A Novel Model Parameter for the Assessment of the Dimerization of n-Butenes over Ni-Containing Aluminosilicate Catalysts
Abstract
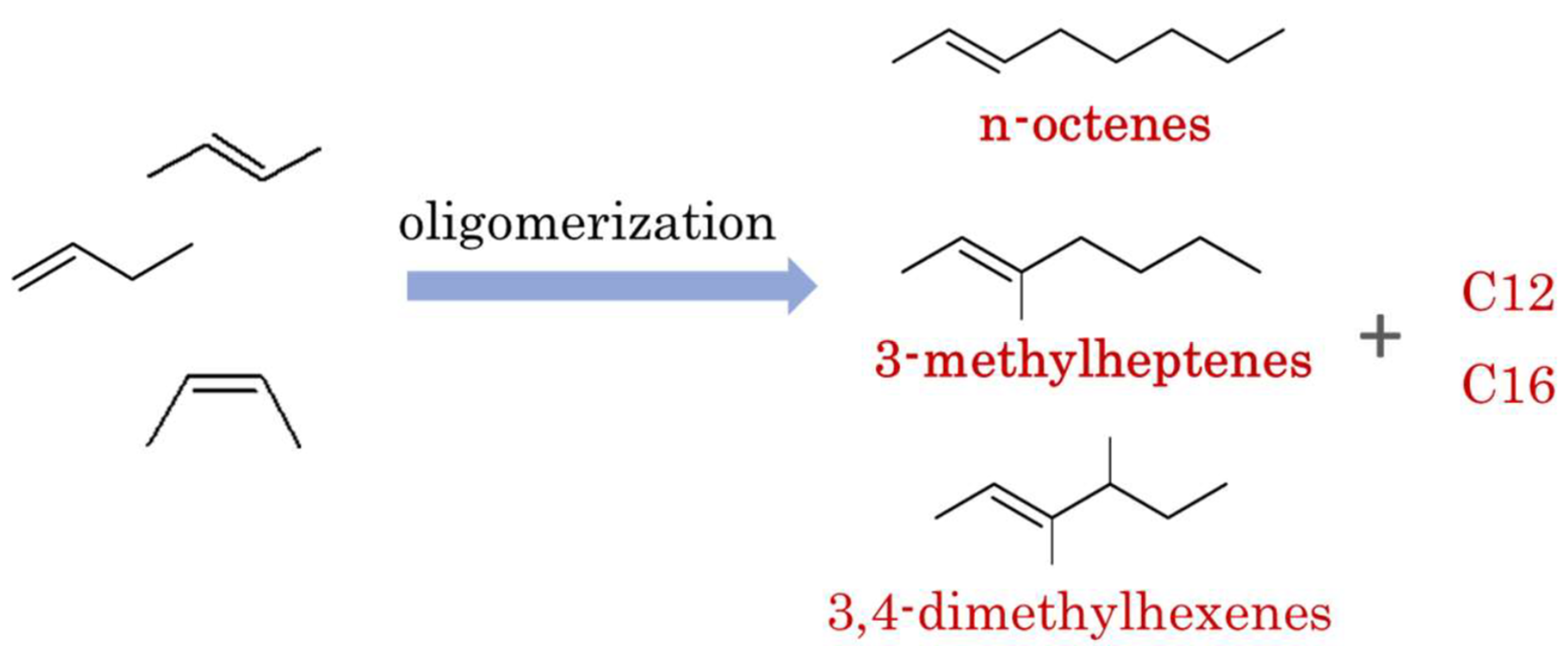
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Catalysts Preparation and Characterization
3.2. Catalysts Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alscher, F.; Nadolny, F.; Frenzel, H.; Knossalla, J.; Peitz, S.; Borovinskaya, E.; Breitkopf, C.; Franke, R.; Reschetilowski, W. Determination of the prevailing n-butenes dimerization mechanism over nickel containing Al-MCM-41/ZSM-5 mixed-phase catalysts. Catal. Sci. Technol. 2019, 9, 2456–2468. [Google Scholar] [CrossRef]
- Brückner, A.; Bentrup, U.; Zanthoff, H.; Maschmeyer, D. The role of different Ni sites in supported nickel catalysts for butene dimerization under industry-like conditions. J. Catal. 2009, 266, 120–128. [Google Scholar] [CrossRef]
- Albrecht, S.; Kießling, D.; Wendt, G.; Maschmeyer, D.; Nierlich, F. Oligomerisierung von n-Butenen. Chem. Ing. Tech. 2005, 77, 695–709. [Google Scholar] [CrossRef]
- Börner, A.; Franke, R. Hydroformylation: Fundamentals, Processes, and Applications in Organic Synthesis; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2016. [Google Scholar]
- Wendt, G.; Fritsch, E.; Schöllner, R.; Siegel, H. Untersuchungen an Nickeloxid-Mischkatalysatoren. II Katalytische Eigenschaften von NiO/SiO2-Katalysatoren. Z. Anorg. Allg. Chem. 1980, 467, 51–60. [Google Scholar] [CrossRef]
- Wendt, G.; Finster, J.; Deininger, D. Zur katalytischen Wirksamkeit von NiO/SiO2- und NiO-Al2O3/SiO2-Katalysatoren bei der Dimerisierung von Olefinen. Wiss. Z. Karl-Marx-Univ. Leipzig, Math.-Naturwiss. Reihe 1981, 30, 346–357. [Google Scholar]
- Reschetilowski, W. Metal-support interaction in bifunctional metal zeolite catalysts and the HSAB principle. Erdöl-Erdgas-Kohle 1996, 112, 467–470. [Google Scholar]
- Masalska, A.; Grzechowiak, J.R.; Jaroszewska, K. Effect of Metal-Support Interactions in Ni/ZSM-5 + Al2O3 Catalysts on the Transformation of n-Paraffins. Top. Catal. 2013, 56, 981–994. [Google Scholar] [CrossRef] [Green Version]
- Nadolny, F.; Alscher, F.; Peitz, S.; Borovinskaya, E.; Franke, R.; Reschetilowski, W. Influence of Remaining Acid Sites of an Amorphous Aluminosilicate on the Oligomerization of n-Butenes after Impregnation with Nickel Ions. Catalysts 2020, 10, 1487. [Google Scholar] [CrossRef]
- Nadolny, F.; Bentrup, U.; Rockstroh, N.; Alscher, F.; Reschetilowski, W.; Peitz, S.; Franke, R.; Brückner, A. Oligomerization of n-butenes over Ni/SiO2–Al2O3: Influence of support modification by steam-treating. A. Catal. Sci. Technol. 2021, 11, 4732–4740. [Google Scholar] [CrossRef]
- Ehrmaier, A.; Liu, Y.; Peitz, S.; Jentys, A.; Chin, Y.-H.; Sanchez-Sanchez, M.; Bermejo-Deval, R.; Lercher, J. Dimerization of Linear Butenes on Zeolite-Supported Ni2+. ACS Catal. 2019, 9, 315–324. [Google Scholar] [CrossRef]
- Beroza, M.; Sarmiento, R. Determination of the Carbon Skeleton and Other Structural Features of Organic Compounds by Gas Chromatography. Anal. Chem. 1963, 35, 1353–1357. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, J.; Wang, J.; Zhang, L.; Gao, J.; Liu, A. Catalytic performance and characterization of Ni-doped HZSM-5 catalysts for selective trimerization of n-butene. Fuel Process. Technol. 2009, 90, 863–870. [Google Scholar] [CrossRef]
- Hoang, D.L.; Berndt, H.; Miessner, H.; Schreier, E.; Völter, J.; Lieske, H. Nickel modified H-ZSM-5 catalysts. Appl. Catal. A Gen. 1994, 114, 295–311. [Google Scholar] [CrossRef]
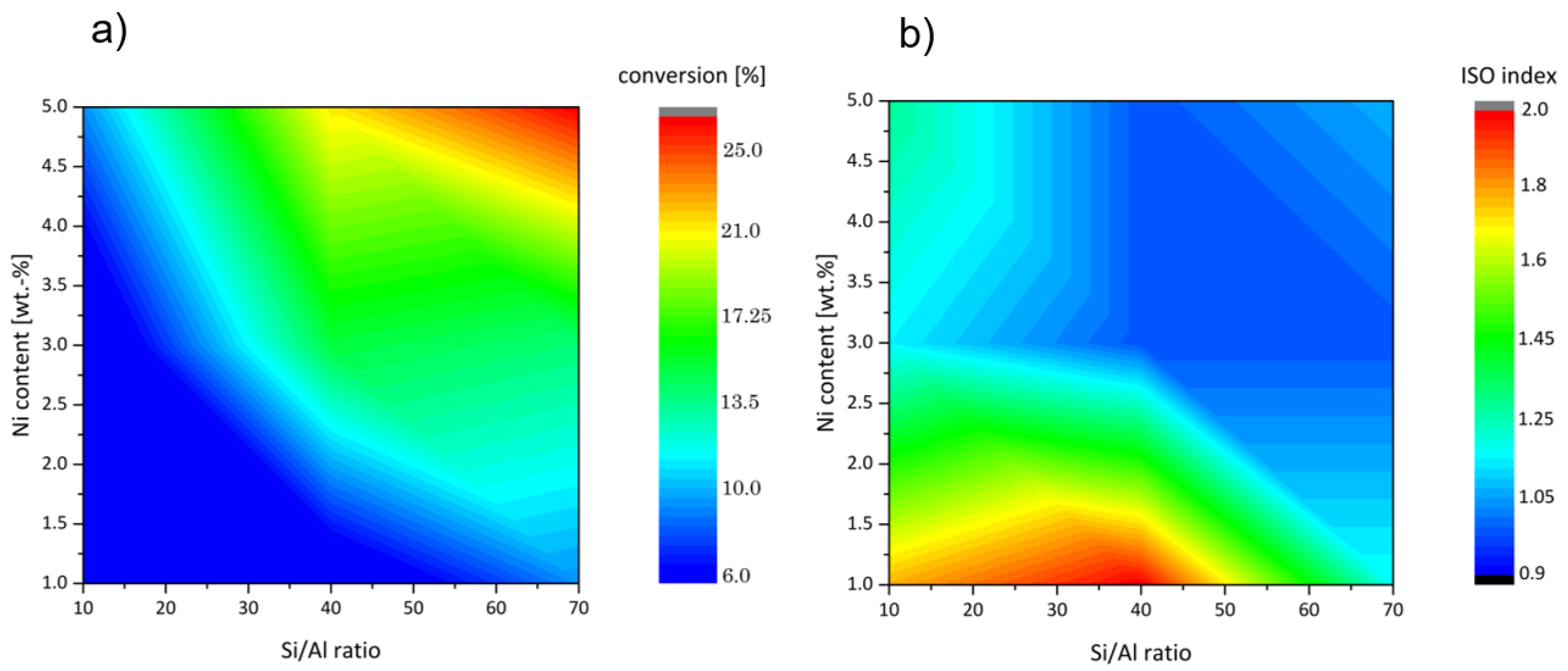
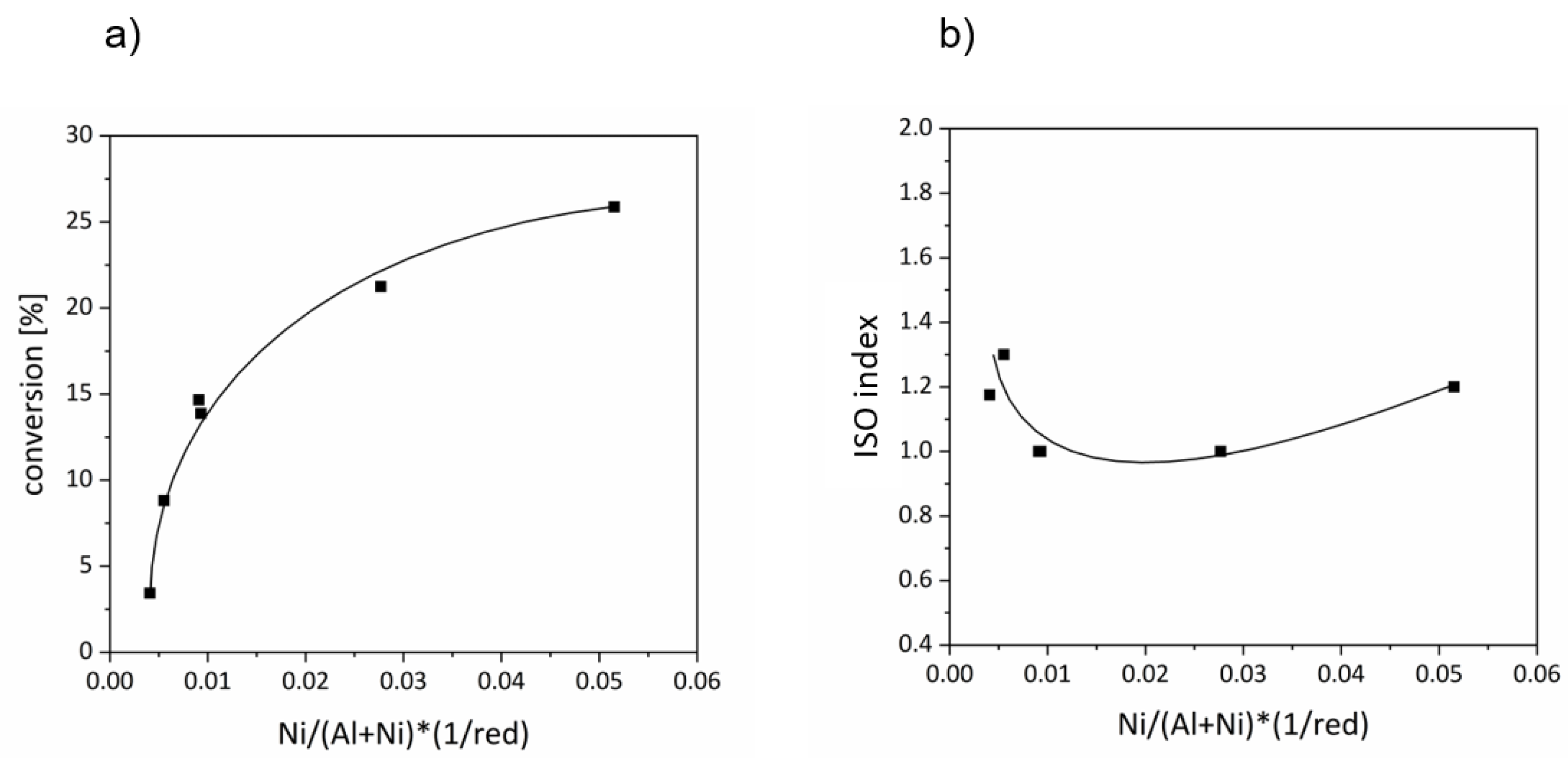
| Catalyst Sample | Si/Al Ratio | Ni Content [wt.-%] | Reduction Degree [%] | Ni/(Al + Ni) Ratio | Ni/(Al + Ni) (1/red) | Conversion [%] | ISO Index |
|---|---|---|---|---|---|---|---|
| OS-10-3 | 9.6 | 4.5 | 83 | 0.34 | 0.004 | 3.4 | 1.2 |
| OS-10-5 | 9.4 | 7.2 | 82 | 0.45 | 0.006 | 8.8 | 1.3 |
| OS-40-3 | 30.6 | 4.1 | 64 | 0.58 | 0.009 | 14.7 | 1.0 |
| OS-40-5 | 29.9 | 6.6 | 25 | 0.69 | 0.028 | 21.2 | 1.0 |
| OS-70-3 | 47.2 | 4.6 | 76 | 0.70 | 0.009 | 13.9 | 1.0 |
| OS-70-5 | 52.7 | 6.6 | 15 | 0.80 | 0.052 | 25.9 | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alscher, F.; Borovinskaya, E.; Peitz, S.; Reschetilowski, W. A Novel Model Parameter for the Assessment of the Dimerization of n-Butenes over Ni-Containing Aluminosilicate Catalysts. Catalysts 2022, 12, 748. https://doi.org/10.3390/catal12070748
Alscher F, Borovinskaya E, Peitz S, Reschetilowski W. A Novel Model Parameter for the Assessment of the Dimerization of n-Butenes over Ni-Containing Aluminosilicate Catalysts. Catalysts. 2022; 12(7):748. https://doi.org/10.3390/catal12070748
Chicago/Turabian StyleAlscher, Felix, Ekaterina Borovinskaya, Stephan Peitz, and Wladimir Reschetilowski. 2022. "A Novel Model Parameter for the Assessment of the Dimerization of n-Butenes over Ni-Containing Aluminosilicate Catalysts" Catalysts 12, no. 7: 748. https://doi.org/10.3390/catal12070748
APA StyleAlscher, F., Borovinskaya, E., Peitz, S., & Reschetilowski, W. (2022). A Novel Model Parameter for the Assessment of the Dimerization of n-Butenes over Ni-Containing Aluminosilicate Catalysts. Catalysts, 12(7), 748. https://doi.org/10.3390/catal12070748








