1. Introduction
Selective catalytic reduction of nitrogen oxides (NO
x, where x is 1 or 2) using NH
3 as the reducing agent (NH
3-SCR) was patented by the Engelhard Corporation (USA) in 1957 [
1]. The first large-scale NH
3-SCR installation was implemented by the IHI Corporation in 1978 [
2]. Nowadays, NH
3-SCR technology is used in the most important commercial methods used for the control of NO
x emissions by electric power stations as well as waste treatment plants [
3]. Monolithic vanadium-based catalysts, such as V
2O
5-WO
3/TiO
2 or V
2O
5-MoO
3/TiO
2, are typically used as industrial NH
3-SCR catalysts for the conversion of NO
x emitted from such stationary sources [
4]. In recent decades, the NH
3-SCR process has been adapted for NO
x conversion in exhaust gases of diesel vehicles [
5]. This technology, called Blue Tec, utilises aqueous urea solution, which is hydrolysed to NH
3 and CO
2 before ammonia is used as a reducing agent for NO
x conversion, similar to a typical NH
3-SCR process. Cu-exchanged zeolites, such as Cu-CHA, are used as catalysts in urea-based SCR systems in diesel vehicles [
6]. In general, NH
3-SCR technology is a mature and well-optimised method utilised for NO
x emission control in electric power stations and industrial boilers. However, there are some drawbacks to this technology that limit its adaptation to current needs. The most important is related to the operation of the NH
3-SCR monolithic converters with dusty gases, which may result in clogging of channels and finally decrease the efficiency of the NO
x conversion. Nowadays, most of the NH
3-SCR converters are located upstream of an electrostatic precipitator (ESP) and therefore operate with a dusty gas stream (high-dust NH
3-SCR). To solve the monolith clogging problem, flue gases de-dusting in the ESP unit should be undertaken prior to the exhaust gas being directed to the NH
3-SCR converter (low-dust NH
3-SCR). The problem is that the ESP units effectively operate only with relatively cold flue gases, at 250 °C or even lower. Thus, in such modified installations that operate with commercial vanadium-based catalysts of the NH
3-SCR process, the de-dusted gas, downstream of the ESP unit, must be additionally heated to above 300 °C prior to being directed to the NH
3-SCR converter [
4,
7]. Of course, such rearrangement of the exhaust gas purification system should result in protection of the monolithic catalysts against clogging by dust particles; however, it increases the operating costs of such an installation due to the additional heating of the exhaust gases downstream of the ESP unit. An optional, possibly cheaper, solution could be an effective NO
x conversion at a temperature below 250 °C without the need to heat the exhaust gases between the ESP and NH
3-SCR units. However, to implement this option, the development of an effective catalyst of the NH
3-SCR process operating at temperatures below 250 °C is necessary.
Mesoporous silica materials are very promising supports of low-temperature NH
3-SCR catalysts [
4,
8,
9,
10]. Such materials, produced by the surfactant directed methods, are characterized by uniform mesoporous structure, high surface area and thermal and hydrothermal stability in the temperature range of the low-temperature NH
3-SCR process. Catalytically active components can be effectively deposited on the silica surface in highly dispersed form by the template ion-exchange (TIE) method [
4,
8,
9,
10,
11]. Our previous studies of MCM-41 modified with copper and iron showed its very promising catalytic performance in the NH
3-SCR process [
4,
8,
9,
10]. Copper modified silica, especially by the TIE method, presented a very good catalytic activity in the low-temperature range [
4,
8,
9,
10], while the iron-doped mesoporous silica samples were catalytically active at higher temperatures [
4,
8,
9,
10]. Moreover, our previous studies [
4,
9] showed that deposition of copper and iron on spherical MCM-41 resulted in the catalysts being more selective to nitrogen and effectively operating in a slightly broader temperature range comparing to the analogous catalysts based on cylindrical MCM-41. This is possibly related to the shorter residence time of reactant molecules inside pores of MCM-41 and therefore also lower risk of the side reactions (e.g., NH
3 to NO oxidation). An important additional conclusion is related to the incorporation of aluminium into silica walls prior to transition metals deposition. It has been shown that the generation of acid sites by aluminium species incorporated into MCM-41 walls is not beneficial for the low-temperature NH
3-SCR process, but results in activation of the catalysts at higher temperatures [
12]. Manganese is another component of the low-temperature NH
3-SCR catalysts broadly reported in the scientific literature [
13,
14,
15]. Manganese may exist in different oxidations states, such as Mn
2+, Mn
3+ and Mn
4+, however it has been postulated that Mn
4+ is the most active in the low-temperature NH
3-SCR process [
16], because it can promote NO oxidation to NO
2, which is necessary for the fast NH
3-SCR reaction (2NH
3 + NO + NO
2 → 2N
2 + 3H
2O) [
17]. On the other hand, such a high activity of manganese in oxidation processes is possibly responsible for direct ammonia oxidation at higher temperatures and therefore a decrease in the NH
3-SCR efficiency in this range [
18].
The main goal of the studies was the determination of the catalytic performance of transition metal pairs (Cu-Fe, Cu-Mn, and Fe-Mn) deposited on spherical MCM-41 by TIE method as well as analysis of the possible synergistic interactions between these metals in the NH3-SCR process. High surface area mesoporous silica materials as well as application of TIE method for metal deposition should result in catalysts with a high contribution of surface available catalytically active monometallic and bimetallic species. This is a very important advantage of this type of catalyst in comparison with unsupported mixed metal oxide catalysts. Moreover, the relatively wide channels (in mesoporous range) should ensure effective reactant diffusion. Thus, such catalytic systems offer some advantages for the effective catalyst design.
2. Results and Discussion
The spherical morphology of MCM-41 was proved by scanning electron microscopy (SEM). The size of the silica spheres was in the range of 600–700 nm with a smaller contribution of spheres with diameters below 300 nm (
Supplementary Materials, Figure S1). Deposition of transition metals into S-MCM-41 (monometallic and bimetallic samples) did not result in significant changes in silica morphology (
Supplementary Materials, Figures S2 and S3). However, as verified by EDS analysis, nanorods of CuO were identified in the CuFe sample. The thickness of such nanorods was about 50–60 nm, while their length was typically above 3000 nm. Moreover, in the case of this sample, small aggregates of CuO with an average diameter of 30–50 nm, deposited on the surface of silica spheres, could be identified (
Supplementary Materials, Figure S3A). The formation of similar CuO nanorods has been reported for copper oxide synthesized by wet chemical-assisted hydrothermal processing using polyethylene glycol (PEG 4000) as the structure-directing template [
19]. On the other hand, the formation of similar nanorod Fe
2O
3 crystallites has been reported in our previous studies [
11,
20] for iron deposition into as-prepared MCM-41 by the TIE method. Hexadecyltrimethylammonium cations extracted from pores of as-prepared S-MCM-41 to the solution of CuCl
2 can possibly play a role of structure-directing agent, similar to PEG 4000 [
19]. This interesting effect needs additional studies to explain the mechanism behind such nanorod metal oxides formation.
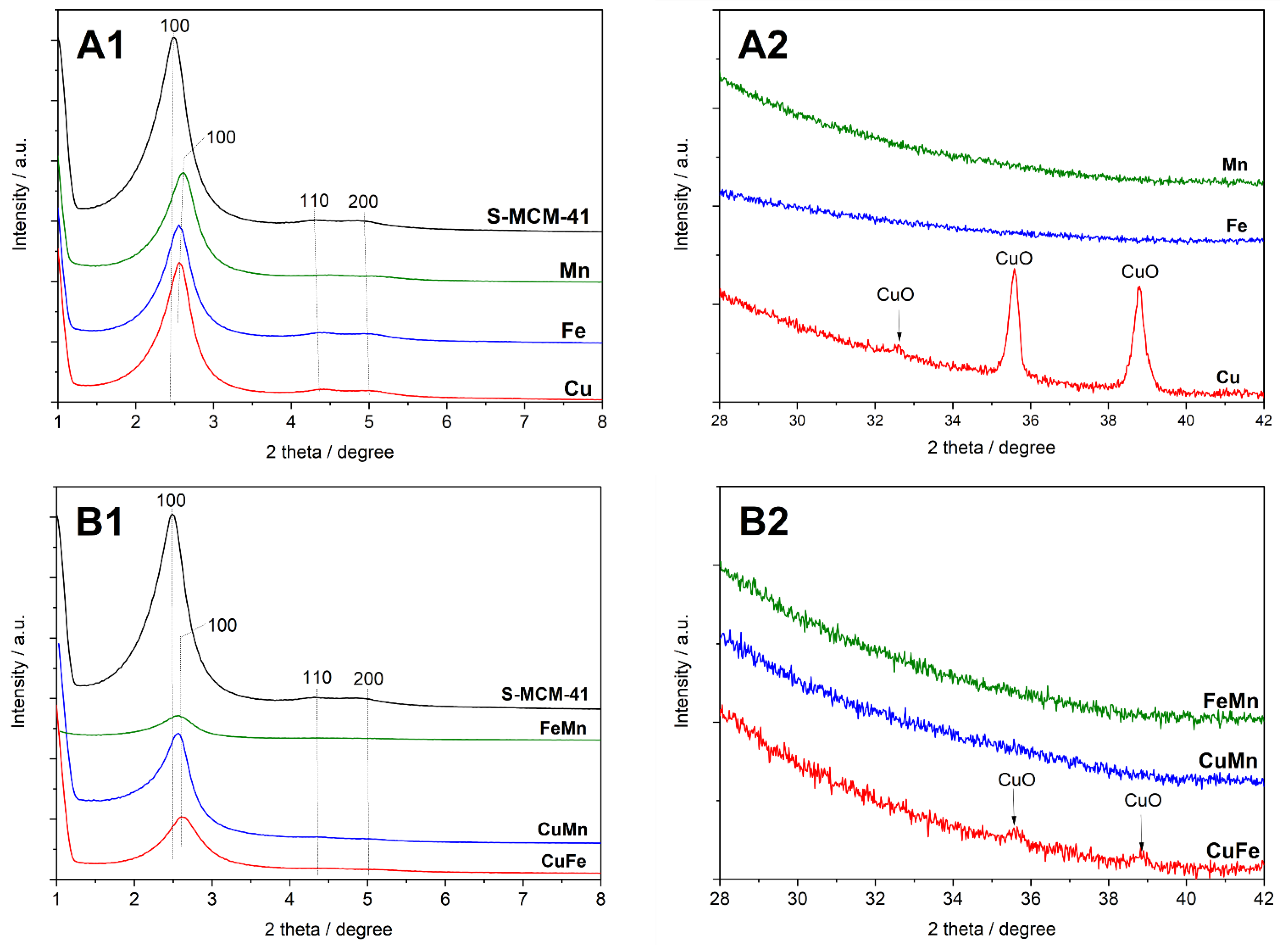
The XRD patterns of spherical MCM-41 (S-MCM-41) and its modifications with transition metals—Cu, Fe and Mn—deposited by the TIE method are shown in
Figure 1(A1,A2). In diffractogram of S-MCM-41 three reflections, (100), (110) and (200), characteristic of the hexagonal structure of MCM-41, are observed. Deposition of transition metals only slightly decreased the intensity of these diffraction peaks (
Figure 1(A1)) indicating that the TIE method did not result in a significant destruction of the ordered porous structure of S-MCM-41. On the other hand, a shift of (100) diffraction peak into higher values of 2θ (
Figure 1(A1)) is possibly related to the deposition of transition metal species inside pores and therefore decreasing their size. In the case of S-MCM-41 modified with copper species, the sample Cu, reflections characteristic of small CuO crystallites were found (
Figure 1(A2)). The estimated size of CuO crystallites (about 30 nm) is significantly larger than the pore diameter of S-MCM-41 (3.25 nm), indicating that copper oxide crystallites are located outside of the mesoporous system of the silica support. Similar reflections, characteristic of iron and manganese oxides were not found in the S-MCM-41 samples modified with these transition metals. Deposition of transition metal pairs by the TIE method significantly decreased the intensity of the (100), (110) and (200) diffraction peaks (
Figure 1(B1)), indicating partial destruction of the ordered porous structure of S-MCM-41. This effect is the most significant for the FeMn sample. Deposition of transition metal pairs, similar to the monometallic samples, shifted the (100) reflection into slightly higher values of 2θ, indicating a possible decrease in pore size due to deposition of metal species inside pores. In the case of the CuFe sample, the low-intensive reflections characteristic of CuO can be identified (
Figure 1(B2)). Such diffraction peaks are not present in the diffraction pattern of the CuMn sample. Thus, deposition of manganese together with copper inhibits aggregation of copper species into CuO crystallites.
The nitrogen adsorption–desorption isotherms of S-MCM-41 and its modifications with manganese, iron, and copper, presented in
Figure S4A (Supplementary Materials), are classified as type IV according to the IUPAC standards and are characteristic of mesoporous materials, such as MCM-41 [
21,
22]. The characteristic feature of the recorded isotherms is a steep increase in nitrogen uptake at relative pressure of 0.15–0.30 assigned to the capillary condensation of N
2 molecules inside mesopores. Deposition of transition metal pairs resulted in a small decrease in nitrogen uptake, indicating a decrease of mesopore volume, possibly related to deposition of the larger amount of transition metal species deposited inside mesopores (
Supplementary Materials, Figure S4B). An additional increase in nitrogen sorption, observed for the CuMn sample in the relative pressure range of 0.5–0.9, is possibly related to the presence of larger pores located between sticked silica spheres. The hysteresis loops in adsorption–desorption isotherms belong to the H3 category according to the IUPAC classification characteristic of the pore network consisting of large pores only partially filled with the condensate [
21,
22].
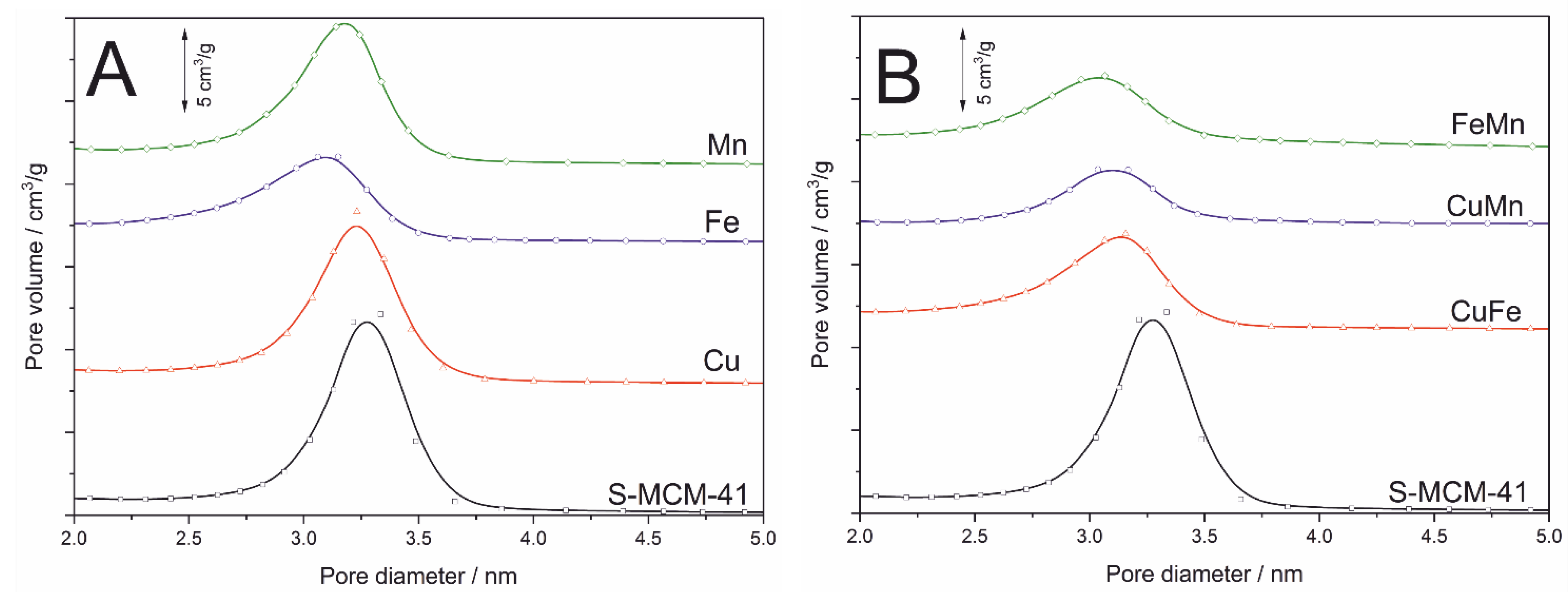
The profiles of pore size distribution (PSD) determined for S-MCM-41 and its modifications are presented in
Figure 2. The PSD profile of S-MCM-41 shows its uniform porous structure with the maximum pore diameter at about 3.3 nm. Deposition of transition metals resulted in a decrease of PSD maximum intensity and shift of the maximum to lower diameters (3.1–3.2 nm), indicating deposition of transition metal species inside pores (
Figure 2A). This effect was more significant for the samples modified with transition metal pairs (
Figure 2B). In this case pore diameter decreased to about 3.0–3.2 nm.
Textural parameters of the samples are compared in
Table 1. The specific surface area (S
BET) determined for S-MCM-41 is 1063 m
2·g
−1. Deposition of transition metals decreased S
BET by less than 3% and pore volume by less than 18%. In the case of the samples modified with pairs of transition metals, the S
BET values decreased by 2–16% and pore volume by about 12–19%.
The content of transition metals deposited to S-MCM-41 is presented in
Table 1. In the case of the monometallic samples, the metal loading is in the range of 1.4–1.9 wt%, while for the bimetallic samples the loading of each deposited transition metal is in the range of 1.3–1.9 wt%.
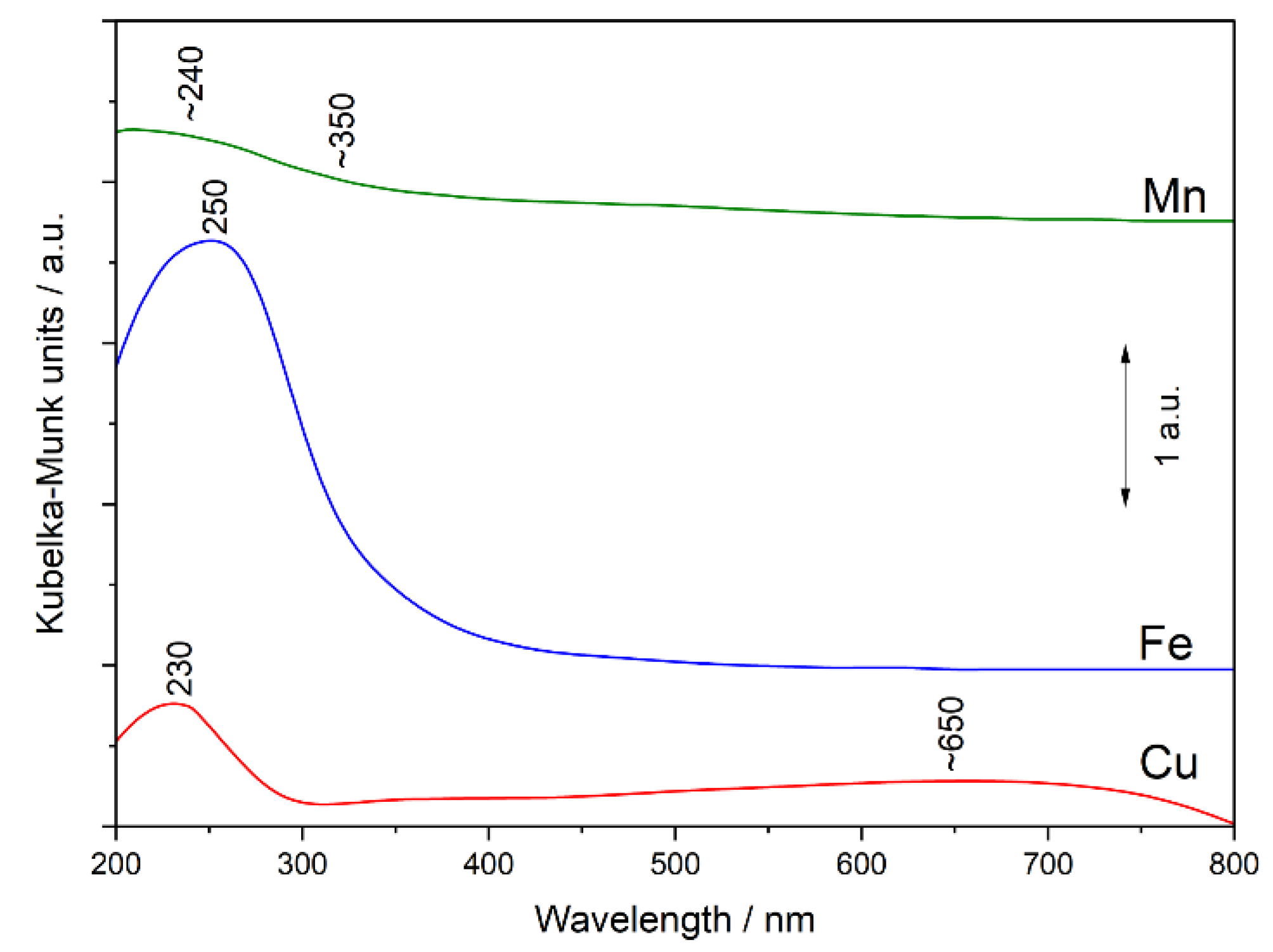
The coordination and aggregation of transition metals introduced into S-MCM-41 were analysed by UV-vis DR spectroscopy.
Figure 3 presents the spectra recorded for the monometallic samples. The spectrum of the Cu sample contains main maximum at about 230 nm assigned to monomeric Cu
2+ ions interacting with oxygens of silica (O
2– → Cu
2+) [
23,
24]. The presence of monomeric Cu
2+ ions is also proved by the presence of the band at about 650 nm related to d-d transition of Cu
2+ ions in pseudo-octahedral coordination (e.g., Cu(H
2O)
62+) [
23,
24]. The increased level of absorbance, observed above 300 nm, is assigned to the aggregated copper oxide species of different sizes (oligomeric and small crystallites) [
24,
25].
The main band in the spectrum of the Fe sample (
Figure 3), located at about 250 nm, is possibly a superposition of three bands. The band assigned to monomeric Fe
3+ cations in tetrahedral coordination is expected at about 210 nm. The band related to mononuclear Fe
3+ ions in octahedral coordination, which possibly dominate in the studied sample, should appear at about 260 nm [
25,
26,
27]. The shoulder at about 300–400 nm is characteristic of small oligonuclear Fe
xO
y clusters [
28]. Thus, the obtained results indicate that Fe
2+ was oxidised to Fe
3+ during iron deposition process, possibly in calcination step. Iron was deposited in the form of highly dispersed species, mainly as mononuclear Fe
3+ ions in octahedral coordination, which is in line with the results of XRD studies (
Figure 1(A2)).
The broad band, located in a spectrum of the Mn sample below 350 nm, is possibly a superposition of two sub-bands. The first, expected at about 240 nm, is assigned to the O-Mn
2+ ligand-to-metal charge-transfer transition and the
6A
1g →
4T
1g ligand field transition of distorted octahedral Mn
2+. The band at 315 nm is related to O-Mn
3+ ligand-to-metal charge-transfer transition [
29]. Moreover, the broad shoulder above 350 nm is possibly related to the O-Mn
2+ ligand-to-metal
6A
1g →
4T
2g transition [
29]. Thus, the obtained results indicate that part of Mn
2+ cations were oxidized to Mn
3+ ions.
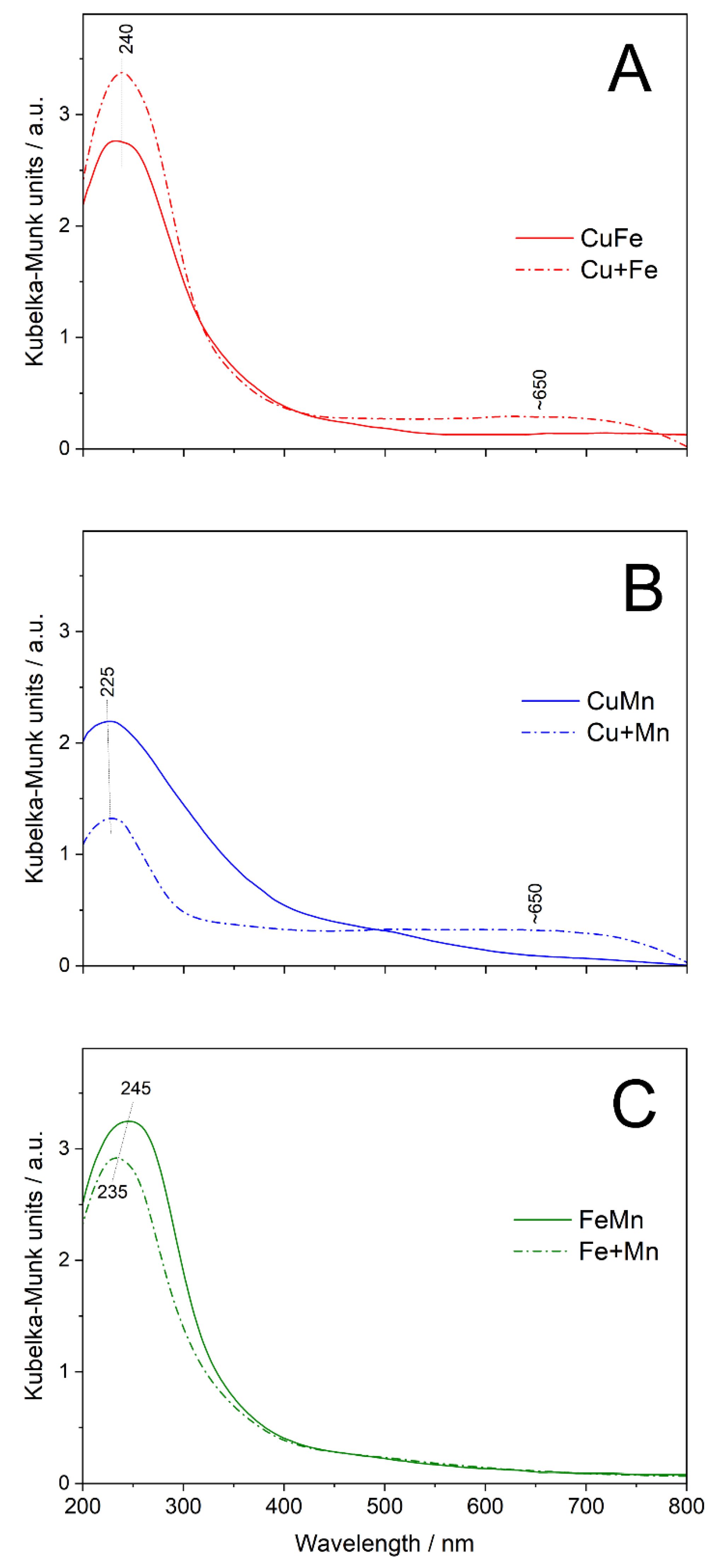
An analysis of UV-vis DR spectra of the bimetallic samples is difficult due to overlapping bands originated from different metal species. To identify the differences in the forms of metal species in the bimetallic and monometallic samples, the original spectra of the bimetallic samples were compared to the sum of the monometallic samples’ spectra (
Figure 4). The main difference in the spectrum of the bimetallic CuFe sample and the sum of the monometallic samples’ spectra (Cu+Fe) is related to the increased level of absorbance observed above 450 nm (
Figure 4A) assigned to the presence of aggregated copper oxide species in the Cu sample [
24,
25]. Thus, the UV-vis DRS studies proved the results of XRD studies (
Figure 1(A2,B2)), showing that deposition of copper and iron significantly limits the formation of CuO crystallites.
The spectrum of the bimetallic CuMn sample consists of the asymmetric band centred at about 225 nm, related to monomeric Cu
2+ and Mn
2+ cations (
Figure 4B). In the case of the cumulative spectrum of the monomeric samples (Cu+Mn), this band is located exactly in the same position. The shoulder above 400 nm in a spectrum of the CuMn sample is possibly related to the presence of Mn
2+ cations [
29]. However, this shoulder could also be assigned to the presence of aggregated, possibly oligomeric, copper oxide species [
24,
25]. The increased level of absorbance at higher wavelength, characteristic of the presence of CuO aggregates, is less intensive in the spectrum of the CuMn sample comparing to the sum of the Cu and Mn spectra (Cu+Mn), indicating limited contribution of such aggregates in the bimetallic sample.
The spectrum of the FeMn sample consists of the band centred at about 240 nm and the shoulder above 340 nm (
Figure 4C). The main peak is related to monomeric Fe
3+ and Mn
2+ cations, while the shoulder is assigned to oligomeric iron oxide species (region about 340 nm), Mn
3+ cations (region about 340 nm) and Mn
2+ (region above 450 nm) [
25,
26,
27,
29]. The cumulative spectrum of the Fe and Mn samples (Fe+Mn) is very similar to the spectrum of the bimetallic FeMn sample.
Reducibility of transition metal species deposited on S-MCM-41 was analysed by a temperature-programmed reduction method (H
2-TPR). The reduction profile of the Cu sample consists of a sharp peak at about 260 °C with a shoulder at 215 °C and a broad peak at about 510 °C (
Figure 5). The low-temperature shoulder at about 215 °C is possibly assigned to the reduction of copper (Cu
2+ → Cu
0) in the form of oligomeric copper oxide species and CuO crystallites [
30]. The peak at 260 °C is related to the reduction of monomeric Cu
2+ cations to Cu
+ species, while the broad maximum of about 510 °C is a possible superposition representing two processes—reduction of monomeric Cu
+ cations to Cu
0 [
31] expected below 450 °C as well as reduction of strongly stabilized copper species (possibly at higher temperatures). Thus, the results of H
2-TPR prove that copper was deposited into S-MCM-41 in the form of monomeric cations together with aggregated metal oxide species.
Reduction profile of the Fe sample consists of a broad peak spread in the range of 120–725 °C with the main maximum at about 420 °C and two shoulders at 270 and 560 °C (
Figure 5). It has been reported that reduction (Fe
3+ → Fe
2+) temperature of iron species deposited on mesoporous silicas decreases with an increase in their aggregation [
32,
33,
34]. Therefore, it seems that the low-temperature peak at 270 °C is assigned to the reduction of Fe
3+ to Fe
2+ cations in aggregated metal oxide species, while the main maximum at 420 °C is related to the reduction of monomeric Fe
3+ cations to Fe
2+. The broad maximum at about 560 °C is related to the reduction of Fe
2+ to Fe
0 [
35]. Thus, iron deposited into S-MCM-41 is present mainly in the form of monomeric cations with the smaller contribution of aggregated metal oxide species.
The reduction profile of the Mn sample is spread in the range of 170–660 °C and consists of at least two overlapping peaks at about 340 and 530 °C (
Figure 5). The low-temperature peak is assigned to the reduction of highly dispersed Mn
3O
4 species to MnO, while the high-temperature maximum is possibly attributed to the reduction of more aggregated Mn
3O
4 species [
36,
37].
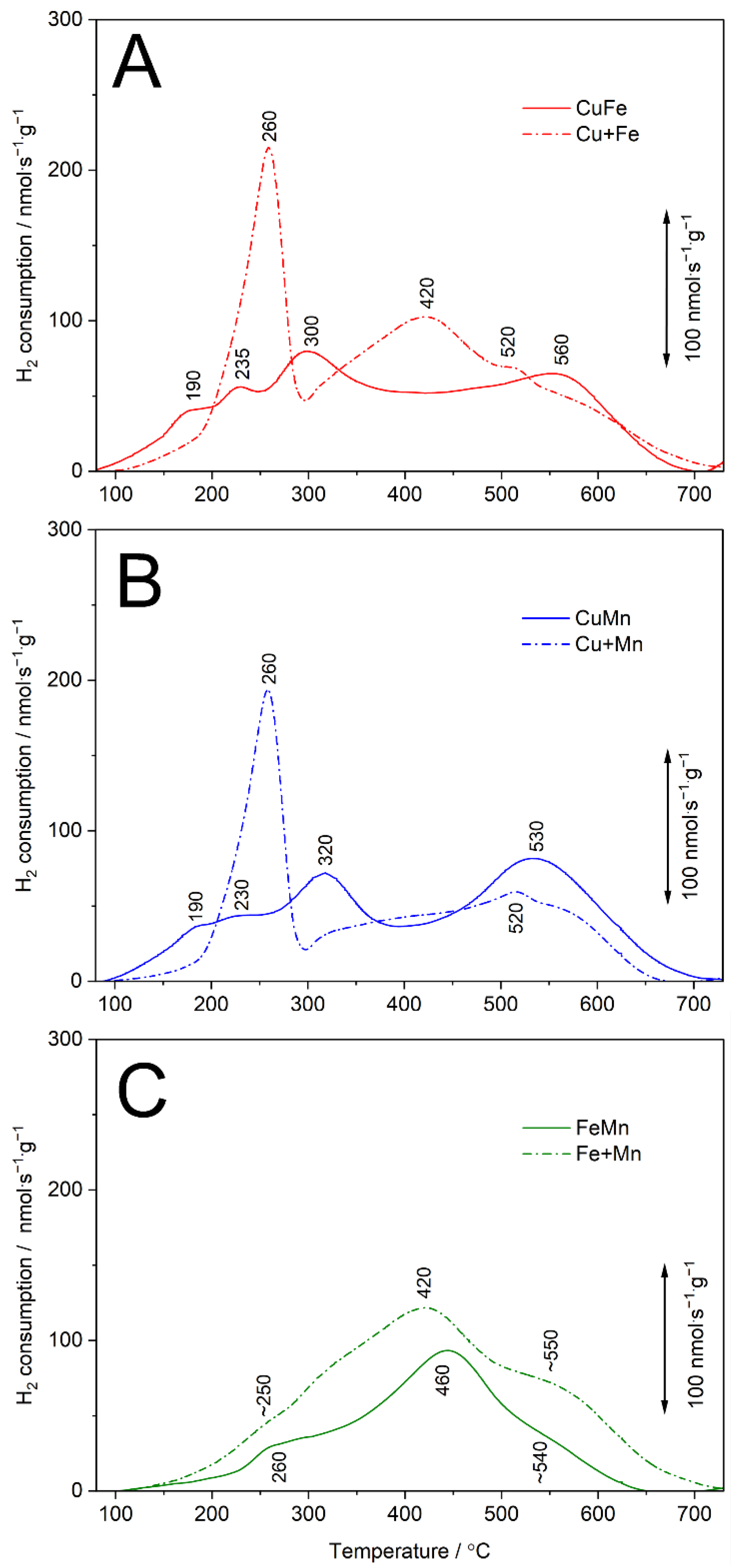
The analysis of H
2-TPR profiles of the bimetallic samples is difficult due to the overlapping of the reduction peaks originating from different metal species. Therefore, the reduction profiles of the bimetallic samples were compared with the cumulative profiles of the monometallic samples (
Figure 6). The reduction profile of the CuFe sample consists of three low-temperature peaks at 190, 235 and 300 °C and a broad high-temperature maximum at about 560 °C (
Figure 6A). The peak at 190 °C is possibly related to the reduction of copper (Cu
2+ → Cu
0) in the form of oligomeric copper oxide species and small CuO crystallites [
30], while the maxima at 235 and 300 °C are assigned to two overlapping processes—reduction of monomeric Cu
2+ cations to Cu
+ [
31] as well as reduction of Fe
3+ to Fe
2+ cations in iron oxide species [
33,
34]. The broad high-temperature shoulder above 350 °C is related to the reduction of monomeric Cu
+ cations to Cu
0 [
31], while at higher temperatures (peak at 560 °C) reduction of Fe
2+ to Fe
0 is observed [
35]. However, the reduction of strongly stabilized copper species at higher temperatures cannot be excluded. There are significant differences in the profiles of the bimetallic CuFe sample and cumulative profile of the monometallic (Cu+Fe) samples (
Figure 6A), indicating the formation of easy-reducible copper species (reduction below 200 °C) as well as the higher contribution of monomeric copper cations in the CuFe sample than in the Cu sample. These results are in line with the conclusions of the XRD (
Figure 1(A2)) and UV-vis DRS studies (
Figure 4A).
The reduction profile of the CuMn sample consists of four overlapping peaks at 190, 230, 320 and 530 °C (
Figure 6B). The peak at 190 °C could be assigned to the reduction of copper cations (Cu
2+ → Cu
0) in oligomeric copper oxide species and small CuO crystallites [
30], while the maximum at 230 °C is possibly related to the reduction of monomeric Cu
2+ cations to Cu
+ ions [
31]. The peak at 320 °C could be assigned to two overlapping processes—reduction of Mn
3+ cations to Mn
2+ in highly dispersed species [
32] and reduction of monomeric Cu
2+ cations to Cu
+ [
31]. Reduction of Mn
3+ to Mn
2+ cations in aggregated metal oxide species is possibly represented by a peak at 530 °C [
32,
36]. However, the reduction of strongly stabilized copper species at higher temperatures cannot be excluded. The differences in the profile of the bimetallic CuMn sample and cumulative profile of the monometallic (Cu+Mn) samples (
Figure 6B), indicate the formation of easy-reducible copper species (reduction below 200 °C) and increased contribution of monomeric copper cations in the CuMn sample in comparison to the Cu sample. Moreover, the obtained results show higher contribution of aggregated manganese species in the bimetallic CuMn sample compared to the monometallic Mn sample.
The reduction profile of the FeMn sample consists of three overlapping peaks at about 260, 460 and 540 °C (
Figure 6C). The first peak is possibly related to two overlapping processes—reduction of Fe
3+ to Fe
2+ in iron oxide species [
32,
33,
34] as well as reduction of Mn
3+ to Mn
2+ cations in highly dispersed species [
36,
37]. The main reduction peak at 460 °C and shoulder at about 540 °C are assigned to two overlapping processes—reduction of Mn
3+ to Mn
2+ in aggregated metal oxide species [
32] and reduction of Fe
2+ to Fe
0 in iron oxide species [
35]. The profile of the bimetallic FeMn sample and cumulative (Fe+Mn) profile of the monometallic samples are very similar and the main difference is related to various intensities of these profiles. Thus, in contrast to the Cu-containing bimetallic samples, in the case of FeMn, deposited transition metal species and their contribution in bimetallic and monometallic samples are very similar.
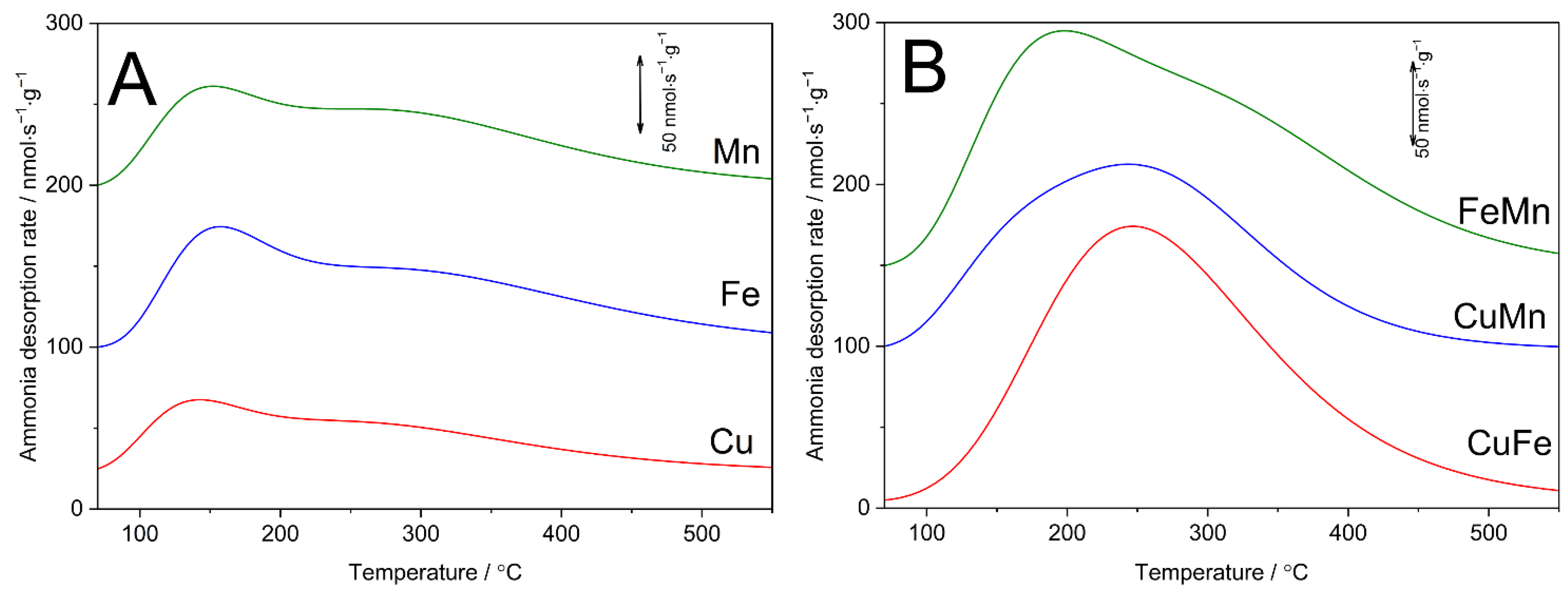
Surface acidity of the samples was studied by the NH
3-TPD method. Ammonia desorption profiles are presented in
Figure 7, while surface concentration (C
a) and density (D
a) of acid sites in the samples are compared in
Table 1. S-MCM-41 non-modified with transition metals do not exhibit any surface acidity (results not shown), thus acid sites are generated by transition metals deposition. Ammonia desorption profiles recorded for the monometallic samples contain two overlapping maxima (
Figure 7A). The first, representing ammonia bounded to weaker acid sites, is centred, depending on deposited transition metal, in the range of 140–155 °C. The second broad maximum, assigned to ammonia molecules bounded to stronger acid sites is centred at about 260–280 °C. The shape of the ammonia desorption profiles recorded for monometallic samples based on mesoporous spherical silica of MCM-41 type is very similar to those reported in scientific literature [
12,
37,
38]. Our previous studies [
11] showed that deposition of copper and manganese to cylindrical MCM-41 resulted exclusively in Lewis acid sites, while in the case of iron deposition the presence of a small amount of Brønsted type acid sites was also identified. Therefore, the formation of mainly donor–acceptor bonds (coordination bounds) between ammonia molecules (donor of electron pair) and transition metal cations (acceptor of electron pair) is expected in these studies.
Ammonia desorption profiles of the bimetallic samples (
Figure 7B) are different from the profiles recorded for the monometallic samples (
Figure 7A). Desorption profile of the CuFe sample consists of a maximum centred at about 250 °C with a shoulder at higher temperatures. In the case of the CuMn sample desorption profile is represented by a broad maximum centred at about 245 °C, which is a superposition of at least two peaks. Ammonia desorption profile of the FeMn sample consists of at least two overlapping maxima centred at about 200 and 315 °C. Thus, in the case of the bimetallic samples, the ammonia desorption profiles are not a simple cumulation of desorption profiles of the monometallic samples but represent different distribution of acid sites with respect to their acid strength.
The concentration and density of acid sites in the bimetallic samples are higher than in monometallic samples (
Table 1). This is related to higher transition metal loading in the samples modified with pairs of transition metals. The concentration and density of acid sites depend on the transition metal loading and aggregation of deposited metal species. The Cu sample presents the lowest surface concentration of acid sites in a series of the monometallic samples. This is not surprising considering the presence of CuO crystallites in this sample.
S-MCM-41 modified with transition metal species were tested as catalysts of the selective catalytic reduction of NO with ammonia (NH
3-SCR). Results of the catalytic studies for the monometallic Cu and Fe samples, as well as mechanical mixture of monometallic samples (Cu+Fe) and bimetallic CuFe catalyst, are compared in
Figure 8A. Monometallic Cu catalyst is active in NO conversion at temperatures above 200 °C, however at higher temperatures the efficiency of the NH
3-SCR process is significantly limited by the side process of direct ammonia oxidation with oxygen present in the reaction mixture. In the case of the Fe catalyst, the NO conversion is observed from about 150 °C and is higher than the conversion obtained for the Cu catalyst in the studied temperature range. Mechanical mixture of monometallic samples (Cu+Fe) presented significantly higher catalytic activity than monometallic catalysts, however the side rection of direct ammonia oxidation decreased the efficiency of the NH
3-SCR process at temperatures above 325 °C. Bimetallic CuFe catalyst presented better catalytic activity than the monometallic samples but was less active than the mechanical mixture of the monometallic catalysts (Cu+Fe). It should be noted that the side process of direct ammonia oxidation was significantly reduced in the case of bimetallic CuFe catalyst in comparison to the mechanical mixture of monometallic catalysts. N
2 and N
2O were the only identified nitrogen-containing products of the NH
3-SCR process. The selectivity to nitrogen, determined for this series of catalysts, is high and did not drop below 90% in the studied temperature range (
Figure 8A). The selectivity to nitrogen was on the level of 98–100% in the case of the Fe and CuFe catalysts, while for the Cu sample and mechanical mixture of monometallic (Cu+Fe) samples was in the range of 91–97% at temperatures below 425 °C.
The monometallic Mn catalyst presented lower activity in the NO conversion than the Cu sample at temperatures below 375 °C, however at higher temperatures the efficiency of this catalyst in NH
3-SCR is higher due to its limited activity in the side process of direct ammonia oxidation (
Figure 8B). The NO conversion profile of the mechanical mixture of the monometallic (Cu+Mn) catalysts is very similar to the sum of the profiles obtained for the monometallic samples. However, bimetallic CuMn catalyst presented much better activity in the NO conversion than mechanical mixture (Cu+Mn) of the monometallic samples. In this case, the NO conversion started at about 150 °C and the level of the NO conversion above 95% was obtained in the range of 275–325 °C, while at higher temperatures the efficiency of the NH
3-SCR process decreased due to the side process of direct ammonia oxidation. The selectivity to nitrogen of the CuMn catalyst is very close to 100% in the studied temperature range and is higher than for the mechanical mixture (Cu+Mn) of the monometallic samples. It should be noted that the content of copper and manganese is the same in the CuMn catalyst and mechanical mixture of the samples (Cu+Mn) (
Table 1). Thus, the simultaneous presence of both copper and manganese species results in the synergistic catalytic effect.
Catalytic activity of the bimetallic FeMn sample is higher than monometallic Cu catalyst and lower than the Fe sample (
Figure 8C). Thus, the simultaneous presence of iron and copper in the catalyst results in decreased catalytic activity in comparison with the monometallic Fe catalyst. Selectivity of the bimetallic FeMn catalyst to nitrogen is close to 100% in the studied temperature range.
The turn-over-frequency (TOF) values determined for the reaction at 275 °C are shown in
Figure 8. Because most of the postulated mechanisms of the NH
3-SCR reaction include chemisorption and activation of ammonia molecules as one of the main reaction steps [
39], it was therefore assumed that each of the acid sites, determined by the NH
3-TPD method (
Figure 7,
Table 1), are active sites of the studied reaction. The TOF value determined for the CuMn is much higher than for other catalysts. Moreover, this value (10.0 × 10
−3 s
−1) is higher than the sum of TOF values determined for the monometallic Cu and Mn samples (7.8 × 10
−3 s
−1). Thus, this proves the cooperative synergistic effect of copper and manganese in the NH
3-SCR reaction.
Thus, simultaneous presence of both copper and manganese species results in a cooperative synergistic catalytic effect of these metals, which is possibly related to the electron transfers: Cu
2+ + Mn
3+ → Cu
+ + Mn
4+ and Cu
2+ + Mn
2+ → Cu
0 + Mn
4+. Wu et al. [
40] have recently reported a significant role of Cu
2+/Cu
+ coexistence in the NH
3-SCR process. Cu
2+ cations are adsorption sites of NH
3 and possibly also NO molecules and promote the formation of NO
+ active species as well as dehydrogenation of ammonia molecules. On the other side, Cu
+ can act as the adsorption site for oxygen, promoting the formation of active oxygen O
− species. A similar role was also recently postulated for Cu
0 species by Liu et al. [
41]. Thus, the synergistic effect between Cu
2+ and Cu
+/Cu
0 can result in the rapid formation of reactive intermediates of the NH
3-SCR process. On the other side Mn
4+ was reported to be active in the dehydrogenation of adsorbed ammonia molecules into reactive -NH
2 intermediate [
42] as well as oxygen adsorption with the formation of active oxygen O
− species necessary for activation of the reactants in the NH
3-SCR process. Thus, such active oxygen species can also be reactive in the oxidation of NO to NO
2, which is necessary for the low-temperature nitrogen oxides conversion by fast SCR process, 2NH
3 + NO + NO
2 → 2N
2 + 3H
2O. To verify the activity of the most active bimetallic CuMn catalyst, as well as monometallic Cu and Mn catalysts, additional experiments of NO to NO
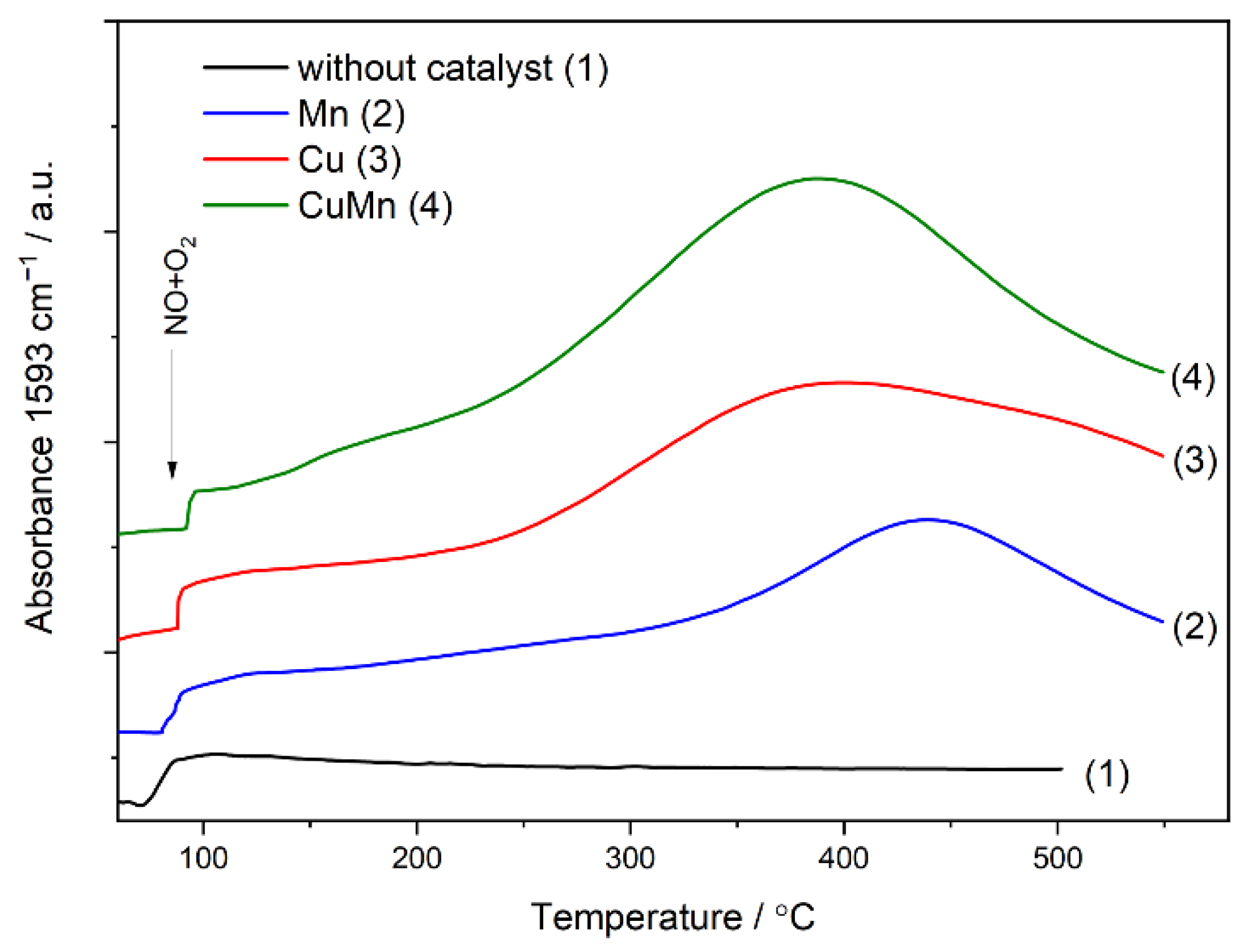
2 oxidation were undertaken (
Figure 9). As can be seen, the NO to NO
2 oxidation is much more effective in the case of bimetallic CuMn catalyst than for monometallic Cu and Mn catalysts. The estimated NO conversion in the maximum (390 °C) of the CuMn profile is about 50%. The maxima in the profiles of the monometallic samples are shifted into higher temperatures, 400 and 440 °C for the Cu and Mn samples, respectively. Moreover, the estimated NO conversions for these maxima are about 34 and 19% for the Cu and Mn catalysts, respectively. In general, these results are in line with the results of the NH
3-SCR catalytic studies (
Figure 8B). The CuMn catalyst presented the highest activity, and the maximum of the NO conversion is at a lower temperature than for the monometallic Cu and Mn samples. Thus, it is postulated that oxidation of NO to NO
2, and therefore the fast SCR pathway, plays an important role in the NO conversion over studied catalysts, especially in the case of bimetallic CuMn catalyst.
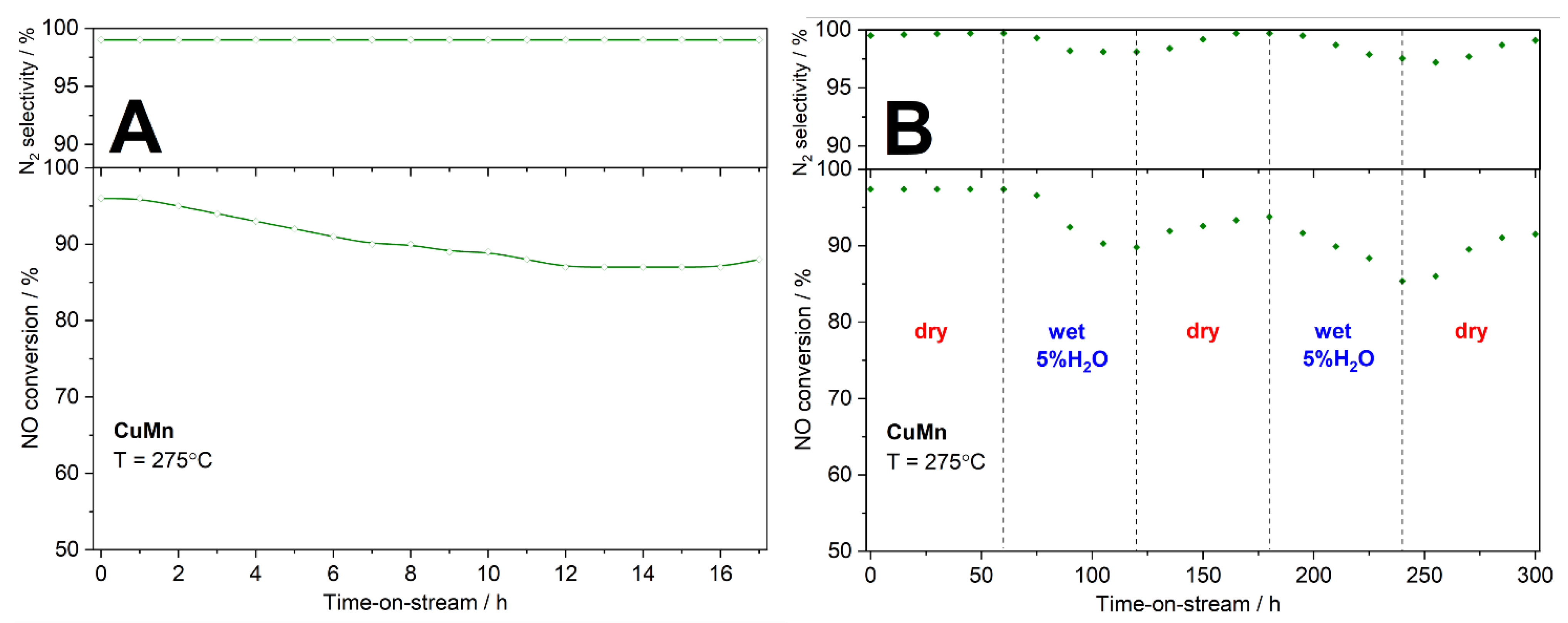
For the most active catalyst, CuMn, additional stability tests were done at 275 °C (
Figure 10). In the long-term stability test (
Figure 10A), the NO conversion decreased from 96 to 87% during the first 12 h of the stability test and was then stable or even slightly increased. Selectivity to nitrogen was on the level of 99% during 17 h of the stability test. In the second stability test the influence of water vapour presence in the reaction mixture on the catalytic efficiency of NH3-SCR was studied (
Figure 10B). In this case water vapour (5 vol.%) was periodically added into the reaction mixture. After 60 min of the test, water vapour was introduced into the rection mixture and the NO conversion decreased from about 97 to 88%. After another 60 min, the water vapour flow was stopped and this resulted in an increase in the NO conversion to about 94%. After the following cycles with wet and dry reaction mixture the NO conversion level of 90% was obtained. The obtained results indicate that the presence of water vapour in the reaction mixture decreased efficiency of the NH3-SCR process, however deactivation of the catalysts is rather limited and partially reversible after removal of water vapour from the reaction mixture.