Abstract
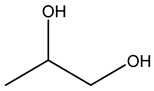
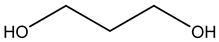
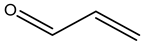
The valuable products produced from glycerol transformation have become a research route that attracted considerable benefits owing to their huge volumes in recent decades (as a result of biodiesel production as a byproduct) as well as a myriad of chemical and biological techniques for transforming glycerol into high-value compounds, such as fuel additives, biofuels, precursors and other useful chemicals, etc. Biodiesel has presented another challenge in the considerable increase in its byproduct (glycerol). This review provides a recent update on the transformation of glycerol with an exclusive focus on the various catalysts’ performance in designing reaction operation conditions. The different products observed and cataloged in this review involved hydrogen, acetol, acrolein, ethylene glycol, and propylene glycol (1,3-propanediol and 1,2-propanediol) from reforming and dehydration and hydrogenolysis reactions of glycerol conversions. The future prospects and critical challenges are finally presented.
1. Introduction
Petroleum fuels became the primary energy source for transport requirements during the twentieth century. Since the beginning of the twenty-first century, primarily all vehicles run with diesel, gasoline, or natural gas [1]. Around the world, the population is increasing, thereby increasing energy requirements simultaneously, and nowadays, the origin of energy is mostly fossil fuels such as oil, coal, or natural gas [2,3,4]. Generally, fossil fuel-based carbon dioxide emissions are unaltered and grant the so-called “greenhouse effect”, which intensively affects the climate changes [5,6]. Furthermore, with the rise in pollution and crude oil prices, oil, coal, and gas burn not only to fulfills energy demands, but also contribute to the global warming crisis. The burning of fossil fuels produces a substantial amount of carbon dioxide, which traps heat in the atmosphere and contributes to global warming [7,8]. Wind, water, biomass, and solar energy are primary alternative energy sources to stop using fossil fuels.
Biomass is currently achieving a significant focus in terms of providing the world’s energy needs [9,10,11]. Due to its eco-friendly nature and low sulfur production, it is estimated that by 2050, biomass will contribute 50% of the total energy demand. The demand for energy worldwide is expected to increase up to 48% by 2040 [10]. Thus, bio-derived fossil fuels are crucial for reducing the carbon footprint [9,10,11].
Biodiesel is commonly manufactured from animal fat or vegetable oil, and through the transesterification technique, glycerol is produced as a waste product (Equation (1)) [12]. Biodiesel production is growing continuously due to the growing fossil fuel demand for transportation and industries. The biodiesel trend enhanced glycerol production as a byproduct [11]. Glycerol contains higher hydrogen moles compared to ethanol and methanol [13]. Each 1 ton of biodiesel production from plants produces 110 kg of glycerol. Worldwide glycerol production was increased five-fold to reach 36 billion liters between 2006–2018 [14]. It is pretty clear that glycerol availability is not in doubt; it is believed that generally, glycerol production will continue to increase due to an upsurge in biodiesel production. Glycerol was raised as an exciting biomass feedstock for high-value-added product conversion due to its modest price and high availability in the market [15]. Hence, glycerol transformation into other useful products is the essence of overall improvement in biodiesel production economics [16].
Glycerides Alcohols Esters Glycerol
Glycerol is crucial for feedstocks in food, pharmaceutical, and other industries and is produced through microbial or biodiesel. Semkiv et al. explained glycerol production by microbes (Saccharomyces cerevisiae, Osmotolerant yeasts, Microalgae, and cyanobacteria). High-added value chemicals produced from glycerol follow different paths such as oxidation, reforming, carbonylation, acetalization, esterification, hydrogenolysis, dehydration, etc., as shown in Table 1 [16,17,18,19,20,21]. Glycerol-based additives have been used for blending agents in gasoline, diesel fuel, and biodiesel [22].

Table 1.
Derivatives of glycerol.
Martin and Richter presented a review for glycerol oligomerization to diglycerol and triglycerol, focusing on utilizing acidic and basic heterogeneous catalysts. Pure glycerol was replaced through its oligomers for numerous uses in the cosmetics and food industries [23]. Razali and Abdullah presented a review for catalyzing glycerol to lactic acid, where mixed metal oxides and bimetallic catalysts were found to be promising candidates [24]. Most of the published reviews have summarized the chemo, as well as biochemocatalytic transesterification and economic aspects of various transformation routes discussed [25,26]. A few comprehensive reviews have been published on the catalytic conversion of glycerol via reforming, dehydration, and hydrogenolysis. Since glycerol transformation is rapidly developing and its critical features are not clear, it is important to gather information on recent developments. Consequently, the objective of this review is to provide an update on the transformation of glycerol derived from biodiesel plants using heterogeneous catalysts into different products (chemicals, fuel additives, fuels). The review is organized well into three sections, dedicated to three major types of reactions (reforming, dehydration, and hydrogenolysis), with an emphasis on the performance of catalysts in designing reaction conditions. The different products observed and cataloged in this review involved hydrogen, acetol, acrolein, ethylene glycol, 1,3-propanediol(1,3-PDO), and 1,2-propanediol (1,2-PDO) from reforming, dehydration and hydrogenolysis reactions of glycerol conversions.
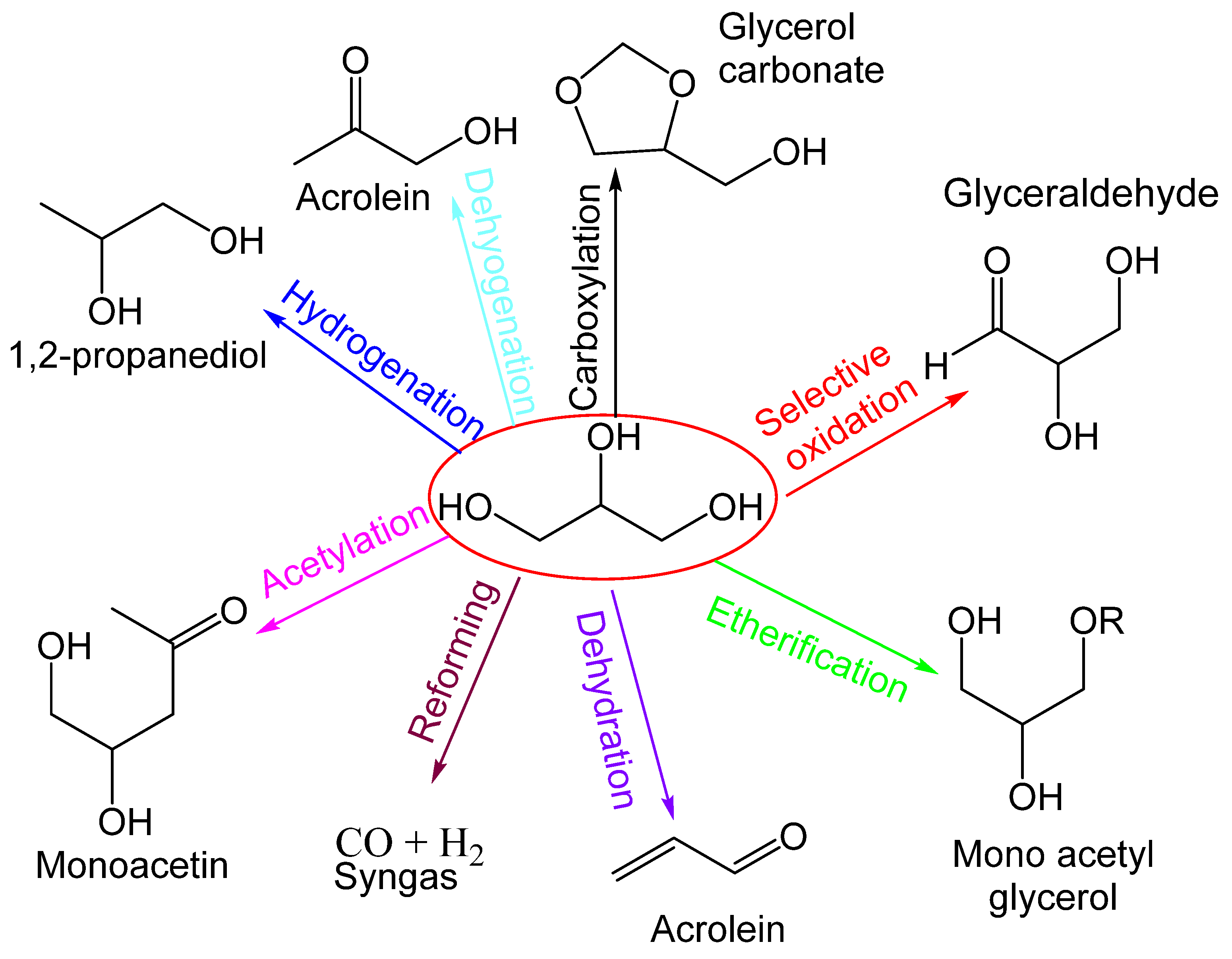
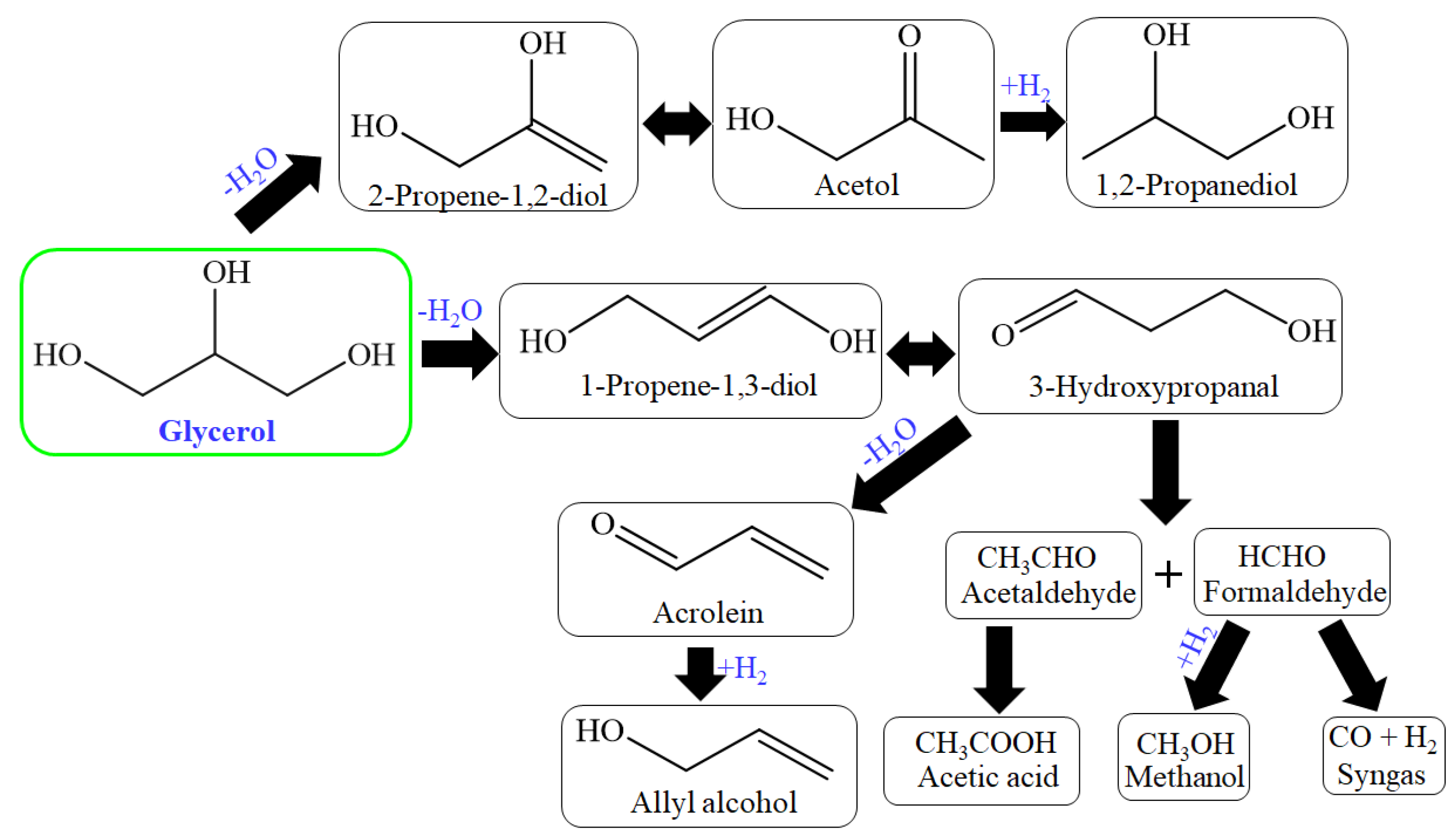
The production of chemical products from the catalytic transformation of glycerol is shown in Figure 1. Glycerol is produced from the transesterification of biodiesel and vegetable oils and animal fats, and the fermentation of carbohydrates. Syngas produced from glycerol yield liquid hydrocarbons, methanol, and other value-added products from the Fischer–Tropsch process. Syngas through the water–gas shift reaction produces liquid hydrocarbons, which include diesel, liquid naphtha, etc. Furthermore, through the methanol synthesis process, syngas produces methanol, and further methanol is used for the transesterification process and to produce chemicals [27].
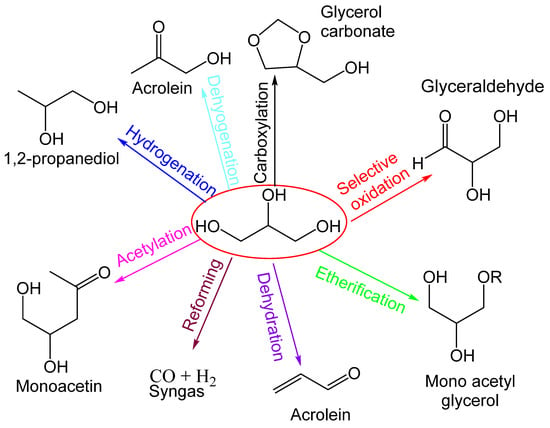
Figure 1.
Catalytic transformation of glycerol to various different products.
2. Glycerol Reforming
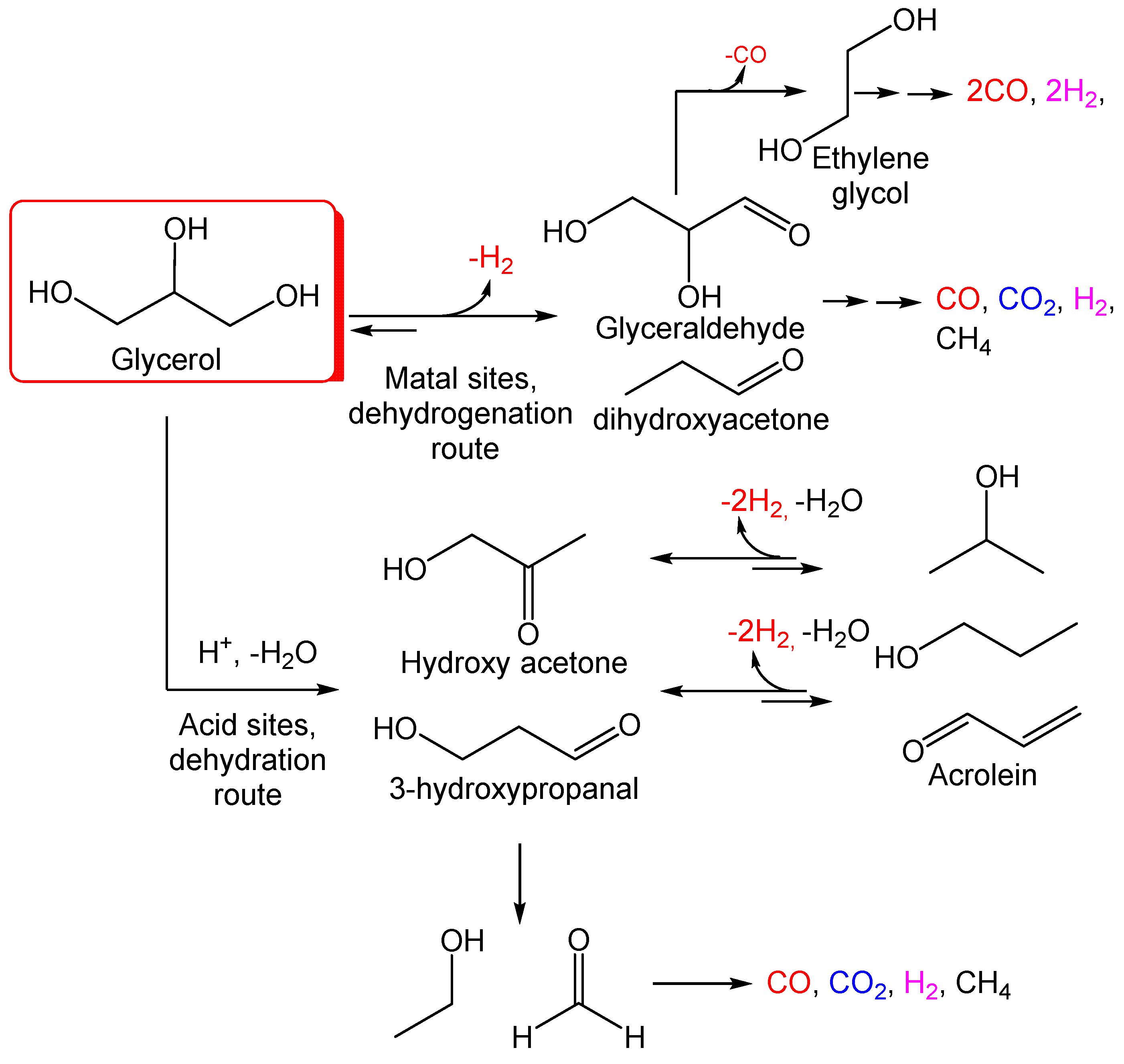
The glycerol transformation carried out through combustion in the past was unsafe, uneconomic, and technically deficient due to the high temperature employed, unwanted gas emission, and complex product [27,28]. Reforming techniques have gained acceptance by industrialists and researchers in view of their efficiencies. Steam, aqueous, and autothermal reforming processes have been primarily investigated in glycerol reforming. The steam reforming reaction is one of the most common reactions used in hydrogen synthesis. Low pressure and very endothermic reactions promote hydrogen selectivity. The aqueous phase reforming (APR) method is regarded as an inexpensive method for producing H2 due to its lower temperature and lack of vaporizing water and fuels [29,30]. Glycerol has a wide product distribution due to its complexity compared to low-chain hydrocarbons or alcohols and to the numerous reactions associated with the reforming process (Figure 2). However, this technique creates H2 without vaporizing the feedstock, resulting in significant energy savings [31]. Supercritical water gasification (SCWG)) is a cost-effective method of turning biomass (such as food waste, paper industrial waste, sewage sludge, agricultural waste, and forestry residue) into hydrogen-rich syngas [32]. Chemical looping steam reforming (CLSR), which uses oxygen carriers (OCs) as a catalyst, has been widely recognized as a highly effective method for generating hydrogen from catalytic steam reforming [33].
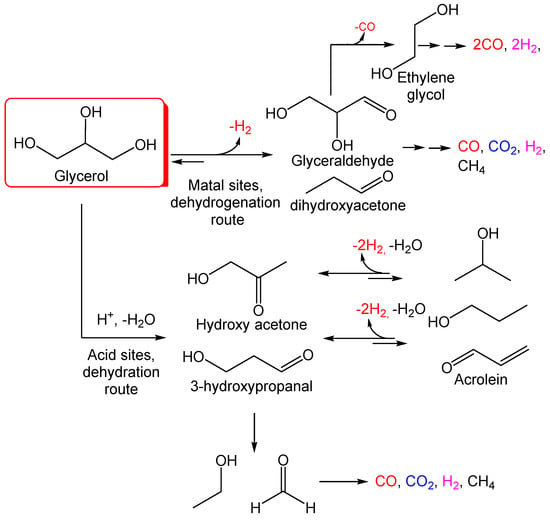
Figure 2.
Potential reaction pathways in glycerol aqueous phase reforming (APR).
Bio-derived liquids steam reforming produces hydrogen and becomes interested due to the environment and raises hydrogen demand, specifically in proton-exchange membrane fuel cells (PEMFC) [34]. The low temperature and high-pressure process conditions, concentrated feed, and acidic catalysts are used for the supercritical water reforming process. High temperature and low pressure, diluted feed, and alkali catalyst are used for gasification [35].
The glycerol reforming and endothermic reactions took place. Hence, the heat needed for the decomposition of glycerol (Equation (2)) is significantly larger than the water–gas shift (WGS) reaction (Equation (3)), where heat is produced as energy. As operated under atmospheric pressure, glycerol steam reforming (Equation (4)) becomes desirable with less fear of protection. Considerably, 1 mol of glycerol converts to produce 7 mol of hydrogen (Equation (3)). Vast amounts of steam and elevated temperature shift a reaction forward to equilibrium, and hence much more H2 is generated [36].
C3H8O3 ↔ 4H2 + 3CO Decomposition Reaction
CO + H2O ↔ H2 + CO2 Water Gas Shift Reaction
C3H8O3 + 3H2O ↔ 7H2 + 3CO2 Steam Reforming Reaction
The mentioned noble metal catalysts, Pt [37,38], Ir [39], Ru [40], and Rh [41,42], have shown good resistance to coke and catalytic activity. Vaidya and Rodrigues studied Ni, Pt, and Ru catalysts that promote hydrogen production but cause deactivation by dehydration [43].
2.1. Platinum Catalystsfor Glycerol Reforming
Doukkali et al. investigated Pt and/or Ni supported over γ-alumina catalyst for the generation of hydrogen by a method of APR through three preparation methods of catalyst: impregnation method, and sol-gel in acidic and basic medium. Total glycerol transformation occurred with maximum hydrogen production by sol-gel in basic method over PtNiAl (T = 250 °C, P = 30 bar, and WHSV = 2.6 hr−1). The acidity of the superficial hydroxyl groups on the -AlOOH surface could be to blame for the lower glycerol conversion into gaseous products, favoring dehydration processes and resulting in more OHCs in the liquid phase. In fact, by blocking surface hydroxyl sites on -Al2O3, structural alterations in hydrothermal settings were explored to create efficient alumina catalysts for APR processes. The drop in H2 production could be explained by Ni particle aggregation and reoxidation of Ni particles. These behaviors combined with the adsorption of carbonaceous molecules/organic compounds identified on several of the catalysts could be the primary causes of their deactivation [37].
Pompeo et al. investigated SiO2 catalyst supported with Pt and Ni through the ion-exchange method for steam reforming to produce hydrogen. The experiment for the steam reforming reaction of glycerol has demonstrated that using 1 Pt and 2 Pt catalysts. The more active and stable catalyst was Pt compared to Ni catalyst. At 350 °C, a complete conversion to gas was achieved with a space-time of 1.66 and 0.88 min, respectively. Due to the fact that the Ni catalyzed the WGS reaction to remove the CO adsorbed on the surface and turn it into CO2, the selectivity of gas products was significantly different. Only 3% CO was obtained using the 5 Ni catalyst at 450 °C for 1.66 min. During 40 hr activity test on stream at 350 °C, the 2 Pt catalyst allowed entire conversion to gas without deactivation. It was also suggested that bimetallic catalyst research is required to obtain a system with high selectivity for H2 and good stability [38].
2.2. Ruthenium Catalysts for Glycerol Reforming
Ru catalyst supported over core-shell metal-ceramic micro composites, created at low temperature and 1 atm pressure through microwave-induced hydrothermal oxidation technique (MW-HTO) of aluminum (Al) metal particles. The highest glycerol turnover ratio was achieved over Al, and Ru-modified Al2O3 at Al compared with Ru-modified MgAl2O4 and Ru-modified Al2O3 catalysts, suggesting that Al metal particles seem to be more effective in preparing MgAl2O4 at Al and Al2O3 at Al composite structure. The highest selectivity of hydrogen was 70% at Ru/MgAl2O4 at Al catalyst at 823 K reaction temperature, 1 atm pressure with 870 mmgcat−1 h−1 feed flow rate. The Ru/MgAl2O4@Al and Ru/Al2O3@Al catalysts have 2–3 times the glycerol conversion rates of their Ru/MgAl2O4 and Ru/Al2O3counterparts, owing to enhanced heat transfer via the metal-ceramic composite catalytic architectures [40]. Glycerol steam reforming process over Ru doped Al2O3 catalyst. In total, 89.1% glycerol transformed to produce 0.49 (mol/mol) yield of hydrogen at T = 500 °C, P = 0.1 MPa and 1.98 W/FA0. This research has aided in developing reactor systems for producing hydrogen from glycerol steam reforming [41].
2.3. Rhodium Catalysts for Glycerol Reforming
Martinez et al. studied hydrogen production from glycerol reforming using fluorite-type oxides CeZr-CoRh prepared by the sol-gel method. 100% glycerol was transformed to produce 86% hydrogen yield at 650 °C. The activity data showed that the catalysts’ ability to activate H2O under reaction circumstances was connected to selective H2 generation. This process guarantees that the by-products are steam reformed to H2. As this capacity deteriorates, H2 generation decreases, steam reforming capability reduces, and glycerol decomposition takes over. In this scenario, the creation of condensable compounds, such as hydroxyacetone, acetaldehyde, and acrolein, was aided by the production of CO, CH4, and C2H4 [44].
Lee and Doohwan synthesized Core−shell MgAl2O4 metal−ceramic composites at Al catalyst by hydrothermal method, and Rh (1, 3, and 5 wt.% loading) modified MeAl2O4 catalysts were synthesized by incipient wetness impregnation method. Then, 100% glycerol transformed to produce Rh/MgAl2O4 at Al catalyst [T = 450 °C, and WHSV = 34,000 mL g−1 h−1]. The high surface area and complex surface morphological properties of MeAl2O4@Al as a catalyst enhance increased Rh cluster dispersion while hindering their thermal sintering during glycerol steam reforming processes at high temperatures. Rh/MeAl2O4@Al had a 20–30% greater glycerol conversion turnover rate than Rh/MeAl2O4 (both Rh cluster sizes are similar), indicating that the facilitated heat and mass transfer through the unique MeAl2O4@Al microstructures cooperated constructively for the reactions. These are being explored as future research topics [45]. Baca et al. studied Rh, Ni, and Co metal-doped over Al2O3–ZrO2–TiO2 (AZT) for syngas production through carbon dioxide reforming of glycerol. Then, 1 wt.% Rh doped AZT, 5 wt.% Ni-doped AZT, and 5 wt.% Co-doped AZT catalysts were synthesized through the incipient impregnation method. The glycerol transformation was 80% achieved with 24% maximum hydrogen yield on Rh doped AZT catalyst at 750 °C. Glycerol Conversion reduced in the given sequence: 1% Rh doped AZT > 5% Ni doped AZT > 5% Co-doped AZT. Rh doped AZT catalyst remained stable due to fewer coke formations and a shortage of sintering. During the 72-h testing, the decrease in CO2 conversions was less than 13%, indicating the remarkable stability of Rh/AZT and Ni/AZT [42]. Delparish et al. produced syngas from glycerol by oxidative steam reforming. The impregnation technique was used to prepare 2 wt.% of Rh doped Al2O3 catalysts. The glycerol was completely converted at 550 °C, and maximum hydrogen yield was achieved at 700 °C. Temperature influenced product distribution by increasing the amount of combustible species in the product mixture that were oxidized. The effect became more severe when C/O was lowered from 1.125 to 0.75. The promotion of WGS in the presence of steam was confirmed by reducing S/C from 5 to 4 and then from 4 to 3, which resulted in monotonically decreased H2 and CO2 yields and an increase in CO yield [46]. Danga et al. studied glycerol and ethanol for dry reforming with CaCO3 to produce syngas. A series of metal components, such as nickel, iron, copper, platinum, palladium, ruthenium, and rhodium, were used to promote dry reforming. In these series of metals, nickel became a promising candidate due to its outstanding performance, as well as its low price. Here, 100% glycerol conversion along with approximate 92% syngas selectivity achieved on 10 wt.% Ni contained in CaCO3catalyst [T = 550 °C, and P = 1 atm.]. The CO2 released from the CaCO3 catalyst eliminated cokes and attained stability. The direct use of CaCO3 in this process was discovered viable, and the CO2 generated from CaCO3 decomposition may be used to modify the H2/CO ratio. Ni was the most promising contender among the active metals, including Ni, Co, Fe, Cu, Rh, Ru, Pt, and Pd, due to its great performance and low price. Over the 10 Ni–CaCO3 catalyst, 100% conversion of glycerol and ethanol, 92% summed H2 and CO selectivity, and an H2/CO ratio of 1.2 could be reached under ideal conditions. Meanwhile, the robustness of this integrated technique was proved over five dry reforming–regeneration cycles. The findings suggest a novel way to use CO2 in the form of carbonates [47].
2.4. Nickel Catalysts for Glycerol Reforming
The catalyst based on Ni becomes a choice for the reason that the metallic sites of Ni work well enough in water gas shift reaction as well as C–H and C–O cleavage. Nevertheless, the tremendous challenge is how to control catalysts’ deactivation triggered by sintering and deposition of carbon at catalyst active sites under optimum operating conditions [13]. Hence, various protocols [40,48] and supports like Al2O3 [49,50,51], ZrO2 [52,53,54], CeO2 [55,56] were generated.
Yancheshmeh et al. suggested a new and simple method for preparing Ni-modified Al2O4 spinel catalyst from the steam reforming technique of glycerol for the production of hydrogen. Solvothermal preparation method was used for catalyst represented as NiAl-G1, and the coprecipitation preparation method was used for catalyst described as NiAl-G2 as well as NiAl-C (NiAl2O4 catalyst synthesized via the coprecipitation method was designated as NiAl-C). The maximum hydrogen yield was 76.38% achieved with 95.42% maximum glycerol transformation on NiAl-G2 catalyst 630 °C, 1 atm, and 19,600 cm3/gcat/h space velocity reaction conditions. By creating an oxidative environment surrounding nickel active sites, the creation of a well-dispersed CeAlO3 phase slowed the growth of filamentous carbon on the nickel surface and aided the gasification of carbon deposits. During the 16-h SRG process, the catalytic activity was maintained, and the rate of coke generation was as low as 0.0004 gcokegcatalh−1. These findings support Ce/NiAl_G2’s potential as a catalyst for the SRG reaction [56]. Ni added to CaO-modified attapulgite (CaO–ATP) catalyst from reforming of glycerol for hydrogen manufacturing. Using the impregnation technique, Ni-Al2O3, Ni-ATP, and Ni-CaO-ATP catalysts can be synthesized. A maximum hydrogen yield of 85.3% was achieved on Ni-CeO-ATP catalyst with 93.71% glycerol conversion at T = 600 °C and GHSV = 1 h−1 reaction condition. The stability of catalyst showed that ATP had better resistance for deposition of carbon compared to alumina and CaO addition further reduced the production of carbon. Due to its unusual intermediate structure, ATP outperformed Al2O3 as a carrier. When CaO was utilized as an ATP modifier, it promoted the water gas shift reaction, resulting in increased hydrogen yield, hydrogen selectivity, and a reduction in CO production. The stability results revealed that ATP is more resistant to carbon deposition than Al2O3, and the addition of CaO lowered carbon formation even more. As a result, a Ni-based catalyst supported on a CaO-modified attapulgite could be a potential material for efficient glycerin steam reforming to create H2 [57].
Wu et al. explored the mesoporous Ni-Cu/CeO2 for the production of renewable hydrogen via APR of glycerol. The C-C breakage of glycerol benefits from Ni, but Cu can amplify the WGS reaction. Cu metal’s surface is amenable to adsorption Carbonyl radical following glycerol’s decarbonylation and hydroxyl free radical in the solution, which facilitates the reaction. Consequently, the addition of Cu element enhanced the proportion of H2 and CO2 in the overall gas. It was discovered that increasing the catalyst’s Cu concentration improved hydrogen production (125.08 to 195.57 μmol min−1 gcat−1). Additionally, the CO2 in overall gas was absorbed by the addition of CaO and it lowers the activation energy. Hence, 1Ni2Cu/CeO2 + 0.2gCaO catalyst shows H2 production rate increased from 168.97 to 301.92 μmol min−1 gcat−1. Therefore, improved H2 production as a result of the inclusion of Cu and CaO [29].
Liu et al. studied MgO supported Ni-Co bimetallic catalyst prepared by sol-gel method followed by calcination. The resulted catalyst examined the APR of glycerol for hydrogen production. After addition MgO support, H2 production activity improves by 1.5 times compared to pure Ni-Co bimetallic catalyst. Due to the in-situ adsorption and removal of CO2, the WGS reaction was further stimulated with the addition of CaO, and the methanation reaction was inhibited, resulting in the catalyst having a high activity of hydrogen production. The stability test also shown that the gelatinous MgO supports had an impact on the catalyst’s activity and stability [30].
Veiga et al. studied crude glycerol steam reforming with oxalic acid to synthesize Ni-doped La-Zr catalysts. Ni-doped La-Zr catalyst was prepared with the coprecipitation method at different calcination temperatures. Ni-doped La-Zr catalysts calcined at 850 °C achieved 90% hydrogen yield and 99.9% glycerol conversion. The catalyst prepared at 850 °C calcination and operated at 650 °C reaction showed higher catalytic stability, activity, and better resistance to carbon formation. The greatest performance seen for Ni-850 catalysts is mostly due to the development of oxygen vacancies caused by nickel substitution into the La-Zr lattice during calcination, favoring the oxidation of carbon deposits throughout the process [58].
Charisiou et al. investigated Ni supported over Y2O3–ZrO2 catalyst for the glycerol steam reforming. The catalyst synthesized through the impregnation technique was Ni modified Zr and Ni modified YZr. The maximum transformation was 90% achieved at 700 °C, and the maximum H2 selectivity was 82% achieved at 450 °C over Ni modified YZr catalyst. The research on spent catalyst revealed the less carbon deposition on Ni/Zr (Ni modified Zr) catalyst as it was more graphitic in nature and higher carbon deposition on Ni/YZr spent catalyst. The addition of Y2O3 stabilized the ZrO2 tetragonal phase, resulted in more easily reducible NiO nanoparticles, increased the O2 storage capacity of the support and the medium strength acid sides of the catalyst, and, despite having a higher concentration of basic sites, the Ni/YZr presented more stable monodentate carbonates. Allyl alcohol, acetaldehyde, acetone, acrolein, acetic acid, and acetol were the primary liquid products detected for both catalysts. Although higher amounts of carbon were deposited on the Ni/YZr spent catalytic sample (0.70 gcoke/gcatal compared to 0.51 gcoke/gcatal for the Ni/Zr catalyst), the addition of Y2O3 on to the ZrO2 support resulted in structures with lower crystallinity (amorphous carbon) that are more easily oxidized during the reaction. The coke deposits found on spent Ni/Zr catalysts, on the other hand, were graphitic structures with little flaws, resulting in the deadly encapsulation of Ni particles [54].
2.5. Cobalt Catalysts for Glycerol Reforming
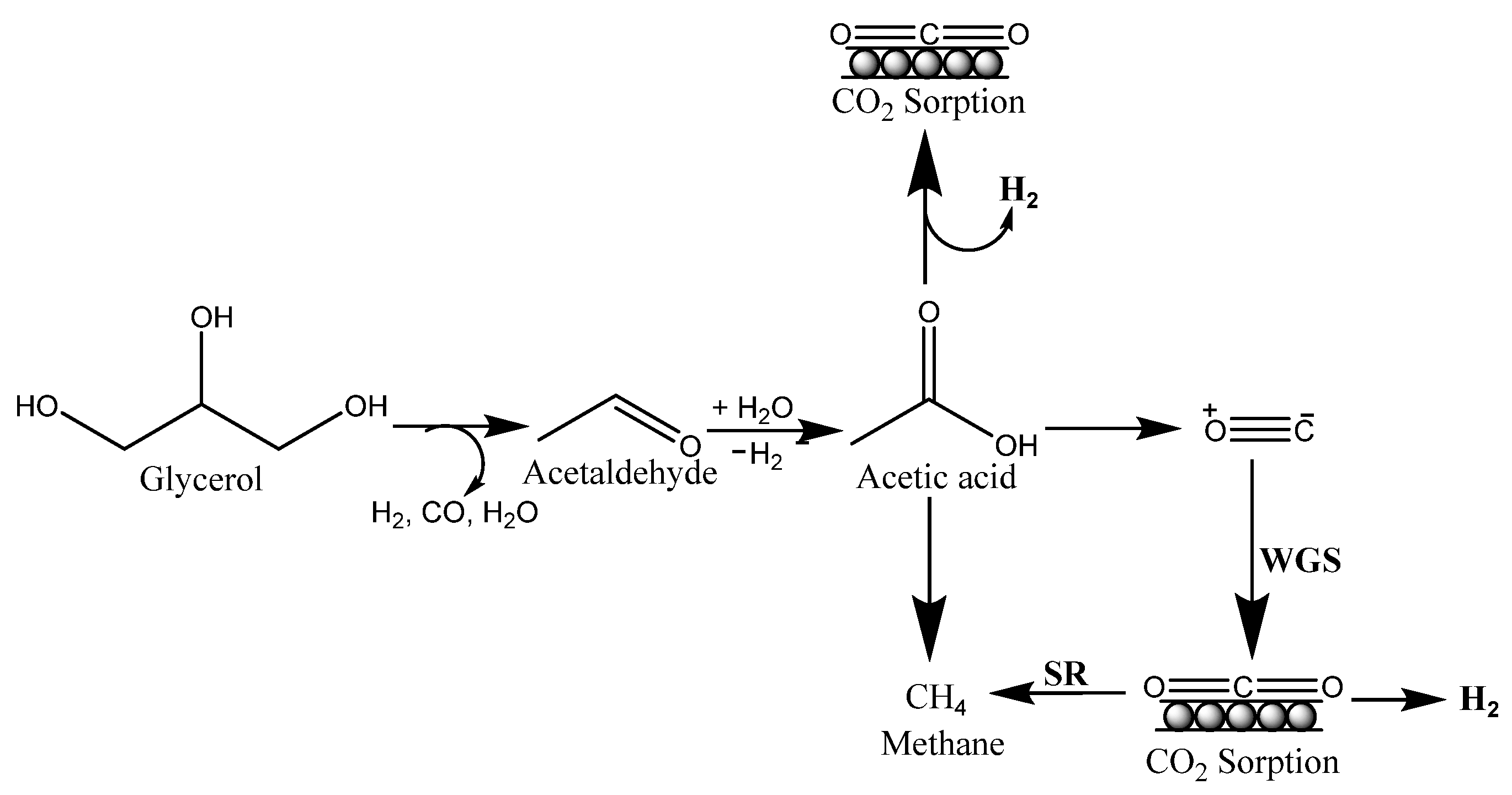
Cobalt (Co) played an extensive role in the synthesis of hydrogen as many researchers represented this glycerol steam reforming. Ghungrud and Vaidya studied the synthesis of hydrogen by sorption–enhanced steam reforming technique (SESGR) on a Co-promoted hydrotalcites catalyst. 92.8% maximum glycerol conversion and 89.7% hydrogen yield were achieved over Co-Ca-HTlc catalyst represented as HM2 at T = 823 K, GHSV = 5600 mL g−1 h−1. Ca, Co, and Zn promoted hydrotalcite catalysts were manufactured through the precipitation method. HM2 contained Cu showed the best performance for the dehydrogenation process, and it was known that basic sites promoted the dehydrogenation process. At 550 °C, Cu-bearing materials showed a long pre-breakthrough duration (40 min) and high H2 purity (93.1 mol%). They were also better adsorbents, removing 1.1 mol CO2/kg sorbent at 550 °C. Over 20 cycles of adsorption and regeneration, the cyclic stability of materials was examined. The potential of customized HTlc-materials for better H2 production from the SESGR process is explored in this work [59].
A feasible reaction pathway for the SESGR process was provided based on the product composition (Figure 3). Glycerol undergoes dehydration hydrogenation reactions upon adsorption, resulting in acetaldehyde. The adsorbed acetaldehyde was then further hydration-dehydrogenated into acetic acid. Over Co metal, acetic acid undergoes C-C bond breakage, yielding H2, CO2, CO, and CH4. The basic catalyst promotes rapid dehydrogenation, which prevents the generation of olefins and carbon deposition. CO and CH4 are converted to H2 and CO2 by WGS reaction and steam reforming processes, with trace amounts of CO remaining [59].
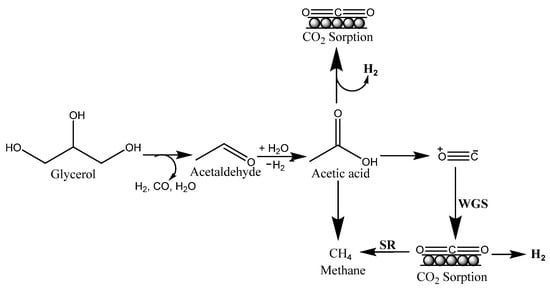
Figure 3.
Plausible reaction mechanism for SESGR.
Zhou et al. investigated NiCo modified CNTs bimetallic catalysts with an appropriate distribution of Ni, as well as Co, metal for synthesizing hydrogen through the impregnation technique in which metal precursors were injected into the cave of CNTs by ultra-radiations. At 450 °C and 1 atm, 100% glycerol transformation was achieved with 91.4% H2 selectivity over Ni(i)Co(i)/CNTs catalyst. Ni(i)Co(i)/CNTs exhibited the best stability and catalyst performance. Although the Ni and Co oxides were only partially reduced, the location of metal species had a significant impact on reducibility. The synergetic effect between Ni and Co species was found in Ni(i)Co(i)/CNTs and Ni(o)Co(o)/CNTs, where they had the same distribution on the internal or external wall. Sintering of active sites was markedly simpler on the external wall of CNTs, causing the catalyst to deactivate quickly and become less reactive for the water gas shift process. The result of this study revealed that there is a new and straightforward method for assembling bimetallic catalyst systems. The Ni-based bimetallic catalysts might be used to reform mono/multi-alcohols such as ethanol, ethylene glycol, sorbitol, and other organic wastes from second-generation biorefineries, as well as biogas and other organic wastes [37].
Al-Salihi et al. investigated Co-Ni-MgO-based SBA-15 nano-catalysts for the glycerol steam reforming for hydrogen manufacturing. The one pot hydrothermal method was used to create different mesoporous catalysts, such as Co-SBA-15, Ni-SBA-15, Co-MgO-SBA-15, Ni-MgO-SBA-15, and Co-Ni-SBA-15 and bimetallic 10 wt.% Co-5 wt.% Ni modified SBA-15 catalyst synthesized by the impregnation method. A glycerol conversion rate of 100% along with 82.8% hydrogen selectivity was achieved over 10 wt.% Co-5 wt.% Ni modified SBA-15 catalyst at 650 °C. At a low temperature (450 °C), the one-pot catalysts showed 50% glycerol conversion compared to 70% for the impregnated catalyst, while both yielded 62% H2 selectivity. Cobalt-based SBA-15 catalysts have superior GSR activity and stability than nickel-based catalysts in monometallic catalysts. The addition of MgO to Co-SBA-15 improved glycerol conversion (up to 99%) and catalyst stability. The addition of MgO to Ni-SBA-15 reduced the quantity of carbon deposition on the catalysts by as much as 66%, according to TGA-TPO analyses of spent catalysts. It’s worth noting that under our experimental conditions, all of the catalysts examined at the optimum temperature of 650 °C demonstrated a remarkable hydrogen selectivity of 70% [36].
Duo et al. developed chemical looping steam reforming process for glycerol conversion to hydrogen over Al-MCM-41 and SBA-15 well-order mesoporous OCs containing NiO and CeO2 nanoparticles [33]. It was discovered that the NiO and CeO2 in the OCs were reduced by glycerol, and the reduced OCs were in charge of producing hydrogen. It was shown remarkable stability in 60 redox cycles (180 h), and the carbon conversion and hydrogen selectivity were both over 60% and 85%, respectively. Oxygen transport was enhanced by heavily loading mesoporous MCM-41 and SBA-15 with NiO and CeO2, which also produced outstanding redox CLSR cycles and long-term stability [33].
The summary of some more reported literature related to glycerol reforming over various catalysts to produce hydrogen is represented in Table 2. Noble metal catalysts, such as Pd, Ru, Ir, Rh, and Pt, are known for their high catalytic activity, which results in good performance and coke resistance. Other catalysts, such as Ni, Co, and Cu, on the other hand, are less expensive and more readily available and have a desirable catalytic activity for boosting hydrogen production. Their stability, on the other hand, is undesirable since the coking process frequently deactivates them. As a result, developing a highly active and stable catalyst requires the employment of metals as catalysts in combination with the proper selection of support (based on porosity, redox characteristics, and thermal stability, among other factors). Alumina and silica are the most common catalytic supports in research, but some catalysts can also be supported on active carbon, nanotubes, mesoporous carbon, nanoparticles, and other materials. Due to its high specific surface and thermal stability, alumina is the preferred and most used support for this purpose. However, carbon deposition tends to render it inactive. The optimal bimetallic catalyst must possess excellent thermal strength, mechanical resistance, and redox characteristics to produce H2 with high selectivity.

Table 2.
Hydrogen production from glycerol reforming using various catalytic systems under optimal reaction conditions.
3. Glycerol Dehydration
Acrolein was obtained by anhydrous glycerol decomposition in the existence of solid catalysts such as alkali–metal acid sulfates and phosphorus pentoxide in the 19th century [91,92]. Hence, the suggested route is illustrated in Figure 4. Acrolein is transformed directly into targeted high-value-added derivative products because of its toxic effects. Acrolein is an intermediate in producing acrylic acid and acrylic polymers in the industry. The consumption of acrolein is also for the production of DL-methionine, and 3-methylthio-propionaldehyde is produced as an intermediate here [93]. Early studies concentrated on homogeneous catalysts in the aqueous phase under near- or supercritical conditions. However, the vapor phase over heterogeneous catalysts are chosen due to the predicted high cost of the reaction vessel and separation concerns [10]. However, until the end of the twentieth century, when cheaper glycerol from the biodiesel synthesis process became accessible, these early works in both the gaseous and liquid phases remained unexplored [93].
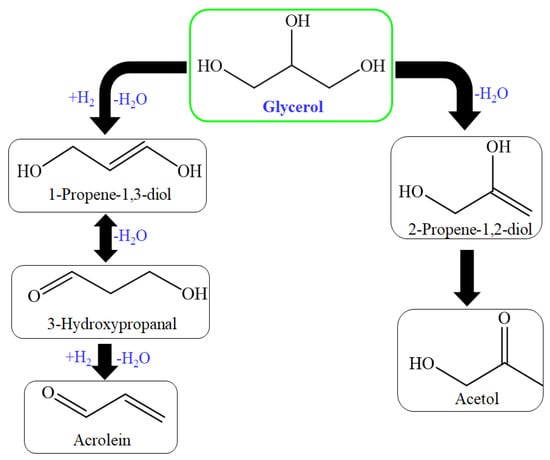
Figure 4.
Reaction route for glycerol dehydration to produce acrolein and acetol.
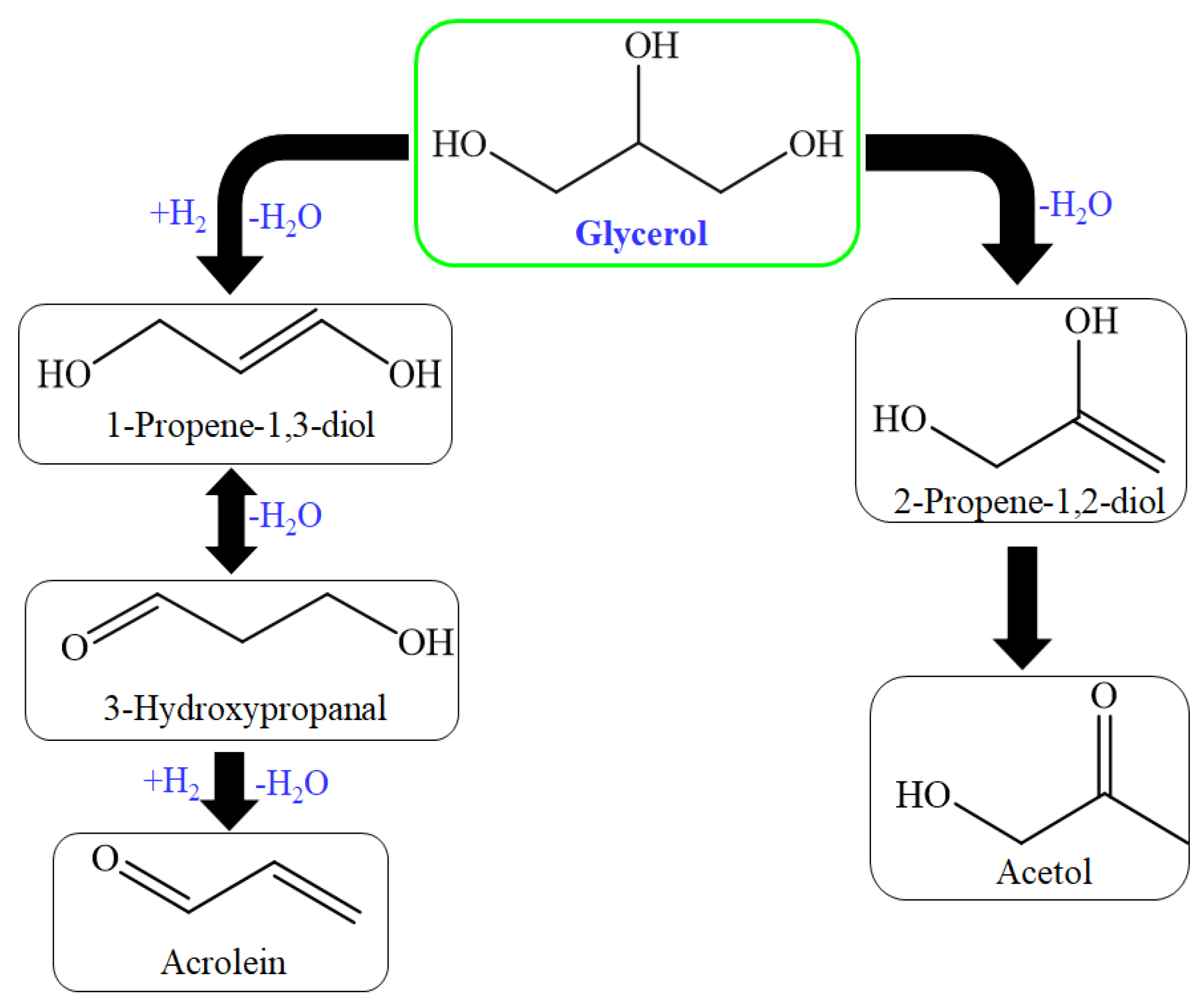
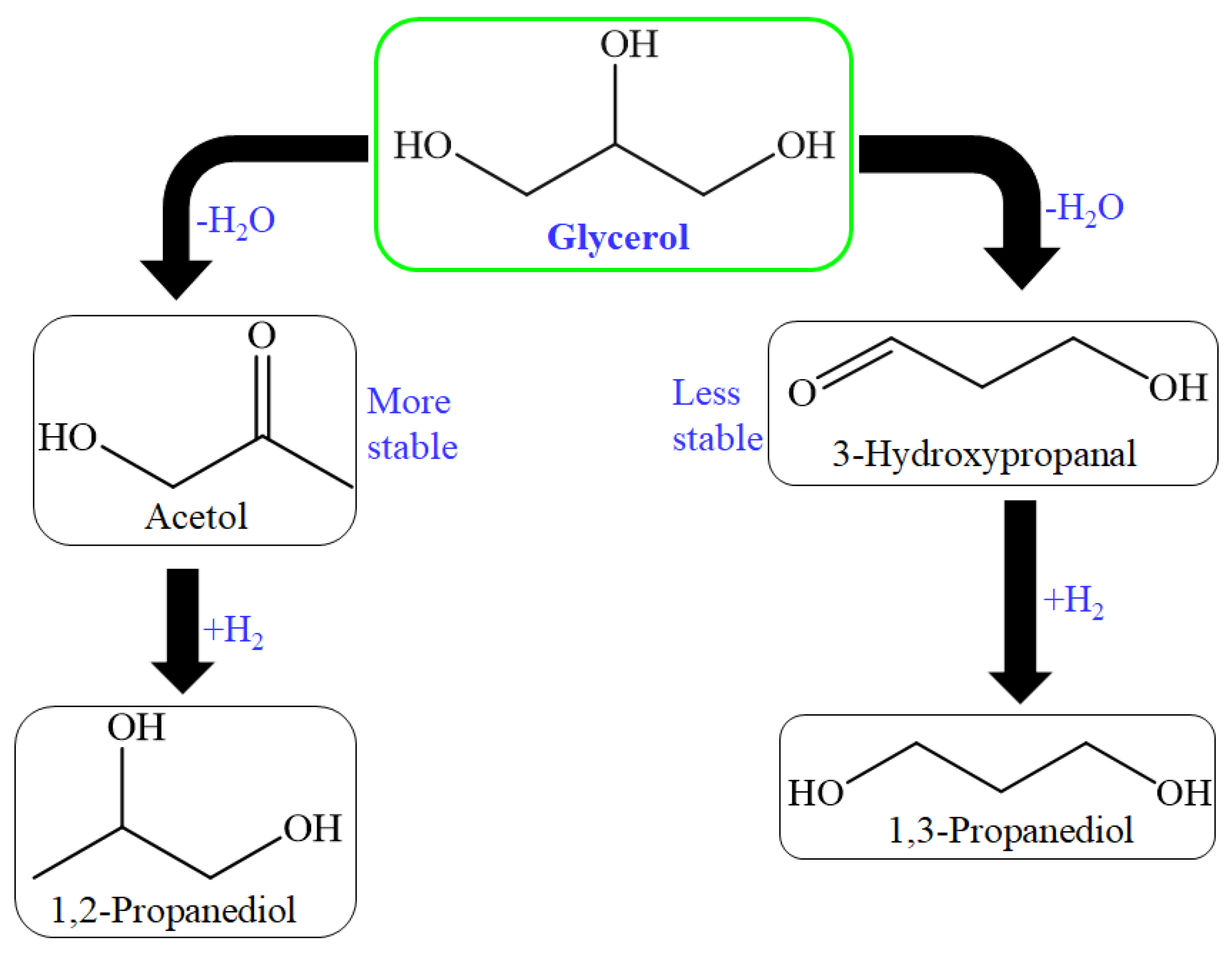
The high selectivity was achieved due to the control of 1st dehydration reaction. Tsukuda et al. proposed this mechanism, where 3-hydroxypropionaldehyde formation was favorable over the acetol formation, which is considered a byproduct of the primary reaction step; further, this mechanism (Figure 5) suggested two hydrogenation steps: first from acrolein and second from acetol to achieve allylic alcohol and 1,2-PDO, simultaneously [94,95]. So many solid acid catalysts have been used for dehydration of glycerol for acrolein production through zirconia, heteropoly acid, niobia, and alumina catalysts [96,97,98].
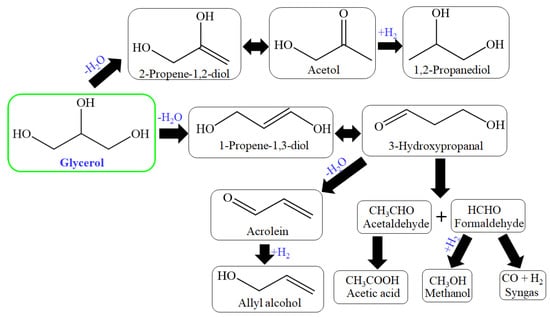
Figure 5.
Reaction route for acrolein formation by acid-base catalysts.
3.1. Zeolite Catalystsfor Glycerol Dehydration
Lin et al. investigated ß zeolites (HNa–ß–κ) for the production of acrolein through glycerol dehydration. In total, 65% glycerol transformed to produce 23.3% acrolein yield at HNa–ß–0 catalysts at 200 °C. It was speculated that smaller pores increased selectivity but reduced activity for acrolein production. An increase in temperature favored the selectivity of acrolein. After being employed in the reaction test (see infra) and recalcination at 450 °C in the air for 4 h, the XRD patterns of these zeolitic catalysts were substantially identical. These results showed that the ion-exchange technique, subsequent calcination, and reaction tests did not result in any visible changes in the zeolitic crystal structure. As the Na/Al ratio increased, the apparent surface area of the HNa-ß–κ zeolites decreased. At the same time, the pore width reduced as the Na+/Al ratio increased, which could explain why Na+ is larger than H+ when residing in the zeolitic channel. According to the pore diameter data, these pore widths correspond to the 12-membered ring in the b-zeolites and the 10-membered ring in the H-MFI zeolite. Na+/H+ ratio increment also enhanced acrolein selectivity and decreased glycerol conversion. The highest acrolein yield was achieved due to the replacement of acidic sites with non-active Na+ sites [99].
Ren et al. investigated MnHPO4 and meso-zeolite (H-ZSM-5) catalyst prepared by the ion-exchange technique and concluded that the less strong acid amount was more beneficial for the production of acrolein and also coke suppressed in glycerol dehydration. A 100% transformation of glycerol was achieved for all reactions and 84.7% highest acrolein yield over meso-zeolite(HZSM-5) supported on MnHPO4 catalysts [100]. Meso and microporous zeolite by one-step synthesis technology for acrolein formation from glycerol dehydration. HZSM-25 catalyst achieved 99% glycerol transformation and 80% acrolein selectivity at 450 °C, 1 atm, and 38.47 h−1 WHSV. The high acidity and fast diffusion of species in synthesized ZSM-5 supported acrolein production from dehydration of glycerol. Diffraction peaks of five types of transition metal hydrogen phosphates supported on meso-HZSM-5 were also shown to exhibit this behavior [101]. Qureshi et al. investigated nano H-ZSM-5 highly crystalline catalyst for glycerol dehydration through hydrothermal technique. A 98% glycerol transformation occurred to produce 87% acrolein selectivity at HZSM-5 (Si/Al = 75) at 320 °C, 1 atm, and 4 h−1 WHSV. According to these findings, the catalysts with a Si/Al ratio of 75–100 had a higher crystallinity than the other samples. The SEM pictures revealed the catalyst’s surface morphology, particle size, and crystal aggregation. The exterior surface of all H-ZSM-5 catalysts produced was uniform and had a highly orientated shape. The catalysts exhibit consecutive crystal lattices and a smooth surface, according to TEM pictures of H-ZSM-5 with a Si/Al ratio of 75 [102].
Yun et al. investigated marigold-like silicon dioxide (silica) functionalized through groups of sulfonic acid (MF–FS) and zeolite (HZSM-5) catalysts for the production of acrolein from glycerol dehydration. The maximum acrolein yield was 73%, with 100% glycerol transformation achieved over MF-CS catalyst at 250 °C. Bronsted acid sites in MF-FS catalyst promoted stability as well as selectivity for acrolein production [103]. Chai et al. studied acrolein production through dehydration on various solid acid-base catalysts synthesized by the impregnation method. A 100% glycerol transformation was achieved with 65% acrolein selectivity over 15 wt.% WO3/ZrO2 catalyst at 315 °C, 1 atm, and 80 h−1 GHSV. For acrolein production, Bronsted acidic sites were superior to Lewis acidic sites [94]. Xie et al. investigated microwave absorbing catalysts for acrolein production from glycerol dehydration. Most notably, the catalyst stability by microwave heating technique was much better than the heating process by electric. WO3/ZrO2 at SiC coated microwave absorbing catalyst was synthesized. The complete glycerol transformation (100%) was achieved with 70% acrolein selectivity by 250 °C microwave heating [104].
Shan et al. investigated fabricated nanosheet MFI zeolite catalyst for glycerol dehydration for acrolein production [105]. A 99.7% transformation of glycerol was achieved with 81.8% acroleinat 320 °C and 1 atm. Compared to conventional ZSM-5 zeolite (CMZ-X), MFI zeolites nanosheets were more active as well as stable for glycerol dehydration reaction in the gas phase. The nanosheet MFI zeolites (NMZ-X) decreased the rate of coke deposition which resulted higher stability as well as activity. The zeolites in CMZ-X produced with tetrapropylammonium hydroxide as the structural guiding agent were shown as uniform 400 nm particles with cylindroid intergrowth. The NMZ-X Si/Al molar ratio has a significant impact on the morphologies of nanosheet MFI zeolites. NMZ-100 appears to be a three-dimensional multi-lamellar stacking of MFI nanosheets with intergrowth. The NMZ-100 particles had a diameter of roughly 1.5 µm and were made up of 30–40 nm thick lamillar stacking. Meanwhile, NMZ-50 demonstrated similar MFI nanosheet lamellar staking, but the particle size (500 nm) and lamellar stacking thickness (approximately 10 nm) were substantially less. NMZ-30, on the other hand, looks like cotton balls and could not tell the MFI nanosheets from the SEM image. NMZ-30 was well-crystallized and exhibited nanosheet MFI zeolite properties, as indicated by the XRD patterns. It was discovered that Al content had a crucial impact on the development of NMZ-X by comparing TEM pictures of nanosheet MFI zeolites with varied Si/Al molar ratios. Due to the abundance of unstable Si–O–Al units, it was difficult to generate large area MFI nanosheets with a low Si/Al molar ratio [105].
3.2. Titania Catalysts for Glycerol Dehydration
Titanium oxide (Titania) has three crystalline phases: brookite, anatase, and rutile. Amid all, the anatase phase has vital applications [106,107,108,109]. Babaei et al. investigated TiO2 catalysts for acrolein production for glycerol dehydration. It was mentioned that the central hydroxyl group adsorption on the surface had the best geometry and energy (30.91 kcal/mol) for dehydration reaction to acrolein. The glycerol dehydration to acrolein is followed by removing water molecules. The TiO2 surface exhibited more activity in proton transfer reaction with 89.1 kcal/mol E (activation energy) comparing the above two routes for acrolein formation [109]. Ulgen et al. synthesized WO3/TiO2 catalyst through the impregnation method for acrolein formation from glycerol dehydration. Maximum acrolein selectivity of 80% and the complete conversion of glycerol was achieved over WO3/TiO2 catalyst at 280 °C. In most cases, a larger BET surface area was associated with better glycerol conversion. Titania-supported tungsten oxide systems were ideal catalysts for the dehydration reaction of glycerol due to their longer service time, decreased deactivation speed, and much lower pricing [110].
3.3. Silica Catalysts for Glycerol Dehydration
Wang et al. focused on low-temperature (210–230 °C) glycerol dehydration to acrolein through temperature-programmed surface reaction (TPSR). A series of acid catalysts (lewis acid catalysts, supported heteropolyacids and superacids, molecular sieves, and metal oxides) synthesized by the impregnation method has been screened for glycerol dehydration at low temperature (210 °C) through TPSR. SiW/SiO2, as well as SO42−/TiO2 achieved high acrolein selectivity (85% and 86%) simultaneously. The maximum glycerol transformation was achieved at 94.4% at 230 °C [111]. Rosas et al. investigated some most active catalysts, namely, NH4 and La modified β zeolites, Pd and La modified Y zeolites, hierarchical zeolite (ZSM-5), WO3 modified ZrO2, WO3 modified TiO2, ZrOx and WOx modified NbOx, WO3-SiO2 modified ZrO2, NbOx-WOxmodified Al2O3, H3PO4 modified MCM-41, SAPO-40, and NbPSi for glycerol dehydration reaction in the gas phase. A 100% conversion was obtained at 300–325 °C. In this, the gas-phase catalytic process was more suitable than the liquid-phase due to high acrolein yields (>70%) [112]. Silica and phosphate supported copper catalyst for glycerol dehydration to produce acetol. Silica and phosphate supported copper catalyst produced through sodium silicate neutralization with the orthophosphoric acid and added copper nitrate. After this, 58.3% acetol selectivity and 100% glycerol transformation were achieved 40 CuSP catalyst at 220 °C, and atmospheric pressure. Lewis acidic sites were responsible for the increase in selectivity of acetol [113]. Basu et al. investigated silica-supported copper chromite catalyst synthesized through a sol-gel technique for dehydration of glycerol for acetol production. The transformation of glycerol reached 100% with 70% maximum selectivity of acetol for 40 wt.% Copper chromite loading on silica at 220 °C and atmospheric pressure. The spent and oxidized catalyst presented lower selectivity and conversion compared with reduced form catalyst due to cupric ion in reduced form catalyst, which worked as Lewis acid sites in dehydration [114].
3.4. Alumina Catalysts for Glycerol Dehydration
Mesoporous alumina was prepared with different precipitants through a continuous precipitation method for gas-phase glycerol dehydration for acrolein production by Lima et al. A 99% glycerol transformation occurred at alumina catalyst prepared at 7 pH with NaOH precipitant at 500 °C. Results revealed high carbon formation and higher olefins production, which showed the production of carbon is related to these by-production formations. The sample synthesized with KOH had the largest specific surface area (432 m2 g−1) among the influences of the precipitating agent, whereas the sample created with Na2CO3 had the lowest specific surface area (432 m2 g−1) (48 m2 g−1). The specific surface area of the produced material was similarly affected by the pH of the precipitation. Due to the type of precipitate generated, samples prepared at pH 5 (389 m2 g−1 of Na2CO3 precipitant and 359 m2 g−1 of NaOH) have a greater specific surface area than those prepared at pH 7. When precipitation was carried out at pH 7, the resulting residue was weakly crystalline, creating pseudo-boehmite. At pH 5, an amorphous precipitate was formed, yielding a microcrystalline boehmite with a higher specific area [115].
Kim et al. investigated silica and alumina catalyst for glycerol dehydration reaction in gas-phase for production of acrolein. The conversion of glycerol was 50% along with 16% acrolein selectivity achieved over Si0.6Al0.4Ox catalyst at 315 °C and 62 h−1 WHSV. It was concluded that the yield of acrolein depended on the brønsted acid sites. As Si mole fraction was increased up to 0.8, the intensity of the Al2O3 XRD peak increased and subsequently declined as Si mole fraction was increased. Due to the tetrahedral interaction between Al and SiO, the degree of crystallinity is determined by the Al concentration contained in SiO2. The specific surface areas of the silica–alumina increased dramatically when Al (or Si) species were added, compared to SiO2 and -Al2O3. The catalyst pore volume followed a volcano-like pattern in relation to the mole fraction of Al, while the average pore diameter shrank as the mole fraction of Al increased. The catalysts’ pore size distributions revealed a noticeable variation in pore diameter with regard to the Al content. For silica–aluminas with Al mole% of less than 0.2, a relatively broad pore size distribution was discovered [116].
A summary of some more reported literature on glycerol dehydration over various catalysts to produce acrolein is represented in Table 3 [92,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136].

Table 3.
Acrolein production from glycerol dehydration using various catalytic systems under optimal reaction conditions.
4. Glycerol Hydrogenolysis
The catalytic hydrogenolysis reaction is completed in two steps: hydrogenation and dehydration. Such a type of reaction system depends mainly on one catalyst particle size along with its structure [134,135]. Fan et al. concluded that the glycerol conversion would be more promising when using an enzymatic approach for glycerol to 1,3-PDO [136]. Sun et al. studied glycerol hydrogenolysis to produce C3 products such as 1,2-PDO and 1,3-PDO [137]. The reaction route for glycerol hydrogenation producing ethylene glycol, methanol, ethanol, and methane [61]:
C3H8O2 + H2O ↔ C2H6O2 + CH3OH
C2H6O2 + H2 ↔ CH3CH2OH + H2O
CH3OH + H2 ↔ CH4 + H2O
The reaction route for glycerol hydrogenation into propylene glycols (1,2-propanediol(1,2-PDO), and 1,3-propanediol (1,3-PDO)) were shown as (Figure 6) [138]. So many heterogeneous catalysts were utilized for hydrogenolysis reaction in which noble metals (Au, Rh, Pd, Pt, and Ru) based catalysts were utilized or non–noble metals (Ni, Co, and Cu) based catalysts were used [139,140,141,142,143,144,145,146,147,148,149,150,151,152,153].
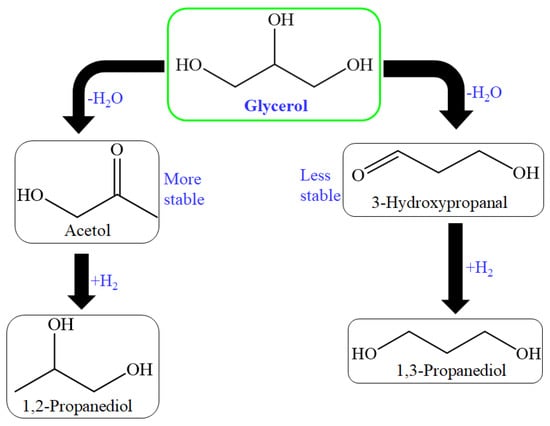
Figure 6.
Production of 1,2-PDO and 1,3-PDO through dehydration and hydrogenation of glycerol.
4.1. Nickel Catalysts for Glycerol Hydrogenolysis
Bulk Ni phosphide catalyst prepared through hydrothermal method for glycerol hydrogenolysis for production of 1,2-PDO. 95% 1,2-PDO selectivity along with 5.4% glycerol conversion was achieved over Ni3P catalyst at 190 °C and 5.5 MPa. It was concluded that low conversion was achieved over the catalysts which were treated at low temperature (190 °C). Hexagonal morphology was shown by bulk nickle phosphide catalyst. After annealing, the sample synthesized at a pH of 9 predominantly showed metallic nickel diffraction peaks, but the sample produced at a higher pH value (pH 12) was found to be pure amorphous metallic nickel as determined by XRD. When the pH was reduced to 7, the comparatively strong diffraction peaks of Ni3P were seen. All diffraction peaks of the annealed sample may be assigned to the Ni3P phase when the pH value is 5. The influence of annealing temperature is also demonstrated. More substantial diffraction peaks and larger particle sizes resulted in a higher annealing temperature (600 °C), resulting in a lower dispersion degree, which is usually undesirable for catalysts. It is discovered that Ni3P has a hexagonal prism shape, and its length-to-diameter ratio is 2 to 1 [141].
Zhang et al. studied macro-mesoporous carbon (MMCs) encapsulated in Ni nanoparticles catalysts synthesized by dual–templating technique for glycerol hydrogenolysis to 1,2-PDO. On hierarchical catalysts, the glycerol conversion of 95.5% was achieved along with 77.1% selectivity of 1,2-PDO over Ni/MMC-6 catalyst at 210 °C and 4 MPa. Ni supported on multiporous carbon was highly active, stable, and selective catalysts for hydrogenolysis of glycerol to PDO. These catalysts’ performance was attributed to their large surface area and pore volume proposed to reduce mass transfer limitation in the system. The total surface area and pore volume of the Ni/MMC-X materials were found to be much greater than those of the Ni/C micro and Ni/CNT (Ni modified carbon nanotubes) materials [154].
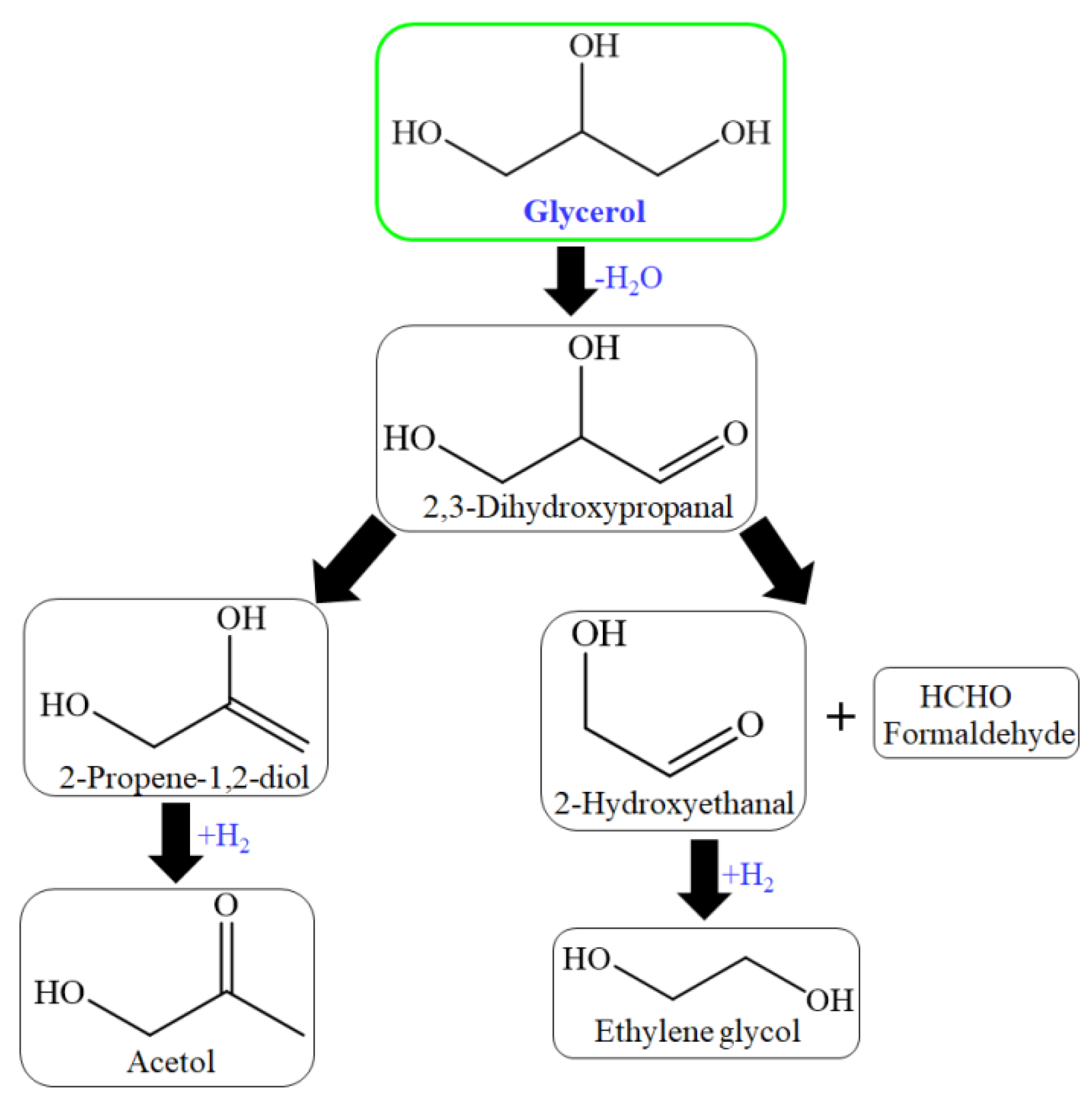
Chimentão et al. investigated zinc–supported Cu and Ni catalysts for glycerol hydrogenolysis. 100% transformation of glycerol was achieved over Ni/ZnAl catalyst. 55% and 49% selectivity of hydroxyacetone and 29% and 26% yield of hydroxy acetone were achieved over Cu/ZnAl and Cu/ZnO catalyst simultaneously at 300 °C. Ni promoted catalysts that formed methane, and Cu promoted catalysts that acetol formation. The thermodynamically stable phase of zinc oxide at ambient conditions is zincite, which crystallizes in the hexagonal wurtzite structural type. It was mentioned that the acidity of ZnAl and ZnO promoted activity. Adsorption and acidity played a vital role in the transformation of glycerol [155]. The catalyst has both acidic as well as basic sites. The basic sites’ influence must also be considered. The mechanism of glycerol dehydration for acetol formation over basic sites was proposed [95]. The reaction mechanism proposed over basic sites began with the dehydrogenation step rather than the dehydration step [156] (Figure 7).
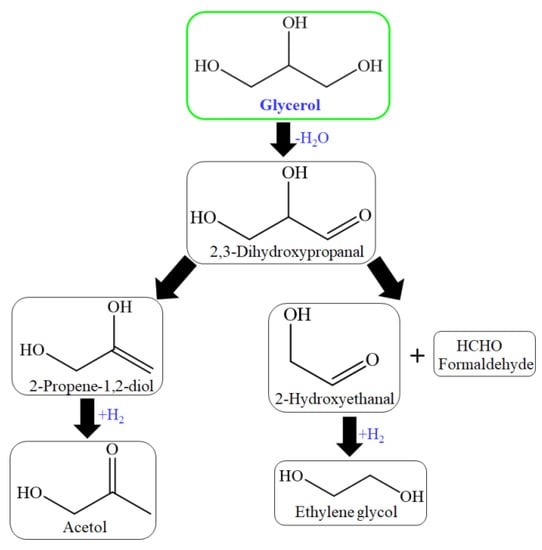
Figure 7.
Reaction process over basic sites of HZSM-5 catalyst for glycerol conversion.
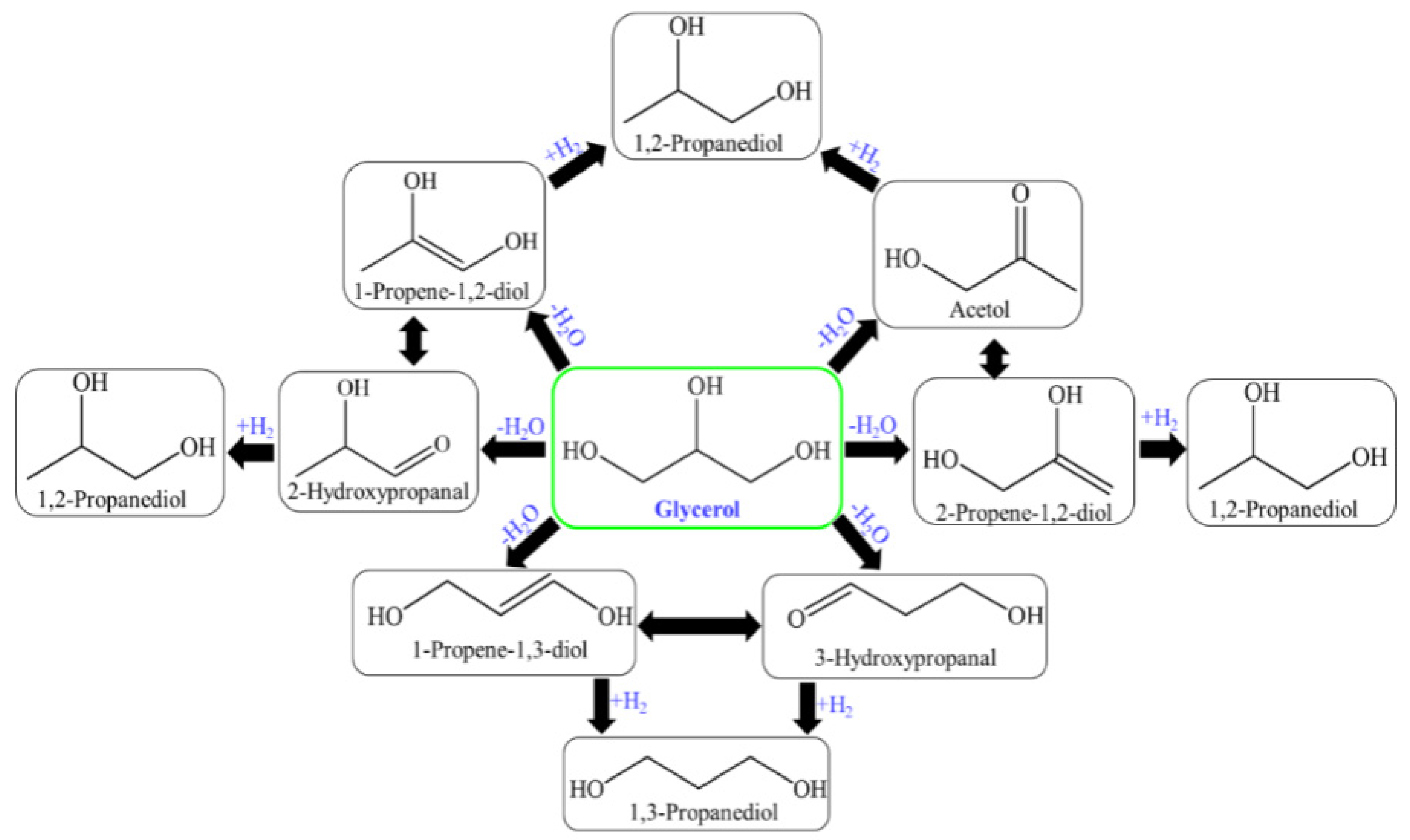
In this, 2,3-dihydroxypropanal, through dehydration and hydrogenation produced acetol and/or through the retro-aldol reaction of formaldehydeand2-hydroxyethanal (hydroxyacetaldehyde) to produce ethylene glycol through hydrogenation [93,95,156]. The glycerol hydrogenolysis mechanism is suggested in two steps for propylene glycol (1,2-PDO, 1,3-PDO) production: dehydration and hydrogenation. The mechanism of glycerol hydrogenolysis involves dehydration first and then hydrogenation. The different dehydration intermediates of glycerol correspond to propylene glycol through hydrogenation. The tautomeric equilibrium is indicated as a double-arrow between the dehydration intermediates (Figure 8) [157,158].
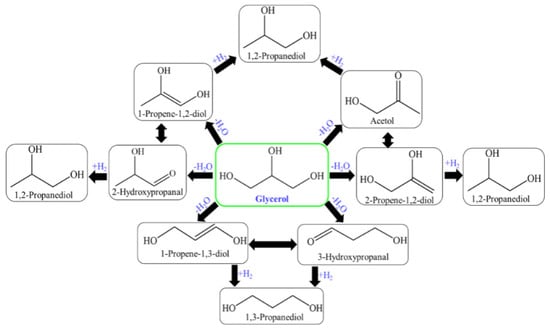
Figure 8.
Glycerol hydrogenolysis for the production of 1,2-PDO and 1,3-PDO through different reaction intermediates.
4.2. Copper Catalysts for Glycerol Hydrogenolysis
Bimetallic Ru–Cu nanoparticles supported over the series of TiO2 catalysts prepared from the impregnation method for hydrogenolysis of glycerol for 1,2-PDO production was studied [142]. 53.9% glycerol conversion and 93.2% 1,2-PDO selectivity were achieved of 2.5Ru-2.5Cu/TiO2 catalyst at 200 °C and 2.5 MPa. The best performance was shown by 2.5Ru-2.5Cu/TiO2 catalyst and concluded that the addition of Ru-Cu-based catalyst enhanced 1,2-PDO selectivity. Copper-supported activated carbon through the impregnation method for glycerol production of 1,2-PDO [159]. The maximum glycerol transformation of 23%, along with 20% 1,2-PDO selectivity, was achieved over copper (Cu) supported on activated carbon treated at 750 °C (Cu/CGran(750)) at 220 °C and 5 MPa. The interaction of Cu species with carbon support was minimized at high temperatures, leading to large Cu particles. Novel bi-functional Cu–Mg/SiO2 catalyst through chemisorptions–hydrolysis technique for production of 1,2-PDO from hydrogenolysis of glycerol and 89.5% transformation of glycerol, as well as 92.1% selectivity of 1,2-PDO achieved over Cu0.1-Mg0.2, supported SiO catalyst at 210 °C and 4 bar [160]. The result revealed that Cu species were enough active sites for maximum activity, the addition of copper reduced the Lewis acid sites and enhanced the Bronsted acid sites. Shan et al. investigated Cu/SBA-15 catalyst through a simply grinding technique and impregnation method for 1,2-PDO production from hydrogenolysis of glycerol. A 90.3% transformation of glycerol along with 97.3% 1,2-PDO selectivity was achieved at 10% Cu/SBA-15 prepared through grinding method at 230 °C and 4 MPa. Cu/SBA-15 catalysts with large surface areas showed excellent stability as well as activity in the hydrogenolysis reaction of glycerol [161].
4.3. Platinum Catalysts for Glycerol Hydrogenolysis
Feng et al. studied tungsten (W) and aluminum (Al) doped into SBA-15 through one-step technology in the acidic medium for glycerol hydrogenolysis for 1,3-PDO production. Diffraction peaks were detected clearly after the metal ions were incorporated into the SBA-15 framework, suggesting that the SBA-15 mesoporous structure was well conserved after the direct synthesis [162]. The glycerol transformation was 66%, and 50% 1,3-PDO selectivity was achieved over Pt supported (W+Al) modified SBA-15 catalyst at 160 °C and 6 MPa. Niu et al. investigated WOx-supported Pt catalysts prepared through hydrothermally and impregnation method for hydrogenolysis of glycerol to 1,3-PDO [163]. Pt doped over HWO3 catalyst produced 63.4% glycerol transformation and 43.1% 1,3-PDO selectivity at 5 MPa and 160 °C. The enhancement in performance showed the Pt nanoparticle dispersion, the high acidic nature, and the interaction of HWO3. The tungsten modification effect on phosphate-supported zirconia Pt catalyst for hydrogenolysis to produce 1-propanol [164].
Wang et al. achieved glycerol conversion 100% and 81% 1–Propanol yield was achieved at Pt/7WOx–ZrP catalyst at 270 °C and 2 MPa. It was concluded that the modification of ZrPWOx increased the catalyst’s total acidity, which was beneficial for glycerol conversion and also enhanced Pt nanoparticles dispersion. The addition of WOx mainly improved Lewis acid sites, while Brønsted acid sites remained the same. Various supported, as well as unsupported, Pt–W-based catalysts to produce 1,3-PDO from the hydrogenolysis of glycerol. Among all, Pt-WOx doped Al2O3 provided the best activity and selectivity and showed strong dispersion of Pt over WOx which promoted strong Bronsted acidic sites [165].
4.4. Iron Catalysts for Glycerol Hydrogenolysis
Heterogeneous iron-containing nanocrystals for the hydrogenolysis of glycerol and mentioned that the incorporation of Fe metal positively affected selectivity and activity of catalysts for glycerol hydrogenolysis towards 1,2-PDO [153]. The recent achievements in Fe–based heterogeneous catalysts in the hydrogenolysis and hydrogenation process are outlined in this review. Lopez et al. studied hydrotalcite-supported transition metals (Fe, Zn, Ni, and Cu) prepared by the coprecipitation technique for hydrogenolysis of glycerol for 1,3-PDO production and 89.7% glycerol transformation along with 82.6% yield and 92% selectivity of 1,2-PDO was achieved over 15 wt.% Cu supported on hydrotalcite (HDTs-Cu) catalyst at 3.4 MPa and 200 °C. The highest activity and 1,2-PDO selectivity were achieved over copper-modified catalysts even with 62% purity of crude glycerol employed.
By comparing these peaks, it was discovered that when the metals iron, nickel, copper, and zinc were replaced in the environment with laminar octahedral brucite coordination, the intensity of the characteristic peak (003) increased, indicating an increase in structural integrity by assuming trilaminar rhombohedral layer stacking. When the surface properties of several HDT-type catalytic materials were characterized, an area drop was seen when the cation ionic radius (ionic radius, Fe, Ni, Cu, and Zn) increased. However, HDT-Ni was an exception because its area value was lower than those achieved with materials containing Cu and Zn. Ni has a shorter ionic radius than Cu and Zn; therefore, it was hypothesized that the catalyst incorporating Ni would have a larger surface area. This can likely be linked to the material’s synthesis or maturation [166].
4.5. Cerium Catalysts for Glycerol Hydrogenolysis
Cerium (Ce) promoted Cu/Mg catalyst through coprecipitation method for production 1,2-PDOthrough hydrogenolysis of glycerol. The activity of catalysts for glycerol hydrogenolysis was achieved in order: Cu/Mg < Cu/Ce1/Mg < Cu/Ce5/Mg < Cu/Ce3/Mg. The markable point was that CeO2 and Cu/Mg with atomic ratio = 3/5 catalyst might be used several times for hydrogenolysis reaction of glycerol without reducing activity. It is worth noting that the Cu/Ce1/Mg and Cu/Ce3/Mg samples revealed varied XRD peaks corresponding to CuO phases, but the metallic Cu phase showed no XRD peaks. When compared to pure CeO2, the CeO2 diffraction peaks of Ce-promoted Cu/Mg materials were moved to the higher angle side. This odd observation could be due to the interaction between CeO2 and Cu/Mg samples [139].
4.6. Silver Catalysts for Glycerol Hydrogenolysis
A series of silver-modified catalysts for hydrogenolysis of glycerol produced 1,2-PDO; 78.8% conversion and54.2% selectivity of 1,2-PDO over Raney-Ni34Ag catalyst at 210 °C and 4 MPa. Ag modified catalyst presented better activity and enhanced C3 product selectivity contrasted with unmodified Raney nickel (R-Ni) catalyst. At low and high magnification, SEM pictures of the R-Ni-based catalysts revealed a prominent porosity zone, indicating that fresh R-Ni is a high-surface-area catalyst made up of reduced Ni particles. R-Ni had a BET surface area of 21.1 m2gcat−1 after drying and passivation, which increased to 42.4 m2 gcat−1 when a modest amount of Ag was added to R-Ni and reduced significantly when more Ag was added [140].
Summary of some more reported literature related to glycerol reforming over heterogeneous catalysts to produce propylene glycol (1,2-PDO, 1,3-PDO), and ethylene glycol is represented in Table 4 and Table 5.

Table 4.
Glycerol hydrogenolysis over various catalytic systems for 1,2-PDO production.

Table 5.
Glycerol hydrogenolysis over various catalytic systems for producing 1,3-PDO, propylene glycol, and ethylene glycol.
5. Conclusions
The knowledge of various catalysts, such as nickel, platinum, ruthenium, copper, cadmium, zeolite, titanium, alumina, iron, cerium, silver, and rhodium, steam reforming process, dehydration process, and hydrogenolysis process of glycerol was reviewed and cataloged. Numerous researchers have described various ways of converting glycerol into value-added products, such as hydrogen, acetol, acrolein, ethylene glycol, propylene glycol (1,2-propanediol, 1,3-propanediol), etc., via several reactions under different reaction conditions. These chemical compounds have a clear monetary value and can help the biodiesel industry.
Many research efforts are ongoing to overcome challenges and explore new frontiers to search for new products. Those challenges involve the selection of catalyst for some products and reaction conditions, severe difficulty in separating the catalyst from desired products, and low yields of the products with long catalytic reaction runs. Today, researchers and engineers are exploring new technologies, highly novel and tolerant catalysts, improving reactor systems and activation methods, and coordinating the chemical and biological catalysts to improve the weaknesses involved with each catalyst. The modern improvement in technology and economical use of crude glycerol for value-added product formulation clearly demonstrate that crude glycerol may play a critical part in the bio-refining industry’s fulfillment.
Author Contributions
Writing—original draft preparation, N.T. and R.P.; data curation, A.P., P.B., and P.C.; formal analysis, R.R., N.M., R.G., A.B.R., and A.M.A.; original draft preparation, supervision and editing, R.B., V.P., and N.A.-Q.; funding acquisition N.A.-Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Qatar University through a National Capacity Building Program Grant (NCBP), [QUCP-CAM-2022-463]. Statements made herein are solely the responsibility of the authors.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Balat, H.; Kırtay, E. Hydrogen from biomass Present scenario and future prospects. Int. J. Hydrogen Energy 2010, 35, 7416–7426. [Google Scholar] [CrossRef]
- Savage, N. Fuel options: The ideal biofuel. Nature 2011, 479, S9–S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tollefson, J. Risky energy research faces uncertain future. Nature 2011, 471, 145–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gielen, D.; Boshell, F.; Saygin, D.; Morgan, D.B.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábalc, G.O.; Pérez-Ramírez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef] [Green Version]
- Aresta, M.; Angelini, A.D.A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef] [Green Version]
- Shirazi, M.A.; Kargari, A.; Tabatabaei, M.; Mostafaeid, B.; Akiae, M.; Barkhi, M.; Shirazi, M.J.A. Acceleration of biodiesel–glycerol decantation through NaCl-assisted gravitational settling: A strategy to economize biodiesel production. Bioresour. Technol. 2013, 134, 401–406. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Ramyakrishna, P.; Raveendra, G.; Rajender, B.; Vijayanand, P.; Ma, J. Recent advances in biomass-derived platform chemicals to valeric acid synthesis. New. J. Chem. 2022, 46, 5907–5921. [Google Scholar]
- Muraza, O. Peculiarities of Glycerol Conversion to Chemicals Over Zeolite-Based Catalysts. Front. Chem. 2019, 7, 233. [Google Scholar] [CrossRef] [PubMed]
- Papageridis, K.N.; Siakavelas, G.; Charisiou, N.D.; Avraam, D.G. Comparative study of Ni, Co, Cu supported on γ-alumina catalysts for hydrogen production via the glycerol steam reforming reaction. Fuel Process. Technol. 2016, 152, 156–179. [Google Scholar] [CrossRef]
- Silva, J.M.; Soria, M.A.; Madeira, L.M. Challenges and strategies for optimization of glycerol steam reforming process. Renew. Sustain. Energy Rev. 2015, 42, 1187–1213. [Google Scholar] [CrossRef] [Green Version]
- Roberto, M.M.; Luis, K.C.; Sanaiotte, P.R.; Otávio, B.M.; Cesar, A.d.S. Glycerol from biodiesel production:Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar]
- Reangchim, P.; Daorattanachai, P.; Laosiripojana, N. Conversion of glycerol waste from biodiesel plant to high-value product. J. Sustain. Energy Environ. 2019, 10, 41–44. [Google Scholar]
- Nda-Umar, U.I.; Ramli, I.; Taufiq-Yap, Y.H.; Muhamad, E.N. An overview of recent research in the conversion of glycerol into biofuels, fuel additives and other bio-based chemicals. Catalysts 2019, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Semkiv, M.V.; Ruchala, J.; Dmytruk, K.V.; Sibirny, A.A. 100 Years Later, What Is New in Glycerol Bioproduction? Trend Biotechnol. 2020, 38, 907–916. [Google Scholar] [CrossRef]
- Tan, H.W.; Aziz, A.R.A.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Len, C.; Luque, R. Continuous flow transformations of glycerol to valuable products:An overview. Sustain. Chem. Process. 2014, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Bos, H.L.; Harmsen, P.F.H.; Annevelink, E. Background Information and Biorefinery Status, Potential and Sustainability: Task 2.1.2 Market and Consumers. Carbohydrates. 2010, pp. 1–28. Available online: https://library.wur.nl/WebQuery/wurpubs/reports/399310 (accessed on 5 May 2022).
- Nanda, R.M.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energy Rev. 2010, 372, 224–238. [Google Scholar] [CrossRef]
- Martin, M.; Richter, A. Oligomerization of glycerol—A critical review. Eur. J. Lipid Sci. Technol. 2011, 113, 100–117. [Google Scholar] [CrossRef]
- Razali, N.; Abdullah, A.Z. Production of lactic acid from glycerol via chemical conversion using solid catalyst: A review. Appl. Catal. A Gen. 2017, 543, 234–246. [Google Scholar] [CrossRef]
- Teng, W.K.; Ngoh, G.C.; Yusoff, R.; Aroua, M.K. A review on the performance of glycerol carbonate production via catalytic transesterification: Effects of influencing parameters. Energy Convers. Manag. 2014, 88, 484–497. [Google Scholar] [CrossRef]
- Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts 2020, 10, 1279. [Google Scholar] [CrossRef]
- Zakaria, Z.Y.; Amin, N.A.S.; Linnekoski, J. A perspective on catalytic conversion of glycerol to olefins. Biomass Bioenergy 2013, 55, 370–385. [Google Scholar] [CrossRef]
- Anitha, M.; Kamarudina, S.K.; Kofli, N.T. The potential of glycerol as a value-added commodity. Chem. Eng. J. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Wu, K.; Dou, B.; Zhang, H.; Liu, D.; Chen, H.; Xu, Y. Aqueous phase reforming of biodiesel byproduct glycerol over mesoporous Ni-Cu/CeO2 for renewable hydrogen production. Fuel 2022, 308, 122014. [Google Scholar] [CrossRef]
- Liu, D.; Dou, B.; Zhang, H.; Zhao, L.; Wu, K.; Zeng, P.; Chen, H.; Xu, Y. Comparison of gelatinous and calcined magnesia supported Ni or/and Co-based catalysts for aqueous phase reforming of glycerol. Renew. Energy 2022, 186, 656–666. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous phase reforming process for the valorization of wastewater streams: Application to different industrial scenarios. Catal. Today 2022, 387, 224–236. [Google Scholar] [CrossRef]
- Su, H.; Yan, M.; Wang, S. Recent advances in supercritical water gasification of biowaste catalyzed by transition metal-based catalysts for hydrogen production. Renew. Sustain. Energy Rev. 2022, 154, 111831. [Google Scholar] [CrossRef]
- Dou, B.; Zhao, L.; Zhang, H.; Wu, K.; Zhang, H. Renewable hydrogen production from chemical looping steam reforming of biodiesel byproduct glycerol by mesoporous oxygen carriers. Chem. Eng. J. 2021, 416, 127612. [Google Scholar] [CrossRef]
- Felseghi, R.A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen Fuel Cell Technology for the Sustainable Future of Stationary Applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef] [Green Version]
- Markocic, E.; Kramberger, B.; Van Bennekom, J.G.; Heeres, H.J.; Vos, J.; Knez, Z. Glycerol reforming in supercritical water; a short review. Renew. Sustain. Energy Rev. 2013, 23, 40–48. [Google Scholar] [CrossRef]
- Al-Salihi, S.; Abrokwah, R.; Dade, W.; Deshmane, V.; Hossain, T.; Kuila, D. Renewable hydrogen from glycerol steam reforming using Co-Ni-MgO based SBA-15 nanocatalysts. Int. J. Hydrogen Energy 2020, 45, 14183–14198. [Google Scholar] [CrossRef]
- El Doukkali, M.; Iriondo, A.; Cambra, J.F.; Gandarias, I.; Jalowiecki-Duhamel, L.; Dumeignil, F.; Arias, P.L. Deactivation study of the Pt and/or Ni-based -Al2O3 catalysts used in the aqueous phase reforming of glycerol for H2 production. Appl. Catal. A Gen. 2014, 472, 80–91. [Google Scholar] [CrossRef]
- Pompeo, F.; Santori, G.F.; Nichio, N.N. Hydrogen production by glycerol steam reforming with Pt/SiO2 and Ni/SiO2 catalysts. Catal. Today 2011, 172, 183–188. [Google Scholar] [CrossRef]
- Huang, X.; Dang, C.; Yu, H.; Wang, H.; Peng, F. Morphology Effect of Ir/La2O2CO3 Nanorods with Selectively Exposed {110} Facets in Catalytic Steam Reforming of Glycerol. ACS Catal. 2015, 5, 1155–1163. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Lee, D. Glycerol steam reforming on Ru catalysts supported on core-shell metal–ceramic microcomposites developed by a microwave-induced hydrothermal method. Appl. Catal. A Gen. 2015, 449, 197–204. [Google Scholar] [CrossRef]
- Sundari, R.; Vaidya, P.D. Reaction Kinetics of Glycerol Steam Reforming Using a Ru/Al2O3 Catalyst. Energy Fuels 2012, 26, 4195–4204. [Google Scholar] [CrossRef]
- Baca, S.; Sayb, Z.; Kocak, Y.; Ercanb, K.; Harfouched, M.; Ozensoy, E.; Avci, A.K. Exceptionally active and stable catalysts for CO2reforming of glycerol to syngas. Appl. Catal. B Environ. 2019, 256, 117808. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Rodrigues, A.E. Glycerol Reforming for Hydrogen Production: A Review. Chem. Eng. Technol. 2009, 32, 1463–1469. [Google Scholar] [CrossRef]
- Martinez, L.M.T.; Araque, M.; Vargas, J.C.; Roger, A.C. Effect of Ce/Zr ratio in CeZr-CoRh catalysts on the hydrogen production by glycerol steam reforming. Appl. Catal. A Gen. 2013, 132–133, 499–510. [Google Scholar] [CrossRef]
- Lee, J.; Doohwan, K. Synthesis and Properties of Core–Shell Metal–Ceramic Microstructures and their Application as Heterogeneous Catalysts. ChemCatChem 2014, 6, 2642–2647. [Google Scholar]
- Delparish, A.; Koc, S.; Caglayan, B.S.; Avc, A.K. Oxidative steam reforming of glycerol to synthesis gas in a microchannel reactor. Catal. Today 2019, 323, 200–208. [Google Scholar] [CrossRef]
- Danga, C.; Wu, S.; Yang, G.; Cao, Y.; Wang, H.; Peng, F.; Yu, H. Syngas production by dry reforming of the mixture of glycerol and ethanol with CaCO3. J. Energy Chem. 2020, 43, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Rezendea, S.M.D.; Franchini, C.A.; Dieuzeide, M.L.; de Farias, A.M.D.; Amadeo, N.; Fraga, M.A. Glycerol steam reforming over layered double hydroxide-supported Pt catalysts. Chem. Eng. J. 2015, 272, 108–118. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Italiano, C.; Pino, L.; Sebastian, V.; Vita, A.; Goula, M.A. Hydrogen production via steam reforming of glycerol over Rh/γ-Al2O3 catalysts modified with CeO2, MgO or La2O3. Renew. Energy 2020, 162, 908–925. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, C.; Li, S.; Han, Z.; Wang, T.; Ma, X.; Gong, J. Hydrogen Production via Glycerol Steam Reforming over Ni/Al2O3: Influence of Nickel Precursors. ACS Sustain. Chem. Eng. 2013, 1, 1052–1062. [Google Scholar] [CrossRef]
- Sánchez, N.; Encinar, J.M.; González, J.F. Sorption Enhanced Steam Reforming of Glycerol: Use of La-Modified Ni/Al2O3 as Catalyst. Ind. Eng. Chem. Res. 2016, 55, 3736–3741. [Google Scholar] [CrossRef]
- Ruppert, R.A.M.; Niewiadomski, M.; Grams, J.; Kwapiński, W. Optimization of Ni/ZrO2 catalytic performance in thermochemical cellulose conversion for enhanced hydrogen production. Appl. Catal. B Environ. 2014, 145, 85–90. [Google Scholar]
- Yan, Z.; Liu, S.; Zhang, Y.; Wang, T.; Luo, S.; Chu, W.; Jing, F. The role of Zr in NiZrAl oxides catalyst and the evaluation on steam reforming of glycerol for hydrogen product. Catal. Today 2019, 319, 229–238. [Google Scholar] [CrossRef]
- Charisiou, C.N.D.; Siakavelas, G.; Tzounis, L.; Dou, B.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Ni/Y2O3—ZrO2 catalyst for hydrogen production through the glycerol steam reforming reaction. Int. J. Hydrogen Energy 2020, 45, 10442–10460. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, B.; Yan, J.; Hong, J.; Wang, L.; Zhang, Y.; Li, J.; Jing, F.; Chu, W. Plasma assisted preparation of nickel-based catalysts supported on CeO2 with different morphologies for hydrogen production by glycerol steam reforming. Powder Technol. 2019, 345, 324–332. [Google Scholar] [CrossRef]
- Yancheshmeh, M.S.; Alizadeh, O.S.; Aissaoui, M.; Iliuta, M.C. A novel synthesis of NiAl2O4 spinel from a Ni-Al mixed-metal alkoxide as a highly efficient catalyst for hydrogen production by glycerol steam reforming. Appl. Catal. B Environ. 2020, 265, 118535. [Google Scholar] [CrossRef]
- Feng, P.; Huang, K.; Xu, Q.; Qi, W.; Xin, S.; Wei, T.; Liao, L.; Yan, Y. Ni supported on the CaO modified attapulgite as catalysts for hydrogen production from glycerol steam reforming. Int. J. Hydrogen Energy 2020, 45, 8223–8233. [Google Scholar] [CrossRef]
- Veiga, S.; Faccio, R.; Romero, M.; Bussi, J. Utilization of waste crude glycerol for hydrogen production via steam reforming over Ni–La–Zr catalysts. Biomass Bioenerg. 2020, 135, 105508. [Google Scholar] [CrossRef]
- Ghungrud, S.A.; Vaidya, P.D. Sorption-enhanced reaction process for glycerol-tohydrogen conversion over cobalt catalyst supported on promoted hydrotalcites. Int. J. Hydrogen Energy 2020, 45, 9440–9450. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, S.; Jing, F.; Luo, S.Z.; Shen, J.; Pang, Y.; Chu, W. Synergetic Bimetallic NiCo/CNT Catalyst for Hydrogen Production by Glycerol Steam Reforming: Effects of Metal Species Distribution. Ind. Eng. Chem. Res. 2020, 59, 17259–17268. [Google Scholar] [CrossRef]
- Seretis, A.; Tsiakaras, P. Aqueous phase reforming (APR) of glycerol over platinum supported on Al2O3 catalyst. Renew. Energy 2016, 85, 1116–1126. [Google Scholar] [CrossRef]
- Callison, J.; Subramanian, N.D.; Rogers, S.M.; Chutia, A.; Gianoliod, D.; Catlow, C.R.A.; Wells, P.P.; Dimitratos, N. Directed aqueous-phase reforming of glycerol through tailored platinum nanoparticles. Appl. Catal. B Environ. 2018, 238, 618–628. [Google Scholar] [CrossRef]
- Touri, A.E.; Taghizadeh, M. Hydrogen Production via Glycerol Reforming over Pt/SiO2Nanocatalyst in a Spiral-Shaped Microchannel Reactor. Int. J. Chem. React. 2016, 14, 1059–1068. [Google Scholar] [CrossRef]
- Ciftci, A.; Peng, B.; Jentys, A.; Lercher, J.A.; Hensen, E.J.M. Support effects in the aqueous phase reforming of glycerol over supported platinum catalysts. Appl. Catal. A Gen. 2012, 431–432, 113–119. [Google Scholar] [CrossRef]
- Pompeo, F.; Santori, G.; Nichio, N.N. Hydrogen and/or syngas from steam reforming of glycerol. Study of platinum catalysts. Int. J. Hydrogen Energy 2010, 35, 8912–8920. [Google Scholar] [CrossRef]
- Lehnert, K.; Claus, P. Influence of Pt particle size and support type on the aqueous-phase reforming of glycerol. Catal. Commun. 2008, 9, 2543–2546. [Google Scholar] [CrossRef]
- King, D.L.; Zhang, L.; Xia, G.; Karim, A.M.; Heldebrant, D.J.; Wang, X.; Peterson, T.; Wang, Y. Aqueous phase reforming of glycerol for hydrogen production over Pt–Re supported on carbon. Appl. Catal. B Environ. 2010, 99, 206–213. [Google Scholar] [CrossRef]
- Demsash, H.D.; Kondamudi, K.V.K.; Upadhyayula, S.; Mohan, R. Ruthenium doped nickel-alumina-ceria catalyst in glycerol steam reforming. Fuel Process. Technol. 2018, 169, 150–156. [Google Scholar] [CrossRef]
- Dahdah, E.; Aouad, S.; Gennequin, C.J.E.; Nsouli, B.; Aboukais, A.; Abi-Aad, E. Glycerol steam reforming over Ru-Mg-Al hydrotalcite-derived mixed oxides: Role of the preparation method in catalytic activity. Int. J. Hydrogen Energy 2018, 43, 19864–19872. [Google Scholar] [CrossRef]
- Kousi, K.; Kondarides, D.I.; Verykios, X.E.; Papadopoulou, C. Glycerol steam reforming over modified Ru/Al2O3 catalysts. Appl. Catal. A Gen. 2017, 542, 201–211. [Google Scholar] [CrossRef]
- Gallegos-Suárez, E.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Efficient hydrogen production from glycerol by steam reforming with carbon supported ruthenium catalysts. Carbon 2016, 96, 578–587. [Google Scholar] [CrossRef]
- Galloa, A.; Pirovano, C.; Ferrini, P.; Marelli, M.; Psaroc, R.; Santangelo, S.; Faggio, G.; Santo, V.D. Influence of reaction parameters on the activity of ruthenium based catalysts for glycerol steam reforming. Appl. Catal. B Environ. 2012, 121–122, 40–49. [Google Scholar] [CrossRef]
- Simonetti, D.A.; Kunkes, E.L.; Dumesic, J.A. Gas-phase conversion of glycerol to synthesis gas over carbon-supported platinum and platinum–rhenium catalysts. J. Catal. 2007, 247, 298–306. [Google Scholar] [CrossRef]
- Bulutoglu, P.S.; Say, Z.; Bac, S.; Ozensoy, E.; Avci, A.K. Dry Reforming of Glycerol Over Rh-Based Ceria and Zirconia Catalysts: New Insights on Catalyst Activity and Stability. Appl. Catal. A Gen. 2018, 564, 157–171. [Google Scholar] [CrossRef]
- Larimi, A.; Khorasheh, F. Renewable hydrogen production over Pt/Al2O3 nano-catalysts: Effect of M-promoting (M=Pd, Rh, Re, Ru, Ir, Cr). Int. J. Hydrogen Energy 2019, 44, 8243–8251. [Google Scholar] [CrossRef]
- Oliveira, E.V.; Seixas, A.C.M.; Jordão, E. Performance of Pt and Pt-Rh Catalyst in the Hydrogen Production from Glycerol. Can. J. Chem. Eng. 2017, 95, 2018–2023. [Google Scholar] [CrossRef]
- Chiodo, V.; Freni, S.; Galvagno, A.; Mondelloa, N.; Frusteri, F. Catalytic features of Rh and Ni supported catalysts in the steam reforming of glycerol to produce hydrogen. Appl. Catal. A Gen. 2010, 381, 1–7. [Google Scholar] [CrossRef]
- Veiga, S.; Faccio, R.; Segobia, D.; Apesteguia, C.; Bussi, J. Hydrogen production by crude glycerol steam reforming over NieLaeTi mixed oxide catalysts. Int. J. Hydrogen Energy 2017, 42, 30525–30534. [Google Scholar] [CrossRef]
- Suffredini, D.F.P.; Thyssen, V.V.; de Almeida, P.M.M.; Gomes, R.S.; Borges, M.C.; de Farias, A.M.D.; Assaf, E.M.; Fraga, M.A.; Brandão, S.T. Renewable hydrogen from glycerol reforming over nickel aluminate-based catalysts. Catal. Today 2016, 289, 96–104. [Google Scholar] [CrossRef]
- Siew, K.W.; Lee, H.C.; Gimbun, J.; Chin, S.Y.; Khan, M.R.; Taufiq-Yap, Y.H.; Cheng, C.K. Syngas production from glycerol-dry (CO2) reforming over La-promoted Ni/Al2O3 catalyst. Renew. Energy 2015, 74, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Li, S.; Zhang, C.; Wang, T.; Gong, J. Glycerol steam reforming over perovskite-derived nickel-based catalysts. Appl. Catal. B Environ. 2014, 144, 277–285. [Google Scholar] [CrossRef]
- Sanchez, E.A.; Comelli, R.A. Hydrogen production by glycerol steam-reforming over nickel and nickel-cobalt impregnated on alumina. Int. J. Hydrogen Energy 2014, 39, 8650–8655. [Google Scholar] [CrossRef]
- Buffoni, I.N.; Pompeo, F.; Santori, G.F.; Nichio, N.N. Nickel catalysts applied in steam reforming of glycerol for hydrogen production. Catal. Commun. 2009, 10, 1656–1660. [Google Scholar] [CrossRef]
- Aman, D.; Radwan, D.; Ebaid, M.; Mikhail, M.S.; van Steen, S.E. Comparing nickel and cobalt perovskites for steam reforming of glycerol. Mol. Catal. 2018, 425, 60–67. [Google Scholar] [CrossRef]
- Reynoso, A.J.; Ayastuy, J.L.; Iriarte-Velasco, U.; Gutierrez-Ortiz, M.A.; Chemical Technologies for the Environmental Sustainability Group. Cobalt aluminate spinel-derived catalysts for glycerol aqueous phase reforming. Appl. Catal. B Environ. 2018, 239, 86–101. [Google Scholar] [CrossRef]
- Dobosz, J.; Cichy, M.; Zawadzki, M.; Borowieck, T. Glycerol steam reforming over calcium hydroxyapatite supported cobalt and cobalt-cerium catalysts. J. Energy Chem. 2018, 27, 404–412. [Google Scholar] [CrossRef]
- Carrero, A.; Vizcaíno, A.J.; Calles, J.A.; García-Moreno, L. Hydrogen production through glycerol steam reforming using Co catalysts supported on SBA-15 doped with Zr, Ce and La. J. Energy Chem. 2016, 26, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Surendar, M.; Sagar, T.V.; Babu, B.H.; Lingaiah, B.; Rao, K.S.R.; Prasad, P.S.S. Glycerol steam reforming over La-Ce-Co mixed oxide-derived cobalt catalysts. ACS Catal. 2015, 5, 45184–45193. [Google Scholar] [CrossRef]
- Cheng, C.K.; Foo, S.Y.; Adesina, A.A. H2-rich synthesis gas production over Co/Al2O3 catalyst via glycerol steam reforming. Catal. Commun. 2010, 12, 292–298. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, X.; Li, Y.; Xu, Y.; Shen, W. Hydrogen production from steam reforming of ethanol and glycerol over ceria-supported metal catalysts. Int. J. Hydrogen Energy 2007, 32, 2367–2373. [Google Scholar] [CrossRef]
- Groll, H.P.A.; Oakland; Hearne, G. Process of Converting a Polyhydric Alcohol to a Carbony Compound. U.S. Patent US2042224A, 26 May 1934. [Google Scholar]
- Mahendran, S.; Nithya, T.; Raju, K.P.; Viswanathan, B.; Selvam, P. Dehydration of glycerol over high surface area γ-alumina supported heteropoly acid catalysts. In Proceedings of the Chemeca 2011: Engineering a Better World, Sydney Hilton Hotel, Sydney, NSW, Australia, 18–21 September 2011; Barton, A.C.T., Ed.; Engineers Australia: Redcliffe, Australia, 2011; pp. 1263–1273. [Google Scholar]
- Katryniok, B.; Paul, S.; Belliere-Baca, V.; Rey, P.; Dumeignil, F. Glycerol dehydration to acrolein in the context of new uses of glycerol. Green Chem. 2010, 12, 2079–2098. [Google Scholar] [CrossRef]
- Chai, S.H.; Wang, H.P.; Liang, Y.; Xu, B.Q. Sustainable production of acrolein: Investigation of solid acid–base catalysts for gas-phase dehydration of glycerol. Green Chem. 2007, 9, 1130–1136. [Google Scholar] [CrossRef]
- Tsukuda, E.; Sato, S.; Takahashi, R.J.; Sodesawa, T. Production of acrolein from glycerol over silica-supported heteropoly acids. Catal. Commun. 2007, 8, 1349–1353. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Alhanash, A.; Kozhevnikov, V.E.; Ivan, F.K. Gas-phase dehydration of glycerol to acrolein catalysed by caesium heteropoly salt. Appl. Catal. A. Gen. 2010, 378, 11–18. [Google Scholar] [CrossRef]
- Foo, G.S.; Wei, D.; Sholl, D.S.; Sievers, C. Role of Lewis and Brønsted Acid Sites in the Dehydration of Glycerol over Niobia. ACS Catal. 2014, 4, 3180–3192. [Google Scholar] [CrossRef]
- Lin, X.; Lv, Y.; Qu, Y.; Zhang, G.; Xi, Y.; Phillips, D.L.; Liu, C. A combined experimental and computational study of the catalytic dehydration of glycerol on microporous zeolites: An investigation of the reaction mechanism and acrolein selectivity. Phys. Chem. Chem. Phys. 2013, 15, 20120–20133. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, F.; Sudhakar, M.; Wang, N.; Dai, J.; Liu, L. Gas-phase dehydration of glycerol to acrolein catalyzed by hybrid acid sites derived from transition metal hydrogen phosphate and meso-HZSM-5. Catal. Today 2019, 332, 20–27. [Google Scholar] [CrossRef]
- Neves, T.M.; Fernandes, J.O.; Lião, L.M.; da Silva, E.D.; da Rosa, C.A.; Mortola, V.B. Glycerol dehydration over micro- and mesoporous ZSM-5 synthesized from a onestep method. Microporous Mesoporous Mater. 2019, 275, 244–252. [Google Scholar] [CrossRef]
- Qureshi, B.A.; Lan, X.; Arslan, M.T.; Wang, T. Highly Active and Selective Nano H-ZSM-5 Catalyst with Short Channels along b-Axis for Glycerol Dehydration to Acrolein. Ind. Eng. Chem. Res. 2019, 58, 12611–12622. [Google Scholar] [CrossRef]
- Yun, D.; Kim, T.Y.; Park, D.S.; Yun, Y.S.; Han, J.W.; Yi, J. A Tailored Catalyst for the Sustainable Conversion of Glycerol to Acrolein: Mechanistic Aspect of Sequential Dehydration. ChemSusChem 2014, 7, 2193–2201. [Google Scholar] [CrossRef]
- Xie, Q.; Li, S.; Gong, R.; Zheng, G.; Wang, Y.; Xu, P.; Duan, Y.; Yu, S.; Lu, M.; Ji, W.; et al. Microwave-assisted catalytic dehydration of glycerol for sustainable production of acrolein over a microwave absorbing catalyst. Appl. Catal. B Environ. 2019, 243, 455–462. [Google Scholar] [CrossRef]
- Shan, J.; Li, Z.; Zhu, S.; Liu, H.; Li, J.; Wang, J.; Fan, W. Nanosheet MFI Zeolites for Gas Phase Glycerol Dehydration to Acrolein. Catalysts 2019, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Drew, K.; Girishkumar, G.; Vinodgopal, K.; Kamat, P.V. Boosting Fuel Cell Performance with a Semiconductor Photocatalyst: TiO2/Pt-Ru Hybrid Catalyst for Methanol Oxidation. Phys. Chem. B Lett. 2005, 109, 11851–11857. [Google Scholar] [CrossRef]
- Parra, R.; Goes, M.S.; Castro, M.S.; Longo, E.; Bueno, P.R.; Varela, J.A. Reaction Pathway to the Synthesis of Anatase via the Chemical Modification of Titanium Isopropoxide with Acetic Acid. Chem. Mater. 2008, 20, 143–150. [Google Scholar] [CrossRef]
- Long, B.; Zhang, J.; Luo, L.; Ouyang, G.; Balogun, M.S.; Song, S.; Tong, Y. High pseudocapacitance boosts the performance of monolithic porous carbon cloth/closely packed TiO2 nanodots as an anode of an all-flexible sodium-ion battery. J. Mater. Chem. A 2019, 7, 2626–2635. [Google Scholar] [CrossRef]
- Babaei, Z.; Chermahini, A.N.; Dinari, M. Glycerol adsorption and mechanism of dehydration to acrolein over TiO2 surface: A density functional theory study. J. Colloid Interface Sci. 2020, 563, 1–7. [Google Scholar] [CrossRef]
- Ulgen, A.; Hoelderich, W.F. Conversion of glycerol to acrolein in the presence of WO3/TiO2 catalysts. Appl. Catal. A Gen. 2011, 400, 34–38. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, F.; Huang, L. Low Temperature Dehydration of Glycerol to Acrolein in Vapor Phase with Hydrogen as Dilution: From Catalyst Screening via TPSR to Real-Time Reaction in a Fixed-Bed. Catalysts 2020, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Rosas, I.P.; Larios, J.L.C.; Zeifert, B.; Blásquez, J.S. Catalytic Dehydration of Glycerine to Acrolein. In Glycerine Production and Transformation—An Innovative Platform for Sustainable Biorefinery and Energy; IntechOpen: London, UK, 2019; pp. 1–19. [Google Scholar]
- Basu, S.; Sen, A.K. Dehydration of glycerol with silica–phosphate-supported copper catalyst. Res. Chem. Intermed. 2020, 46, 3545–3568. [Google Scholar] [CrossRef]
- Basu, S.; Sen, A.K.; Mukherjee, M. Synthesis and performance evaluation of silica-supported copper chromite catalyst for glycerol dehydration to acetol. J. Chem. Sci. 2019, 131, 82. [Google Scholar] [CrossRef] [Green Version]
- Lima, D.S.; Perez-Lopez, O.W. Preparation of alumina with different precipitants for the gas phase dehydration of glycerol and their characterization by thermal analysis. J. Therm. Anal. Calorim. 2020, 142, 1387–1398. [Google Scholar] [CrossRef]
- Kim, Y.T.; Jung, K.D.; Park, E.D. Gas-phase dehydration of glycerol over silica–alumina catalysts. Appl. Catal. B Environ. 2011, 107, 177–187. [Google Scholar] [CrossRef]
- Corma, A.; Huber, G.W.; Sauvanaud, L.; O’Connor, P. Biomass to chemicals: Catalytic conversion of glycerol/water mixtures into acrolein, reaction network. J. Catal. 2008, 257, 163–171. [Google Scholar] [CrossRef]
- Cavani, F.; Guidetti, S.; Marinelli, L.; Piccinini, M.; Ghedini, E.; Signoretto, M. The control of selectivity in gas-phase glycerol dehydration to acroleincatalysed by sulfated zirconia. Appl. Catal. B Environ. 2010, 100, 197–204. [Google Scholar] [CrossRef]
- Kim, Y.T.; Jung, K.D.; Park, E.D. Gas-phase dehydration of glycerol over ZSM-5 catalysts. Microporous Mesoporous Mater. 2010, 131, 28–36. [Google Scholar] [CrossRef]
- De Oliveira, A.S.; Vasconcelos, S.J.S.; de Sousa, J.R.; de Sousa, F.F.; Filho, J.M.; Oliveira, A.C. Catalytic conversion of glycerol to acrolein over modified molecular sieves: Activity and deactivation studies. Chem. Eng. J. 2011, 168, 765–774. [Google Scholar] [CrossRef]
- Tresatayawed, A.; Glinrun, P.; Jongsomjit, B. Ethanol Dehydration over WO3/TiO2 Catalysts Using Titania Derived from Sol-Gel and Solvothermal Methods. Int. J. Chem. Eng. 2019, 2019, 4936292. [Google Scholar] [CrossRef] [Green Version]
- Dalil, M.; Carnevali, D.; Edakea, M.; Auroux, A.; Dubois, J.L.; Patience, G.S. Gas phase dehydration of glycerol to acrolein: Coke on WO3/TiO2 reduces by-products. J. Mol. Catal. A Chem. 2016, 421, 146–155. [Google Scholar] [CrossRef]
- Akizuki, M.; Sano, K.; Oshima, Y. Effect of supercritical water on the stability of WOX/TiO2 and NbOX/TiO2 catalysts during glycerol dehydration. J. Supercrit. Fluids 2016, 113, 158–165. [Google Scholar] [CrossRef]
- Stošić, D.; Bennici, S.; Couturier, J.L.; Dubois, J.L.; Auroux, A. Influence of surface acid–base properties of zirconia and titania based catalysts on the product selectivity in gas phase dehydration of glycerol. Catal. Commun. 2012, 17, 23–28. [Google Scholar] [CrossRef]
- Massa, M.; Andersson, A.; Finocchio, E.; Busca, G. Gas-phase dehydration of glycerol to acrolein over Al2O3−, SiO2−, and TiO2−supported Nb− and W− oxide catalysts. J. Catal. 2013, 307, 170–184. [Google Scholar] [CrossRef]
- Ding, J.; Ma, T.; Yan, C.; Shao, R.; Xu, W.; Yun, Z. Vapour Phase Dehydration of Glycerol to Acrolein Over Wells–Dawson Type H6P2W18O62 Supported on Mesoporous Silica Catalysts Prepared by Supercritical Impregnation. J. Nanosci. Nanotechnol. 2018, 18, 2463–2471. [Google Scholar] [CrossRef] [PubMed]
- Cecilia, J.A.; García-Sancho, C.; Mérida-Robles, M.J.; Santamaría-González, J.; Infantes-Molina, A.; Moreno-Tost, R.; Maireles-Torres, P. Aluminum doped mesoporous silica SBA-15 for glycerol dehydration to value-added chemicals. J. Sol—Gel Sci. Technol. 2017, 83, 342–354. [Google Scholar] [CrossRef]
- Cecilia, J.A.; García-Sancho, C.; Mérida-Robles, J.M.; González-Santamaría, J.; Moreno-Tost, R.; Maireles-Torres, P. WO3 Supported on Zr Doped Mesoporous SBA-15 Silica for Glycerol Dehydration to Acrolein. Appl. Catal. A Gen. 2016, 516, 30–40. [Google Scholar] [CrossRef]
- Herbon, M.; Lange, A.; Weiß, M.; Suprun, W.; Enke, D. Silica-Supported Phosphotungstic Acid for Gas Phase Dehydration of Glycerol. Chem. Eng. Technol. 2015, 38, 431–440. [Google Scholar] [CrossRef]
- Chai, S.H.; Yan, B.; Tao, L.Z.; Liang, Y.; Xu, B.Q. Sustainable production of acrolein: Catalytic gas-phase dehydration of glycerol over dispersed tungsten oxides on alumina, zirconia and silica. Catal. Today 2014, 234, 215–222. [Google Scholar] [CrossRef]
- Huang, L.; Qin, F.; Huang, Z.; Zhuang, Y.; Ma, J.; Xu, H.; Shen, W. Metal Organic Framework-mediated Synthesis of Small-sized γalumina as highly active Catalyst for Dehydration of Glycerol to Acrolein. ChemCatChem 2018, 23, 381–386. [Google Scholar] [CrossRef]
- Danov, S. Esipovich, A.; Belousov, A.; Rogozhi, A. Gas-Phase Dehydration of Glycerol over Commercial Pt/γ-Al2O3 Catalyst. Chin. J. Chem. Eng. 2015, 23, 1138–1146. [Google Scholar] [CrossRef]
- Haider, M.H.; Agostino, C.D.; Dummer, N.F.; Mantle, M.D.; Gladden, L.F.; Knight, D.W.; Willock, D.J.; Morgan, D.J.; Taylor, S.H.; Hutchings, G.J. The Effect of Grafting Zirconia and Ceria onto Alumina as a Support for Silicotungstic Acid for the Catalytic Dehydration of Glycerol to Acrolein. Chem. Eur. J. 2014, 3, 1743–1752. [Google Scholar] [CrossRef]
- Yfanti, V.L.; Lemonidou, A.A. Effect of hydrogen donor on glycerol hydrodeoxygenation to 1,2-propanediol. Catal. Today 2019, 355, 727–736. [Google Scholar] [CrossRef]
- Milewski, A.; Dydo, P.; Jakóbik-Kolon, A.; Czechowicz, D.; Babilas, D.; Burek, M.; Waśkiewicz, S.; Byczek-Wyrostek, A.; Krawczyk, T.; Kasprzycka, A. Preparation of Triglycerol from Glycerol and Epichlorohydrin at Room Temperature: Synthesis Optimization and Toxicity Studies. ACS Sustain. Chem. Eng. 2018, 6, 13208–13216. [Google Scholar] [CrossRef]
- Fan, X.; Burton, R.; Zhou, Y. Glycerol (Byproduct of Biodiesel Production) as a Source for Fuels and Chemicals—Mini Review. Open Fuels Energy Sci. J. 2010, 3, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol hydrogenolysis into useful C3 chemicals. Appl. Catal. B Environ. 2016, 193, 75–92. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic materials for the hydrogenolysis of glycerol to 1,3-propanediol. J. Mater. Chem. 2014, 2, 6688–6702. [Google Scholar] [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Venkat, B.S.R.; Reddy, B.M. Development of Cerium Promoted Copper–Magnesium Catalysts for Biomass Valorization: Selective Hydrogenolysis of Bioglycerol. Appl. Catal. B Environ. 2016, 181, 47–57. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, D.B.; Zhang, D.Y.; Yang, P.X. Bimetallic Effects of Silver-Modified Nickel Catalysts and their Synergy in Glycerol Hydrogenolysis. ChemCatChem 2016, 8, 1929–1936. [Google Scholar] [CrossRef]
- Shi, G.; Su, L.; Jin, K. New bulk nickel phosphide catalysts for glycerol hydrogenolysis to 1,2-propanediol. Catal. Commun. 2015, 59, 180–183. [Google Scholar] [CrossRef]
- Salazar, J.B.; Falcone, D.D.; Pham, H.N.; Datye, A.K.; Passos, F.B.; Davis, R.J. Selective production of 1,2-propanediol by hydrogenolysis of glycerol over bimetallic Ru–Cu nanoparticles supported on TiO2. Appl. Catal. A Gen. 2014, 482, 137–144. [Google Scholar] [CrossRef]
- Liu, L.; Asano, T.; Nakagawa, Y.; Tamura, M.; Okumura, K.; Tomishige, K. Selective Hydrogenolysis of Glycerol to 1,3-Propanediol over Rhenium-Oxide-Modified Iridium Nanoparticles Coating Rutile Titania Support. ACS Catal. 2019, 9, 10913–10930. [Google Scholar] [CrossRef]
- Skuhrovcova, L.; Kolena, J.; Zdenek, T.; Kocik, J. Cu–Zn–Al mixed oxides as catalysts for the hydrogenolysis of glycerol to 1,2-propanediol. React. Kinet. Mech. Catal. 2019, 127, 241–257. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, L.; Li, X.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol to 1,2-propanediol over Cu-based catalysts: A short review. Catal. Today 2019, 107, 2411–2502. [Google Scholar] [CrossRef]
- Modvig, A.; Kumpidet, C.; Riisager, A.; Albert, J. Ru-Doped Wells–Dawson Polyoxometalate as Efficient Catalyst for Glycerol Hydrogenolysis to propanediols. Materials 2019, 12, 2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raju, N.; Rekha, V.; Abhishek, B.; Kumar, P.M.; Sumana, C.; Lingaiah, N. Studies on continuous selective hydrogenolysis of glycerol over supported Cu-Co bimetallic catalysts. New J. Chem. 2020, 44, 3122–3128. [Google Scholar] [CrossRef]
- Li, X.; Wu, D. Synthesis of Co-doped micro-mesoporous SAPO-11 zeolite for glycerol hydrogenolysis. Korean J. Chem. Eng. 2020, 37, 216–223. [Google Scholar] [CrossRef]
- Liang, Y.; Shi, G.; Jin, K. Promotion Effect of Al2O3 on Pt–WOx/SiO2 Catalysts for Selective Hydrogenolysis of Bioglycerol to 1,3-Propanediol in Liquid Phase. Catal. Lett. 2020, 150, 2365–2376. [Google Scholar] [CrossRef]
- Li, X.; Xiang, M.; Wu, D. Hydrogenolysis of glycerol over bimetallic Cu–Ni catalysts supported on hierarchically porous SAPO-11 zeolite. Catal. Commun. 2019, 119, 170–175. [Google Scholar] [CrossRef]
- Xi, Z.; Hong, Z.; Huang, F.; Zhu, Z.; Jia, W.; Li, J. Hydrogenolysis of Glycerol on the ZrO2-TiO2 Supported Pt-WOx Catalyst. Catalysts 2020, 10, 312. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Shang, Y.; Wang, S.; Liu, X.; Wang, X.; Gui, J.; Zhang, C.; Zhu, Y.; Li, Y. Boron oxide modified bifunctional Cu/Al2O3 catalysts for the selective hydrogenolysis of glucose to 1,2-propanediol. Mol. Catal. 2020, 485, 110514. [Google Scholar] [CrossRef]
- Shesterkin, A.A.; Kustov, L.M.; Strekalovaa, A.A.; Kazansky, V.B. Heterogeneous iron-containing nanocatalysts—Promising systems for selective hydrogenation and hydrogenolysis. Catal. Sci. Technol. 2020, 10, 3160–3174. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, B.; Douthwaite, M.; Tang, L.; Zhang, Y.; Wang, M.; Ma, D. Multiporous Carbon Encapsulated Ni Nanoparticles Promote Glycerol Valorisation towards Hydrogenation against Rearrangement. Chin. J. Catal. 2020, 38, 439–444. [Google Scholar]
- Chimentão, R.J.; Miranda, B.C.; Ruiz, D.; Guiradoc, G.; Medina, F.; Llorca, J.; Santos, J.B.O. Catalytic performance of zinc-supported copper and nickel catalysts in the glycerol hydrogenolysis. J. Energy Chem. 2020, 42, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Yoda, E.; Ootawa, A. Dehydration of glycerol on H-MFI zeolite investigated by FT-IR. Appl. Catal. A Gen. 2009, 360, 66–70. [Google Scholar] [CrossRef]
- Coll, D.; Delbecq, F.; Aray, Y.; Sautet, P. Stability of intermediates in the glycerol hydrogenolysis on transition metal catalysts from first principles. Phys. Chem. Chem. Phys. 2011, 13, 1448–1456. [Google Scholar] [CrossRef]
- Dasari, A.M.; Kiatsimkul, P.; Sutterlin, R.W.; Suppes, J.G. Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl. Catal. A Gen. 2005, 281, 225. [Google Scholar] [CrossRef]
- Seguel, J.; García, R.; Chimentão, R.J.; García-Fierro, J.L.; Ghampson, I.T.; Escalona, N.; Sepúlveda, C. Thermal Modification Effect on Supported Cu-Based Activated Carbon Catalyst in Hydrogenolysis of Glycerol. Materials 2020, 13, 603. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Shah, A.K.; Lee, J.H.; Park, Y.H.; Stangar, U.L. Selective hydrogenolysis of glycerol over bifunctional Copper-Magnesium supported catalysts for propanediols synthesis. Ind. Eng. Chem. Res. 2020, 59, 6506–6516. [Google Scholar] [CrossRef]
- Shan, J.; Liu, H.; Lu, K.; Zhu, S.; Li, J.; Wang, J.; Fan, W. Identification of the dehydration active sites in glycerol hydrogenolysis to 1,2-propanediol over Cu/SiO2 catalysts. J. Catal. 2020, 383, 13–23. [Google Scholar] [CrossRef]
- Feng, S.; Zhao, B.; Liang, Y.L.; Liu, L.; Dong, J. Improving Selectivity to 1,3-Propanediol for Glycerol Hydrogenolysis Using W− and Al−Incorporated SBA-15 as Support for Pt Nanoparticles. Ind. Eng. Chem. Res. 2019, 58, 2661–2671. [Google Scholar] [CrossRef]
- Niu, Y.; Zhao, B.; Liang, Y.; Liu, L.; Dong, J. Promoting Role of Oxygen Deficiency on a WO3-Supported Pt Catalyst for Glycerol Hydrogenolysis to 1,3-Propanediol. Ind. Eng. Chem. Res. 2020, 59, 7389–7397. [Google Scholar] [CrossRef]
- Luo, R.; Zhao, X.; Gong, H.; Qian, W.; Li, D.; Chen, M.; Cui, K.; Wang, J.; Hou, Z. Effect of Tungsten Modification on Zirconium Phosphate-supported Pt Catalyst for Selective Hydrogenolysis of Glycerol to 1-Propanol. Energy Fuels 2020, 34, 8707–8717. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Wang, A. Selective hydrogenolysis of glycerol to 1,3-propanediol over Pt-W based catalysts. Chin. J. Catal. 2020, 41, 1311–1319. [Google Scholar] [CrossRef]
- López, A.; Aragón, J.A.; Hernández-Cortez, J.G.; Mosqueira, M.L.; Martínez-Palou, R. Study of hydrotalcite-supported transition metals as catalysts for crude glycerol hydrogenolysis. Mol. Catal. 2019, 468, 9–18. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L.; Fernández, S.G.; Requies, J.; Doukkali, M.E.; Güemez, M.B. Hydrogenolysis through catalytic transfer hydrogenation: Glycerol conversion to 1,2-propanediol. Catal. Today 2012, 195, 22–31. [Google Scholar] [CrossRef]
- Hosgu, H.L.; Yıldız, M.; Gercel, H.F. Hydrogenolysis of Aqueous Glycerol over Raney Nickel Catalyst: Comparison of Pure and Biodiesel By-Product. Ind. Eng. Chem. Res. 2012, 51, 3863–3869. [Google Scholar] [CrossRef]
- Akiyama, M.; Sato, S.; Takahashi, R.; Inui, K.; Yokota, M. Dehydration–hydrogenation of glycerol into 1,2-propanediol at ambient hydrogen pressure. Appl. Catal. A Gen. 2009, 371, 60–66. [Google Scholar] [CrossRef]
- Wu, Z.; Mao, Y.; Song, M.; Yin, X.; Zhang, M. Cu/boehmite: A highly active catalyst for hydrogenolysis of glycerol to 1,2-propanediol. Catal. Commun. 2013, 32, 52–57. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Zhu, Y.; Zheng, H.; Li, Y. Promoting effect of boron oxide on Cu/SiO2 catalyst for glycerol hydrogenolysis to 1,2-propanediol. J. Catal. 2013, 303, 70–79. [Google Scholar] [CrossRef]
- Soares, A.H.; Atia, H.; Armbruster, U.; Passos, F.B.; Martin, A. Platinum, palladium and nickel supported on Fe3O4 as catalysts for glycerol aqueous-phase hydrogenolysis and reforming. Appl. Catal. A Gen. 2017, 548, 179–190. [Google Scholar] [CrossRef]
- Soares, A.V.-H.; Perez, G.; Passos, F.B. Alumina supported bimetallic Pt–Fe catalysts applied to glycerol hydrogenolysis and aqueous phase reforming. Appl. Catal. A Gen. 2016, 185, 77–87. [Google Scholar] [CrossRef]
- Xia, S.; Du, W.; Zheng, L.; Chen, P.; Hou, Z. A thermally stable and easily recycled core–shell Fe2O3@CuMgAl catalyst for hydrogenolysis of glycerol. Catal. Sci. Technol. 2014, 4, 912–916. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Vila, F.; Mariscal, R.; Jiménez-López, A. Hydrogenolysis of glycerol to obtain 1,2-propanediol on Ce-promoted Ni/SBA-15 catalysts. Appl. Catal. B Environ. 2012, 117, 253–259. [Google Scholar] [CrossRef]
- Menchavez, R.N.; Morra, M.J.; He, B.B. Glycerol Hydrogenolysis using a Ni/Ce-Mg Catalyst for Improved Ethanol and 1,2-Propanediol Selectivities. Can. J. Chem. Eng. 2017, 95, 1332–1339. [Google Scholar] [CrossRef]
- Jiang, T.; Kong, D.; Xu, K.; Cao, F. Hydrogenolysis of glycerol aqueous solution to glycols over Ni–Co bimetallic catalyst: Effect of ceria promoting. Appl. Pet. Res. 2016, 6, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Rekha, R.V.; Raju, N.; Sumana, C.; Lingaiah, N. Continuous Hydrogenolysis of Glycerol to 1,2-Propanediol Over Bi-metallic Ni–Ag Supported on γ-Al2O3 Catalysts. Catal. Lett. 2017, 147, 1441–1452. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Guo, X.; Mao, J.; Zhang, S. Ag/Al2O3 for glycerol hydrogenolysis to 1,2-propanediol: Activity, selectivity and deactivation. Green Chem. 2012, 14, 156–163. [Google Scholar] [CrossRef]
- Yadav, G.D.; Chandan, P.A.; Tekale, D.P. Hydrogenolysis of Glycerol to 1,2-Propanediol over Nano-Fibrous Ag-OMS-2 Catalysts. Ind. Eng. Chem. Res. 2012, 51, 1549–1562. [Google Scholar] [CrossRef]
- Oh, J.; Dash, S.; Lee, H. Selective conversion of glycerol to 1,3-propanediol using Pt-sulfated zirconia. Green Chem. 2011, 13, 2004–2007. [Google Scholar] [CrossRef]
- Qin, L.Z.; Song, M.J.; Chen, C.L. Aqueous-phase deoxygenation of glycerol to 1,3-propanediol over Pt/WO3/ZrO2 catalysts in a fixed-bed reactor. Green Chem. 2010, 12, 1466–1472. [Google Scholar] [CrossRef]
- Jiang, T.; Huai, Q.; Geng, T.; Ying, W.; Xiao, T.; Cao, F. Catalytic performance of Pd–Ni bimetallic catalyst for glycerol hydrogenolysis. Biomass Bioenerg. 2015, 78, 71–79. [Google Scholar] [CrossRef]
- Isabelle, C.F.; Manfro, R.L.; Souza, M.M.V.M. Hydrogenolysis of glycerol to propylene glycol in continuous system without hydrogen addition over Cu-Ni catalysts. Appl. Catal. B Environ. 2018, 220, 31–41. [Google Scholar]
- Vasiliadoua, E.S.; Lemonidou, A.A. Investigating the performance and deactivation behaviour of silica-supported copper catalysts in glycerol hydrogenolysis. Appl. Catal. A Gen. 2011, 396, 177–185. [Google Scholar] [CrossRef]
- Mane, R.B.; Ghalwadkar, A.A.; Hengne, A.M.; Suryawanshi, Y.R.; Rode, C.V. Role of promoters in copper chromite catalysts for hydrogenolysis of glycerol. Catal. Today 2011, 164, 447–450. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).