Ozone as a Catalyst of Surplus Activated Sludge Hydrolysis for the Biogas Production Enhancement
Abstract
:1. Introduction
- Thermal;
- Mechanical;
- Chemical.
2. Results and Discussion
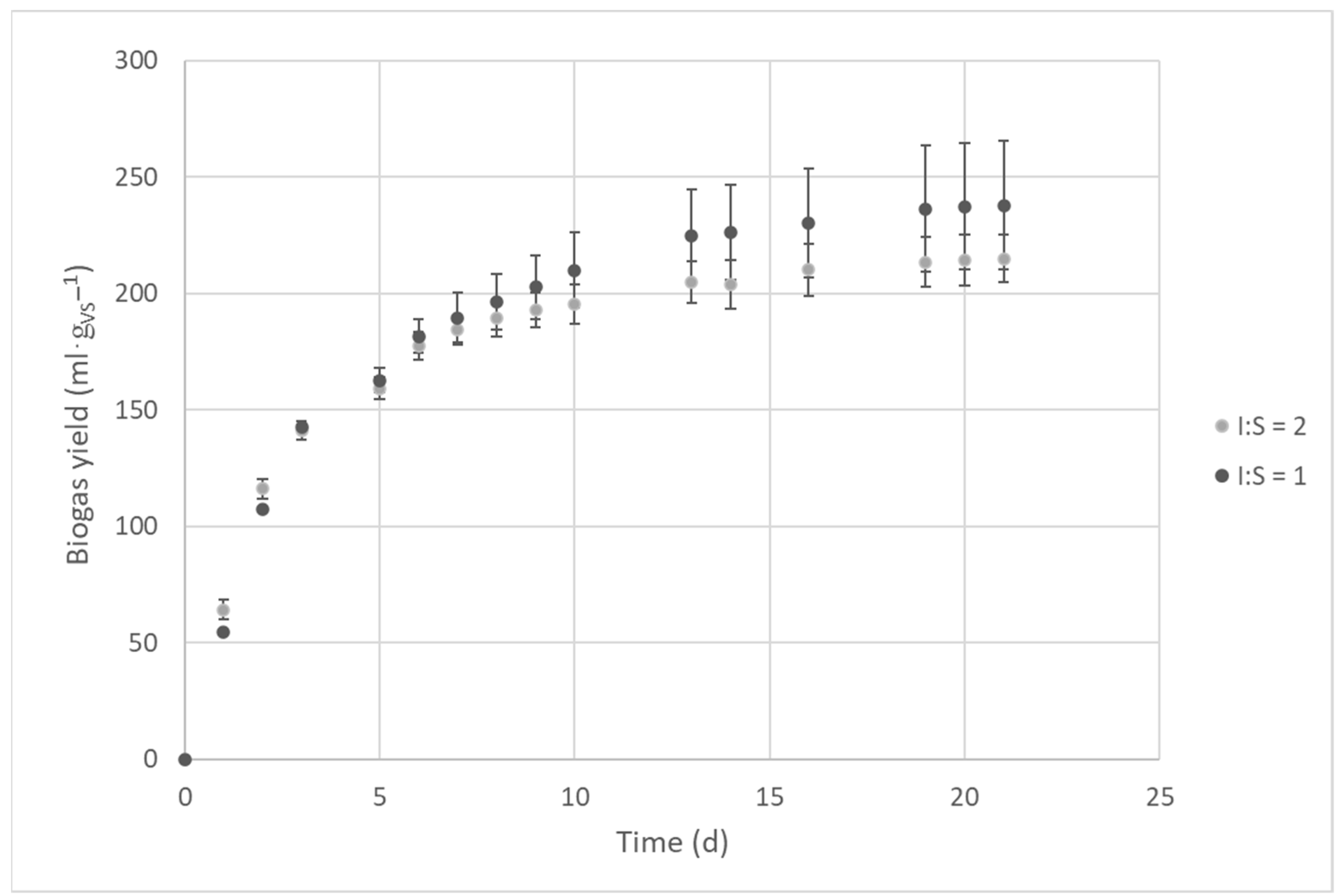
2.1. The Influence of the Inoculum to Substrate Ratio
- Inoculum—anaerobic sludge from a municipal wastewater treatment plant;
- Strictly anaerobic conditions;
- Constant temperature in the mesophilic range;
- Volumetric biogas quantity measurements;
- Automatic stirring devices;
- Bottles capacity between 100 and 500 ml;
- Duration of the test between 13 and 87 d;
- pH in the range from 7 to 7.8;
- Alkalinity at a minimum of 2500 mgCaCO3·L−1;
- Inoculum to substrate ratio (ISR) on the basis of volatile solids between 1 and 2 [20].
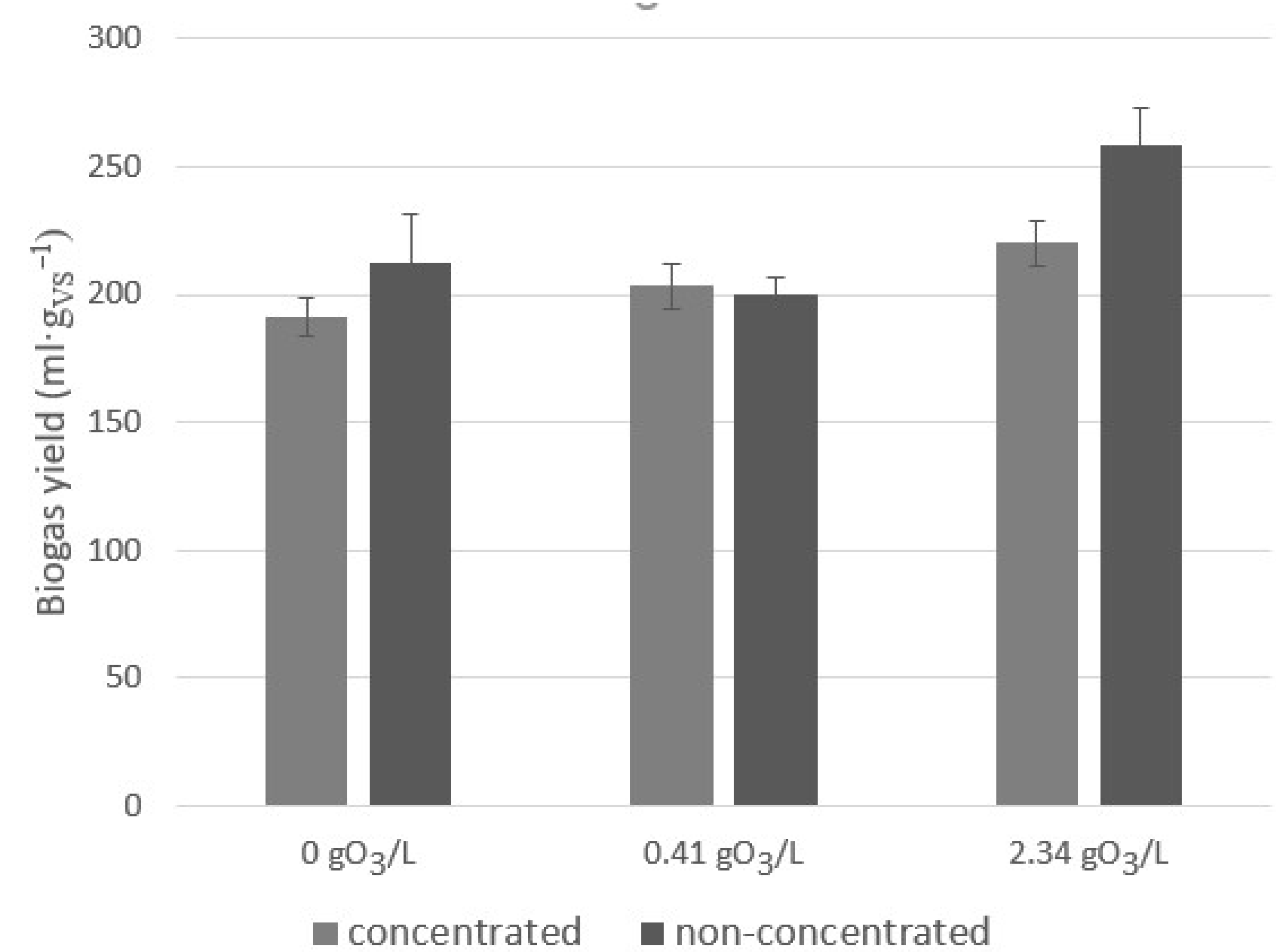
2.2. The Influence of Ozonation on the Biogas Yield and Composition
2.3. SAS Chemical Composition
2.4. The Influence of Ozonation on BMP
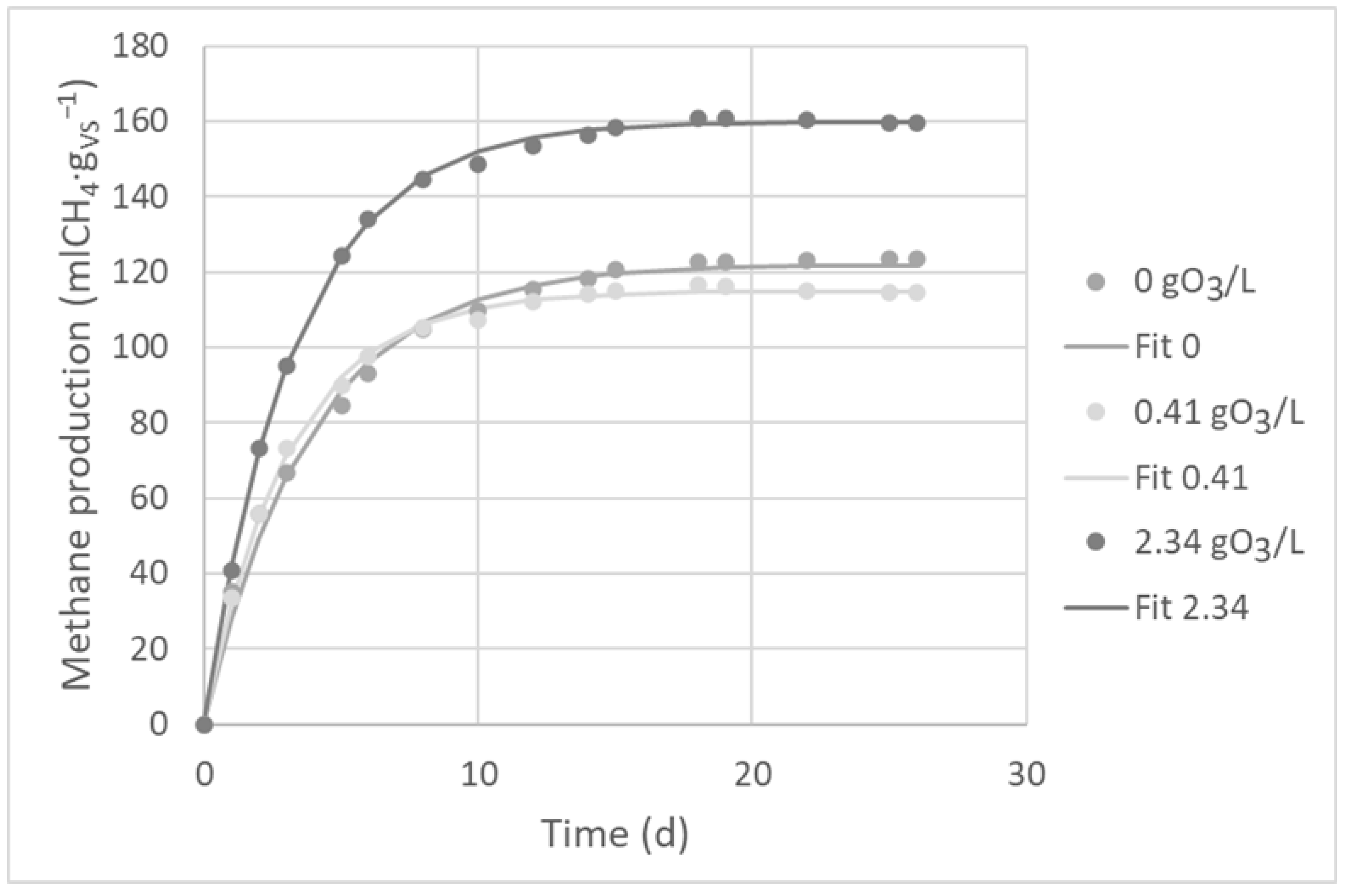
2.5. The Influence of Ozonation on Methane Fermentation Kinetics
- CH4—volume of methane produced in time t (mlCH4);
- CH4max—volume of methane at the end of the process (mlCH4);
- K—kinetic constant (d−1).
3. Conclusions
4. Materials and Methods
4.1. Substrate and Inoculum
4.2. Experimental Set-Up and Procedure
4.3. Analytical Methods
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tarpani, R.R.Z.; Alfonsín, C.; Hospido, A.; Azapagic, A. Life Cycle Environmental Impacts of Sewage Sludge Treatment Methods for Resource Recovery Considering Ecotoxicity of Heavy Metals and Pharmaceutical and Personal Care Products. J. Environ. Manag. 2020, 260, 109643. [Google Scholar] [CrossRef] [PubMed]
- EU. Council Directive of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge Is Used in Agriculture (86/278/EEC); EU: Brussels, Belgium, 1986. [Google Scholar]
- Tansel, B. Morphology, Composition and Aggregation Mechanisms of Soft Bioflocs in Marine Snow and Activated Sludge: A Comparative Review. J. Environ. Manag. 2018, 205, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.K.; Lo, S.L. Application of Physico-Chemical Pretreatment Methods to Enhance the Sludge Disintegration and Subsequent Anaerobic Digestion: An up to Date Review. Rev. Environ. Sci. Biotechnol. 2011, 10, 215–242. [Google Scholar] [CrossRef]
- Foladori, P.; Andreottola, G.; Ziglio, G. Sludge Reduction Technologies in Wastewater Treatment Plants; IWA Publishing: London, UK, 2010; ISBN 9781780401706. [Google Scholar]
- Salsabil, M.R.; Laurent, J.; Casellas, M.; Dagot, C. Techno-Economic Evaluation of Thermal Treatment, Ozonation and Sonication for the Reduction of Wastewater Biomass Volume before Aerobic or Anaerobic Digestion. J. Hazard. Mater. 2010, 174, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, G.; Ruiz, B.; Fiter, M.; Ferrer, C.; Berlanga, J.G.; Alonso, S.; Canut, A. Ozonation as a Pre-Treatment for Anaerobic Digestion of Waste-Activated Sludge: Effect of the Ozone Doses. Ozone Sci. Eng. 2015, 37, 316–322. [Google Scholar] [CrossRef]
- Tuncay, S.; Akcakaya, M.; Icgen, B. Ozonation of Sewage Sludge Prior to Anaerobic Digestion Led to Methanosaeta Dominated Biomethanation. Fuel 2022, 313, 122690. [Google Scholar] [CrossRef]
- Cheng, C.J.; Hong, P.K.A. Anaerobic Digestion of Activated Sludge after Pressure-Assisted Ozonation. Bioresour. Technol. 2013, 142, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Tokutomi, T.; Yasui, H.; Noike, T. Optimal Process Configuration for Anaerobic Digestion with Ozonation. Water Sci. Technol. 2003, 48, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Bougrier, C.; Albasi, C.; Delgenès, J.P.; Carrère, H. Effect of Ultrasonic, Thermal and Ozone Pre-Treatments on Waste Activated Sludge Solubilisation and Anaerobic Biodegradability. Chem. Eng. Process. Process Intensif. 2006, 45, 711–718. [Google Scholar] [CrossRef]
- Dumontet, S.; Dinel, H.; Baloda, S. Pathogen Reduction in Sewage Sludge by Composting and Other Biological Treatments: A Review. Biol. Agric. Hortic. 1999, 16, 409–430. [Google Scholar] [CrossRef]
- Michalska, K.; Pazera, A.; Bizukojć, M. CBI Proakademia; Łódź: Łódź, Poland, 2014; pp. 32–54. [Google Scholar]
- Guarino, G.; Carotenuto, C.; Di Cristofaro, F.; Papa, S.; Morrone, B.; Minale, M. Does the C/N Ratio Really Affect the Bio-Methane Yield? A Three Years Investigation of Buffalo Manure Digestion. Chem. Eng. Trans. 2016, 49, 463–468. [Google Scholar] [CrossRef]
- Zhu, B.; Gikas, P.; Zhang, R.; Lord, J.; Jenkins, B.; Li, X. Characteristics and Biogas Production Potential of Municipal Solid Wastes Pretreated with a Rotary Drum Reactor. Bioresour. Technol. 2009, 100, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Stelmach, E.; Stelmach, J.; Krzystek, L.; Ledakowicz, S. Wpływ Zawartości Węgla i Azotu Na Szybkość Produkcji Biogazu Organicznej Frakcji Stałych Odpadów Komunalnych. Inż. Ap. Chem. 2010, 49, 107–108. [Google Scholar]
- Barber, W.P.F. Influence of Wastewater Treatment on Sludge Production and Processing. Water Environ. J. 2014, 28, 1–10. [Google Scholar] [CrossRef]
- Lafratta, M.; Thorpe, R.B.; Ouki, S.K.; Shana, A.; Germain, E.; Willcocks, M.; Lee, J. Development and Validation of a Dynamic First Order Kinetics Model of a Periodically Operated Well-Mixed Vessel for Anaerobic Digestion. Chem. Eng. J. 2021, 426, 131732. [Google Scholar] [CrossRef]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical Methane Potential (BMP) Assay Method for Anaerobic Digestion Research. Water 2019, 11, 921. [Google Scholar] [CrossRef]
- Raposo, F.; Fernández-Cegrí, V.; de la Rubia, M.A.; Borja, R.; Béline, F.; Cavinato, C.; Demirer, G.; Fernández, B.; Fernández-Polanco, M.; Frigon, J.C.; et al. Biochemical Methane Potential (BMP) of Solid Organic Substrates: Evaluation of Anaerobic Biodegradability Using Data from an International Interlaboratory Study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA-AWWA-WEF: Washington, DC, USA, 2005. [Google Scholar]



| Sample | Nitrogen(%TS) | Carbon(%TS) | Hydrogen(%TS) | Sulfur(%TS) |
|---|---|---|---|---|
| Process with concentrated SAS | ||||
| SAS | 4.40 ± 0.01 | 36.17 ± 0.05 | 4.73 ± 0.01 | 0.00 |
| SAS ozonated 0.41 gO3·L−1 | 4.20 ± 0.01 | 35.48 ± 0.08 | 4.66 ± 0.00 | 0.00 |
| SAS ozonated 2.34 gO3·L−1 | 4.28 ± 0.00 | 36.45 ± 0.15 | 4.76 ± 0.01 | 0.00 |
| Process with non-concentrated SAS | ||||
| SAS | 3.91 ± 0.01 | 36.10 ± 0.08 | 4.65 ± 0.00 | 0.00 |
| SAS ozonated 0.41 gO3·L−1 | 3.78 ± 0.04 | 35.01 ± 0.19 | 4.53 ± 0.02 | 0.00 |
| SAS ozonated 2.34 gO3·L−1 | 3.94 ± 0.02 | 35.11 ± 0.03 | 4.70 ± 0.05 | 0.00 |
| Mean values for all samples | ||||
| 4.16 ± 0.29 | 35.67 ± 0.55 | 4.68 ± 0.08 | 0.00 | |
| Sample | A | b | c | d | f 1 | g 2 | h 3 |
|---|---|---|---|---|---|---|---|
| Process with concentrated SAS | |||||||
| SAS | 3.01 ± 0.00 | 4.73 ± 0.01 | 1.12 ± 0.02 | 0.32 ± 0.00 | 1.50 ± 0.01 | 1.31 ± 0.00 | 1.70 ± 0.01 |
| SAS ozonated 0.41 gO3·L−1 | 2.95 ± 0.01 | 4.66 ± 0.00 | 1.16 ± 0.00 | 0.30 ± 0.00 | 1.44 ± 0.01 | 1.30 ± 0.00 | 1.66 ± 0.00 |
| SAS ozonated 2.34 gO3·L−1 | 3.04 ± 0.01 | 4.76 ± 0.01 | 1.18 ± 0.08 | 0.31 ± 0.00 | 1.49 ± 0.03 | 1.33 ± 0.02 | 1.71 ± 0.01 |
| Process with non-concentrated SAS | |||||||
| SAS | 3.01 ± 0.01 | 4.65 ± 0.00 | 1.22 ± 0.06 | 0.28 ± 0.00 | 1.44 ± 0.03 | 1.33 ± 0.02 | 1.67 ± 0.01 |
| SAS ozonated 0.41 gO3·L−1 | 2.92 ± 0.02 | 4.53 ± 0.02 | 1.31 ± 0.04 | 0.27 ± 0.00 | 1.33 ± 0.00 | 1.32 ± 0.02 | 1.60 ± 0.01 |
| SAS ozonated 2.34 gO3·L−1 | 2.93 ± 0.00 | 4.70 ± 0.05 | 1.24 ± 0.02 | 0.28 ± 0.00 | 1.34 ± 0.02 | 1.29 ± 0.00 | 1.64 ± 0.00 |
| Mean values for all samples | |||||||
| 2.97 ± 0.05 | 4.68 ± 0.08 | 1.20 ± 0.07 | 0.30 ± 0.02 | 1.43 ± 0.07 | 1.31 ± 0.02 | 1.66 ± 0.04 | |
| Sample | BMP—Theor. (mlCH4·gVS−1) | BMP—NIR (mlCH4·gVS−1) | BMP—Bottles (mlCH4·gVS−1) | Bottles/Theor. (%) |
|---|---|---|---|---|
| Process with concentrated SAS | ||||
| SAS | 602 ± 4 | 213 ± 11 | 120 ± 15 | 20 |
| SAS ozonated 0.41 gO3·L−1 | 592 ± 1 | 204 ± 9 | 126 ± 7 | 21 |
| SAS ozonated 2.34 gO3·L−1 | 594 ± 17 | 220 ± 5 | 146 ± 12 | 25 |
| Process with non-concentrated SAS | ||||
| SAS | 584 ± 14 | 228 ± 20 | 124 ± 22 | 21 |
| SAS ozonated 0.41 gO3·L−1 | 556 ± 9 | 213 ± 5 | 121 ± 10 | 22 |
| SAS ozonated 2.34 gO3·L−1 | 576 ± 3 | 229 ± 20 | 160 ± 20 | 28 |
| Sample | CH4max (mlCH4·gVS−1) | k (d−1) | R2 (–) |
|---|---|---|---|
| Process with concentrated SAS | |||
| SAS | 121 ± 1.59 | 0.219 ± 0.008 | 0.997 |
| SAS ozonated 0.41 gO3·L−1 | 126 ± 2.53 | 0.228 ± 0.013 | 0.992 |
| SAS ozonated 2.34 gO3·L−1 | 149 ± 2.70 | 0.231 ± 0.012 | 0.993 |
| Process with non-concentrated SAS | |||
| SAS | 122 ± 1.29 | 0.259 ± 0.011 | 0.993 |
| SAS ozonated 0.41 gO3·L−1 | 115 ± 0.54 | 0.323 ± 0.007 | 0.998 |
| SAS ozonated 2.34 gO3·L−1 | 160 ± 0.46 | 0.300 ± 0.004 | 0.999 |
| Parameters | SAS Substrate | Anaerobic Sludge Inoculum |
|---|---|---|
| pH (−) | 7.24 ± 0.16 | 7.38 ± 0.10 |
| Total solids (g·L−1) | 5.34 ± 1.03 | 34.22 ± 0.85 |
| Volatile solids (g·L−1) | 3.88 ± 1.18 | 20.70 ± 0.57 |
| Total COD (g·L−1) | 12.92 ± 0.56 | 29.43 ± 3.59 |
| Soluble COD (g·L−1) | 0.36 ± 0.01 | 2.83 ± 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paździor, K.; Domińska, M.; Olak-Kucharczyk, M. Ozone as a Catalyst of Surplus Activated Sludge Hydrolysis for the Biogas Production Enhancement. Catalysts 2022, 12, 1060. https://doi.org/10.3390/catal12091060
Paździor K, Domińska M, Olak-Kucharczyk M. Ozone as a Catalyst of Surplus Activated Sludge Hydrolysis for the Biogas Production Enhancement. Catalysts. 2022; 12(9):1060. https://doi.org/10.3390/catal12091060
Chicago/Turabian StylePaździor, Katarzyna, Marlena Domińska, and Magdalena Olak-Kucharczyk. 2022. "Ozone as a Catalyst of Surplus Activated Sludge Hydrolysis for the Biogas Production Enhancement" Catalysts 12, no. 9: 1060. https://doi.org/10.3390/catal12091060
APA StylePaździor, K., Domińska, M., & Olak-Kucharczyk, M. (2022). Ozone as a Catalyst of Surplus Activated Sludge Hydrolysis for the Biogas Production Enhancement. Catalysts, 12(9), 1060. https://doi.org/10.3390/catal12091060






