Quantification of Internal Resistance Contributions of Sediment Microbial Fuel Cells Using Petroleum-Contaminated Sediment Enriched with Kerosene
Abstract
:1. Introduction
2. Results and Discussion
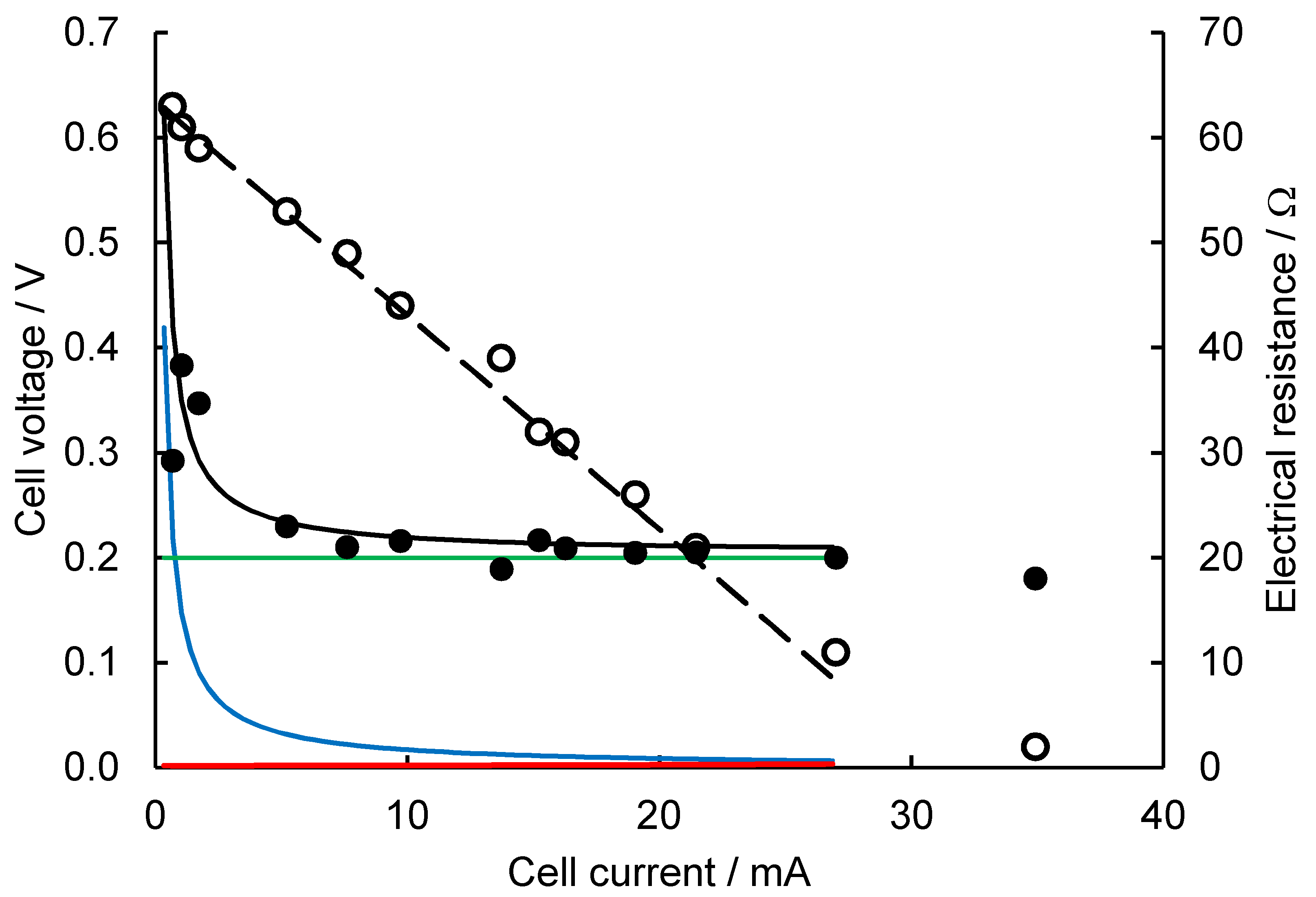
2.1. Validation of the Electrochemical Model
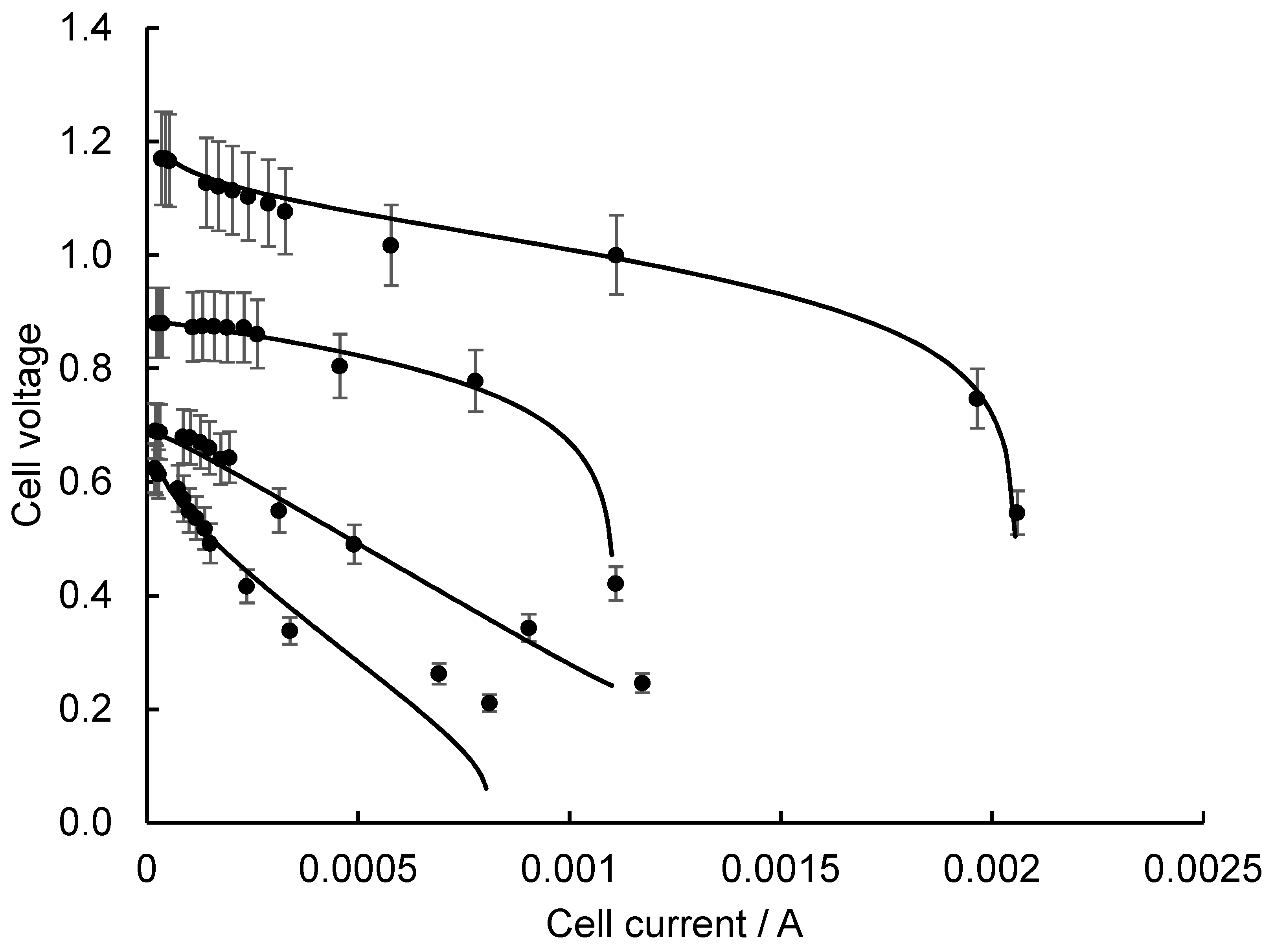
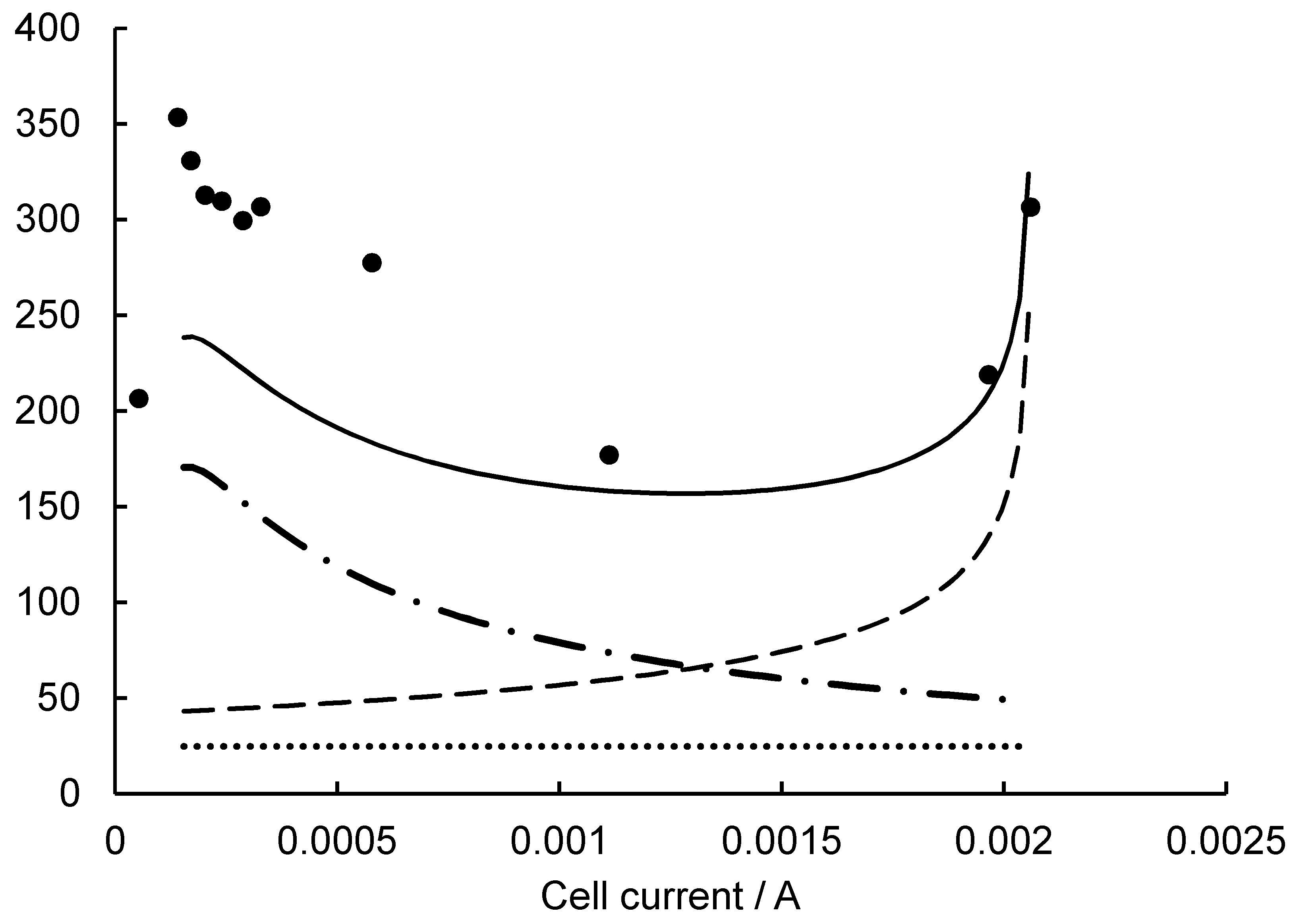
2.2. Evaluation of SMFC Ri from Initial PCS
2.3. Lowering SMFC Ri
3. Materials and Methods
3.1. SMFC Construction
3.2. Data Acquisition
3.3. Electrochemical Model Equations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, G.; Tian, F.; Ding, K.; Wang, L.; Liu, T.; Yang, F. Effect of a bacterial consortium on the degradation of polycyclic aromatic hydrocarbons and bacterial community composition in Chinese soils. Int. Biodeterior. Biodegrad. 2017, 123, 56–62. [Google Scholar] [CrossRef]
- Srinivasarao Naik, B.; Mishra, I.M.; Bhattacharya, S.D. Biodegradation of total petroleum hydrocarbons from oily sludge. Bioremediat. J. 2011, 15, 140–147. [Google Scholar] [CrossRef]
- Lu, L.; Yazdi, H.; Jin, S.; Zuo, Y.; Fallgren, P.H.; Ren, Z.J. Enhanced bioremediation of hydrocarbon-contaminated soil using pilot-scale bioelectrochemical systems. J. Hazard. Mater. 2014, 274, 8–15. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A Review. J. Power Source 2017, 356, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, R.; Zhang, X.; Liu, Z.; Zhu, R.; Zhang, X. A novel exoelectrogen from microbial fuel cell: Bioremediation of marine petroleum hydrocarbon pollutants. J. Environ. Manag. 2019, 235, 70–76. [Google Scholar] [CrossRef]
- Prasad, J.; Tripathi, R.K. Voltage control of sediment microbial fuel cell to power the AC load. J. Power Source 2020, 450, 227721. [Google Scholar] [CrossRef]
- Yu, B.; Tian, J.; Feng, L. Remediation of PAH polluted soils using a soil microbial fuel cell: Influence of electrode interval and role of microbial community. J. Hazard. Mater. 2017, 336, 110–118. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Logeshwaran, P.; Megharaj, M.; Chadalavada, S.; Bowman, M.; Naidu, R. Petroleum hydrocarbons (PH) in groundwater aquifers: An overview of environmental fate, toxicity, microbial degradation and risk-based remediation approaches. Environ. Technol. Innov. 2018, 10, 175–193. [Google Scholar] [CrossRef]
- Varjani, S.J.; Rana, D.P.; Jain, A.K.; Bateja, S.; Upasani, V.N. Synergistic ex-situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int. Biodeterior. Biodegrad. 2015, 103, 116–124. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, R.; Ji, M.; Ren, Z.J. Organic content influences sediment microbial fuel cell performance and community structure. Bioresour. Technol. 2016, 220, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Tang, S.; Xie, S.; Wang, P.; Huang, C.; Geng, X. The oil removal and the characteristics of changes in the composition of bacteria based on the oily sludge bioelectrochemical system. Sci. Rep. 2020, 10, 15474. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Holmes, D.E.; Tender, L.M.; Lovley, D.R. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 2002, 295, 483–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondaveeti, S.K.; Seelam, J.S.; Mohanakrishna, G. Anodic electron transfer mechanism in bioelectrochemical systems. In Microb Fuel Cell a Bioelectrochemical System That Convert Waste to Watts; Das, D., Ed.; Springer: Cham, Switzerland, 2017; pp. 87–100. [Google Scholar] [CrossRef]
- Hong, S.W.; Chang, I.S.; Choi, Y.S.; Chung, T.H. Experimental evaluation of influential factors for electricity harvesting from sediment using microbial fuel cell. Bioresour. Technol. 2009, 100, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- Rismani-Yazdi, H.; Carver, S.M.; Christy, A.D.; Tuovinen, O.H. Cathodic limitations in microbial fuel cells: An overview. J. Power Source 2008, 180, 683–694. [Google Scholar] [CrossRef]
- Koo, B.; Jung, S.P. Improvement of air cathode performance in microbial fuel cells by using catalysts made by binding metal-organic framework and activated carbon through ultrasonication and solution precipitation. Chem. Eng. J. 2021, 424, 130388. [Google Scholar] [CrossRef]
- Sallam, E.R.; Khairy, H.M.; Elnouby, M.S.; Fetouh, H.A. Sustainable electricity production from seawater using Spirulina platensis microbial fuel cell catalyzed by silver nanoparticles-activated carbon composite prepared by a new modified photolysis method. Biomass Bioenergy 2021, 148, 106038. [Google Scholar] [CrossRef]
- Aleman-Gama, E.; Cornejo-Martell, A.J.; Ortega-Martínez, A.; Kamaraj, S.K.; Juárez, K.; Silva-Martínez, S. Oil-contaminated sediment amended with chitin enhances power production by minimizing the sediment microbial fuel cell internal resistance. J. Electroanal. Chem. 2021, 894, 115365. [Google Scholar] [CrossRef]
- Hindatu, Y.; Annuar, M.S.M.; Gumel, A.M. Mini-review: Anode modification for improved performance of microbial fuel cell. Renew Sustain. Energy Rev. 2017, 73, 236–248. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Bakeri, G.; Najafpour, G.; Ghasemi, M.; Oh, S.E. A review on the effect of proton exchange membranes in microbial fuel cells. Biofuel Res. J. 2014, 1, 7–15. [Google Scholar] [CrossRef]
- Clauwaert, P.; Aelterman, P.; Pham, T.H.; De Schamphelaire, L.; Carballa, M.; Rabaey, K.; Verstraete, W. Minimizing losses in bio-electrochemical systems: The road to applications. Appl. Microbiol. Biotechnol. 2008, 79, 901–913. [Google Scholar] [CrossRef]
- Liang, P.; Huang, X.; Fan, M.Z.; Cao, X.X.; Wang, C. Composition and distribution of internal resistance in three types of microbial fuel cells. Appl. Microbiol. Biotechnol. 2007, 77, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Slade, R.C.T.; Varcoe, J.R. Techniques for the study and development of microbial fuel cells: An electrochemical perspective. Chem. Soc. Rev. 2009, 38, 1926–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N. Characterization of membrane electrode assemblies in polymer electrolyte fuel cells using a.c. impedance spectroscopy. J. Appl. Electrochem. 2002, 32, 859–863. [Google Scholar] [CrossRef]
- He, Z.; Wagner, N.; Minteer, S.D.; Angenent, L.T. An upflow microbial fuel cell with an interior cathode: Assessment of the internal resistance by impedance spectroscopy. Environ. Sci. Technol. 2006, 40, 5212–5217. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Ahn, Y.H.; Oh, S.E.; Lee, J.; Cho, K.T.; Kim, Y. Impedance and thermodynamic analysis of bioanode, abiotic anode, and riboflavin-amended anode in microbial fuel cells. Bull. Korean Chem. Soc. 2012, 33, 3349–3354. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Girguis, P.; Liang, P.; Shi, H.; Huang, G.; Cai, L. Biological capacitance studies of anodes in microbial fuel cells using electrochemical impedance spectroscopy. Bioprocess Biosyst. Eng. 2015, 38, 1325–1333. [Google Scholar] [CrossRef]
- Fan, Y.; Sharbrough, E.; Liu, H. Quantification of the internal resistance distribution of microbial fuel cells. Environ. Sci. Technol. 2008, 42, 8101–8107. [Google Scholar] [CrossRef]
- Jemeï, S.; Hissel, D.; Péra, M.C.; Kauffmann, J.M. On-board fuel cell power supply modeling on the basis of neural network methodology. J. Power Source 2003, 124, 479–486. [Google Scholar] [CrossRef]
- Wei, L.I.; Xin-Jian, Z.; Zhi-Jun, M.O. Estimation of equivalent internal-resistance of PEM fuel cell using artificial neural networks. J. Cent. South Univ. Technol. 2007, 14, 690–695. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Liu, Z.L. Experimental study of the microbial fuel cell internal resistance. J. Power Source 2010, 195, 8013–8018. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Cario, B.P.; Santoro, C.; Yang, W.; Saikaly, P.E.; Logan, B.E. Evaluation of Electrode and Solution Area-Based Resistances Enables Quantitative Comparisons of Factors Impacting Microbial Fuel Cell Performance. Environ. Sci. Technol. 2019, 53, 3977–3986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xochitl, D.B.; Sevda, S.; Vanbroekhoven, K.; Pant, D. The accurate use of impedance analysis for the study of microbial electrochemical systems. Chem. Soc. Rev. 2012, 41, 7228–7246. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, J.; Ivanov, I.; Hatzell, M.C.; Yang, W.; Ahn, Y. Reference and counter electrode positions affect electrochemical characterization of bioanodes in different bioelectrochemical systems. Biotechnol. Bioeng. 2014, 111, 1931–1939. [Google Scholar] [CrossRef]
- Park, S.; Yoo, J.-S. Electrochemical impedance spectroscopy for better electrochemical measurements. Am. Chem. Soc. 2003, 75, 455A–461A. [Google Scholar]
- Fuentes-Albarrán, C.; Juárez, K.; Gamboa, S.; Tirado, A.; Alvarez-Gallegos, A. Improving the power density of a Geobacter consortium-based microbial fuel cell by incorporating a highly dispersed birnessite/C cathode. J. Chem. Technol. Biotechnol. 2020, 95, 3169–3178. [Google Scholar] [CrossRef]
- Bartha, R. Biotechnology of petroleum pollutant biodegradation. Microb. Ecol. 1986, 12, 155–172. [Google Scholar] [CrossRef]
- Shabir, G.; Afzal, M.; Anwar, F.; Tahseen, R.; Khalid, Z.M. Biodegradation of kerosene in soil by a mixed bacterial culture under different nutrient conditions. Int. Biodeterior. Biodegrad. 2008, 61, 161–166. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; Wiley: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Ma, S.B.; Ahn, K.Y.; Lee, E.S.; Oh, K.H.; Kim, K.B. Synthesis and characterization of manganese dioxide spontaneously coated on carbon nanotubes. Carbon 2007, 45, 375–382. [Google Scholar] [CrossRef]
- Karra, U.; Huang, G.; Umaz, R.; Tenaglier, C.; Wang, L.; Li, B. Stability characterization and modeling of robust distributed benthic microbial fuel cell (DBMFC) system. Bioresour. Technol. 2013, 144, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Weinstein, A.; Kolln, M.; Garrett, C.; Wang, L.; Bagtzoglou, A.; Karra, U.; Li, Y.; Li, B. Distributed multiple-anodes benthic microbial fuel cell as reliable power source for subsea sensors. J. Power Source 2015, 286, 210–216. [Google Scholar] [CrossRef]
- Salgado-Dávalos, V.; Osorio-Avilés, S.; Kamaraj, S.K.; Vega-Alvarado, L.; Juárez, K.; Silva-Martínez, S.; Alvarez-Gallegos, A. Sediment Microbial Fuel Cell Power Boosted by Natural Chitin Degradation and Oxygen Reduction Electrocatalysts. Clean-Soil Air Water 2021, 49, 200465. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells. Theory and Practice; Elsevier Academic Press: Burlington, MA, USA, 2005. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Benítez, L.; Silva-Martínez, S.; Hernandez-Perez, A.; Kamaraj, S.K.; Abbas, S.Z.; Alvarez-Gallegos, A. Quantification of Internal Resistance Contributions of Sediment Microbial Fuel Cells Using Petroleum-Contaminated Sediment Enriched with Kerosene. Catalysts 2022, 12, 871. https://doi.org/10.3390/catal12080871
Alvarez-Benítez L, Silva-Martínez S, Hernandez-Perez A, Kamaraj SK, Abbas SZ, Alvarez-Gallegos A. Quantification of Internal Resistance Contributions of Sediment Microbial Fuel Cells Using Petroleum-Contaminated Sediment Enriched with Kerosene. Catalysts. 2022; 12(8):871. https://doi.org/10.3390/catal12080871
Chicago/Turabian StyleAlvarez-Benítez, Luisa, Susana Silva-Martínez, Alfredo Hernandez-Perez, Sathish K. Kamaraj, Syed Zaghum Abbas, and Alberto Alvarez-Gallegos. 2022. "Quantification of Internal Resistance Contributions of Sediment Microbial Fuel Cells Using Petroleum-Contaminated Sediment Enriched with Kerosene" Catalysts 12, no. 8: 871. https://doi.org/10.3390/catal12080871
APA StyleAlvarez-Benítez, L., Silva-Martínez, S., Hernandez-Perez, A., Kamaraj, S. K., Abbas, S. Z., & Alvarez-Gallegos, A. (2022). Quantification of Internal Resistance Contributions of Sediment Microbial Fuel Cells Using Petroleum-Contaminated Sediment Enriched with Kerosene. Catalysts, 12(8), 871. https://doi.org/10.3390/catal12080871









