Al2O3 | 220 | Batch | 78 | 48 | 32.8 | Numpilai et al. [122] |
| Rh/C + H2WO4 | 180 | Batch | 21 | 6 | 1.3 | Chaminand et al. [130] |
| Ru-Ir-ReOx/SiO2 | 120 | Batch | 60.7 | 33.7 | 20 | Tamura et al. [131] |
| Catalyst | Temp. (°C) | Reactor | X (%) | S a (%) | Y (%) | Ref. |
|---|---|---|---|---|---|---|
| Cu-Al | 220 | Continuous | 65 | 75 | Not reported | Mane and Rode [105] |
| Cu-Ni | 220 | Batch | 76.6 | 55.3 | 42.4 | Yun et al. [133] |
| Cu-Zn/Al2O3 | 200 | Batch | 43 | 69 | Not reported | Mishra et al. [134] |
| Ni/SiO2-Al2O3 | 240 | Batch | 80~ | ~64 | 22.0 | Seretis and Tsiakaras [135] |
| Pt/Fe2O3-Al2O3 | 250 | Batch | 93.8 | 43.3 | 39 | Yfanti et al. [136] |
| Pt/NaY | 239 | Batch | 85.4 | 76.19 b | Not reported | D’Hondt et al. [137] |
| Pt-HT c | 250 | Batch | 74.4 | 77.5 | Not reported | Pendem et al. [138] |
| Pt-Ir-ReOx/SiO2 | 190 | Batch | 81 | 32 | 53 | Liu et al. [139] |
| Pt-Ni/Al2O3 | 220 | Batch | 71.4 | 52.4 | Not reported | Yan et al. [140] |
| Pt-Sn | 200 | Batch | 49 | 63 | 31 | Barbelli et al. [118] |
| Ru-Pt/Al2O3 | 227 | Batch | 50.1 | 83.5 d | Not reported | Roy et al. [141] |
| Raney-Ni | 180 | Batch | 100 | 43 | Not reported | Yin et al. [142] |
| Catalyst | Temp. (°C) | Reactor | X (%) | S a (%) | Ref. |
|---|---|---|---|---|---|
| Pt/Al2O3 | 240 | Batch | 22.5 | Trace | Soares et al. [145] |
| Pt2-Fe/Al2O3 | 26.2 | Trace | |||
| Pt-Fe/Al2O3 | 29.5 | Trace | |||
| Pt-Fe2/Al2O3 | 33.5 | Trace | |||
| Pt/Al2O3 | 240 | Batch | 12 | Trace | Callison et al. [146] |
| PtCo/CNT | 230 | Fixed-bed | ~60 | Trace | Dietrich et al. [147] |
| PtMo/CNT | |||||
| Ni/ZrO2 | 230 | Batch | 50.8 | 23.8 | Syuhada et al. [143] |
| Ca-Ni/ZrO2 | 48.44 | 14.2 | |||
| Ni/CeO2 | 230 | Batch | 54.26 | 52.73 | Syuhada et al. [144] |
| Ca-Ni/CeO2 | 48.2 | 28.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md Radzi, M.R.; Manogaran, M.D.; Yusoff, M.H.M.; Zulqarnain; Anuar, M.R.; Shoparwe, N.F.; Rahman, M.F.A. Production of Propanediols through In Situ Glycerol Hydrogenolysis via Aqueous Phase Reforming: A Review. Catalysts 2022, 12, 945. https://doi.org/10.3390/catal12090945
Md Radzi MR, Manogaran MD, Yusoff MHM, Zulqarnain, Anuar MR, Shoparwe NF, Rahman MFA. Production of Propanediols through In Situ Glycerol Hydrogenolysis via Aqueous Phase Reforming: A Review. Catalysts. 2022; 12(9):945. https://doi.org/10.3390/catal12090945
Chicago/Turabian StyleMd Radzi, Mohamad Razlan, M. Devendran Manogaran, Mohd Hizami Mohd Yusoff, Zulqarnain, Mohd Razealy Anuar, Noor Fazliani Shoparwe, and Mohd Fikri Ab Rahman. 2022. "Production of Propanediols through In Situ Glycerol Hydrogenolysis via Aqueous Phase Reforming: A Review" Catalysts 12, no. 9: 945. https://doi.org/10.3390/catal12090945
APA StyleMd Radzi, M. R., Manogaran, M. D., Yusoff, M. H. M., Zulqarnain, Anuar, M. R., Shoparwe, N. F., & Rahman, M. F. A. (2022). Production of Propanediols through In Situ Glycerol Hydrogenolysis via Aqueous Phase Reforming: A Review. Catalysts, 12(9), 945. https://doi.org/10.3390/catal12090945






