Facile Synthesis of n-Fe3O4/ACF Functional Cathode for Efficient Dye Degradation through Heterogeneous E-Fenton Process
Abstract
:1. Introduction
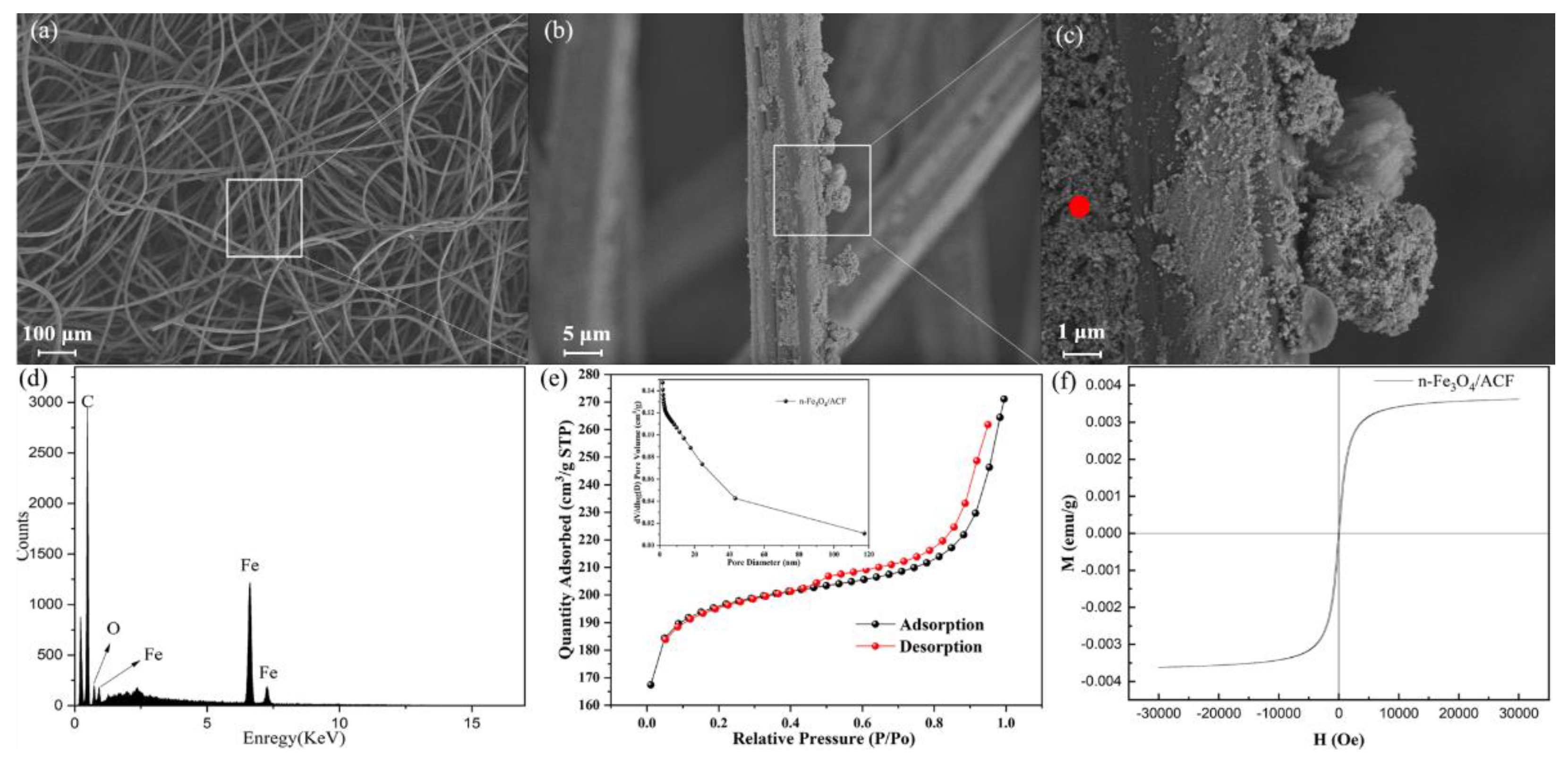
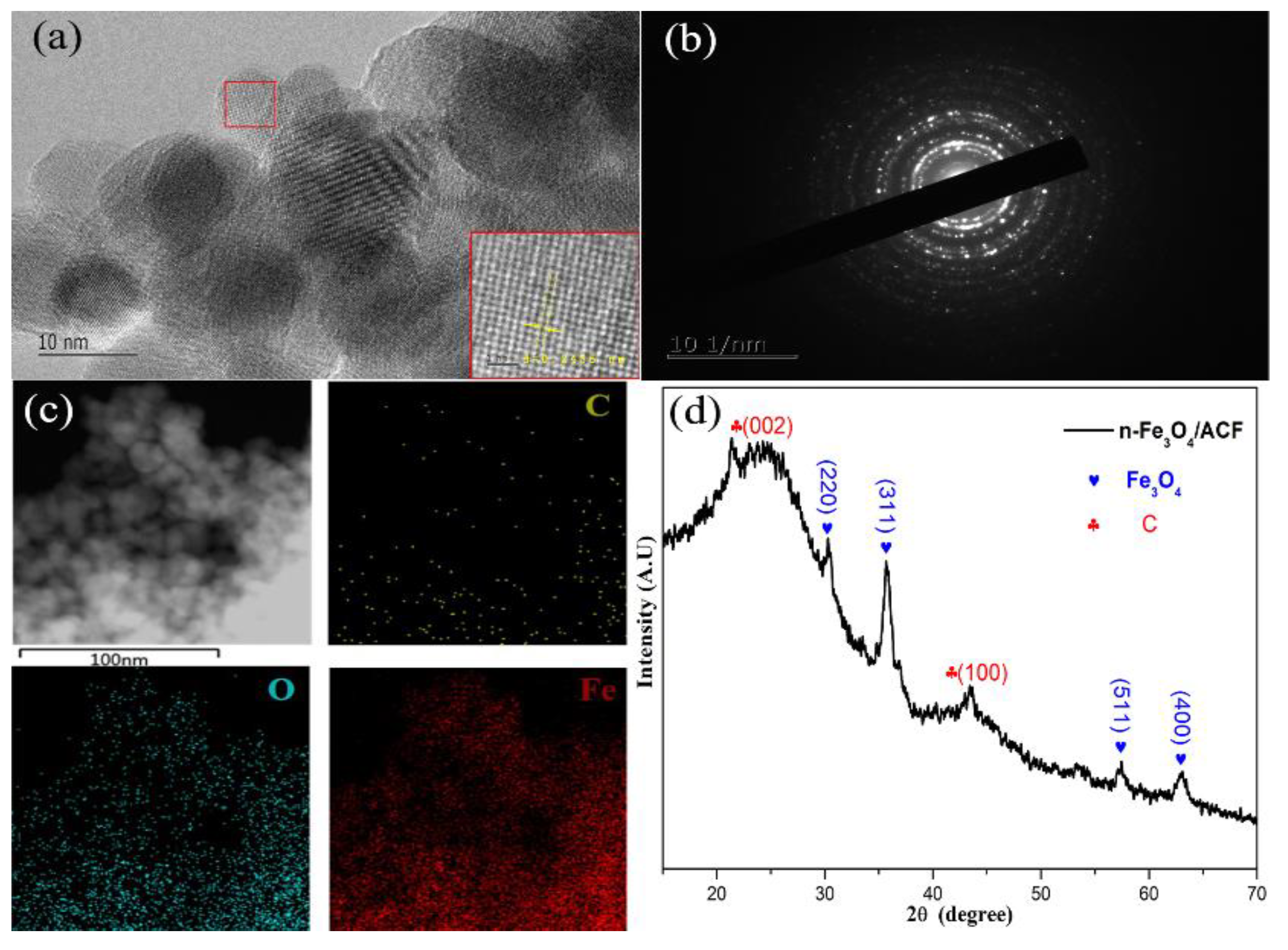
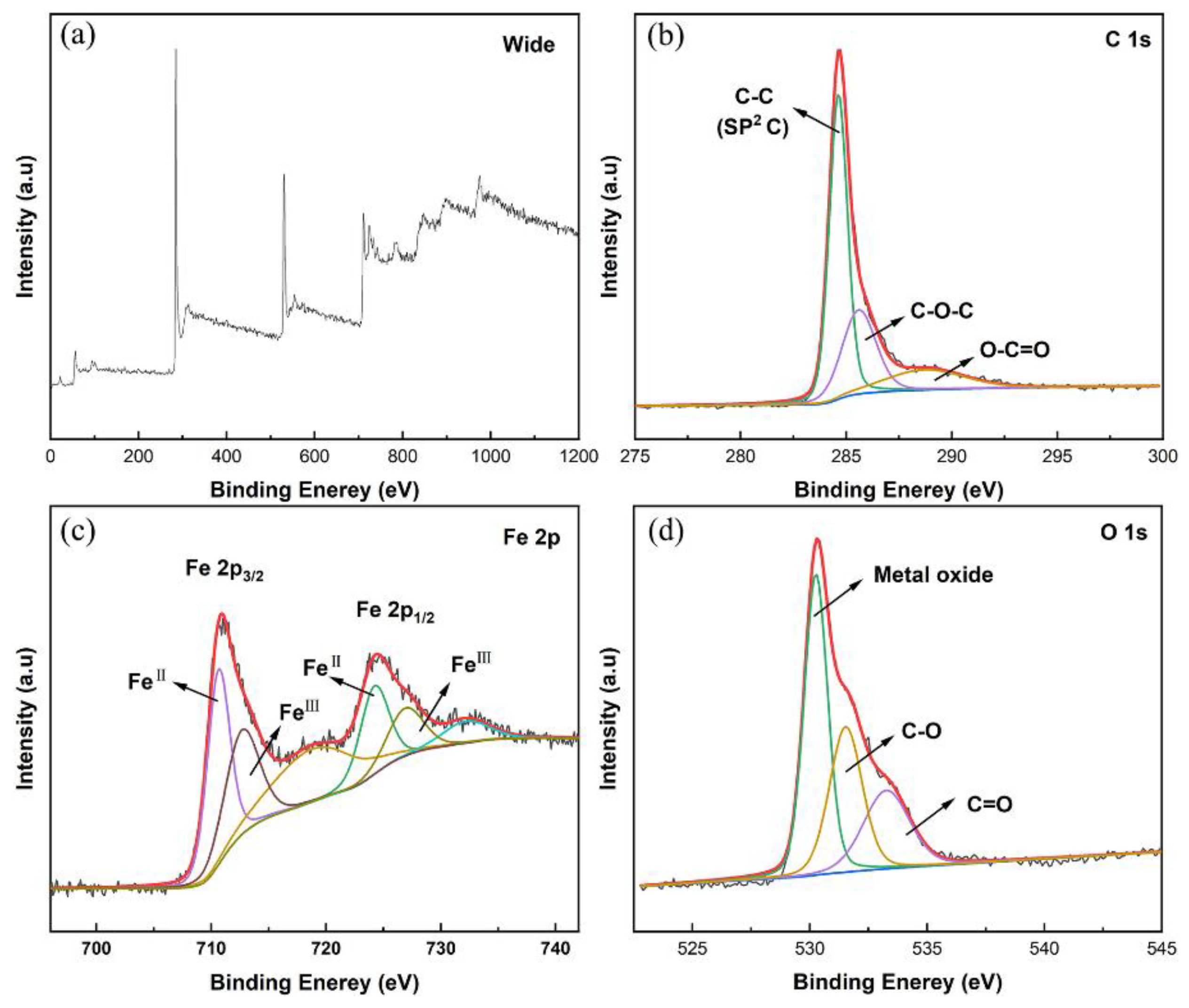
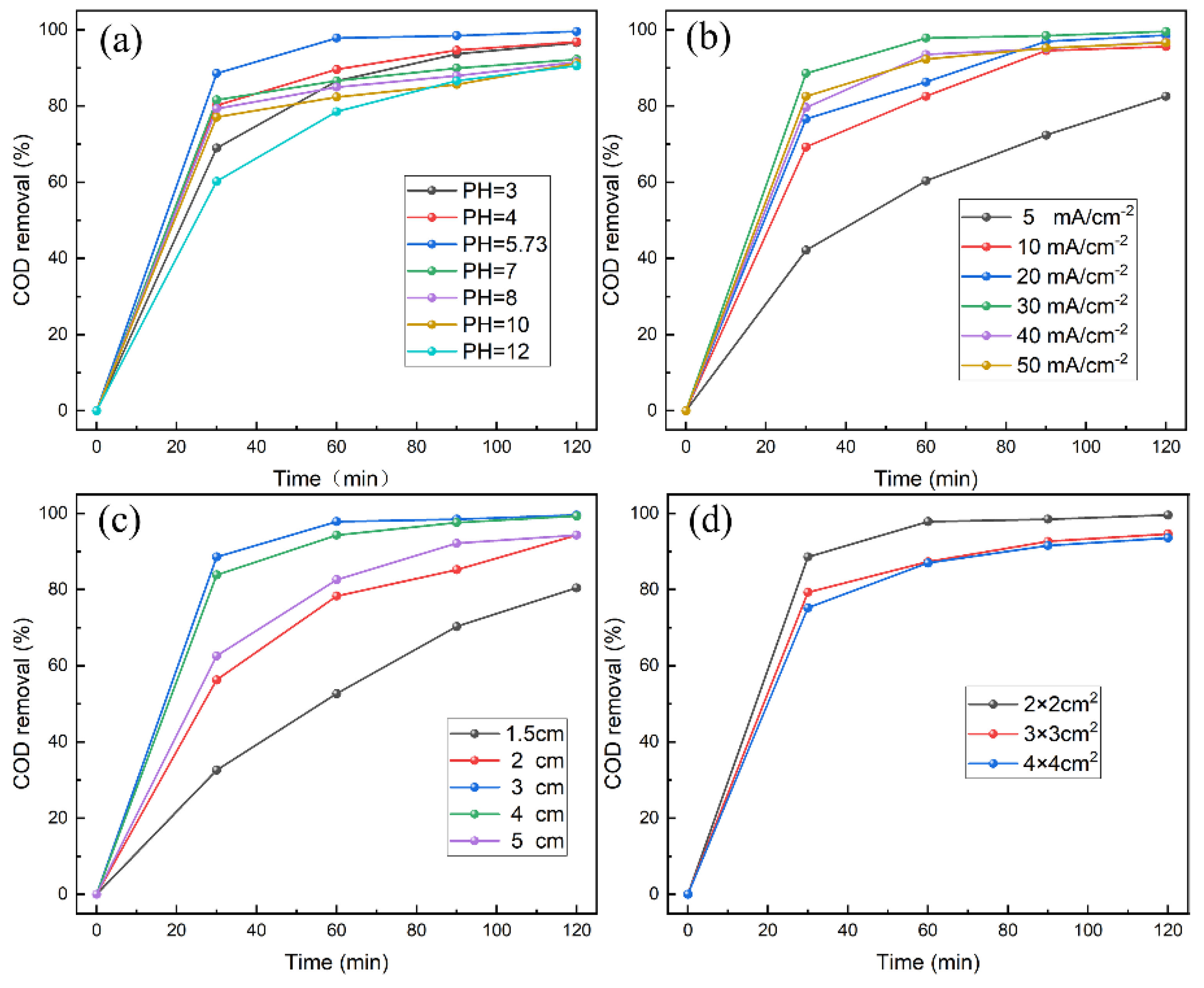
2. Results and Discussion
3. Materials and Methods
3.1. Raw Materials and Reagents
3.2. Preparation of Activated Carbon Fibers
3.3. Deposition of n-Fe3O4 on ACF
3.4. Characterization
3.5. Heterogeneous E-Fenton Performance Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arshad, A.; Iqbal, J.; Ahmad, I.; Israr, M. Graphene/Fe3O4 nanocomposite: Interplay between photo-Fenton type reaction, and carbon purity for the removal of methyl orange. Ceram. Int. 2018, 44, 2643–2648. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling-an overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Ma, J.; Tian, Z.; Li, L.; Lu, Y.; Xu, X.; Hou, J. Loading Nano-CuO on TiO2 Nanomeshes towards efficient photodegradation of methylene blue. Catalysts 2022, 12, 383. [Google Scholar] [CrossRef]
- El-Khouly, S.M.; Fathy, N.A. Multi-walled carbon nanotubes supported amorphous Fe2O3 and Ag2O-Fe2O3 as Fenton catalysts for degradation of maxilon red dye. Asia-Pac. J. Chem. Eng. 2018, 13, e2184. [Google Scholar] [CrossRef]
- Iglesias, O.; Gómez, J.; Pazos, M.; Sanromán, M.Á. Electro-Fenton oxidation of imidacloprid by Fe alginate gel beads. Appl. Catal. B 2014, 144, 416–424. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Zhao, N.; Liu, H.; Wei, Y. Citrate modified ferrihydrite microstructures: Facile synthesis, strong adsorption and excellent fenton-like catalytic properties. RSC Adv. 2014, 4, 21575–21583. [Google Scholar] [CrossRef]
- Padamati, S.K.; Vedelaar, T.A.; Perona Martínez, F.; Nusantara, A.C.; Schirhagl, R. Insight into a Fenton-like reaction using Nanodiamond based relaxometry. Nanomaterials 2022, 12, 2422. [Google Scholar] [CrossRef]
- Li, J.P.; Ai, Z.H.; Zhang, L.Z. Design of a neutral electro-Fenton system with Fe@Fe2O3/ACF composite cathode for wastewater treatment. J. Hazard. Mater. 2009, 164, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zheng, D.Y.; Xie, Z.Y.; Ma, J.W.; Yu, J.; Hou, B.L. The mechanism and oxidation efficiency of bio-electro-Fenton system with Fe@Fe2O3/ACF composite cathode. Sep. Purif. Technol. 2020, 234, 116103. [Google Scholar] [CrossRef]
- Tang, J.T.; Wang, J.L. Metal organic framework with coordinatively unsaturated sites as efficient Fenton-like catalyst for enhanced degradation of sulfamethazine. Environ. Sci. Technol. 2018, 52, 5367–5377. [Google Scholar] [CrossRef]
- Sano, N.; Yamamoto, T.; Takemori, I.; Kim, S.I.; Eiad-ua, A.; Yamamoto, D.; Nakaiwa, M. Degradation of phenol by simultaneous use of gas-phase corona discharge and catalyst-supported mesoporous carbon gels. Ind. Eng. Chem. Res. 2006, 45, 2897–2900. [Google Scholar] [CrossRef]
- Oturan, M.A.; Pimentel, M.; Oturan, N.; Sires, I. Reaction sequence for the mineralization of the short-chain carboxylic acids usually formed upon cleavage of aromatics during electrochemical Fenton treatment. Electrochim. Acta 2008, 54, 173–182. [Google Scholar] [CrossRef]
- Bounab, L.; Iglesias, O.; Gonzalez-Romero, E.; Pazos, M.; Sanroman, M.A. Effective heterogeneous electro-Fenton process of m-cresol with iron loaded actived carbon. Rsc Adv. 2015, 5, 31049–31056. [Google Scholar] [CrossRef] [Green Version]
- Brillas, E.; Sires, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Plewa, J.M.; Eichwald, O.; Ducasse, O.; Dessante, P.; Jacobs, C.; Renon, N.; Yousfi, M. 3D streamers simulation in a pin to plane configuration using massively parallel computing. J. Phys. D 2018, 51, 095206. [Google Scholar] [CrossRef]
- Mohseni, M.; Demeestere, K.; Du Laing, G.; Yüce, S.; Keller, R.G.; Wessling, M. Cnt microtubes with entrapped Fe3O4 nanoparticles remove micropollutants through a heterogeneous electro-Fenton process at neutral ph. Adv. Sustain. Syst. 2021, 5, 2100001. [Google Scholar] [CrossRef]
- Zhou, W.; Rajic, L.J.; Chen, L.; Kou, K.K.; Ding, Y.N.; Meng, X.X.; Wang, Y.; Mulaw, B.; Gao, J.H.; Qin, Y.K.; et al. Activated carbon as effective cathode material in iron-free electro-Fenton process: Integrated H2O2 electrogeneration, activation, and pollutants adsorption. Electrochim. Acta 2019, 296, 317–326. [Google Scholar] [CrossRef]
- Wang, S.B.; Sun, H.Q.; Ang, H.M.; Tade, M.O. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem. Eng. J. 2013, 226, 336–347. [Google Scholar] [CrossRef]
- Roth, H.; Gendel, Y.; Buzatu, P.; David, O.; Wessling, M. Tubular carbon nanotube-based gas diffusion electrode removes persistent organic pollutants by a cyclic adsorption-electro-Fenton process. J. Hazard. Mater. 2016, 307, 1–6. [Google Scholar] [CrossRef]
- Zhao, H.; Qian, L.; Guan, X.; Wu, D.; Zhao, G. Continuous bulk FeCuC aerogel with ultradispersed metal nanoparticles: An efficient 3d heterogeneous electro-Fenton cathode over a wide range of pH 3–9. Environ. Sci. Technol. 2016, 50, 5225–5233. [Google Scholar] [CrossRef]
- Jia, J.; Yang, J.; Liao, J.; Wang, W.; Wang, Z. Treatment of dyeing wastewater with ACF electrodes financially supported by the state key laboratory of environmental aquatic chemistry, Chinese academic of sciences. Water Res. 1999, 33, 881–884. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. Fenton-like degradation of 2,4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl. Catal. B 2012, 123, 117–126. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, G.; Guo, Y.; Yu, J.C. Graphene oxide-Fe2O3 hybrid material as highly efficient heterogeneous catalyst for degradation of organic contaminants. Carbon 2013, 60, 437–444. [Google Scholar] [CrossRef]
- Ding, C.; Zhao, H.; Zhu, X.; Liu, X. Preparation of cotton linters’ aerogel-based C/NiFe2O4 photocatalyst for efficient degradation of methylene blue. Nanomaterials 2022, 12, 2021. [Google Scholar] [CrossRef]
- Huang, H.H.; Lu, M.C.; Chen, J.N. Catalytic decomposition of hydrogen peroxide and 2-chlorophenol with iron oxides. Water Res. 2001, 35, 2291–2299. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R.; Velmathi, S.; Sanjini, N.S. Magnetite as a heterogeneous electro Fenton catalyst for the removal of rhodamine b from aqueous solution. RSC Adv. 2014, 4, 5698–5708. [Google Scholar] [CrossRef]
- Kalantary, R.R.; Farzadkia, M.; Kermani, M.; Rahmatinia, M. Heterogeneous electro-Fenton process by nano-Fe3O4 for catalytic degradation of amoxicillin: Process optimization using response surface methodology. J. Environ. Chem. Eng. 2018, 6, 4644–4652. [Google Scholar] [CrossRef]
- Es’haghzade, Z.; Pajootan, E.; Bahrami, H.; Arami, M. Facile synthesis of Fe3O4 nanoparticles via aqueous based electro chemical route for heterogeneous electro-Fenton removal of azo dyes. J. Taiwan Inst. Chem. Eng. 2017, 71, 91–105. [Google Scholar] [CrossRef]
- Costa, R.C.C.; Lelis, M.F.F.; Oliveira, L.C.A.; Fabris, J.D.; Ardisson, J.D.; Rios, R.R.V.A.; Silva, C.N.; Lago, R.M. Novel active heterogeneous Fenton system based on Fe3-xMxO4 (Fe, Co, Mn, Ni): The role of M2+ species on the reactivity towards H2O2 reactions. J. Hazard. Mater. 2006, 129, 171–178. [Google Scholar] [CrossRef]
- Görmez, F.; Görmez, Ö.; Gözmen, B.; Kalderis, D. Degradation of chloramphenicol and metronidazole by electro-Fenton process using graphene oxide-Fe3O4 as heterogeneous catalyst. J. Environ. Chem. Eng. 2019, 7, 102990. [Google Scholar] [CrossRef]
- Saleh, R.; Taufik, A. Ultraviolet-light-assisted heterogeneous Fenton reaction of Ag-Fe3O4/graphene composites for the degradation of organic dyes. J. Environ. Chem. Eng. 2019, 7, 102895. [Google Scholar] [CrossRef]
- Wang, Y.B.; Zhao, H.Y.; Zhao, G.H. Highly ordered mesoporous Fe3O4@carbon embedded composite: High catalytic activity, wide pH range and stability for heterogeneous electro-Fenton. Electroanalysis 2016, 28, 169–176. [Google Scholar] [CrossRef]
- Anderson, J.F.; Kuhn, M.; Diebold, U. Epitaxially grown Fe3O4 thin films: An XPS study. Surf. Sci. Spectra 1996, 4, 266–272. [Google Scholar] [CrossRef]
- Hou, J.W.; Yang, H.D.; He, B.Y.; Ma, J.G.; Lu, Y.; Wang, Q.Y. High photocatalytic performance of hydrogen evolution and dye degradation enabled by CeO2 modified TiO2 nanotube arrays. Fuel 2022, 310, 122364. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.; Niu, W.; Paerhati, S.; Guo, W.; Ma, J.; Hou, J. Facile Synthesis of n-Fe3O4/ACF Functional Cathode for Efficient Dye Degradation through Heterogeneous E-Fenton Process. Catalysts 2022, 12, 879. https://doi.org/10.3390/catal12080879
Peng W, Niu W, Paerhati S, Guo W, Ma J, Hou J. Facile Synthesis of n-Fe3O4/ACF Functional Cathode for Efficient Dye Degradation through Heterogeneous E-Fenton Process. Catalysts. 2022; 12(8):879. https://doi.org/10.3390/catal12080879
Chicago/Turabian StylePeng, Wei, Wenjun Niu, Sidike Paerhati, Wenjian Guo, Jingui Ma, and Junwei Hou. 2022. "Facile Synthesis of n-Fe3O4/ACF Functional Cathode for Efficient Dye Degradation through Heterogeneous E-Fenton Process" Catalysts 12, no. 8: 879. https://doi.org/10.3390/catal12080879
APA StylePeng, W., Niu, W., Paerhati, S., Guo, W., Ma, J., & Hou, J. (2022). Facile Synthesis of n-Fe3O4/ACF Functional Cathode for Efficient Dye Degradation through Heterogeneous E-Fenton Process. Catalysts, 12(8), 879. https://doi.org/10.3390/catal12080879





