A Novel Tri-Coordination Zinc Complex Functionalized Silicotungstate with ROS Catalytic Ability and Anti-Tumor Cells Activity
Abstract
:1. Introduction
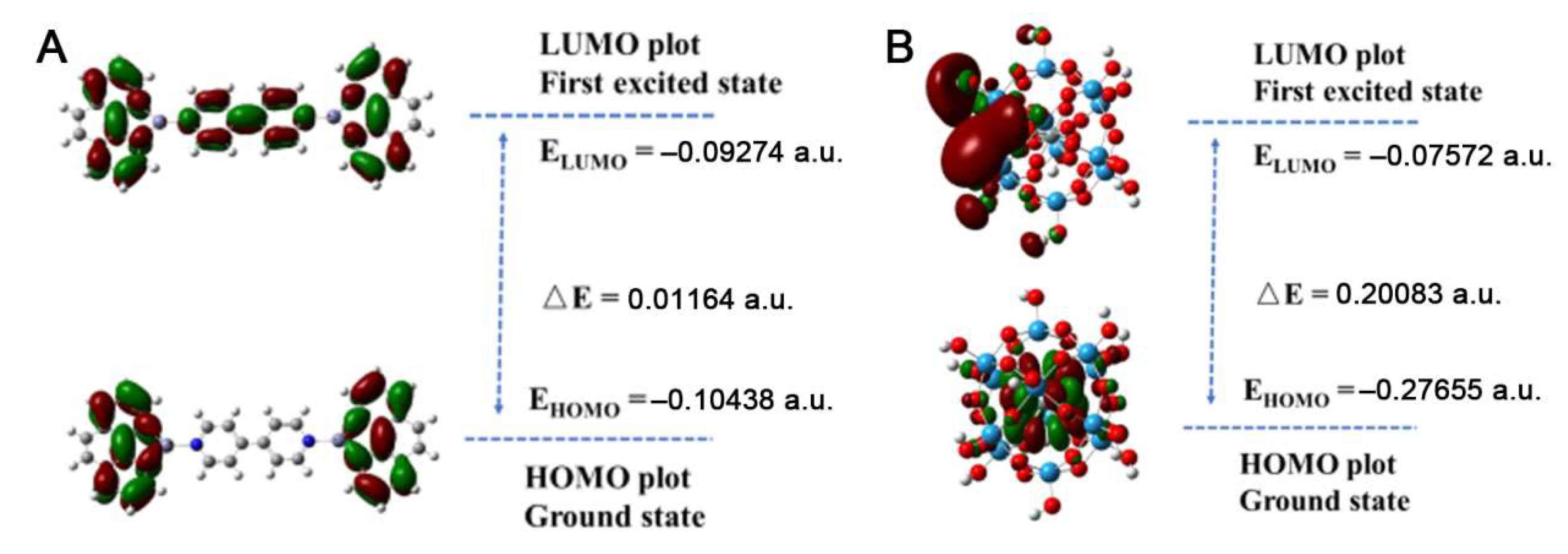
2. Results and Discussion
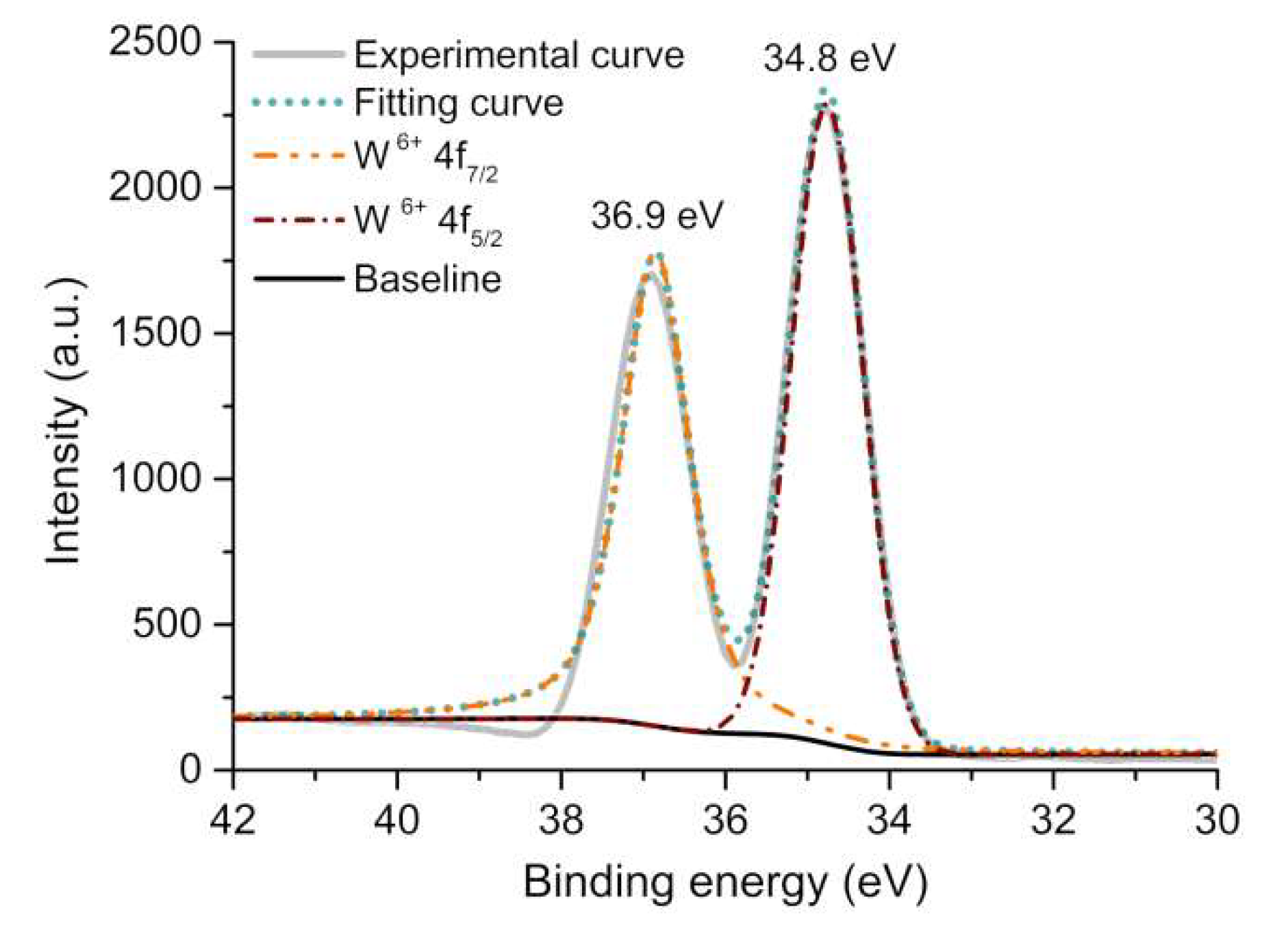
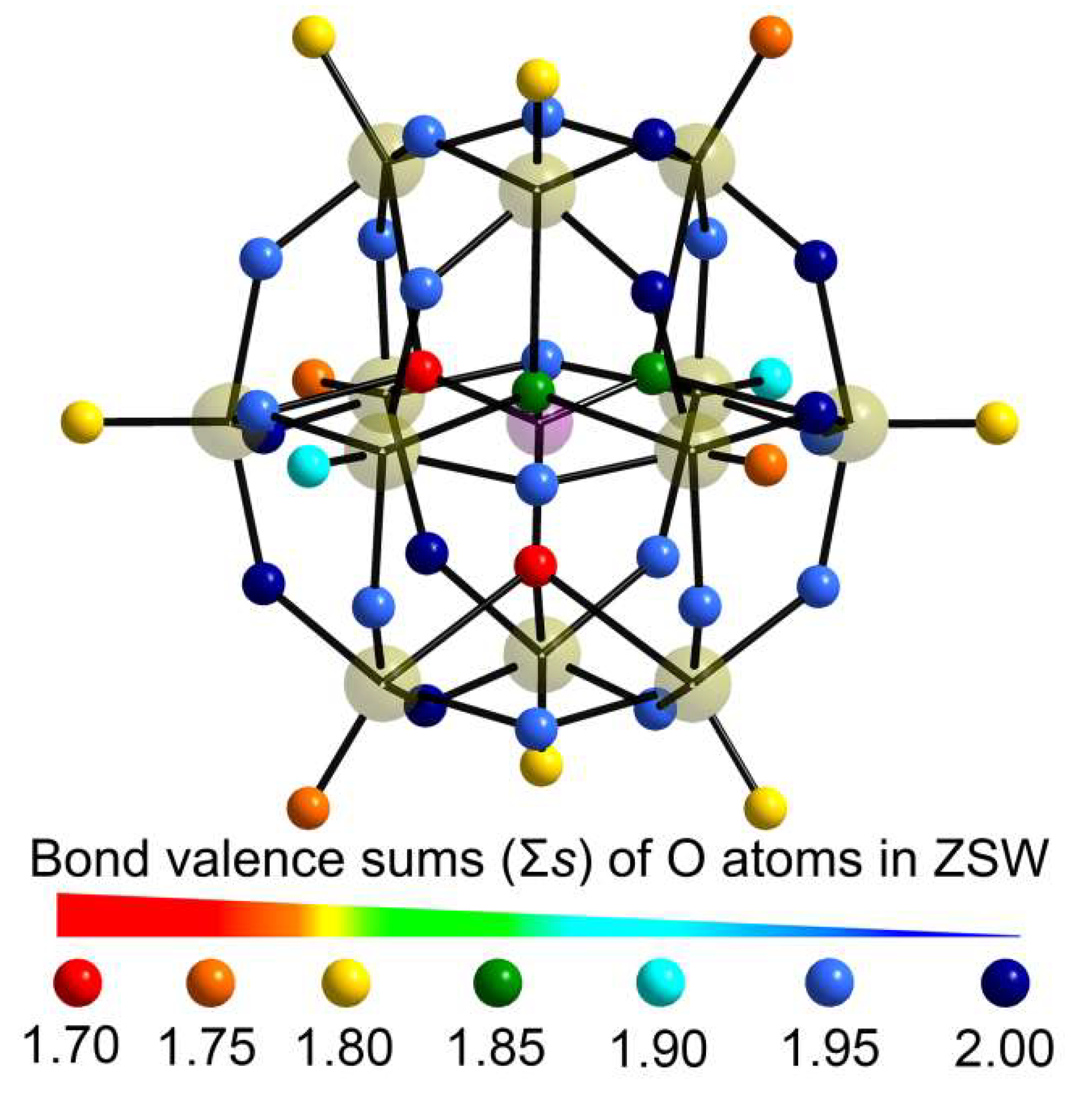
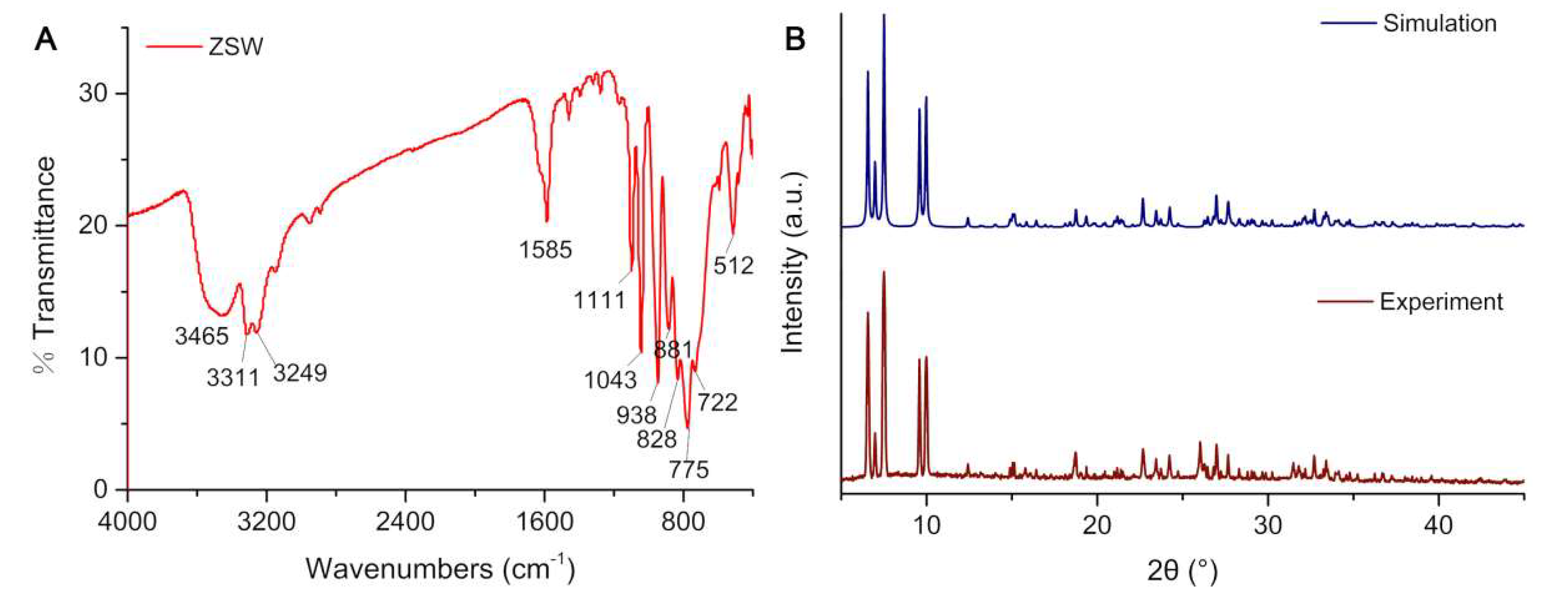
2.1. X-ray Single-Crystal Structures
2.2. Catalytic Property
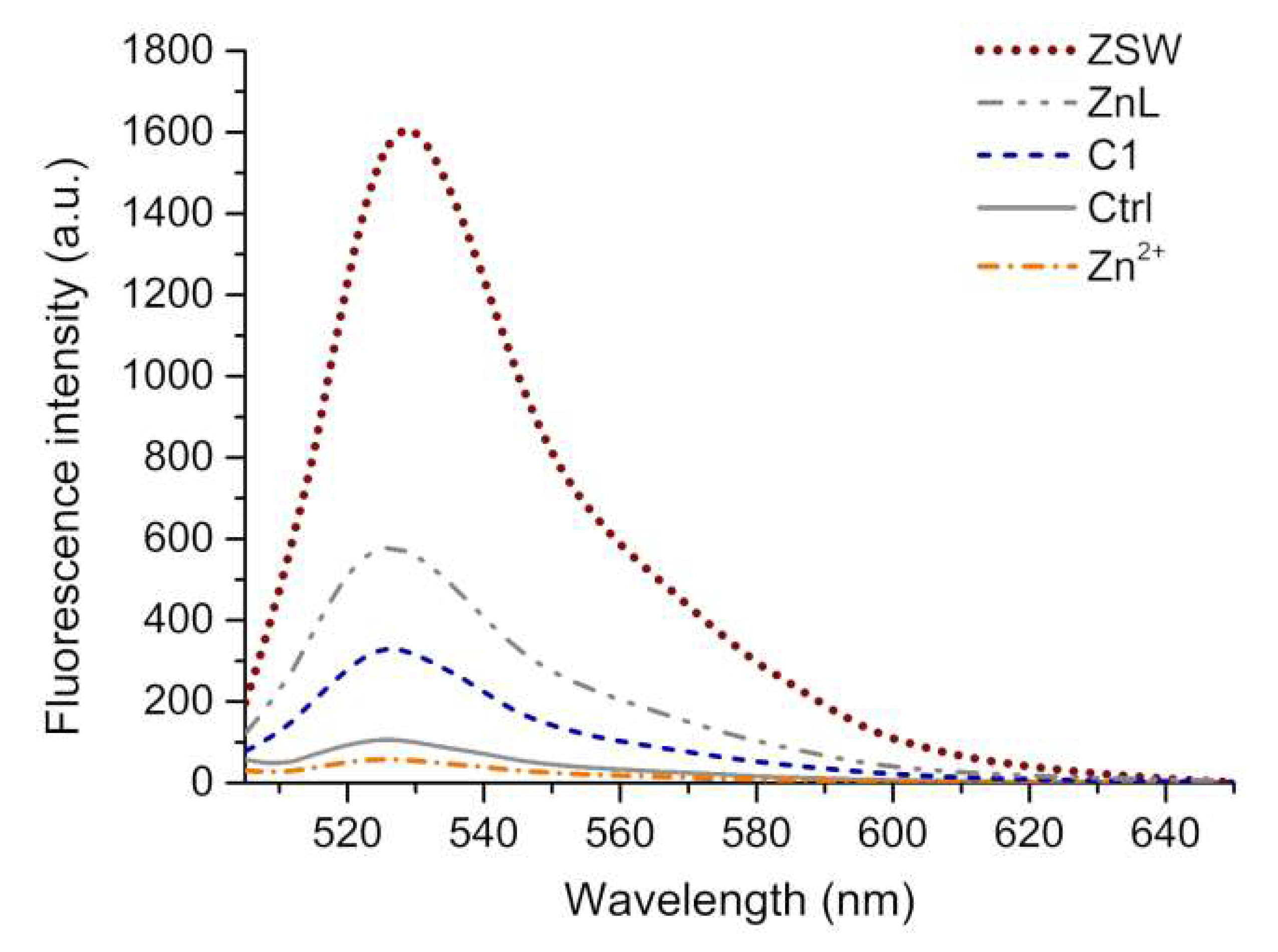
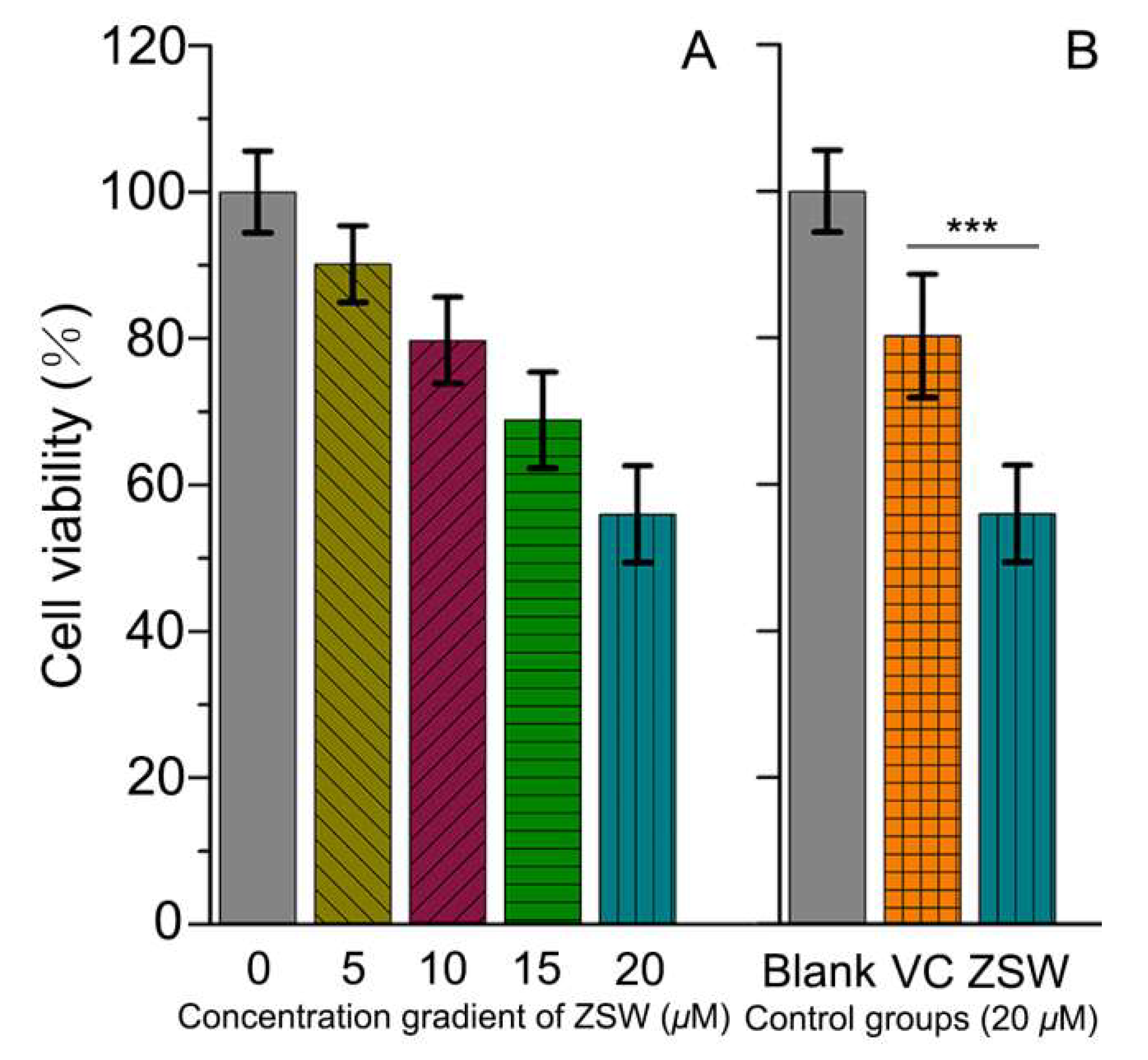
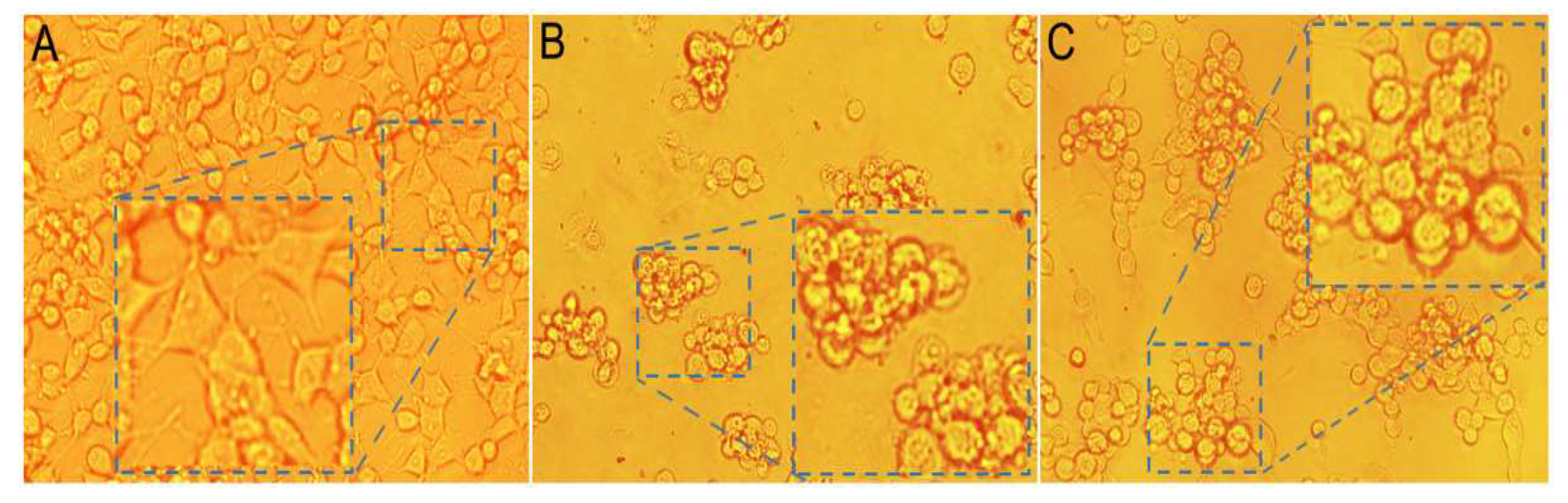
2.3. Anti-ROS-Sensitive Tumor Activity
3. Materials and Methods
3.1. Materials and Methods
3.2. Synthesis of ZSW
3.3. X-ray Data Collection and Structure Refinement
3.4. Catalytic ROS Production of ZSW
3.5. Anti-ROS Sensitive Tumor Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kepp, K.P. Bioinorganic chemistry of Alzheimer’s disease. Chem. Rev. 2012, 112, 5193–5239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.J.; Song, J.B.; Nie, L.M.; Chen, X.Y. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.W.; Ni, D.L.; Rosenkrans, Z.T.; Huang, P.; Yan, X.Y.; Cai, W.B. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.X.; Wang, X.Y.; Wang, Q.; Lou, Z.P.; Li, S.R.; Zhu, Y.Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [PubMed]
- Yao, Y.J.; Zhang, H.L.; Wang, Z.Y.; Ding, J.; Wang, S.Q.; Huang, B.Q.; Ke, S.F.; Gao, C.Y. Reactive oxygen species (ROS)-responsive biomaterials mediate tissue microenvironments and tissue regeneration. J. Mater. Chem. B 2019, 7, 5019–5037. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, L.C.; Symons, M.C.R. Electron spin resonance of haemoglobin and myoglobin. Chem. Soc. Rev. 1983, 12, 387–414. [Google Scholar] [CrossRef]
- Xu, L.H.; Ji, X.H.; Zhao, N.; Song, C.X.; Wang, F.S.; Liu, C.H. The conjugation of Cu/Zn superoxide dismutase (SOD) to O-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride (O-HTCC) enhances its therapeutic potential against radiationinduced oxidative damage. Polym. Chem. 2016, 7, 1826–1835. [Google Scholar] [CrossRef]
- Mishra, P.; Satpati, S.; Baral, S.K.; Dixit, A.; Sabat, S.C. S95C substitution in CuZn-SOD of ipomoea carnea: Impact on the structure, function and stability. Mol. Biosyst. 2016, 12, 3017–3031. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, C.; Hua, J.A.; Ma, P.T.; Wang, J.P.; Niu, J.Y. A binuclear copper-substituted phosphomolybdate with reactive oxygen species catalytic ability and antimicrobial activity. CrystEngComm 2019, 21, 394–398. [Google Scholar] [CrossRef]
- Hua, J.A.; Yuan, X.; Ma, X.; Ma, P.T.; Wang, J.P.; Niu, J.Y. A silver-substituted phosphomolybdate prevents the growth of bacteria without affecting the balance of reactive oxygen species. CrystEngComm 2020, 22, 7832–7837. [Google Scholar] [CrossRef]
- Ma, X.; Hua, J.A.; Xu, C.Z.; Zhang, L.M.; Wang, Y.Q.; Zhang, J.; Cao, L.H.; Niu, Y.L.; Ma, P.T. A heterogeneous catalyzed oxidase consists of zinc-substituted arsenomolybdate with reactive oxygen species catalytic ability. J. Clust. Sci. 2021. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, F.T.; Yue, H.; Hua, J.A.; Ma, P.T. A nano-linear zinc-substituted phosphomolybdate with reactive oxygen species catalytic ability and antibacterial activity. J. Mol. Struct. 2019, 1198, 126865–126867. [Google Scholar] [CrossRef]
- Du, D.Y.; Qin, J.S.; Li, S.L.; Su, Z.M.; Lan, Y.Q. Recent advances in porous polyoxometalate-based metal-organic framework materials. Chem. Soc. Rev. 2014, 43, 4615–4632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.L.; Lin, L.Q.; Sun, D.H.; Chen, H.M.; Yang, D.P.; Li, Q.B. Bio-inspired synthesis of metal nanomaterials and applications. Chem. Soc. Rev. 2015, 44, 6330–6374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.F.; Tsunashima, R. Recent advances on polyoxometalate-based molecular and composite materials. Chem. Soc. Rev. 2012, 41, 7384–7402. [Google Scholar] [CrossRef]
- Yang, K.; Ying, Y.X.; Cui, L.L.; Sun, J.C.; Luo, H.; Hu, Y.Y.; Zhao, J.W. Stable aqueous Zn−Ag and Zn−polyoxometalate hybrid battery driven by successive Ag+ cation and polyoxoanion redox reactions. Energy Storage Mater. 2021, 34, 203–210. [Google Scholar] [CrossRef]
- Gong, C.H.; Zeng, X.H.; Zhu, C.F.; Shu, J.H.; Xiao, P.X.; Xu, H.; Liu, L.C.; Zhang, J.Y.; Zeng, Q.D.; Xie, J.L. A series of organic–inorganic hybrid materials consisting of flexible organic amine modified polyoxomolybdates: Synthesis, structures and properties. RSC Adv. 2016, 6, 106248–106259. [Google Scholar] [CrossRef]
- Jin, H.J.; Zhou, B.B.; Yu, Y.; Zhao, Z.F.; Su, Z.H. Inorganic–organic hybrids constructed from heteropolymolybdate anions and copper–organic fragments: Syntheses, structures and properties. CrystEngComm 2011, 13, 585–590. [Google Scholar] [CrossRef]
- Weng, J.B.; Hong, M.C.; Liang, Y.C.; Shi, Q.; Cao, R. A nucleobase–inorganic hybrid polymer consisting of copper bis(phosphopentamolybdate) and cytosine. J. Chem. Soc. Dalton Trans. 2002, 3, 289–290. [Google Scholar] [CrossRef]
- Meng, J.X.; Lu, Y.; Li, Y.G.; Fu, H.; Wang, E.B. Controllable self-assembly of four new metal–organic frameworks based on different phosphomolybdate clusters by altering the molar ratio of H3PO4 and Na2MoO4. CrystEngComm 2011, 13, 2479–2486. [Google Scholar] [CrossRef]
- Xu, X.X.; Gao, X.; Lu, T.T.; Liu, X.X.; Wang, X.L. Hybrid material based on a coordination -complex-modified polyoxometalate nanorod (CC/POMNR) and PPy: A new visible light activated and highly efficient photocatalyst. J. Mater. Chem. A 2015, 3, 198–206. [Google Scholar] [CrossRef]
- Feng, S.L.; Lu, Y.; Zhang, Y.X.; Su, F.; Sang, X.J.; Zhang, L.C.; You, W.S.; Zhu, Z.M. Three new strandberg-type phenylphosphomolybdate supports for immobilizing horseradish peroxidase and their catalytic oxidation performances. Dalton Trans. 2018, 47, 14060–14069. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xue, Q.; Min, S.T.; Zhang, Y.P.; Hu, H.M.; Gao, S.L.; Xue, G.L. An unusual fan-type polyanion with a silver cation located at the axial center, [AgAsIII2(AsIIIAsVMo4O18(OH)2)3]11–. Dalton Trans. 2013, 42, 3410–3416. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Y.; Xu, L. Coordination assemblies of polyoxomolybdate cluster framework: From labile building blocks to stable functional materials. Dalton Trans. 2011, 40, 4024–4034. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Wang, Z.; Zhang, R.R.; Xu, L.Q.; Sun, D. A novel polyoxometalate-based hybrid containing a 2D [CoMo8O26]∞ structure as the anode for lithium-ion batteries. Chem. Commun. 2017, 53, 10560–10563. [Google Scholar] [CrossRef]
- Zhao, S.; Jia, Y.Q.; Song, Y.F. Acetalization of aldehydes and ketones over H4[SiW12O40] and H4[SiW12O40 ]/SiO2. Catal. Sci. Technol. 2014, 4, 2618–2625. [Google Scholar] [CrossRef]
- Sun, X.J.; Zhang, J.; Yuan, X.Z.; Fu, Z.Y. A silicotungstatebased copper–viologen hybrid photocatalytic compound for efficient degradation of organic dyes under visible light. CrystEngComm 2019, 21, 5563–5567. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Y.J.; Yuan, X.R.; Miao, Y.J.; Zhao, Q.; Hua, J.A.; Ma, P.T. An organic-inorganic hybrid nanoscale phosphotungstate with reactive oxygen species catalytic ability. Inorg. Nano-Met. Chem. 2021, 51, 332–339. [Google Scholar] [CrossRef]
- Hua, J.A.; Wei, X.M.; Bian, Y.J.; Ma, X.; Hao, L.; Sun, J.R.; Fan, J.J.; Niu, Y.L.; Wang, Y.Q. A nanoscale polymolybdate built by two hexavacant Keggin-type fragments via a novel {Ca6P6O38} cluster with β-sheet conformation modulation ability. CrystEngComm 2022, 24, 3153–3159. [Google Scholar] [CrossRef]
- Faller, P.; Hureau, C.; La Penna, G. Metal ions and intrinsically disordered proteins and peptides: From Cu/Zn amyloid-β to general principles. Acc. Chem. Res. 2014, 47, 2252–2259. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, Q.; Wang, B.; Li, D.N.; Zhou, Y.J.; Hua, J.A.; Ma, P.T. A hybrid silicotungstate based on tri-coordination copper complex and Keggin type cluster with reactive oxygen species catalytic ability. J. Mol. Struct. 2020, 1206, 127714–127716. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Hange, F.; Madarász, J.; Pokol, G. In situ HT-XRD study on the formation of hexagonal ammonium tungsten bronze by partial reduction of ammonium paratungstate tetrahydrate. Eur. J. Inorg. Chem. 2006, 17, 3413–3418. [Google Scholar] [CrossRef]
- Zheng, S.T.; Zhang, J.; Juan, J.M.; Yuan, D.Q.; Yang, G.Y. Poly(polyoxotungstate)s with 20 nickel centers: From nanoclusters to one-dimensional chains. Angew. Chem. Int. Ed. Engl. 2009, 48, 7176–7179. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Crystallogr. B Struct. Sci. 1985, 41, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Shields, G.P.; Raithby, P.R.; Allen, F.H.; Motherwell, W.D.S. The assignment and validation of metal oxidation states in the Cambridge structural database. Acta Crystallogr. B Struct. Sci. 2000, 56, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Hua, J.A.; Wang, K.; Zhang, H.M.; Zhang, C.L.; He, Y.F.; Guo, Z.J.; Wang, X.Y. Modulating conformation of Aβ peptide: An effective way to prevent protein-misfolding disease. Inorg. Chem. 2018, 57, 13533–13543. [Google Scholar] [CrossRef]
- Streb, C.; Ritchie, C.; Long, D.L.; Kögerler, P.; Cronin, L. Modular assembly of a functional polyoxometalate-based open framework constructed from unsupported AgI–AgI interactions. Angew. Chem. Int. Ed. Engl. 2007, 46, 7579–7582. [Google Scholar] [CrossRef]
- Ma, X.; Bian, Y.J.; Zhou, Y.J.; Zhao, Q.; Tian, Y.; Hua, J.A.; Ma, P.T. Synthesis, characterization, and catalytic property of a hybrid nanoscale polyoxoniobate. J. Clust. Sci. 2021, 32, 613–620. [Google Scholar] [CrossRef]
- MacDonald, L.; Rausch, B.; Symes, M.D.; Cronin, L. Selective hydrogenation of nitroarenes using an electrogenerated polyoxometalate redox mediator. Chem. Commun. 2018, 54, 1093–1096. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.T.; Yuan, D.Q.; Zhang, J.; Yang, G.Y. Combination of lacunary polyoxometalates and high-nuclear transition metal clusters under hydrothermal conditions. 3. Structure and characterization of [Cu(enMe)2]2{[Cu(enMe)2(H2O)]2[Cu6(enMe)2(B-α-SiW9O34)2]}·4H2O. Inorg. Chem. 2007, 46, 4569–4574. [Google Scholar] [CrossRef]
- Wang, L.M.; Wang, Y.; Fan, Y.; Xiao, L.N.; Hu, Y.Y.; Gao, Z.M.; Zheng, D.F.; Cui, X.B.; Xu, J.Q. The design, syntheses and characterization of a series of hybrids based on polyoxometalates and metal complexes. CrystEngComm 2014, 16, 430–440. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, X.Y.; Zhang, C.L.; Jiao, Y.; Guo, Z.J. Inhibitory action of macrocyclic platiniferous chelators on metalinduced Aβ aggregation. Chem. Sci. 2012, 3, 1304–1312. [Google Scholar] [CrossRef]
- Zhao, J.S.; Wang, Y.; Zhou, J.W.; Qi, P.F.; Li, S.W.; Zhang, K.X.; Feng, X.; Wang, B.; Hu, C.W. A copper(ii)-based MOF film for highly efficient visible-light-driven hydrogen production. J. Mater. Chem. A 2016, 4, 7174–7177. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision D. Available online: https://gaussian.com/ (accessed on 29 April 2022).
- Padmanabhan, J.; Parthasarathi, R.; Subramanian, V.; Chattaraj, P.K. Group philicity and electrophilicity as possible descriptors for modeling ecotoxicity applied to chlorophenols. Chem. Res. Toxicol. 2006, 19, 356–364. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Han, Q.Q.; Zhang, H.M.; Yan, Y.Y. Evaluation of the binding interactions of p-acetylaminophenol, aspirin, ibuprofen and aminopyrine with norfloxacin from the view of antipyretic and anti-inflammatory. J. Mol. Liq. 2020, 312, 113397. [Google Scholar] [CrossRef]
- Hua, J.A.; Wei, X.M.; Ma, X.; Jiao, J.Z.; Chai, B.H.; Wu, C.B.; Zhang, C.L.; Niu, Y.L. A {Cd4Cl2O14} cluster functionalized sandwich-type tungstoarsenate as a conformation modulator for misfolding Aβ peptides. CrystEngComm 2022, 24, 1171–1176. [Google Scholar] [CrossRef]
- Liu, J.C.; Wang, J.F.; Han, Q.; Shangguan, P.; Liu, L.L.; Chen, L.J.; Zhao, J.W.; Streb, C.; Song, Y.F. Multicomponent self-assembly of a giant heterometallic polyoxotungstate supercluster with antitumor activity. Angew. Chem. Int. Ed. 2021, 60, 11153–11157. [Google Scholar] [CrossRef]
- Bruker S.M.A.R.T. SAINT (Version 6.02); Bruker AXS Inc.: Madison, WI, USA, 2000. [Google Scholar]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. B Struct. Sci. 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHEXTL-97, Programs for Crystal Structure Refinements; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Ma, X.; Wang, Y.Q.; Hua, J.A.; Xu, C.Y.; Yang, T.; Yuan, J.; Chen, G.Q.; Guo, Z.J.; Wang, X.Y. A β-sheet-targeted theranostic agent for diagnosing and preventing aggregation of pathogenic peptides in Alzheimer’s disease. Sci. China Chem. 2020, 63, 73–82. [Google Scholar] [CrossRef]

| Empirical Formula | C68H48N12O40SiW12Zn4 |
|---|---|
| Formula weight | 4168.95 |
| Crystal system | Orthorhombic |
| Space group | Ibam |
| a/Å | 13.297(3) |
| b/Å | 25.304(5) |
| c/Å α/deg | 26.922(5) 90 |
| β/deg γ/deg | 90 90 |
| V/Å3 | 9058(3) |
| Z | 4 |
| Dc/g cm–3 | 3.057 |
| μ/mm–1 | 16.308 |
| T/K | 296(2) |
| Limiting indices | –15 ≤ h ≤ 15 |
| –25≤ k ≤ 30 | |
| –32 ≤ l ≤ 28 | |
| Measured reflections | 22599 |
| Independent reflections | 4084 |
| Rint | 0.0403 |
| Data/restraints/parameters | 4084/12/326 |
| GOF on F2 | 1.051 |
| Final R indices (I > 2σ(I)) | R1 = 0.0310 |
| wR2 = 0.0956 | |
| R indices (all data) | R1 = 0.0415 |
| wR2 = 0.1019 |
| Bond | Valence | Bond | Valence | Bond | Valence | Atom | Σs |
|---|---|---|---|---|---|---|---|
| Si (1)-O (4) | 0.9246 (1) | Si (1)-O (8) | 1.1293 (2) | Si (1)-O (4)# | 0.9246 (1) | ||
| Si (1)-O (8)# | 1.1293 (2) | Si (1) | 4.1078 (8) | ||||
| W (1)-O (1) W (1)-O (3)# W (2)-O (3) W (2)-O (6) W (3)-O (7) W (3)-O (8)# W (4)-O (2)# W (4)-O (10) | 1.8122 (9) 1.0000 (0) 1.0357 (5) 1.0844 (5) 1.0218 (5) 0.2762 (3) 1.0218 (5) 1.0583 (9) | W (1)-O (2) W (1)-O (2)# W (2)-O (8) W (2)-O (7) W (3)-O (7)# W (3)-O (10) W (4)-O (4)# W (4)-O (11) | 1.0027 (1) 1.0027 (1) 0.2165 (9) 1.0136 (1) 1.0218 (5) 0.9602 (7) 0.2868 (9) 1.8122 (9) | W (1)-O (3) W (1)-O (4) W (2)-O (5) W (2)-O (12)# W (3)-O (8) W (3)-O (10)# W (4)-O (6)# W (4)-O (12) | 1.0000 (0) 0.2393 (7) 1.7928 (1) 1.0246 (2) 0.2762 (3) 0.9602 (7) 1.0218 (5) 1.0413 (7) | W (1) W (2) W (3) W (4) | 6.0570 (1) 6.2320 (1) 6.1277 (7) 6.2426 (7) |
| Zn (1)-N (1) | 0.5185 (3) | Zn (1)-N (2) | 0.7191 (1) | Zn (1)-N (3) | 0.4965 (8) | Zn (1) | 1.7342 (3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Hua, J.; Wang, M.; Zhang, D.; Pei, X.; Zhao, X.; Niu, Y.; Wang, Y. A Novel Tri-Coordination Zinc Complex Functionalized Silicotungstate with ROS Catalytic Ability and Anti-Tumor Cells Activity. Catalysts 2022, 12, 695. https://doi.org/10.3390/catal12070695
Ma X, Hua J, Wang M, Zhang D, Pei X, Zhao X, Niu Y, Wang Y. A Novel Tri-Coordination Zinc Complex Functionalized Silicotungstate with ROS Catalytic Ability and Anti-Tumor Cells Activity. Catalysts. 2022; 12(7):695. https://doi.org/10.3390/catal12070695
Chicago/Turabian StyleMa, Xiang, Jiai Hua, Man Wang, Deqiang Zhang, Xinyao Pei, Xiaoyu Zhao, Yulan Niu, and Yanqing Wang. 2022. "A Novel Tri-Coordination Zinc Complex Functionalized Silicotungstate with ROS Catalytic Ability and Anti-Tumor Cells Activity" Catalysts 12, no. 7: 695. https://doi.org/10.3390/catal12070695
APA StyleMa, X., Hua, J., Wang, M., Zhang, D., Pei, X., Zhao, X., Niu, Y., & Wang, Y. (2022). A Novel Tri-Coordination Zinc Complex Functionalized Silicotungstate with ROS Catalytic Ability and Anti-Tumor Cells Activity. Catalysts, 12(7), 695. https://doi.org/10.3390/catal12070695







