Pd-Supported Co3O4/C Catalysts as Promising Electrocatalytic Materials for Oxygen Reduction Reaction
Abstract
:1. Introduction
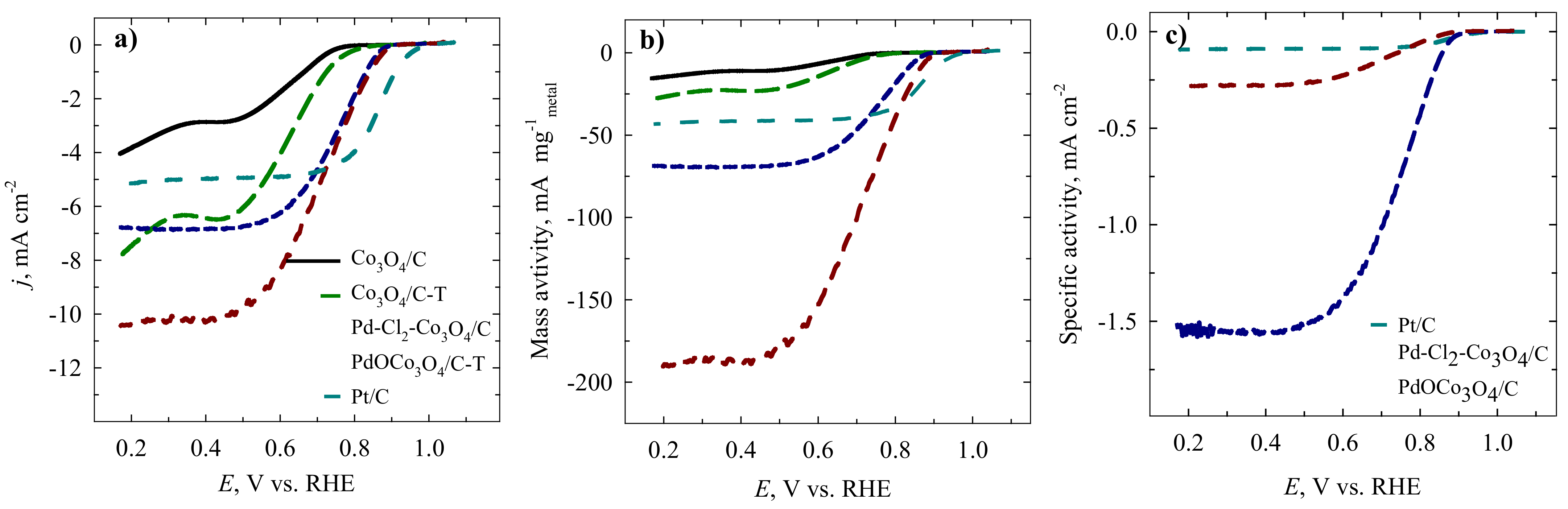
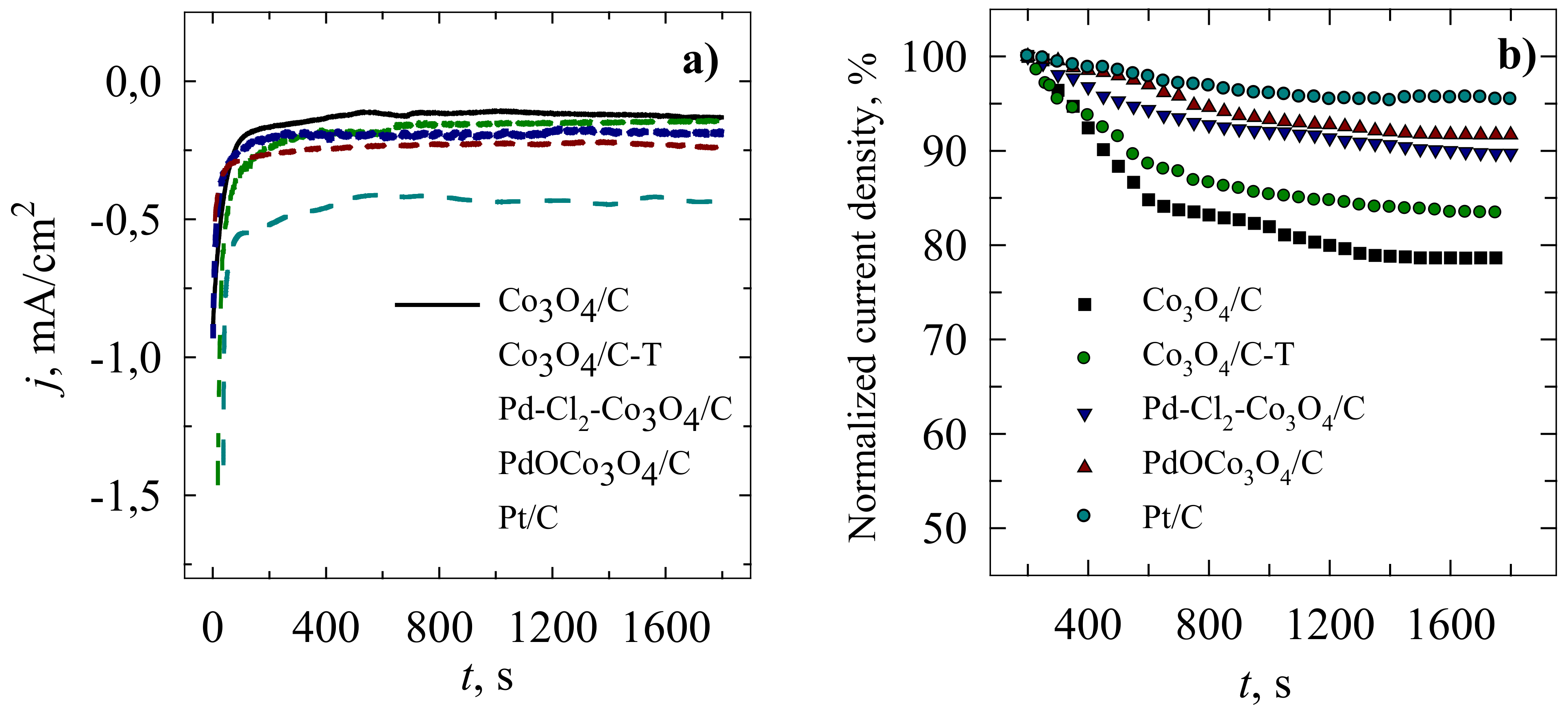
2. Results
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Catalysts
3.3. Characterization of Catalysts
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- A 1912 News Article Ominously Forecasted the Catastrophic Effects of Fossil Fuels on Climate Change. Available online: https://qz.com/817354/scientists-have-been-forecasting-that-burning-fossil-fuels-will-cause-climate-change-as-early-as-1882/ (accessed on 7 April 2022).
- Ogungbemi, E.; Ijaodola, O.; Khatib, F.N.; Wilberforce, T.; Hassan, Z.E.; Thompson, J.; Ramadan, M.; Olabid, A.G. Fuel cell membranes-Pros and cons. Energy 2019, 172, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C.-J. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, G.-M.; Yao, R.; Yu, T.; Han, M.-F.; Huang, R.-S. High oxygen reduction activity of Pt-Ni alloy catalyst for proton exchange membrane fuel cells. Catalysts 2022, 12, 250. [Google Scholar] [CrossRef]
- Chiodoni, A.; Salvador, G.P.; Massaglia, G.; Belmondo, L.; Munoz-Tabares, J.A.; Sacco, A.; Garino, N.; Castellino, M.; Margarita, V.; Ahmed, D.; et al. MnxOy-based cathodes for oxygen reduction reaction catalysis in microbial fuel cells. Int. J. Hydrogen Energy 2019, 44, 4432–4441. [Google Scholar] [CrossRef]
- Uskaikar, P.H.; Shett, P.N.; Malode, J.S. Electrocatalytic reduction of oxygen on Co3O4: Effects of processing method. Mater. Sci. Technol. 2018, 1, 129–135. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Zularisam, A.W.; Hai, I.F. Potential of porous Co3O4nanorods as cathode catalyst for oxygen reduction reaction in microbial fuel cells. Bioresour. Technol. 2016, 220, 537–542. [Google Scholar] [CrossRef] [Green Version]
- Gunji, T.; Wakabayashi, R.H.; Noh, S.H.; Han, B.; Matsumoto, F.; Di Salvo, F.J.; Abruna, H.D. The effect of alloying of transition metals (M = Fe, Co, Ni) with palladium catalysts on the electrocatalytic activity for the oxygen reduction reaction in alkaline media. Electrochim. Acta 2018, 283, 1045–1052. [Google Scholar] [CrossRef]
- Lee, Y.; Jang, J.; Lee, G.J.; Jeon, O.S.; Kim, H.S.; Hwang, H.J.; Shul, Y.G. Optimization of the Pd-Fe-Mo Catalysts for Oxygen Reduction Reaction in Proton-Exchange Membrane Fuel Cells. Electrochim. Acta 2016, 220, 29–35. [Google Scholar] [CrossRef]
- Xie, X.; Shen, W. Morphology control of cobalt oxide nanocrystals for promoting their catalytic performance. Nanoscale 2009, 1, 50–60. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cui, X.; Yang, Y.; Xiao, P.; Cao, L.; Zhang, Y. Hierarchical structures of nickel, cobalt-based nanosheets and iron oxyhydroxide nanorods arrays for electrochemical capacitors. Electrochim. Acta 2015, 161, 137–143. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Zularisam, A.W. Enhanced oxygen reduction reaction in air-cathode microbial fuel cells using flower-like Co3O4 as an efficient cathode catalyst. Int. J. Hydrogen Energy 2017, 42, 19287–19295. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Docampo, M.; Rivas-Murias, B.; Rodriguez-Gonzalez, B.; Salgueinio, V. Thermodynamically driven oxidation–induced Kirkendall effect in octahedron-shaped cobalt oxide nanocrystals. CrystEngComm 2017, 19, 5542–5548. [Google Scholar] [CrossRef]
- Qian, C.; Guo, X.; Zhang, W.; Yang, H.; Qian, Y.; Xu, F.; Qian, S.; Lin, S.; Fan, T. Co3O4 nanoparticles on porous bio-carbon substrate as catalyst for oxygen reduction reaction. Microporous Mesoporous Mater. 2019, 277, 45–51. [Google Scholar] [CrossRef]
- Shahid, M.M.; Rameshkumar, P.; Huang, N.M. Morphology dependent electrocatalytic properties of hydrothermally synthesized cobalt oxide nanostructures. Ceramics Int. 2015, 41, 13210–13217. [Google Scholar] [CrossRef]
- Tabong, C.D.; Ondoh, A.M.; Yufanyi, D.M.; Foba, J. Cobalt(II) and Zinc(II) Complexes of Hexamethylenetetramine as Single Source Precursors for their Metal Oxide Nanoparticles. J. Mater. Sci. Res. 2015, 4, 70–81. [Google Scholar] [CrossRef]
- Jia, W.; Li, J.; Lu, Z.; Juan, Y.; Jiang, Y. Synthesis of Honeycomb-Like Co3O4 Nanosheets with Excellent Supercapacitive Performance by Morphological Controlling Derived from the Alkaline Source Ratio. Materials 2018, 11, 1560. [Google Scholar] [CrossRef]
- Liang, H.; Raitano, J.M.; Zhang, L.; Chan, S.-W. Controlled Synthesis of Co3O4 Nanopolyhedrons and Nanosheets at Low Temperature. Chem. Commun. 2009, 7569–7571. [Google Scholar] [CrossRef]
- Kepenienė, V.; Stagniūnaitė, R.; Tamašauskaitė-Tamašiūnaitė, L.; Pakštas, V.; Jasulaitienė, V.; Léger, B.; Rousseau, J.; Ponchel, A.; Monflier, E.; Norkus, E. Co3O4/C and Au supported Co3O4/C nanocomposites–Peculiarities of fabrication and application towards oxygen reduction reaction. Mat. Chem. Phys. 2020, 241, 122332. [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, J.H.; Wang, J.; Li, Q.-Y.; Xu, C.-W.; Lu, X. Three-dimensional ordered mesoporous Co3O4 enhanced by Pd for oxygen evolution reaction. Sci. Rep. 2017, 7, 41542. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.Y.; Sui, L.X.; Zhao, L.; Gu, D.M.; Huang, G.S.; Wang, Z.B. Controlling the surface roughness of chain-like Pd nanowires by pH values as excellent catalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2019, 44, 6551–6559. [Google Scholar] [CrossRef]
- Erikson, H.; Sarapuu, A.; Gullón, S.J.; Tammeveski, K. Recent progress in oxygen reduction electrocatalysis on Pd-based catalysts. J. Electroanal. Chem. 2016, 780, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Chen, W.; Wang, W.; Li, J.; Chen, S. Pt-Pd nanodendrites as oxygen reduction catalyst in polymer-electrolyte-membrane fuel cell. Int. J. Hydrogen Energy 2017, 42, 25234–25243. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, C.; Gao, X.; Du, C.; Zhuang, Z.; Chen, W. Immobilizing Pd nanoclusters into electronically conductive metal-organic frameworks as bi-functional electrocatalysts for hydrogen evolution and oxygen reduction reactions. Electrochim. Acta 2019, 306, 627–634. [Google Scholar] [CrossRef]
- Liao, M.; Li, W.; Xi, X.; Luo, C.; Fu, Y.; Gui, S.; Mai, Z.; Yan, H.; Jiang, C. Highly active Pt decorated Pd/C nanocatalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 24090–24098. [Google Scholar] [CrossRef]
- Hoflund, B.; Li, Z. Surface characterization study of a Pd/Co3O4 methane oxidation catalyst. Appl. Surf. Sci. 2006, 253, 2830–2834. [Google Scholar] [CrossRef]
- Yang, N.; Ni, S.; Sun, Y.; Zhu, Y. A facial strategy to synthesize Pd/Co3O4nanosheets with enhanced performance for methane catalytic oxidation. Mol. Catal. 2018, 452, 28–35. [Google Scholar] [CrossRef]
- Angerstein-Kozlowska, H.; Conway, B.E.; Sharp, W.B.A. The real condition of electrochemically oxidized platinum surfaces: Part I. Resolution of component processes. J. Electroanal. Chem. 1973, 43, 9–36. [Google Scholar] [CrossRef]
- Grden, M.; Łukaszewski, M.; Jerkiewicz, G.; Czerwinski, A. Electrochemical behaviour of palladium electrode: Oxidation, electrodissolution and ionic adsorption. Electrochim. Acta. 2008, 53, 7583–7598. [Google Scholar] [CrossRef]
- Kepenienė, V.; Tamašauskaitė-Tamašiūnaitė, L.; Vaičiūnienė, J.; Pakštas, V.; Norkus, E. PtCeO2/C and PtNb2O5/C as electrocatalysts for ethanol oxidation. Chemija 2016, 27, 31–36. [Google Scholar]
- Jolm, F.; William, M.F.; Peter, S.E. SobolKennetlf D. Bomben. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Minesota, MN, USA, 1992. [Google Scholar]
- Militello, M.C.; Simko, S.J. NIST Standard Reference Database 20, Version 4.1, Web Version. Surf. Sci. Spectra 1994, 3. Available online: http://srdata.nist.gov/xps/ (accessed on 12 July 2022).
- Yao, X.; Xin, X.; Zhang, Y.; Wang, J.; Liu, Z.; Xu, X. Co3O4 nanowires as high capacity anode materials for lithium ion batteries. J. Alloy Compd. 2012, 521, 95–100. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, L.; Shang, Y.; Gao, J.; Li, J.; He, X. Facile synthesis of monodisperse Co3O4 mesoporous microdisks as an anode material for lithium ion batteries. Electrochim. Acta 2015, 151, 109–117. [Google Scholar] [CrossRef]
- Mc Intyre, N.S.; Johnston, D.D.; Coatsworth, L.L.; Davidson, R.D.; Brown, J.R. NIST Standard Reference Database 20, Version 4.1, Web Version. Surf. Interface Anal. 1990, 15, 265. [Google Scholar]
- Hsieh, P.-T.; Chen, Y.-C.; Kao, K.S.; Wang, C.M. Luminescence mechanism of ZnO thin film investigated by XPS measurement. Appl. Phys. A 2008, 90, 317–321. [Google Scholar] [CrossRef]
- Zhu, Y.; Yue, M.; Natarajan, V.; Kong, L.; Ma, L.; Zhang, Y.; Zhao, Q.; Zhan, J. Efficient activation of persulfate by Fe3O4@β-cyclodextrin nanocomposite for removal of bisphenol A. RSC Adv. 2018, 8, 14879–14887. [Google Scholar] [CrossRef] [Green Version]
- Shi, K.-M.; Cheng, X.; Jia, Z.-Y.; Guo, J.-W.; Wang, C.; Wang, J. Oxygen reduction reaction of Fe-polyaniline/carbon nanotube and Pt/C catalysts in alkali media. Int. J. Hydrogen Energy 2016, 41, 16903–16912. [Google Scholar] [CrossRef]
- Ge, X.; Sumboja, A.; Wuu, D.; An, T.; Li, B.; Goh, T.F.W.; Hor, A.T.S.; Zong, Y.; Liu, Z. Oxygen reduction in alkaline media: From mechanisms to recent advances of catalysts. ACS Catal. 2015, 5, 4643–4667. [Google Scholar] [CrossRef]
- He, Q.; Li, Q.; Khene, S.; Ren, X.; López-Suárez, F.E.; Lozano-Castelló, D.; Bueno-López, A.; Wu, G. High-loading cobalt oxide coupled with nitrogen-doped graphene for oxygen reduction in anion-exchange-membrane alkaline fuel cells. J. Phys. Chem. C. 2013, 117, 8697–8707. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Lu, L.; He, Y.; Qi, X.; Deng, Y. Carbon supported MnOx-Co3O4 as cathode catalyst for oxygen reduction reaction in alkaline media. Int. J. Hydrogen Energy 2013, 38, 13611–13616. [Google Scholar] [CrossRef]
- Wang, T.; Chutia, A.; Brett, D.J.L.; Shearing, P.R.; He, G.; Chai, G.; Parkin, I.P. Palladium alloys used as electrocatalysts for the oxygen reduction reaction. Energy Environ. Sci. 2021, 14, 2639–2669. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Y.; Zhang, L.; Tang, T.; Xiao, H.; Chen, W.; Zhao, M.; Jia, J.; Zhu, H. Facile synthesis of PdO-doped Co3O4 nanoparticles as an efficient bifunctional oxygen electrocatalyst. App. Catal. B Environ. 2019, 243, 175–182. [Google Scholar] [CrossRef]
- Paulus, U.; Schmidt, T.; Gasteiger, H.; Behm, R. Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst: A thin-film rotating ring-disk electrode study. J. Electroanal. Chem. 2001, 495, 134–145. [Google Scholar] [CrossRef]
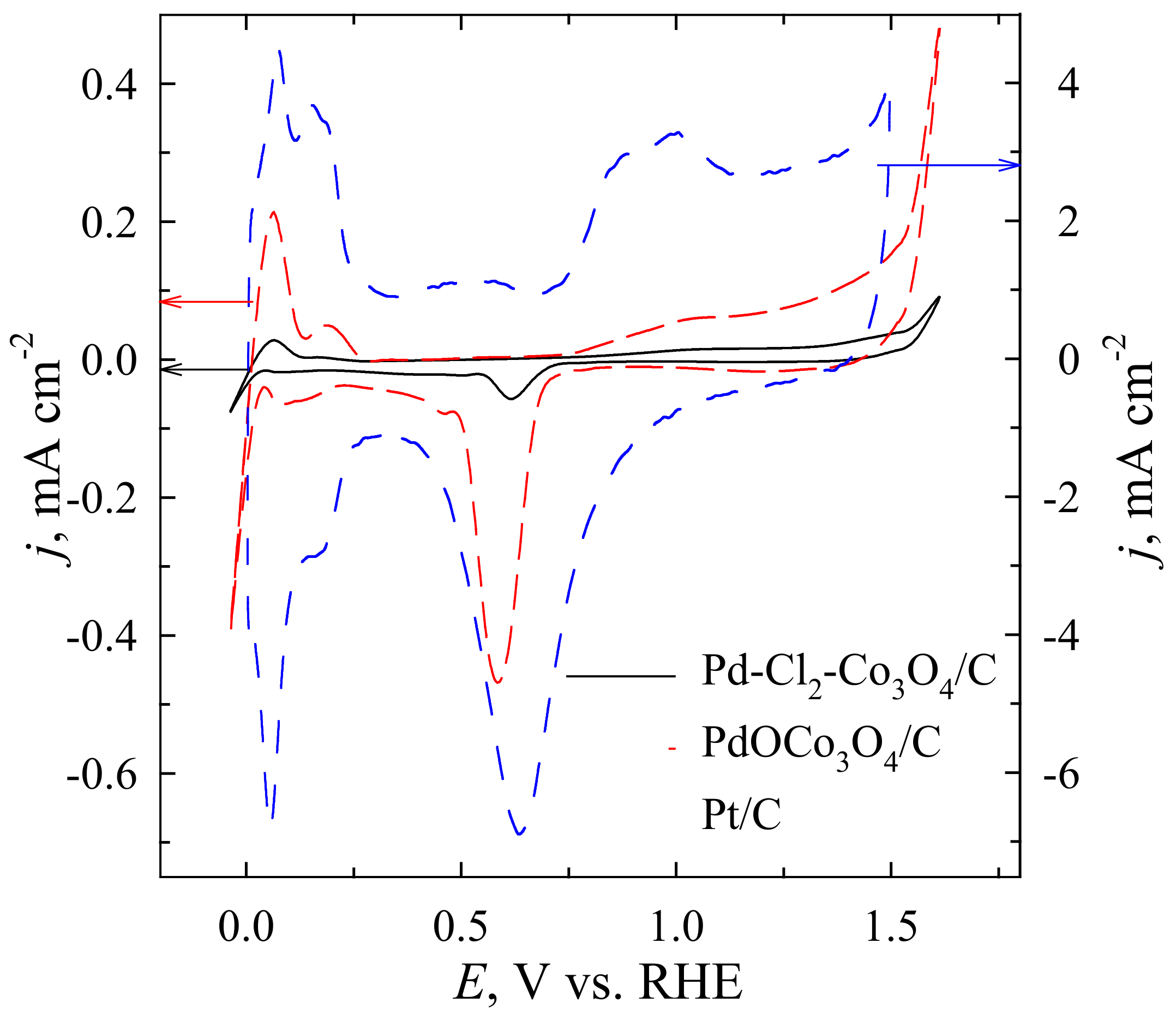

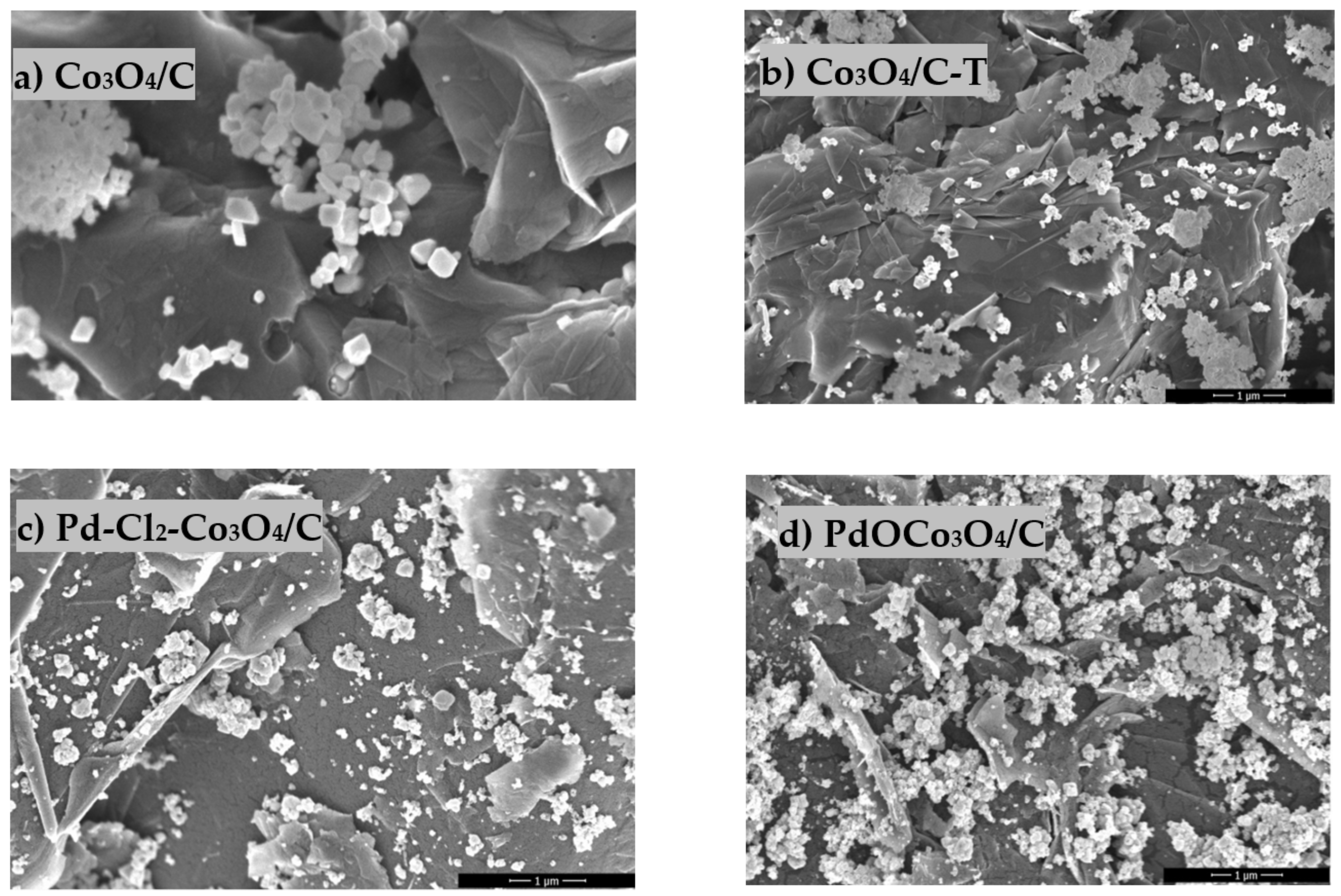

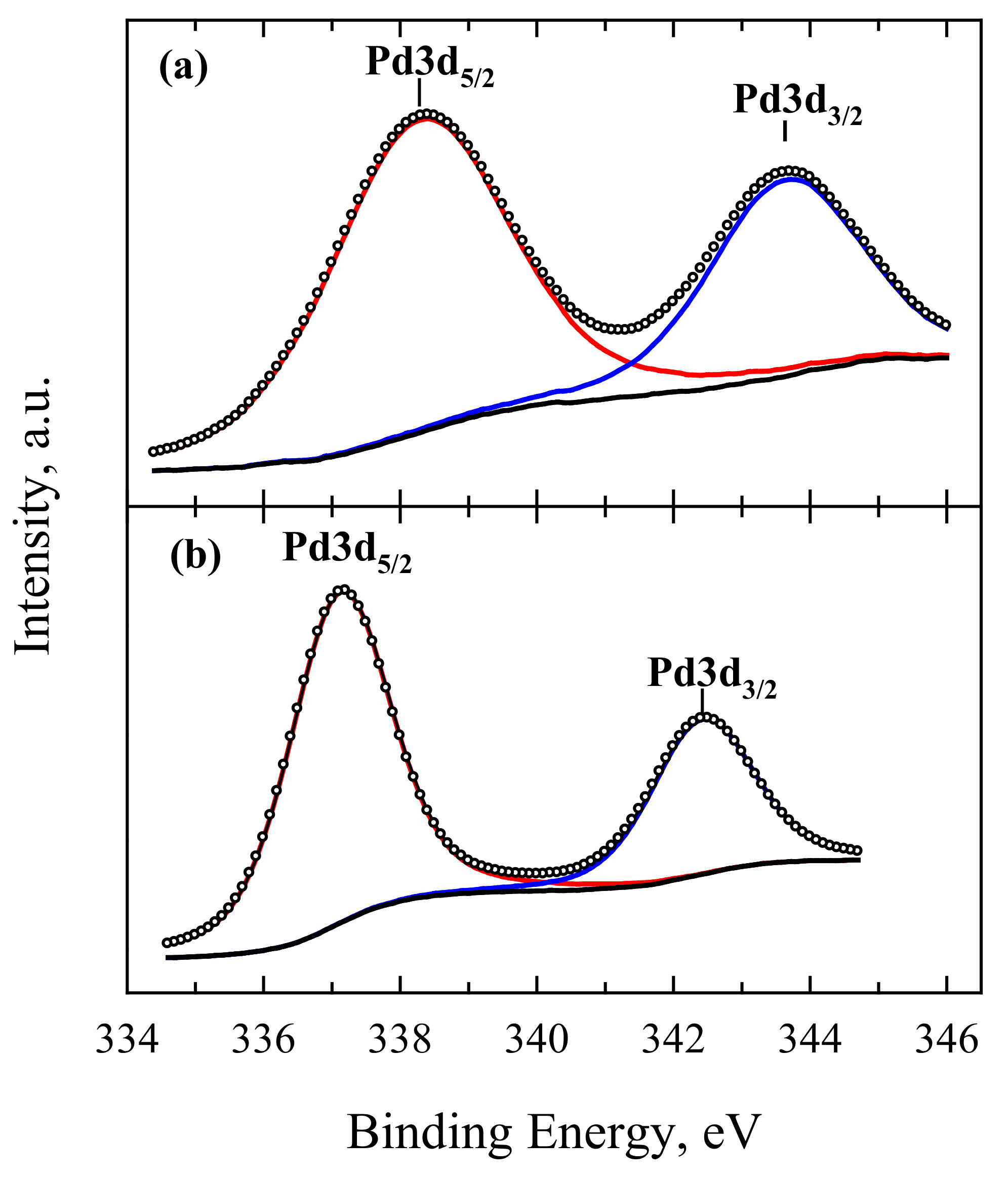

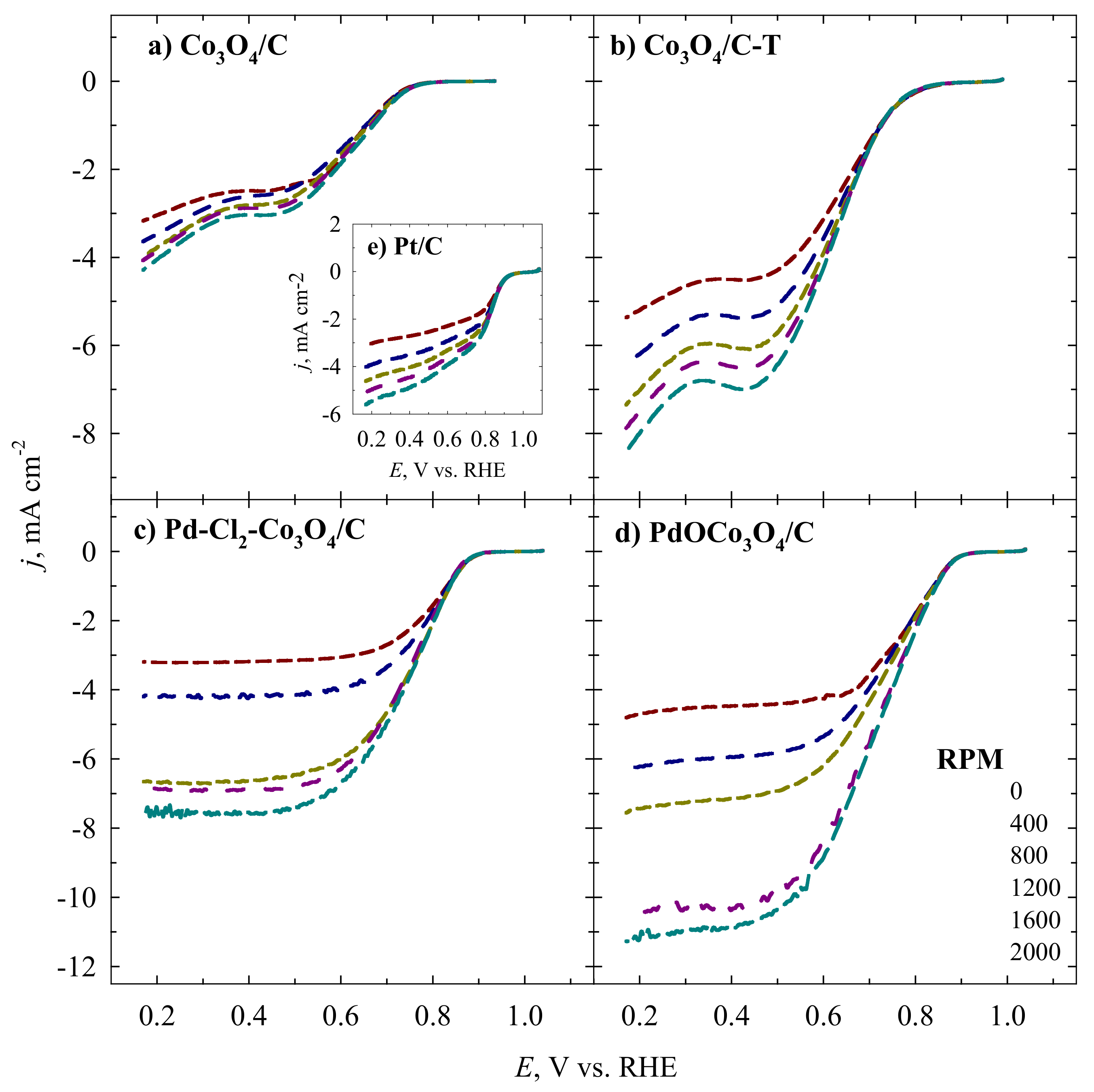

| Catalyst | Loading, µg cm−2 | Mass Weight, wt.% | ESA | ||||
|---|---|---|---|---|---|---|---|
| Pt | Pd | Co | PdCl2/PdO | Co3O4 | cm−2 | m2 g−1 | |
| Co3O4/C | - | - | 254.29 | - | 58 | - | - |
| Co3O4/C-T | - | - | 273.48 | - | 74 | - | - |
| Pd-Cl2-Co3O4/C | - | 83.00 | 98.43 | 7.7 | 22.5 | 0.34 | 10.04 |
| PdOCo3O4/C | 47.05 | 54.46 | 3.03 | 12.46 | 2.54 | 74.97 | |
| Pt/C | 118.40 | - | - | - | - | 11.04 | 47.52 |
| Catalyst | Crystallite—Average Size Value, nm | ||
|---|---|---|---|
| XRD | TEM | ||
| PdO/Pt | Co3O4 | ||
| Co3O4/C | - | ~12.0 | ~25.0 |
| Co3O4/C-T | - | 18.1 ± 0.4 | ~25.0 |
| Pd-Cl2-Co3O4/C | - | ~12.0 | ~6.0 |
| PdOCo3O4/C | 4.0 | 13.8 ± 0.5 | ~3.0–44 |
| Pt/C | 4–5 [30] | - | 4–5 [30] |
| Catalyst | Pd3d5/2 | Pd3d3/2 | Co2p3/2 | O1s | C1s | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eb, eV | at.% | Eb, eV | at.% | Eb, eV | at.% | Eb, eV | at.% | Eb, eV | at.% | |
| Pd-Cl2-Co3O4/C | 338.28 | 65.63 | 343.63 | 34.37 | 780.15 | 41.64 | 529.91 | 29.42 | 284.26 | 50.25 |
| 783.09 | 39.13 | 531.86 | 24.35 | 284.87 | 28.16 | |||||
| 786.30 | 19.23 | 532.82 | 19.98 | 286.41 | 14.64 | |||||
| 533.92 | 26.25 | 287.73 | 6.95 | |||||||
| PdOCo3O4/C | 337.11 | 67.93 | 342.42 | 32.07 | 779.55 | 57.03 | 529.70 | 8.39 | 284.19 | 34.73 |
| 782.22 | 28.19 | 531.27 | 24.84 | 284.54 | 24.46 | |||||
| 532.69 | 44.72 | 285.84 | 26.36 | |||||||
| 786.17 | 14.78 | 533.98 | 17.82 | 286.94 | 11.74 | |||||
| 535.03 | 3.22 | 288.49 | 2.71 | |||||||
| Catalyst | Eonset, V vs. RHE | Ehalf-wave V vs. RHE | Electrolyte | References |
|---|---|---|---|---|
| Co3O4/C | 0.78 | 0.62 | 0.1 M NaOH | This work |
| Co3O4/C-T | 0.83 | 0.63 | 0.1 M NaOH | This work |
| Pd-Cl2-Co3O4/C | 0.91 | 0.75 | 0.1 M NaOH | This work |
| PdOCo3O4/C | 0.92 | 0.72 | 0.1 M NaOH | This work |
| Pt/C | 0.95 | 0.86 | 0.1 M NaOH | This work |
| PdYNPs | 0.90 | 0.85 | 0.1 M KOH | [42] |
| Pd3Fe NPs/CB | 0.90 | Not presented | 0.1 M NaOH | [8] |
| Pd3Co NPs/CB | 0.90 | Not presented | 0.1 M NaOH | [8] |
| Pd3Ni NPs/CB | 0.87 | Not presented | 0.1 M NaOH | [8] |
| Pd@Zncore shell | 0.85 | 0.82 | 0.1 M KOH | [42] |
| Co3O4/CIMP-MW | 0.83 | 0.67 | 0.1 M NaOH | [19] |
| Co3O4/C | 0.83 | 0.78 | 1 M KOH | [43] |
| PdCo-300 | 0.81 | 0.83 | 0.1 M KOH | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kepenienė, V.; Stagniūnaitė, R.; Rafique, S.; Vaičiūnienė, J.; Jasulaitiene, V.; Pakštas, V.; Sukackienė, Z.; Vilkauskaite, R.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Pd-Supported Co3O4/C Catalysts as Promising Electrocatalytic Materials for Oxygen Reduction Reaction. Catalysts 2022, 12, 920. https://doi.org/10.3390/catal12080920
Kepenienė V, Stagniūnaitė R, Rafique S, Vaičiūnienė J, Jasulaitiene V, Pakštas V, Sukackienė Z, Vilkauskaite R, Tamašauskaitė-Tamašiūnaitė L, Norkus E. Pd-Supported Co3O4/C Catalysts as Promising Electrocatalytic Materials for Oxygen Reduction Reaction. Catalysts. 2022; 12(8):920. https://doi.org/10.3390/catal12080920
Chicago/Turabian StyleKepenienė, Virginija, Raminta Stagniūnaitė, Sidra Rafique, Jūratė Vaičiūnienė, Vitalija Jasulaitiene, Vidas Pakštas, Zita Sukackienė, Rasa Vilkauskaite, Loreta Tamašauskaitė-Tamašiūnaitė, and Eugenijus Norkus. 2022. "Pd-Supported Co3O4/C Catalysts as Promising Electrocatalytic Materials for Oxygen Reduction Reaction" Catalysts 12, no. 8: 920. https://doi.org/10.3390/catal12080920
APA StyleKepenienė, V., Stagniūnaitė, R., Rafique, S., Vaičiūnienė, J., Jasulaitiene, V., Pakštas, V., Sukackienė, Z., Vilkauskaite, R., Tamašauskaitė-Tamašiūnaitė, L., & Norkus, E. (2022). Pd-Supported Co3O4/C Catalysts as Promising Electrocatalytic Materials for Oxygen Reduction Reaction. Catalysts, 12(8), 920. https://doi.org/10.3390/catal12080920









