1. Introduction
The world faces serious environmental problems which are mainly embodied by solid waste accumulation and air pollution. The construction and demolition wastes (C&DW) that generate billions of tons each year consitute a considerable proportion of solid waste. Recycled concrete (RCs) and recycled clay bricks (RCBs) are two main compositions of C&DWs in China due to the fact that old buildings that have been demolished are mostly composed of a brick–concrete structure [
1]. Those RCs are traditionally landfilled, exerting a heavy burden on the environment. Some researchers have attempted to crush and sieve RCs into recycled concrete aggregates (RCA) for the preparation of new concrete [
2,
3,
4] and think of new approaches to raise the added value of RCAs, particularly recycled fine aggregates (RFA) that have a grading size of less than 5 mm. Relative to natural fine aggregates, RFAs have two main features of high porosity [
5] and high alkalinity [
6,
7]. The former is attributed to micro cracks brought about by the mechanical crushing process; whilst the latter originates from calcium hydroxide [Ca(OH)
2] that exists in the old mortar attached to the surface of virgin aggregates. Starting with the above two distinguishing features, the high added value of RFA can be obtained by the combined use of photocatalytic technology, which is always blamed for high costs.
Photocatalysis refers to a phenomenon in which the photocatalyst, which is in a general N-type semiconductor such as nanoscale titanium dioxide (TiO
2) (NT) under the irradiation of ultraviolet (UV) light, triggers a photocatalytic effect that degrades pollutant materials. It was reported that an anodic TiO
2 nanotube layer with a large area can be used for photocatalysis in the gaseous phase [
8]. The entire process is light-driven and thus is environmentally friendly. The photon flux affects the kinetic process; whilst a lifted irradiance boosts the efficiency of photocatalysts [
9]. The principle of photocatalysis is to use light to excite compound semiconductors such as titanium dioxide and to use their electrons and holes to participate in oxidation–reduction reactions. When light with energy greater than or equal to the energy gap irradiates semiconductor nanoparticles, the electrons in the valence band will be excited to transition to the conduction band, leaving relatively stable holes in the valence band, thus forming electron hole pairs. Because there are many defects and dangling bonds in nano materials, these defects and dangling bonds can capture electrons or holes and prevent the recombination of electrons and holes. These trapped electrons and holes diffuse to the surface of the particles, respectively, resulting in a strong redox potential. The superoxide anion and the hydroxyl radical are predominant species that trigger the synergistic effect between TiO
2 photocatalysis and dye photosensitization [
10]. Once RFAs are endowed with high added value through combination with NT, the high production cost of RFAs can be offset, and the large-scale reuse of RFAs can be realized.
Previous studies widely researched cementitious-materials-based photocatalytic mortar (PCM) containing NT and proved a myriad of value-added functions including air-purification, self-cleaning [
11,
12], and antialgae fouling [
13]. A key limit of the traditional intermixing method, however, is the waste of photocatalysts. Attributing to the covering effect and the agglomeration effect, the content of NT in most cases is about 3–5 wt.% of cementitious materials to realize evident photocatalytic performance [
14]. On the contrary, the combined use of RFA and NT in the form of a composite photocatalyst (NT@RFA) is a step further towards maximizing the value of both NT and the carrier of RFA. It was reported that an increased nitrogen oxides (NOx) removal was realized when the NT was loaded onto the porous carrier [
15,
16,
17,
18]. The alkaline medium was also proven to coordinate with photocatalysts in boosting the degradation of acidic pollutant gases such as sulfur dioxide (SO
2) [
19]. The above forms the theoretical basis for the upcycling of RFAs. Here, upcycling means that the value extracted from recycled wastes (RFAs) is improved through recycling. The added value of NT@RFA is comprehensively gained from the beneficial properties of the NT-induced photocatalytic activity and the RFA augmented effect.
Recycled glass (RG) was also used as a carrier to load NT; whilst the prepared composite photocatalyst (NT@RG) was verified to promote photocatalytic activity due to its light-transmitting property [
20,
21]. That research, however, only studied the photocatalytic performance of NT@RG alone rather than that of photocatalytic mortar using NT@RG as an aggregate. A huge gap evidently exists within the above two research objects. This study to some degree fills the gap. The combined use of NT@RFA and NT@RG as aggregates in photocatalytic mortar is quite interesting and meaningful. On one hand, the NT@RFA with a porous structure is of high water absorption and crushing index. It leads the end product (photocatalytic mortar) to have deteriorated properties, such as a reduced compressive strength, an increased drying shrinkage, and an increased water absorption. On the other hand, the near-zero porosity of NT@RG endows it with a higher hardness relative to NT@RFA and a better dimensional stability when the system is losing water. NT@RG is thus expected to compensate for the defects of NT@RFA and make extra light penetrate into deep pores of NT@RFA via its light-transmitting property.
The use of NT@RG as aggregate potentially triggers alkali–silica reaction (ASR). A principal countermeasure is to use pozzolanic materials to control that reaction. The use of pozzolanic materials at the same time can also save the use of cement and abate carbon dioxide emission. Apart from traditional pozzolanic materials such as fly ash and furnace slag, many researchers have tried to find new materials that are more adaptable to the local situation. For example, it was reported that marine clay can be utilized with high added value as a supplementary binding material in manufacturing sustainable concrete products [
22,
23]. Recycled fine aggregates and recycled glass powder can be co-used in concrete production, which reduces the total carbon dioxide emission by about 19% [
24,
25]. Incinerated sewage sludge ash sourced from the incineration of sewage sludge could be used with lime to produce building materials that are eco-friendly [
26]. By investigating the incineration bottom ash sourced from municipal solid wastes, the constituents of ceramic and glass were proven to be of a similar efficiency strength index to fly ash and ground-granulated blast-furnace slag [
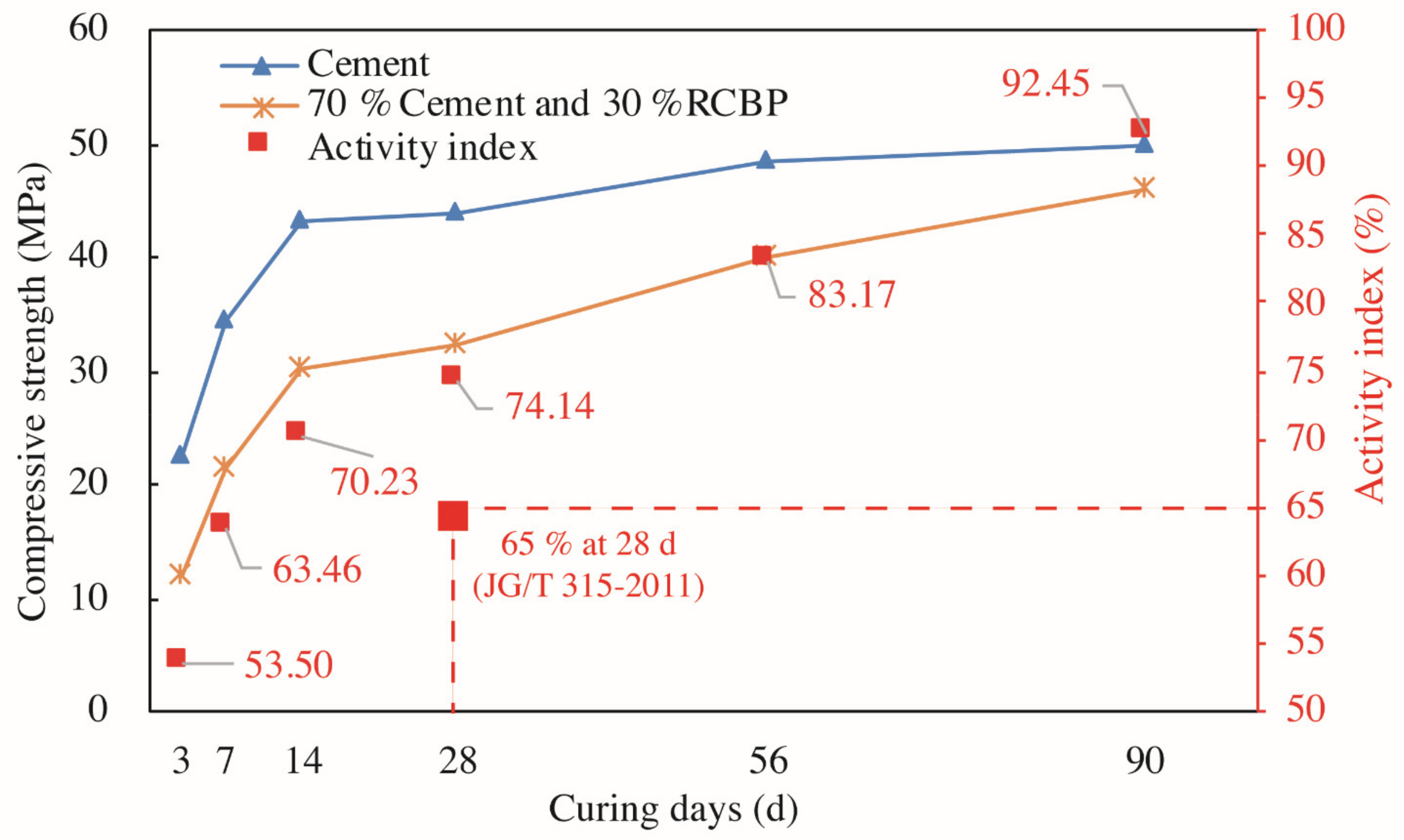
27]. Those mineral admixtures, attributing to the filling effect and the pozzolanic activity, can both save the use of cement and promote properties of the final product. The above cases, however, are not applicable to Shenzhen, a rising modern megacity of China, because Shenzhen possesses no heat-engine plants or steel works locally. Transporting those byproducts evidently causes extra costs and thus the use of fly ash is costly. By contrast, the fact that many buildings have been demolished provides Shenzhen with a tremendous amount of recycled clay brick powder (RCBP) [
25]. Reusing RCBP in building materials such as photocatalytic mortars is therefore significant, especially considering the practical situation of Shenzhen, namely, its large-scale urban renewal and extreme shortage of natural resources.
Besides the problem of solid waste accumulation, air pollution is also a difficult problem confronted by many developing countries including China. Photocatalytic technology is generally regarded as a potential efficient approach to solving this problem, especially when the technology is combined with cement-based materials such as mortar. Most of the previous studies related to photocatalytic degradation focused on NOx removal. Sulfur dioxide (SO
2), another typical composition of haze, is less studied. The control of SO
2 emissions, however, has been a crucial goal of China’s in the previous 20 years [
28]. This is because SO
2 produced by coal-fired power plants contributes to acid rain and affects climate patterns. Its high atmospheric level in China, relating to the country’s reliance on coal power, has caused significant infrastructural and environmental damage. Relative to the traditional flue-gas desulfurization, photocatalytic mortar can degrade SO
2 that has already been discharged into the atmosphere.
The novelty of this study is the use of prepared composite photocatalysts (NT@RG and NT@RFA) as aggregates in photocatalytic mortar. It realizes the upcycling of RFA and RG. The high alkalinity of NT@RFA is relied on to boost SO
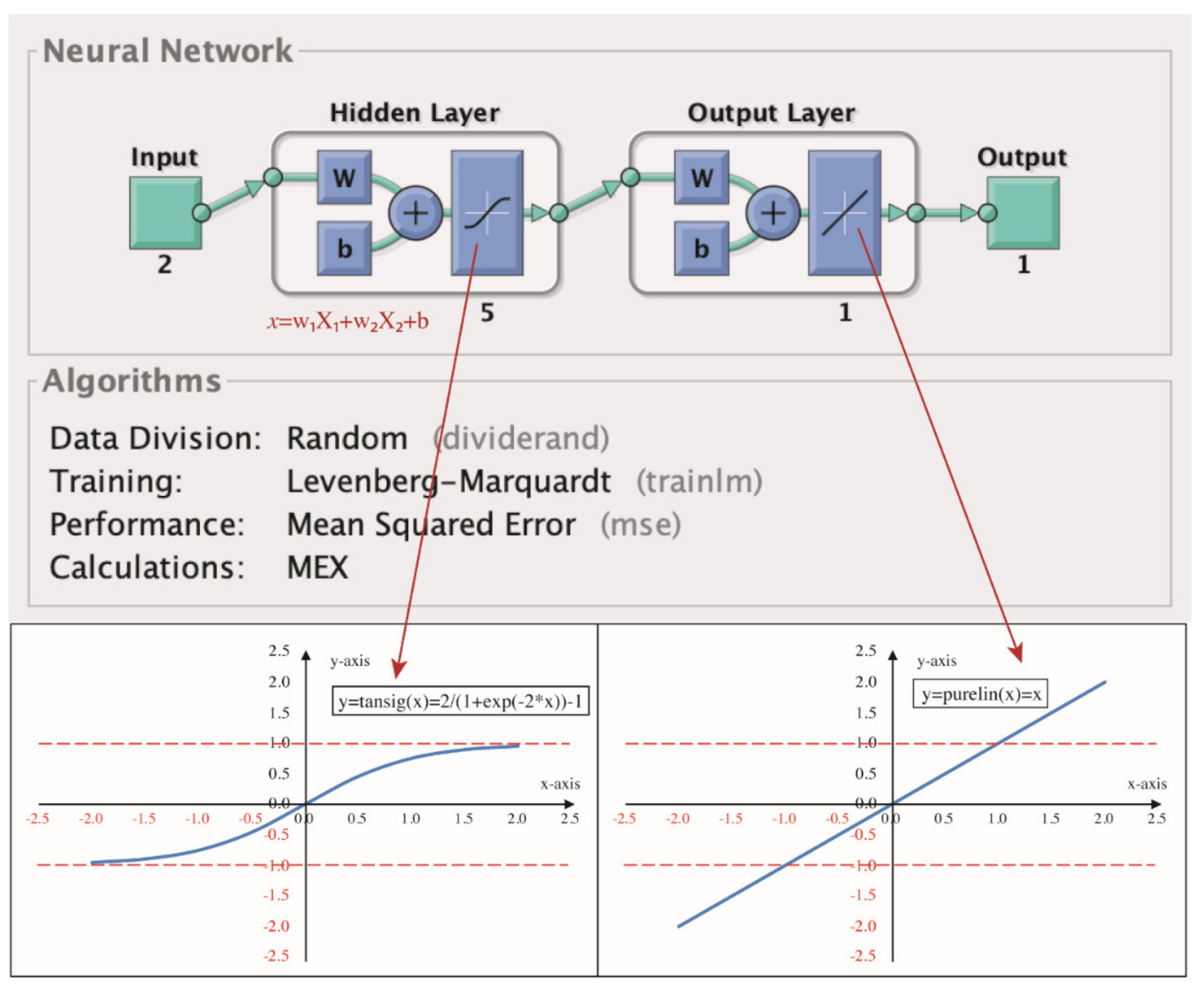
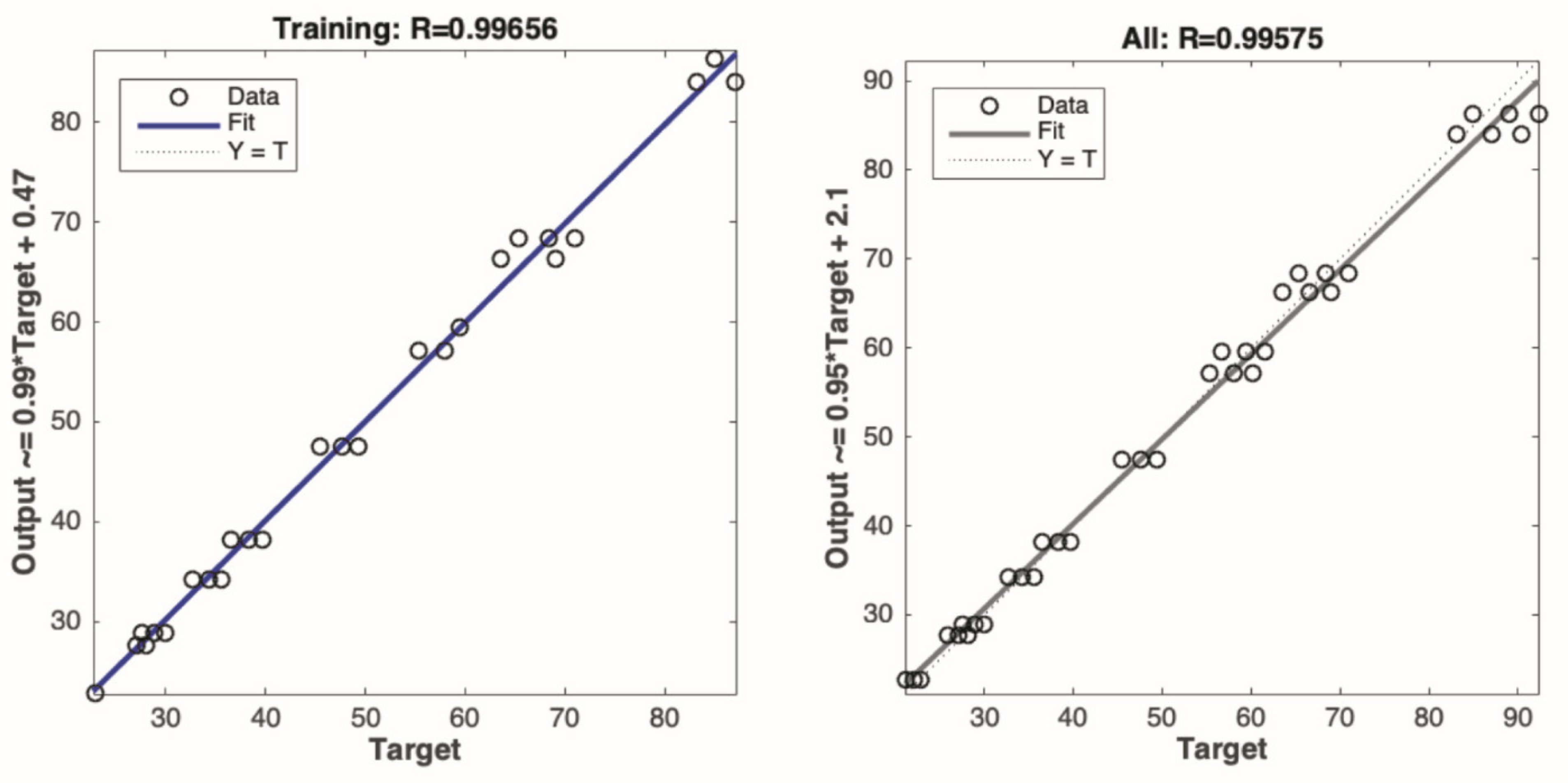
2 removal. The NT@RG, via its near-zero water absorption, compensates for defects (e.g., poor dimensional stability) of the mortar containing NT@RFA. The NT@RG is also targeted to introduce some external light into deep pores of the NT@RFA because of its light-transmitting property. A typical local resource (RCBP) is also developed and researched for its potential use in controlling ASR associated with NT@RG. Furthermore, the response surface methodology (RSM) and an artificial neural network (ANN), which are considered as two excellent models to optimize issues relating to multi-influence factors [
29], are used to predict the photocatalytic performance. RSM and ANN are promising modeling approaches since the photocatalytic process is typically characterized by various testing conditions and the associated changeable testing results. The initial concentration and the flow rate of the pollutant are two critical parameters in determining the final photocatalytic efficiency. This paper therefore co-uses RSM and ANN to predict the photocatalytic performance of photocatalytic mortar containing high-content construction wastes.