CO Oxidation over Alumina-Supported Copper Catalysts
Abstract
1. Introduction
2. Results and Discussion
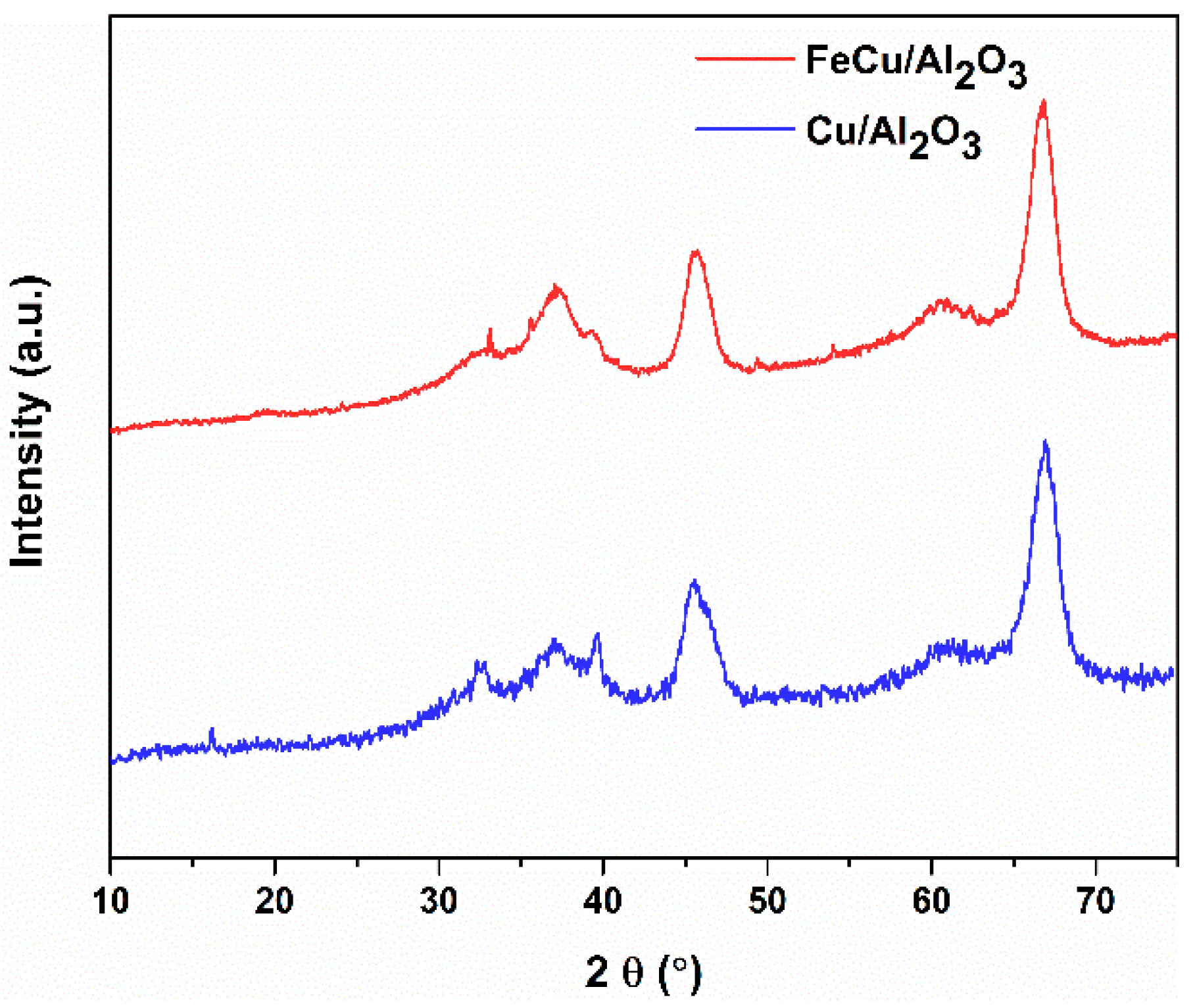
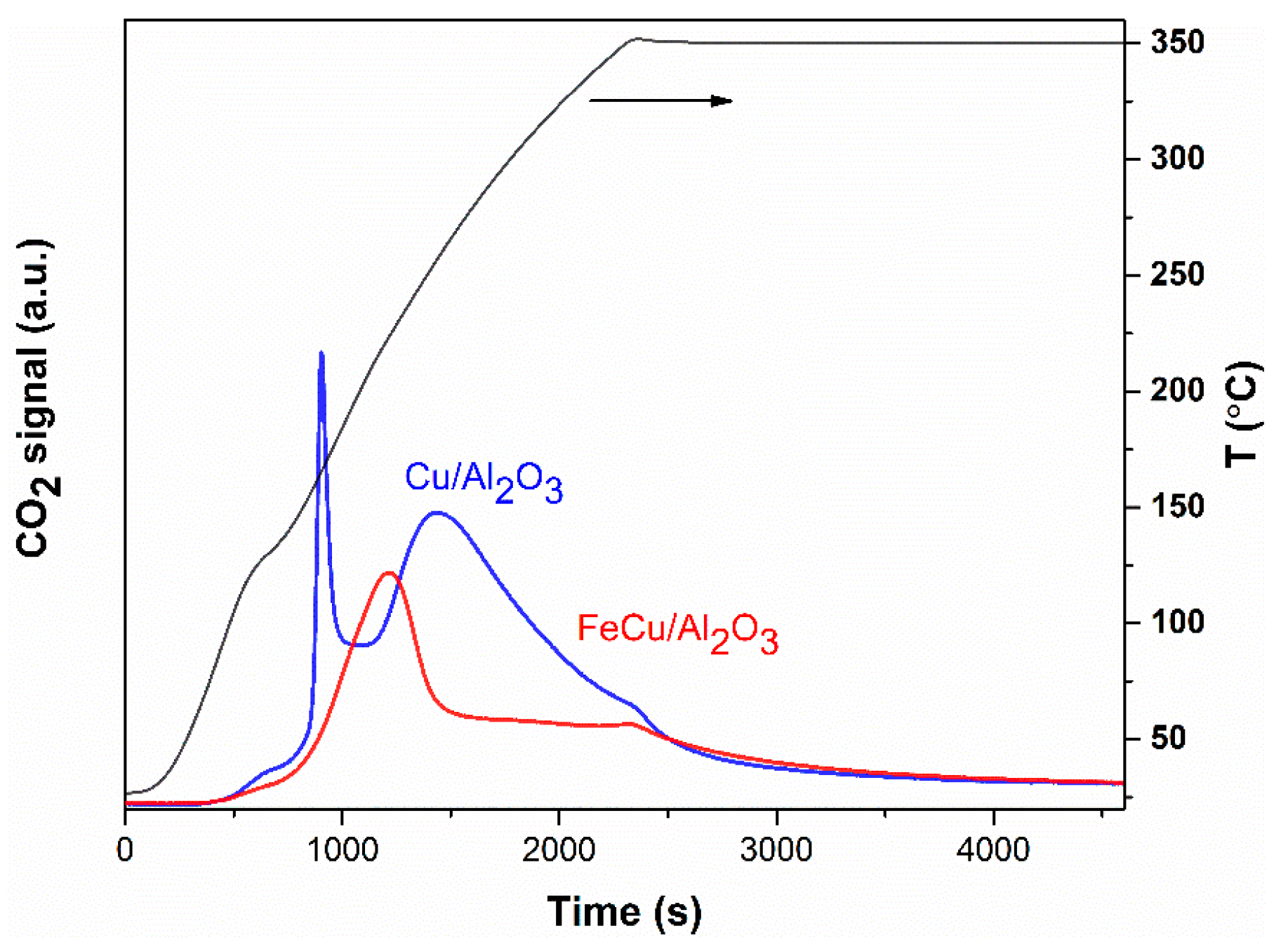
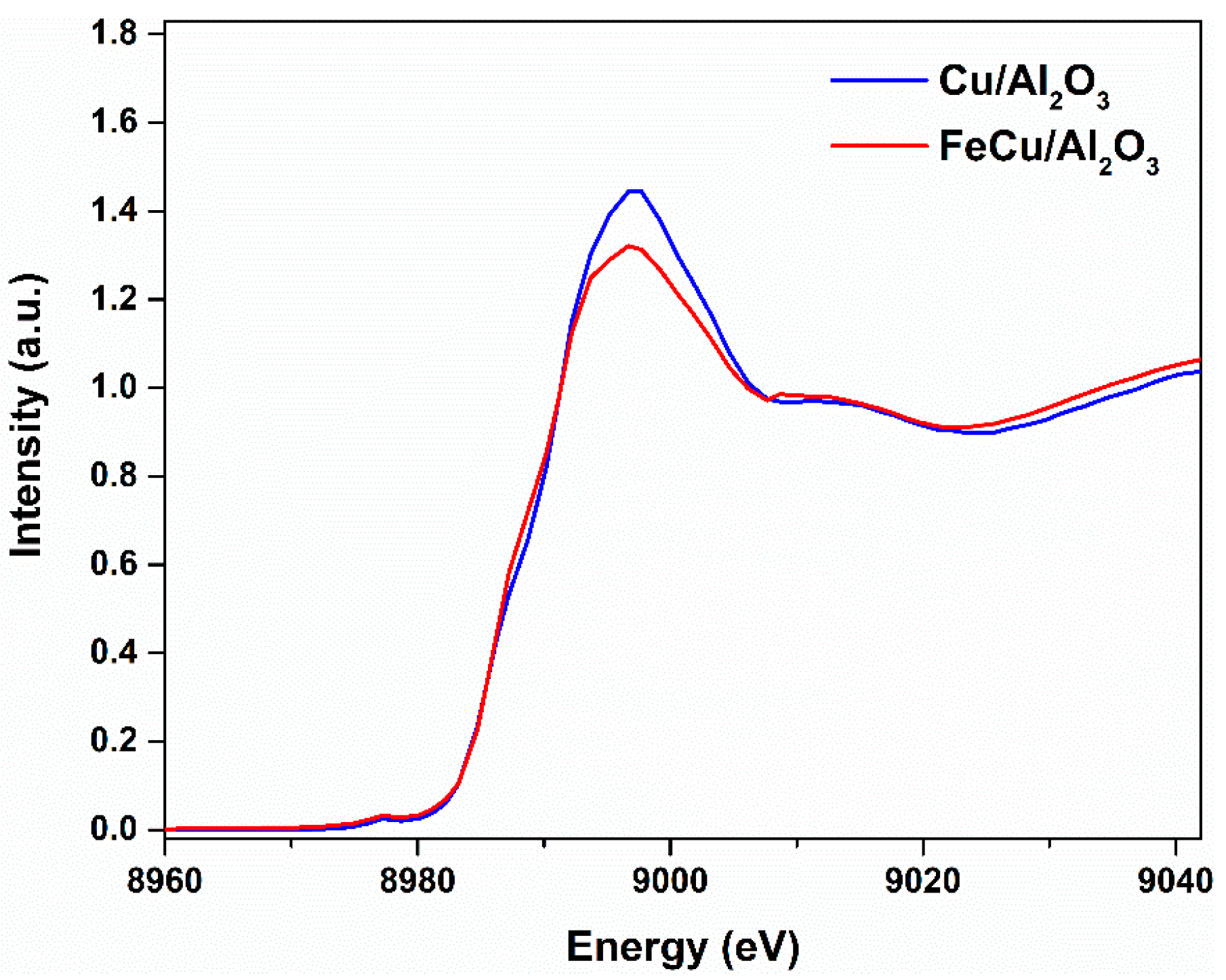
2.1. Catalyst Properties
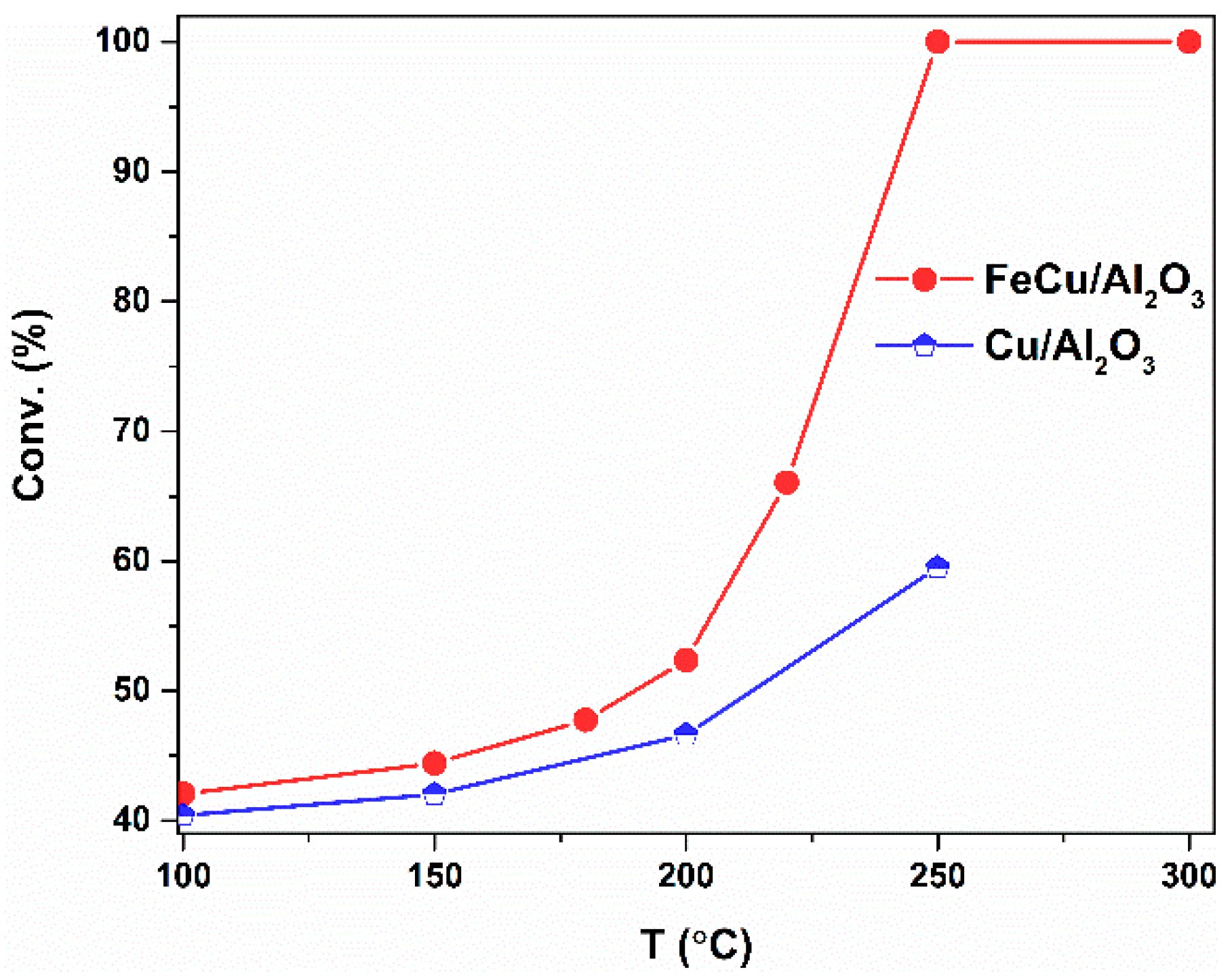
2.2. Catalytic Evaluation of the CO Oxidation
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. X-Ray Absorption Spectroscopy Measurement
3.4. Catalytic Evaluation of CO Oxidation
3.5. Carbon Monoxide Temperature-Programmed Reduction (CO-TPR)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Freund, H.-J.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO Oxidation as a Prototypical Reaction for Heterogeneous Processes. Angew. Chem. Int. Ed. 2011, 50, 10064–10094. [Google Scholar] [CrossRef] [PubMed]
- Van Spronsen, M.A.; Frenken, J.W.M.; Groot, I.M.N. Surface science under reaction conditions: CO oxidation on Pt and Pd model catalysts. Chem. Soc. Rev. 2017, 46, 4347–4374. [Google Scholar] [CrossRef] [PubMed]
- Stamenković, V.; Arenz, M.; Blizanac, B.; Mayrhofer, K.; Ross, P.; Marković, N. In situ CO oxidation on well characterized Pt3Sn (hkl) surfaces: A selective review. Surf. Sci. 2005, 576, 145–157. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Z.; Jia, P. Pd-doped C3N monolayer: A promising low-temperature and high-activity single-atom catalyst for CO oxidation. Appl. Surf. Sci. 2020, 537, 147881. [Google Scholar] [CrossRef]
- Meunier, F.C.; Cardenas, L.; Kaper, H.; Šmíd, B.; Vorokhta, M.; Grosjean, R.; Aubert, D.; Dembélé, K.; Lunkenbein, T. Synergy between metallic and oxidized Pt sites unravelled during room temperature CO oxidation on Pt/ceria. Angew. Chem. Int. Ed. 2021, 60, 3799–3805. [Google Scholar] [CrossRef]
- Widmann, D.; Behm, R.J. Activation of Molecular Oxygen and the Nature of the Active Oxygen Species for CO Oxidation on Oxide Supported Au Catalysts. Accounts Chem. Res. 2014, 47, 740–749. [Google Scholar] [CrossRef]
- Min, B.K.; Friend, C.M. Heterogeneous Gold-Based Catalysis for Green Chemistry: Low-Temperature CO Oxidation and Propene Oxidation. Chem. Rev. 2007, 107, 2709–2724. [Google Scholar] [CrossRef]
- Lin, J.; Wang, X.; Zhang, T. Recent progress in CO oxidation over Pt-group-metal catalysts at low temperatures. Chin. J. Catal. 2016, 37, 1805–1813. [Google Scholar] [CrossRef]
- Xu, S.; Chansai, S.; Xu, S.; Stere, C.E.; Jiao, Y.; Yang, S.; Hardacre, C.; Fan, X. CO poisoning of Ru catalysts in CO2 hydrogenation under thermal and plasma conditions: A combined kinetic and diffuse reflectance infrared fourier transform spectroscopy–mass spectrometry study. ACS Catal. 2020, 10, 12828–12840. [Google Scholar] [CrossRef]
- Knudsen, J.; Nilekar, A.U.; Vang, R.T.; Schnadt, J.; Kunkes, E.L.; Dumesic, J.A.; Mavrikakis, M.; Besenbacher, F. A Cu/Pt Near-Surface Alloy for Water-Gas Shift Catalysis. J. Am. Chem. Soc. 2007, 129, 6485–6490. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Wang, X.; Mei, B.; Luo, E.; Li, Y.; Meng, Q.; Jin, Z.; Jiang, Z.; Liu, C.; et al. CO-Tolerant PEMFC Anodes Enabled by Synergistic Catalysis between Iridium Single-Atom Sites and Nanoparticles. Angew. Chem. 2021, 133, 26381–26387. [Google Scholar] [CrossRef]
- Mosrati, J.; Abdel-Mageed, A.M.; Vuong, T.H.; Grauke, R.; Bartling, S.; Rockstroh, N.; Atia, H.; Armbruster, U.; Wohlrab, S.; Rabeah, J.; et al. Tiny species with big impact: High activity of Cu single atoms on CeO2–TiO2 deciphered by operando spectroscopy. ACS Catal. 2021, 11, 10933–10949. [Google Scholar] [CrossRef]
- Huang, T.-J.; Tsai, D.-H. CO oxidation behavior of copper and copper oxides. Catal. Lett. 2003, 87, 173–178. [Google Scholar] [CrossRef]
- Falsig, H.; Hvolbæk, B.; Kristensen, I.S.; Jiang, T.; Bligaard, T.; Christensen, C.H.; Nørskov, J.K. Trends in the Catalytic CO Oxidation Activity of Nanoparticles. Angew. Chem. 2008, 120, 4913–4917. [Google Scholar] [CrossRef]
- Jia, A.-P.; Hu, G.-S.; Meng, L.; Xie, Y.-L.; Lu, J.-Q.; Luo, M.-F. CO oxidation over CuO/Ce1−xCuxO2−δ and Ce1−xCuxO2−δ catalysts: Synergetic effects and kinetic study. J. Catal. 2012, 289, 199–209. [Google Scholar] [CrossRef]
- Zhang, X.-m.; Tian, P.; Tu, W.; Zhang, Z.; Xu, J.; Han, Y.-F. Tuning the dynamic interfacial structure of copper–ceria catalysts by indium oxide during CO oxidation. ACS Catal. 2018, 8, 5261–5275. [Google Scholar] [CrossRef]
- Liu, Z.-P.; Hu, P.; Alavi, A. Mechanism for the high reactivity of CO oxidation on a ruthenium–oxide. J. Chem. Phys. 2001, 114, 5956–5957. [Google Scholar] [CrossRef]
- Huang, B.; Kobayashi, H.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Nishida, Y.; Sato, K.; Nagaoka, K.; Haneda, M.; Xie, W.; et al. A CO adsorption site change induced by copper substitution in a ruthenium catalyst for enhanced CO oxidation activity. Angew. Chem. Int. Ed. 2019, 131, 2252–2257. [Google Scholar] [CrossRef]
- Kim, Y.; Over, H.; Krabbes, G.; Ertl, G. Identification of RuO2 as the active phase in CO oxidation on oxygen-rich ruthenium surfaces. Top. Catal. 2000, 14, 95–100. [Google Scholar] [CrossRef]
- Joo, S.H.; Park, J.Y.; Renzas, J.; Butcher, D.R.; Huang, W.; Somorjai, G.A. Size Effect of Ruthenium Nanoparticles in Catalytic Carbon Monoxide Oxidation. Nano Lett. 2010, 10, 2709–2713. [Google Scholar] [CrossRef]
- Fedorov, A.; Saraev, A.; Kremneva, A.; Selivanova, A.; Vorokhta, M.; Šmíd, B.; Bulavchenko, O.; Yakovlev, V.; Kaichev, V. Kinetic and mechanistic study of CO oxidation over nanocomposite Cu-Fe-Al oxide catalysts. ChemCatChem 2020, 12, 4911–4921. [Google Scholar] [CrossRef]
- Fedorov, A.V.; Tsapina, A.M.; Bulavchenko, O.A.; Saraev, A.A.; Odegova, G.V.; Ermakov, D.Y.; Zubavichus, Y.V.; Yakovlev, V.A.; Kaichev, V.V. Structure and chemistry of Cu–Fe–Al nanocomposite catalysts for CO oxidation. Catal. Lett. 2018, 148, 3715–3722. [Google Scholar] [CrossRef]
- Engel, T.; Ertl, G. Surface residence times and reaction mechanism in the catalytic oxidation of CO on Pd(111). Chem. Phys. Lett. 1978, 54, 95–98. [Google Scholar] [CrossRef]
- Cisternas, J.; Holmes, P.; Kevrekidis, I.G.; Li, X. CO oxidation on thin Pt crystals: Temperature slaving and the derivation of lumped models. J. Chem. Phys. 2003, 118, 3312–3328. [Google Scholar] [CrossRef]
- Baxter, R.; Hu, P. Insight into why the Langmuir–Hinshelwood mechanism is generally preferred. J. Chem. Phys. 2002, 116, 4379–4381. [Google Scholar] [CrossRef]
- Hopstaken, M.J.P.; Niemantsverdriet, J.W. Structure sensitivity in the CO oxidation on rhodium: Effect of adsorbate coverages on oxidation kinetics on Rh(100) and Rh(111). J. Chem. Phys. 2000, 113, 5457. [Google Scholar] [CrossRef][Green Version]
- Widmann, D.; Behm, R. Dynamic surface composition in a Mars-van Krevelen type reaction: CO oxidation on Au/TiO2. J. Catal. 2018, 357, 263–273. [Google Scholar] [CrossRef]
- Qi, L.; Yu, Q.; Dai, Y.; Tang, C.; Liu, L.; Zhang, H.; Gao, F.; Dong, L.; Chen, Y. Influence of cerium precursors on the structure and reducibility of mesoporous CuO-CeO2 catalysts for CO oxidation. Appl. Catal. B 2012, 119, 308–320. [Google Scholar] [CrossRef]
- Niu, J.; Liland, S.E.; Yang, J.; Rout, K.R.; Ran, J.; Chen, D. Effect of oxide additives on the hydrotalcite derived Ni catalysts for CO2 reforming of methane. Chem. Eng. J. 2018, 377, 119763. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Ma, H.; Sollund, E.S.; Zhang, W.; Fenes, E.; Qi, Y.; Wang, Y.; Rout, K.R.; Fuglerud, T.; Piccinini, M.; Chen, D. Kinetic modeling of dynamic changing active sites in a Mars-van Krevelen type reaction: Ethylene oxychlorination on K-doped CuCl2/Al2O3. Chem. Eng. J. 2021, 407, 128013. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Y.; Zhang, H.; Ma, G.; Zhang, W.; Qi, Y.; Fuglerud, T.; Jiang, Z.; Ding, W.; Chen, D. Facet-Induced Strong Metal Chloride-Support Interaction over CuCl2/γ-Al2O3 Catalyst to Enhance Ethylene Oxychlorination Performance. ACS Catal. 2022, 12, 8027–8037. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Y.; Qi, Y.; Rout, K.R.; Chen, D. Critical Review of Catalysis for Ethylene Oxychlorination. ACS Catal. 2020, 10, 9299–9319. [Google Scholar] [CrossRef]
- Xie, Y.-C.; Tang, Y.-Q. Spontaneous monolayer dispersion of oxides and salts onto surfaces of supports: Applications to heterogeneous catalysis. Adv. Catal. 1990, 37, 1–43. [Google Scholar] [CrossRef]
- Ma, H.; Fenes, E.; Qi, Y.; Wang, Y.; Rout, K.R.; Fuglerud, T.; Chen, D. Understanding of K and Mg co-promoter effect in ethylene oxychlorination by operando UV–vis-NIR spectroscopy. Catal. Today 2020, 369, 227–234. [Google Scholar] [CrossRef]
- Yang, G.; Haibo, Z.; Biying, Z. Monolayer dispersion of oxide additives on SnO2 and their promoting effects on thermal stability of SnO2 ultrafine particles. J. Mater. Sci. 2000, 35, 917–923. [Google Scholar] [CrossRef]
- Yu, X.-F.; Wu, N.-Z.; Xie, Y.-C.; Tang, Y.-Q. A monolayer dispersion study of titania-supported copper oxide. J. Mater. Chem. 2000, 10, 1629–1634. [Google Scholar] [CrossRef]
- Jernigan, G.G.; Somorjai, G.A. Carbon monoxide oxidation over three different oxidation states of copper: Metallic copper, copper (I) oxide, and copper (II) oxide-a surface science and kinetic study. J. Catal. 1994, 147, 567–577. [Google Scholar] [CrossRef]
- Nagase, K.; Zheng, Y.; Kodama, Y.; Kakuta, J. Dynamic Study of the Oxidation State of Copper in the Course of Carbon Monoxide Oxidation over Powdered CuO and Cu2O. J. Catal. 1999, 187, 123–130. [Google Scholar] [CrossRef]
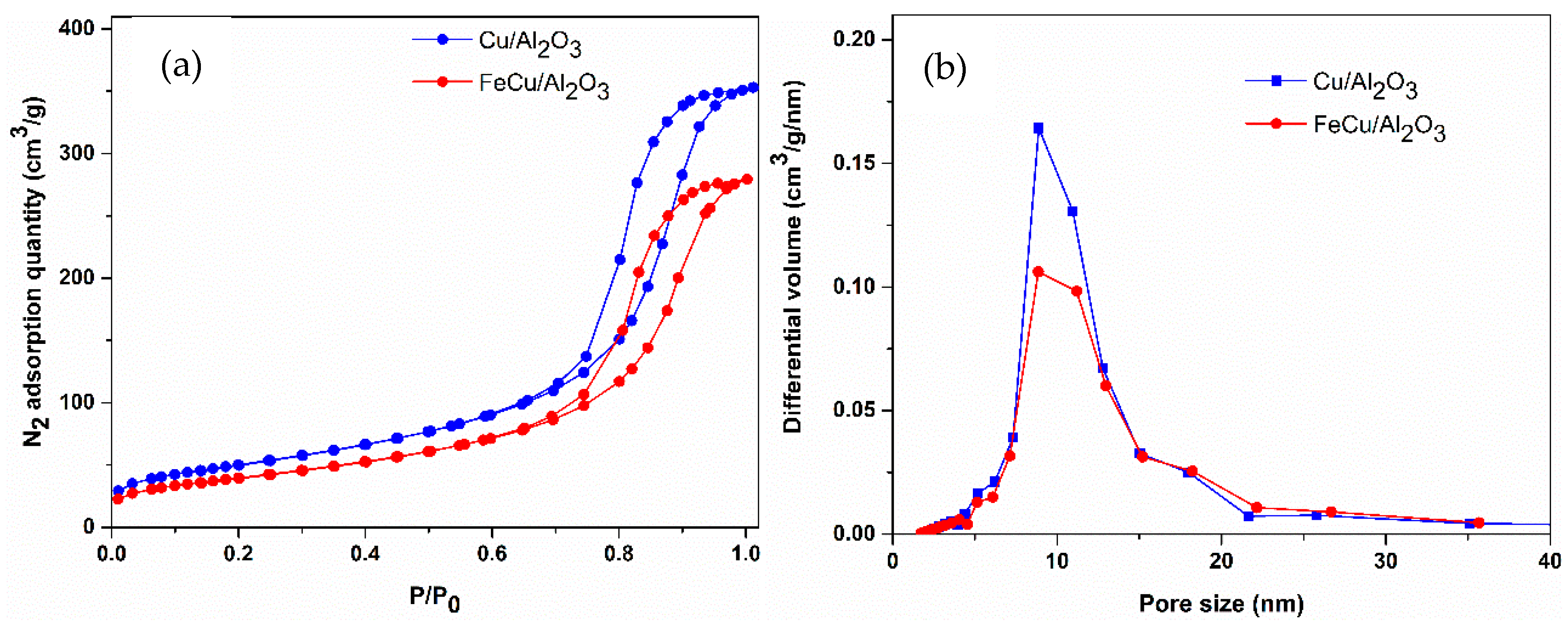


| Catalyst | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Metal Loading (wt%) |
|---|---|---|---|---|
| Cu/γ-Al2O3 | 181 | 0.55 | 9.07 | 5 |
| FeCu/γ-Al2O3 | 144 | 0.43 | 9.10 | 1/5 (Fe/Cu) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; Wang, L.; Wang, X.; Li, L.; Ma, H. CO Oxidation over Alumina-Supported Copper Catalysts. Catalysts 2022, 12, 1030. https://doi.org/10.3390/catal12091030
Ma G, Wang L, Wang X, Li L, Ma H. CO Oxidation over Alumina-Supported Copper Catalysts. Catalysts. 2022; 12(9):1030. https://doi.org/10.3390/catal12091030
Chicago/Turabian StyleMa, Guoyan, Le Wang, Xiaorong Wang, Lu Li, and Hongfei Ma. 2022. "CO Oxidation over Alumina-Supported Copper Catalysts" Catalysts 12, no. 9: 1030. https://doi.org/10.3390/catal12091030
APA StyleMa, G., Wang, L., Wang, X., Li, L., & Ma, H. (2022). CO Oxidation over Alumina-Supported Copper Catalysts. Catalysts, 12(9), 1030. https://doi.org/10.3390/catal12091030