The High Electrocatalytic Performance of NiFeSe/CFP for Hydrogen Evolution Reaction Derived from a Prussian Blue Analogue
Abstract
:1. Introduction
2. Results and Discussion
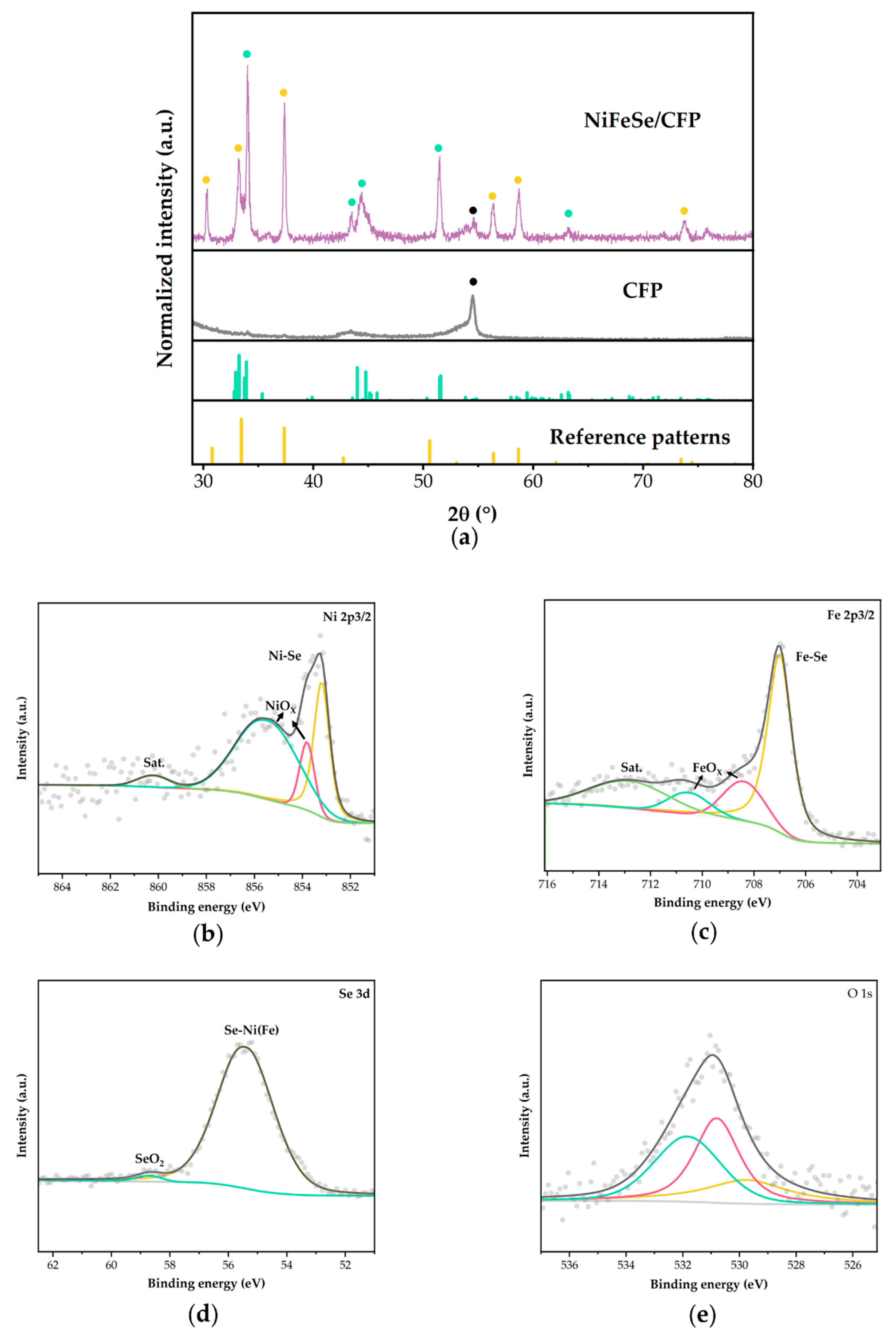
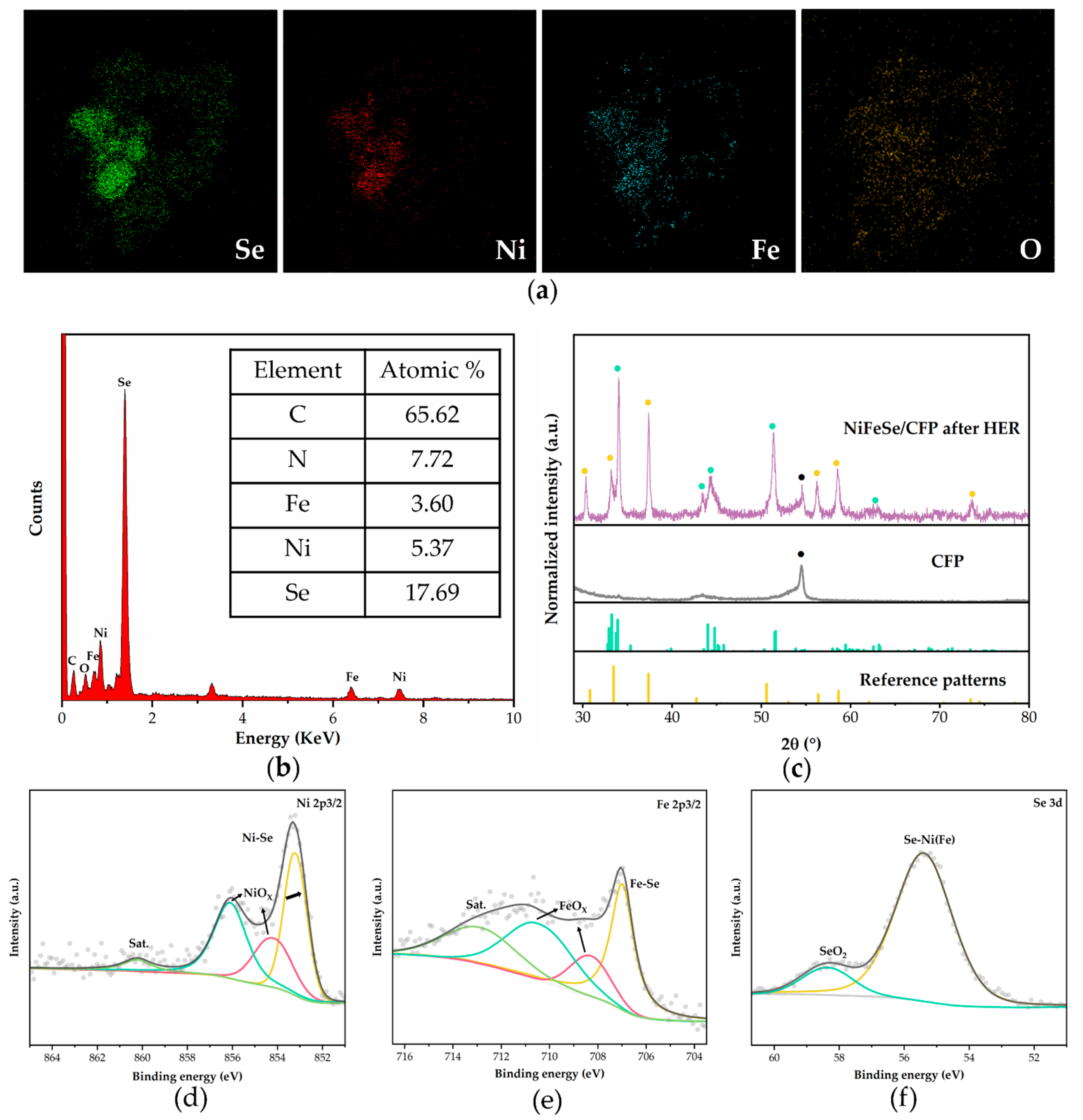
2.1. Microstructure and Phase Identification
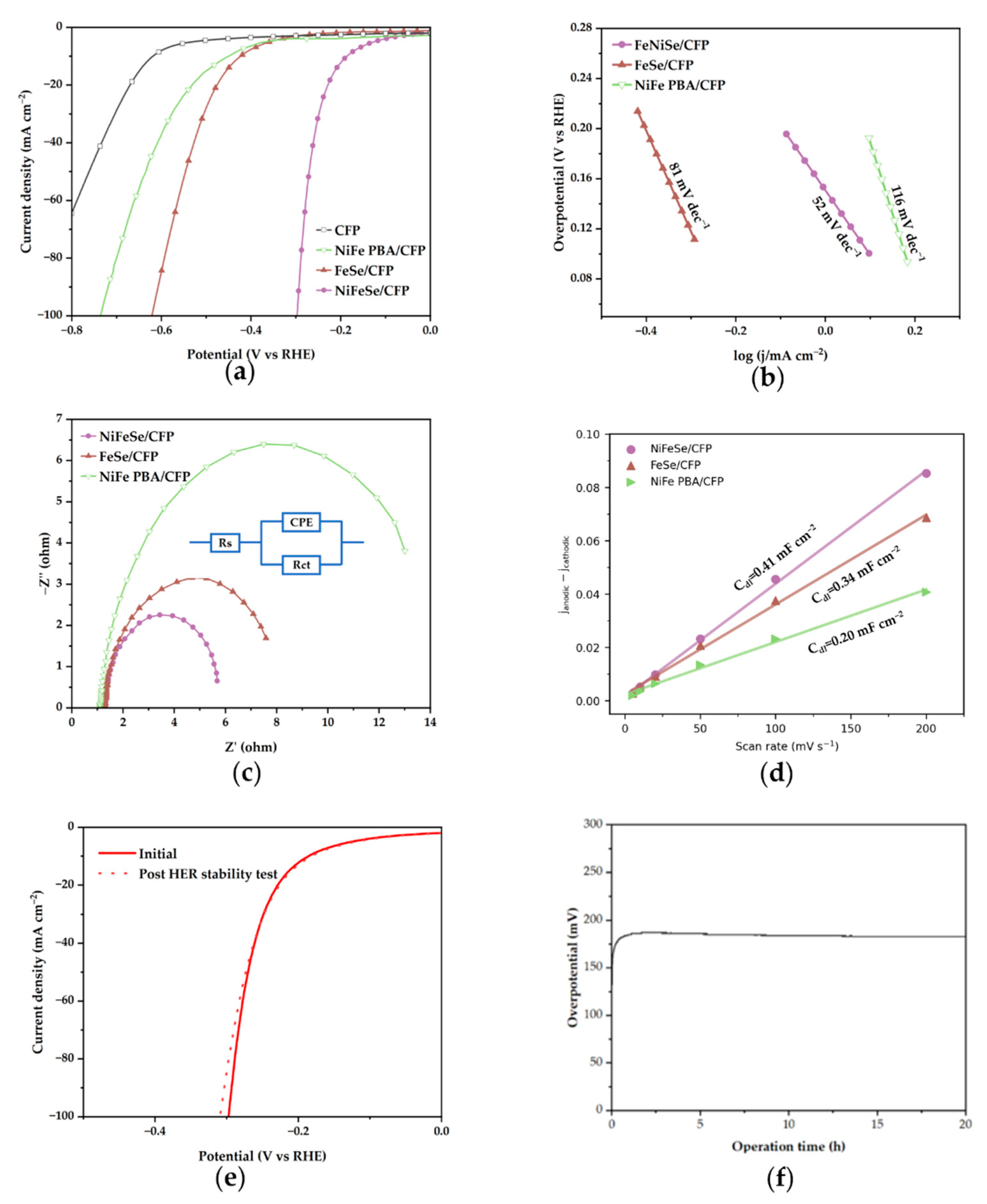
2.2. HER Performance Testing
3. Materials and Methods
3.1. Preparation of Electrodes
3.2. Electrodeposition of NiFe PBA/CFP and Fe PBA/CFP Precursors
3.3. Fabrication of NiFeSe/CFP and FeSe/CFP Electrocatalysts
3.4. Material Characterizations
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, D.; McDonagh, A.; Qiao, S.Z.; Wang, G. High-capacity aqueous potassium-ion batteries for large-scale energy storage. Adv. Mater. 2017, 29, 1604007. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhao, S.; Li, W.; Neville, Y.P.; Akpinar, I.; Shearing, P.R.; Brett, D.J.L.; He, G. Realizing optimal hydrogen evolution reaction properties via tuning phosphorous and transition metal interactions. Green Energy Environ. 2020, 5, 506–512. [Google Scholar] [CrossRef]
- Liu, X.; Liu, W.; Ko, M.S.; Park, M.J.; Kim, M.G.; Oh, P.G.; Chae, S.J.; Park, S.; Casimir, A.; Wu, G. Metal (Ni, Co)-metal oxides/graphene nanocomposites as multifunctional electrocatalysts. Adv. Funct. Mater. 2015, 25, 5799–5808. [Google Scholar] [CrossRef]
- Jian, J.; Jiang, G.S.; van de Krol, R.; Wei, B.Q.; Wang, H.Q. Recent advances in rational engineering of multinary semiconductors for photoelectrochemical hydrogen generation. Nano Energy 2018, 51, 457–480. [Google Scholar] [CrossRef]
- Zhang, S.; Han, B.; Wang, J. Special topic on ionic liquids: Energy, materials & environment. Sci. China Chem. 2016, 59, 505–506. [Google Scholar]
- Liang, H.; Gandi, A.N.; Anjum, D.H.; Wang, X.; Schwingenschlogl, U.; Alshareef, H.N. Plasma-assisted synthesis of NiCoP for efficient overall water splitting. Nano Lett. 2016, 16, 7718–7725. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R.; Liu, S.; Zhuang, X.; Feng, X. Interface engineering of MoS2/Ni3S2 heterostructures for highly enhanced electrochemical overall—water—splitting activity. Angew. Chem. 2016, 128, 6814–6819. [Google Scholar] [CrossRef]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef]
- Bentley, C.L.; Kang, M.; Maddar, F.M.; Li, F.; Walker, M.; Zhang, J.; Unwin, P.R. Electrochemical maps and movies of the hydrogen evolution reaction on natural crystals of molybdenite (MoS2): Basal vs. edge plane activity. Chem. Sci. 2017, 8, 6583–6593. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Cheng, N.; Pu, Z.; Xing, W.; Sun, X. NiSe nanowire film supported on nickel foam: An efficient and stable 3D bifunctional electrode for full water splitting. Angew. Chem. 2015, 127, 9483–9487. [Google Scholar] [CrossRef]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef] [PubMed]
- Djire, A.; Wang, X.; Xiao, C.; Nwamba, O.C.; Mirkin, M.V.; Neale, N.R. Basal plane hydrogen evolution activity from mixed metal nitride MXenes measured by scanning electrochemical microscopy. Adv. Funct. Mater. 2020, 30, 2001136. [Google Scholar] [CrossRef]
- Ologunagba, D.; Kattel, S. Pt-and Pd-modified transition metal nitride catalysts for the hydrogen evolution reaction. Phys. Chem. Chem. Phys. 2022, 24, 12149–12157. [Google Scholar] [CrossRef] [PubMed]
- Majhi, K.C.; Yadav, M. Palladium oxide decorated transition metal nitride as efficient electrocatalyst for hydrogen evolution reaction. J. Alloys Compd. 2021, 855, 157511. [Google Scholar] [CrossRef]
- Hou, Y.; Qiu, M.; Zhang, T.; Zhuang, X.; Kim, C.S.; Yuan, C.; Feng, X. Ternary porous cobalt phosphoselenide nanosheets: An efficient electrocatalyst for electrocatalytic and photoelectrochemical water splitting. Adv. Mater. 2017, 29, 1701589. [Google Scholar] [CrossRef]
- Yang, J.; Lei, C.; Wang, H.; Yang, B.; Li, Z.; Qiu, M.; Zhuang, X.; Yuan, C.; Lei, L.; Hou, Y. High-index faceted binary-metal selenide nanosheet arrays as efficient 3D electrodes for alkaline hydrogen evolution. Nanoscale 2019, 11, 17571–17578. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, Y.; An, T.; Cao, M. Increasing the active sites and intrinsic activity of transition metal chalcogenide electrocatalysts for enhanced water splitting. J. Mater. Chem. A 2020, 8, 25465–25498. [Google Scholar] [CrossRef]
- Peng, X.; Yan, Y.; Jin, X.; Huang, C.; Jin, W.; Gao, B.; Chu, P.K. Recent advance and prospectives of electrocatalysts based on transition metal selenides for efficient water splitting. Nano Energy 2020, 78, 105234. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, D.; Ye, F.; Wang, K.; Shi, Z.; Chen, X.; Zhao, C. Self-supported NiSe2 nanowire arrays on carbon fiber paper as efficient and stable electrode for hydrogen evolution reaction. ACS Sustain. Chem. Eng. 2018, 6, 11884–11891. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, D.; Ye, F.; Wang, K.; Shi, Z. Synthesis of lawn-like NiS2 nanowires on carbon fiber paper as bifunctional electrode for water splitting. Int. J. Hydrogen Energy 2017, 42, 17038–17048. [Google Scholar] [CrossRef]
- Chae, S.H.; Muthurasu, A.; Kim, T.; Kim, J.S.; Khil, M.S.; Lee, M.; Kim, H.; Lee, J.Y.; Kim, H.Y. Templated fabrication of perfectly aligned metal-organic framework-supported iron-doped copper-cobalt selenide nanostructure on hollow carbon nanofibers for an efficient trifunctional electrode material. Appl. Catal. B 2021, 293, 120209. [Google Scholar] [CrossRef]
- Ming, F.; Liang, H.; Shi, H.; Xu, X.; Mei, G.; Wang, Z. MOF-derived Co-doped nickel selenide/C electrocatalysts supported on Ni foam for overall water splitting. J. Mater. Chem. A 2016, 4, 15148–15155. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, X.; Huang, J.; Wu, Z.; Gao, F. An Fe-doped nickel selenide nanorod/nanosheet hierarchical array for efficient overall water splitting. J. Mater. Chem. A 2019, 7, 2233–2241. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S.L.; Lu, X.F.; Wu, Z.P.; Luan, D.; Lou, X.W. Trimetallic Spinel NiCo2−xFexO4 Nanoboxes for Highly Efficient Electrocatalytic Oxygen Evolution. Angew. Chem. Int. Ed. 2021, 60, 11841–11846. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Zhang, L.; Liu, C.S.; Pang, H. Core–shell-type ZIF-8@ ZIF-67@ POM hybrids as efficient electrocatalysts for the oxygen evolution reaction. Inorg. Chem. Front. 2019, 6, 2514–2520. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Xie, J.-Q.; Ye, H.; Fu, X.-Z.; Sun, R.; Wong, C.-P. Co-Fe-P nanotubes electrocatalysts derived from metal-organic frameworks for efficient hydrogen evolution reaction under wide pH range. Nano Energy 2019, 56, 225–233. [Google Scholar] [CrossRef]
- Nivetha, R.; Gothandapani, K.; Raghavan, V.; Jacob, G.; Sellappan, R.; Bhardwaj, P.; Pitchaimuthu, S.; Kannan, A.N.M.; Jeong, S.K.; Grace, A.N. Highly porous MIL-100 (Fe) for the hydrogen evolution reaction (HER) in acidic and basic media. ACS Omega 2020, 5, 18941–18949. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, X.; Niu, Y.; Shao, L.; Zhu, C.; Hou, L. Cobalt iron selenide/sulfide porous nanocubes as high-performance electrocatalysts for efficient dye-sensitized solar cells. J. Power Sources 2017, 369, 35–41. [Google Scholar] [CrossRef]
- Xuan, C.; Zhang, J.; Wang, J.; Wang, D. Rational design and engineering of nanomaterials derived from prussian blue and its analogs for electrochemical water splitting. Chem. Asian J. 2020, 15, 958–972. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, W.; Shi, Z.; Che, M.; Yu, X.; Tang, Y.; Gao, Q. Electrospinning hetero-nanofibers of Fe3C-Mo2C/nitrogen-doped-carbon as efficient electrocatalysts for hydrogen evolution. ChemSusChem 2017, 10, 2597–2604. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, P.; Cao, S.; Chen, H.; Zhou, S.; Liu, H.; Wang, H.; Zhang, J.; Liu, S.; Wei, S. Contemporaneous inverse manipulation of the valence configuration to preferred Co2+ and Ni3+ for enhanced overall water electrocatalysis. Appl. Catal. B 2021, 284, 119725. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Wang, Z.; Sugahara, Y.; Yamauchi, Y. Hollow porous heterometallic phosphide nanocubes for enhanced electrochemical water splitting. Small 2018, 14, 1802442. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, X.; Jiang, X.; Shen, B.; Teng, Z.; Sun, D.; Fu, G.; Tang, Y. Surface carbon layer controllable Ni3Fe particles confined in hierarchical N-doped carbon framework boosting oxygen evolution reaction. Adv. Powder Mater. 2022, 1, 100020. [Google Scholar] [CrossRef]
- Martinez Garcia, R.; Knobel, M.; Reguera, E. Thermal-induced changes in molecular magnets based on prussian blue analogues. J. Phys. Chem. B 2006, 110, 7296–7303. [Google Scholar] [CrossRef] [PubMed]
- Varnell, J.A.; Tse, E.C.M.; Schulz, C.E.; Fister, T.T.; Haasch, R.T.; Timoshenko, J.; Frenkel, A.I.; Gewirth, A.A. Identification of carbon-encapsulated iron nanoparticles as active species in non-precious metal oxygen reduction catalysts. Nat. Commun. 2016, 7, 12582. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Zou, H.H.; Yuan, C.Z.; Zou, H.Y.; Cheang, T.Y.; Zhao, S.J.; Qazi, U.Y.; Zhong, S.L.; Wang, L.; Xu, A.W. Bimetallic phosphide hollow nanocubes derived from a prussian-blue-analog used as high-performance catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2017, 7, 1549–1555. [Google Scholar] [CrossRef]
- Karakaya, C.; Solati, N.; Savacı, U.; Keleş, E.; Turan, S.; Çelebi, S.; Kaya, S. Mesoporous thin-film NiS2 as an idealized pre-electrocatalyst for a hydrogen evolution reaction. Acs Catal. 2020, 10, 15114–15122. [Google Scholar] [CrossRef]
- Colli, A.N.; Girault, H.H.; Battistel, A. Non-Precious Electrodes for Practical Alkaline Water Electrolysis. Materials 2019, 12, 1336. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Yu, F.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Conway, B.; Tilak, B. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta 2002, 47, 3571–3594. [Google Scholar] [CrossRef]
- Long, X.; Li, G.; Wang, Z.; Zhu, H.; Zhang, T.; Xiao, S.; Guo, W.; Yang, S. Metallic iron–nickel sulfide ultrathin nanosheets as a highly active electrocatalyst for hydrogen evolution reaction in acidic media. J. Am. Chem. Soc. 2015, 137, 11900–11903. [Google Scholar] [CrossRef]
- Ahn, W.; Park, M.G.; Lee, D.U.; Seo, M.H.; Jiang, G.; Cano, Z.P.; Hassan, F.M.; Chen, Z. Hollow multivoid nanocuboids derived from ternary Ni-Co-Fe prussian blue analog for dual—electrocatalysis of oxygen and hydrogen evolution reactions. Adv. Funct. Mater. 2018, 28, 1802129. [Google Scholar] [CrossRef]
- Ge, Y.; Dong, P.; Craig, S.R.; Ajayan, P.M.; Ye, M.; Shen, J. Transforming nickel hydroxide into 3D prussian blue analogue array to obtain Ni2P/Fe2P for efficient hydrogen evolution reaction. Adv. Energy Mater. 2018, 8, 1800484. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Lu, Z.; Cui, Y. CoSe2 nanoparticles grown on carbon fiber paper: An efficient and stable electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, W.; Song, E.; Gu, F.; Zheng, Z.; Zhao, X.; Zhao, Y.; Liu, J.; Zhang, W. Adsorption-energy-based activity descriptors for electrocatalysts in energy storage applications. Natl. Sci. Rev. 2018, 5, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen-and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent trends and perspectives in electrochemical water splitting with an emphasis on sulfide, selenide, and phosphide catalysts of Fe, Co, and Ni: A review. Acs Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Xuan, C.; Xia, K.; Lei, W.; Xia, W.; Xiao, W.; Chen, L.; Xin, H.L.; Wang, D. Composition-dependent electrocatalytic activities of NiFe-based selenides for the oxygen evolution reaction. Electrochim. Acta 2018, 291, 64–72. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the activity origin of a copper-based electrocatalyst for selective nitrate reduction to ammonia. Angew. Chem. Int. Ed. 2020, 59, 5350–5354. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, Z.; Gao, S.; Su, J.; Lun, Z.; Xia, G.; Chen, J.; Zhang, R.; Chen, Q. Tuning electronic structures of nonprecious ternary alloys encapsulated in graphene layers for optimizing overall water splitting activity. Acs Catal. 2017, 7, 469–479. [Google Scholar] [CrossRef]
- Lee, J.; Son, N.; Shin, J.; Pandey, S.; Joo, S.W.; Kang, M. Highly efficient hydrogen evolution reaction performance and long-term stability of spherical Ni100−xFex alloy grown directly on a carbon paper electrode. J. Alloys Compd. 2021, 869, 159265. [Google Scholar] [CrossRef]
- Yang, C.C.; Zai, S.F.; Zhou, Y.T.; Du, L.; Jiang, Q. Fe3C-Co nanoparticles encapsulated in a hierarchical structure of N-doped carbon as a multifunctional electrocatalyst for ORR, OER, and HER. Adv. Funct. Mater. 2019, 29, 1901949. [Google Scholar] [CrossRef]
- Zhai, P.; Zhang, Y.; Wu, Y.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Sun, L.; Hou, J. Engineering active sites on hierarchical transition bimetal oxides/sulfides heterostructure array enabling robust overall water splitting. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Cui, Z.; Li, J.; Yang, F.; Huang, L.; Ma, C.; Zeng, M. CoMn phosphide encapsulated in nitrogen-doped graphene for electrocatalytic hydrogen evolution over a broad pH range. Chem. Commun. 2021, 57, 2400–2403. [Google Scholar] [CrossRef]
- Hei, J.; Xu, G.; Wei, B.; Zhang, L.; Ding, H.; Liu, D. NiFeP nanosheets on N-doped carbon sponge as a hierarchically structured bifunctional electrocatalyst for efficient overall water splitting. Appl. Surf. Sci. 2021, 549, 149297. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, H.; Liu, P.; Shen, Y.; Cheng, C.; Hirata, A.; Fujita, T.; Tang, Z.; Chen, M. Versatile nanoporous bimetallic phosphides towards electrochemical water splitting. Energy Environ. Sci. 2016, 9, 2257–2261. [Google Scholar] [CrossRef]
- Kou, T.; Smart, T.; Yao, B.; Chen, I.; Thota, D.; Ping, Y.; Li, Y. Theoretical and experimental insight into the effect of nitrogen doping on hydrogen evolution activity of Ni3S2 in alkaline medium. Adv. Energy Mater. 2018, 8, 1703538. [Google Scholar] [CrossRef]
- Wu, X.; He, D.; Zhang, H.; Li, H.; Li, Z.; Yang, B.; Lin, Z.; Lei, L.; Zhang, X. Ni0.85Se as an efficient non-noble bifunctional electrocatalyst for full water splitting. Int. J. Hydrogen Energy 2016, 41, 10688–10694. [Google Scholar] [CrossRef]
- Zheng, X.; Han, X.; Liu, H.; Chen, J.; Fu, D.; Wang, J.; Zhong, C.; Deng, Y.; Hu, W. Controllable synthesis of NixSe (0.5 ≤ x ≤ 1) nanocrystals for efficient rechargeable zinc—air batteries and water splitting. ACS Appl. Mater. Interfaces 2018, 10, 13675–13684. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Liu, Y.; Liu, Y.; Zhang, C.; Jia, K.; Su, J.; Wang, K. The High Electrocatalytic Performance of NiFeSe/CFP for Hydrogen Evolution Reaction Derived from a Prussian Blue Analogue. Catalysts 2022, 12, 739. https://doi.org/10.3390/catal12070739
Guo Y, Liu Y, Liu Y, Zhang C, Jia K, Su J, Wang K. The High Electrocatalytic Performance of NiFeSe/CFP for Hydrogen Evolution Reaction Derived from a Prussian Blue Analogue. Catalysts. 2022; 12(7):739. https://doi.org/10.3390/catal12070739
Chicago/Turabian StyleGuo, Yajie, Yongjie Liu, Yanrong Liu, Chunrui Zhang, Kelun Jia, Jibo Su, and Ke Wang. 2022. "The High Electrocatalytic Performance of NiFeSe/CFP for Hydrogen Evolution Reaction Derived from a Prussian Blue Analogue" Catalysts 12, no. 7: 739. https://doi.org/10.3390/catal12070739
APA StyleGuo, Y., Liu, Y., Liu, Y., Zhang, C., Jia, K., Su, J., & Wang, K. (2022). The High Electrocatalytic Performance of NiFeSe/CFP for Hydrogen Evolution Reaction Derived from a Prussian Blue Analogue. Catalysts, 12(7), 739. https://doi.org/10.3390/catal12070739





