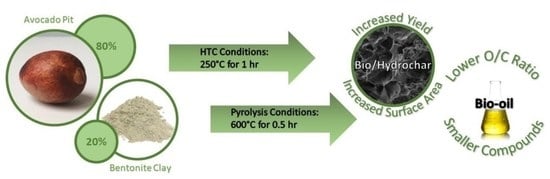
Impact of Bentonite Clay on In Situ Pyrolysis vs. Hydrothermal Carbonization of Avocado Pit Biomass
Abstract
:1. Introduction
2. Results and Discussion
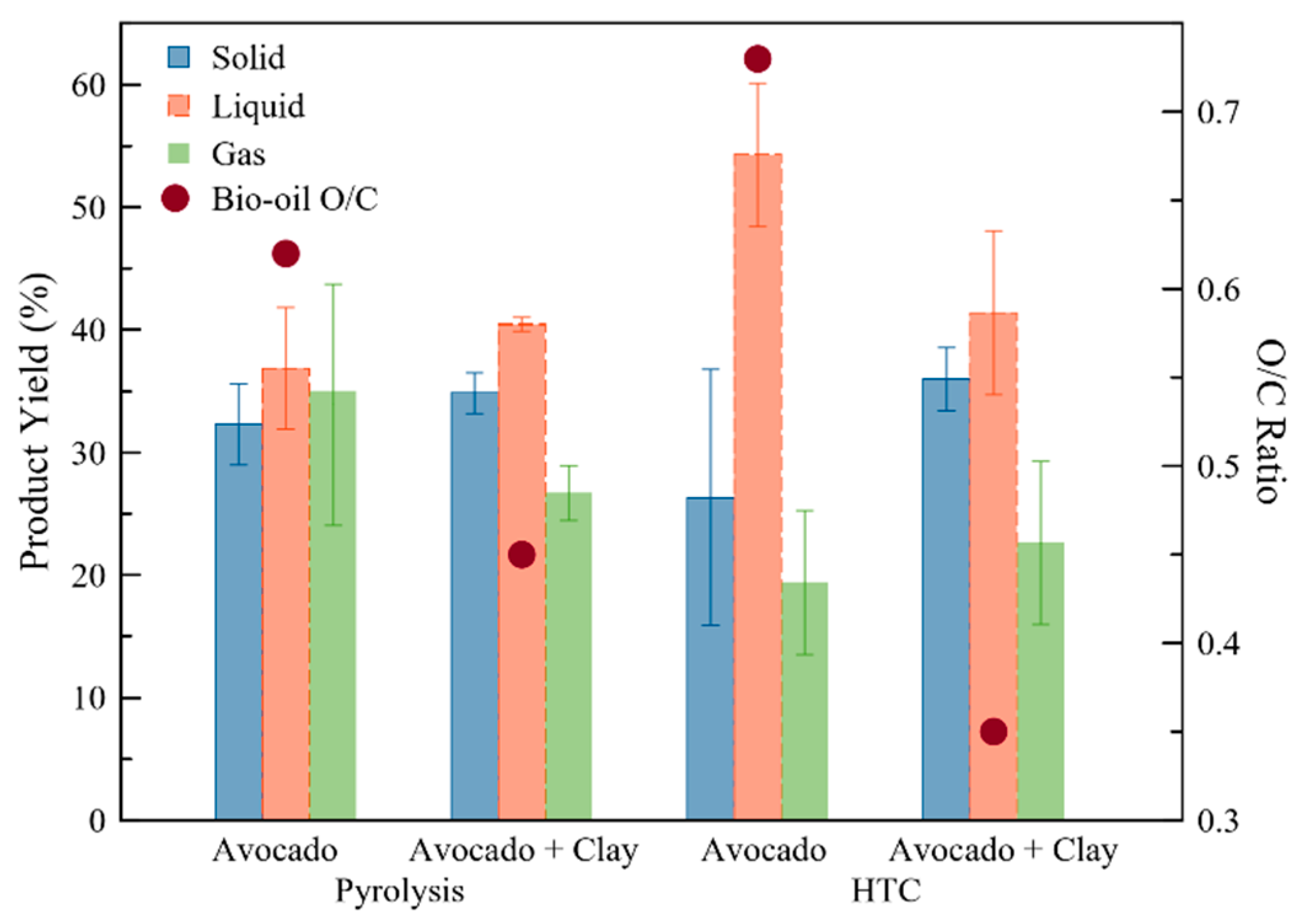
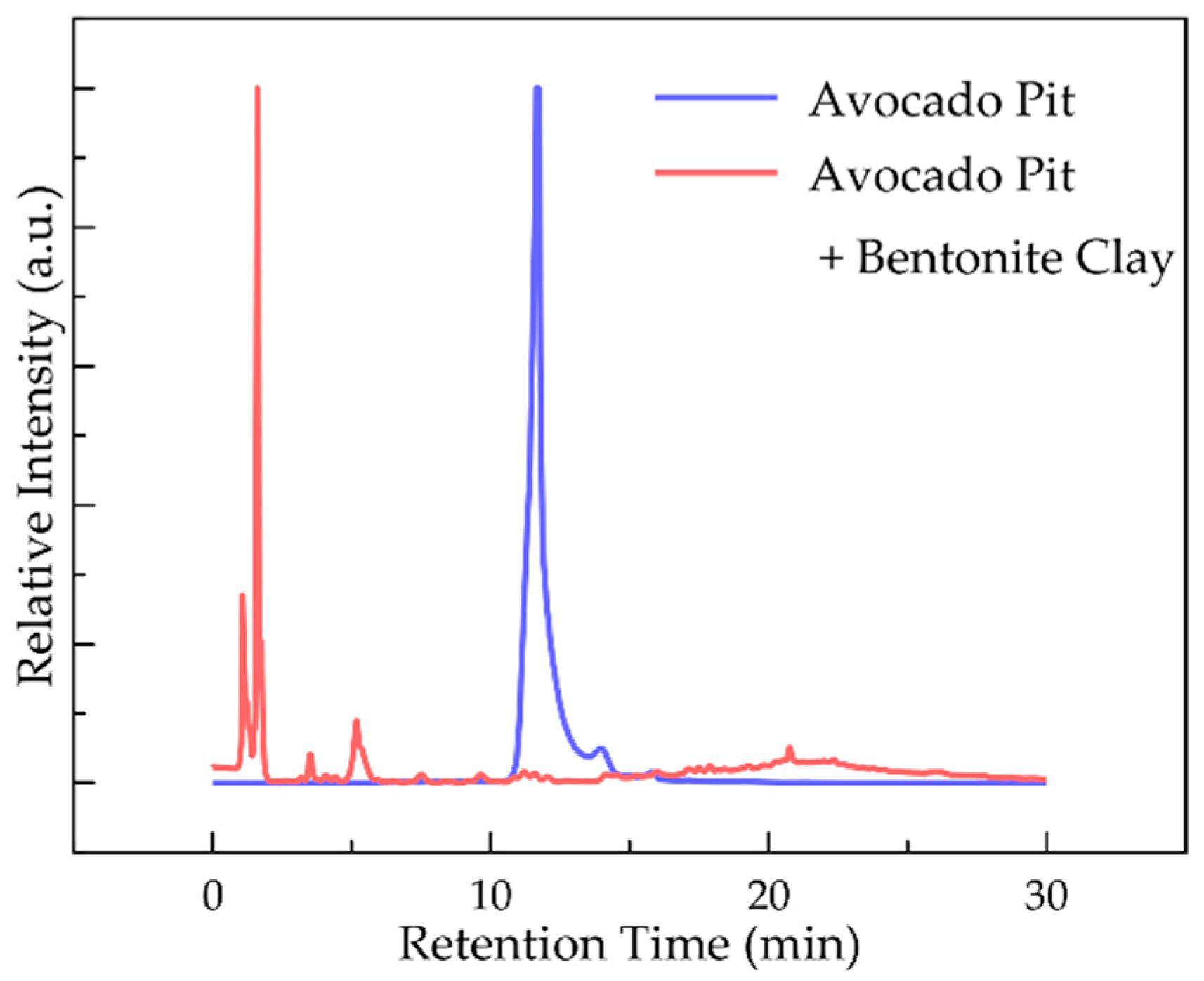
2.1. Bio-Oils and Biogas from Pyrolysis and Hydrothermal Carbonization
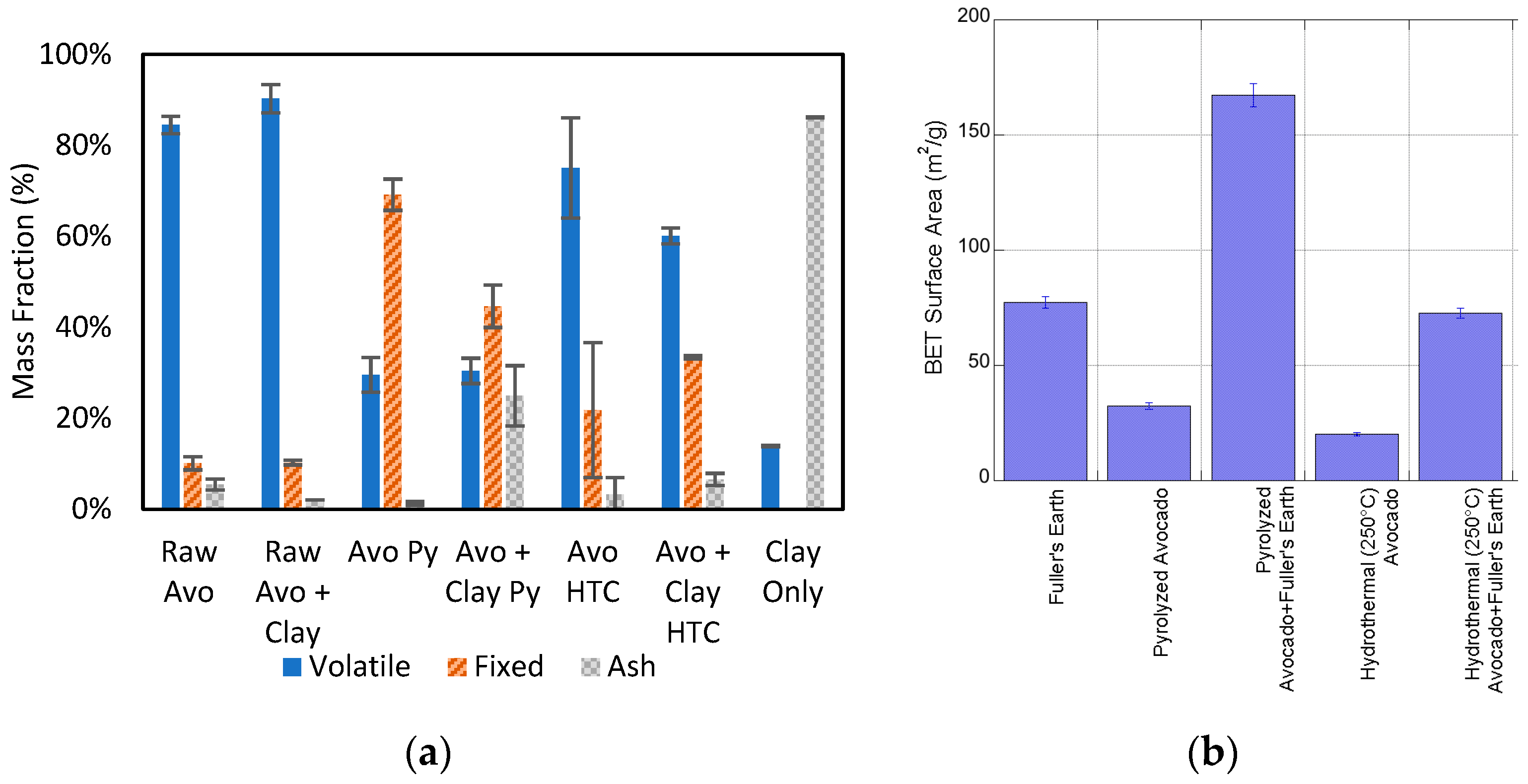
2.2. Characterization of Bio- and Hydrochar
2.3. Summary: Impact of Bentonite on Pyrolysis and Hydrothermal Carbonization
3. Materials and Methods
3.1. Materials
3.2. Pyrolytic Conversion of Biomass
3.3. Hydrothermal Conversion of Biomass
3.4. Analysis of Condensable Bio-Oils and Liquid Phase of HTC
3.5. Characterization of the Resulting Biochars and Hydrochars
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ruhul Kabir, M.; Kumar, A. Comparison of the energy and environmental performances of nine biomass/coal co-firing pathways. Bioresour. Technol. 2012, 124, 394–405. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Goal 14 | Department of Economic and Social Affairs. Available online: https://sdgs.un.org/goals/goal14 (accessed on 7 June 2022).
- Pham, T.N.; Shi, D.; Resasco, D.E. Environmental Evaluating strategies for catalytic upgrading of pyrolysis oil in liquid phase. Appl. Catal. B Environ. 2014, 145, 10–23. [Google Scholar] [CrossRef]
- Mante, O.D.; Agblevor, F.A. Catalytic pyrolysis for the production of refinery-ready biocrude oils from six different biomass sources. Green Chem. 2014, 16, 3364. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Poornima, D.T.; Sivashanmugam, P.; Kavitha, S.; Yukesh, K.R.; Varjani, S.; AdishKumar, S.; Kumar, G.; Banu, R.J. Lignocellulosic biomass-based pyrolysis: A comprehensive review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef]
- Lyu, G.; Wu, S.; Zhang, H. Estimation and Comparison of Bio-Oil Components from Different Pyrolysis Conditions. Front. Energy Res. 2015, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Yang, Z.; Hse, C.; Su, Q.; Wang, K.; Jiang, J.; Xu, J. In situ catalytic hydrogenation of model compounds and biomass-derived phenolic compounds for bio-oil upgrading. Renew. Energy 2017, 105, 140–148. [Google Scholar] [CrossRef]
- He, J.; Kunitake, T.; Nakao, A. Facile In Situ Synthesis of Noble Metal Nanoparticles in Porous Cellulose Fibers. Chem. Mater. 2003, 15, 4401–4406. [Google Scholar] [CrossRef]
- Iliopoulou, E.; Stefanidis, S.; Kalogiannis, K. Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Appl. Catal. B 2012, 127, 281–290. [Google Scholar] [CrossRef]
- Gökdai, Z.; Sınağ, A.; Yumak, T. Comparison of the catalytic efficiency of synthesized nano tin oxide particles and various catalysts for the pyrolysis of hazelnut shell. Biomass Bioenergy 2010, 34, 402–410. [Google Scholar] [CrossRef]
- Li, J.; Yan, R.; Xiao, B.; Liang, D.; Lee, D. Preparation of Nano-NiO Particles and Evaluation of Their Catalytic Activity in Pyrolyzing Biomass Components†. Energy Fuels 2007, 333, 16–23. [Google Scholar] [CrossRef]
- Ischia, M.; Maschio, R.D.; Grigiante, M.; Baratieri, M. Clay-sewage sludge co-pyrolysis. A TG-MS and Py-GC study on potential advantages afforded by the presence of clay in the pyrolysis of wastewater sewage sludge. Waste Manag. 2011, 31, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Dou, G.; Goldfarb, J.J.L. In situ upgrading of pyrolysis biofuels by bentonite clay with simultaneous production of heterogeneous adsorbents for water treatment. Fuel 2017, 195, 273–283. [Google Scholar] [CrossRef]
- De Resende, E.C.; Gissane, C.; Nicol, R.; Heck, R.J.; Guerreiro, M.C.; Coelho, J.V.; De Oliveira, L.C.A.; Palmisano, P.; Berruti, F.; Briens, C.; et al. Synergistic co-processing of Red Mud waste from the Bayer process and a crude untreated waste stream from bio-diesel production. Green Chem. 2013, 15, 496. [Google Scholar] [CrossRef]
- Ooi, X.Y.; Gao, W.; Ong, H.C.; Lee, H.V.; Juan, J.C.; Chen, W.H.; Lee, K.T. Overview on catalytic deoxygenation for biofuel synthesis using metal oxide supported catalysts. Renew. Sustain. Energy Rev. 2019, 112, 834–852. [Google Scholar] [CrossRef]
- Oi, L.E.; Choo, M.Y.; Lee, H.V.; Ong, H.C.; Hamid, S.B.A.; Juan, J.C. Recent advances of titanium dioxide (TiO2) for green organic synthesis. RSC Adv. 2016, 6, 108741–108754. [Google Scholar] [CrossRef]
- Wu, L.M.; Zhou, C.H.; Tong, D.S.; Yu, W.H.; Wang, H. Novel hydrothermal carbonization of cellulose catalyzed by montmorillonite to produce kerogen-like hydrochar. Cellulose 2014, 21, 2845–2857. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Tompsett, G.A.; Murphy, C.M.; Maag, A.R.; Carabillo, N.; Bailey, M.; Hemingway, J.J.; Romo, C.I.; Paulsen, A.D.; Yelvington, P.E.; et al. Synergistic Effects of Inexpensive Mixed Metal Oxides for Catalytic Hydrothermal Liquefaction of Food Wastes. ACS Sustain. Chem. Eng. 2020, 8, 6877–6886. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, K.; Sudibyo, H.; Tester, J.W.; Huang, G.; Han, L.; Goldfarb, J.L. Production of upgraded biocrude from hydrothermal liquefaction using clays as in situ catalysts. Energy Convers. Manag. 2021, 247, 114764. [Google Scholar] [CrossRef]
- Rahman, T.; Jahromi, H.; Roy, P.; Adhikari, S.; Hassani, E.; Oh, T.S. Hydrothermal liquefaction of municipal sewage sludge: Effect of red mud catalyst in ethylene and inert ambiences. Energy Convers. Manag. 2021, 245, 114615. [Google Scholar] [CrossRef]
- Maged, A.; Kharbish, S.; Ismael, I.S.; Bhatnagar, A. Characterization of activated bentonite clay mineral and the mechanisms underlying its sorption for ciprofloxacin from aqueous solution. Environ. Sci. Pollut. Res. Int. 2020, 27, 32980. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.; Yuan, P.; Liu, H.; Liu, D.; Liu, J.; He, H.; Zhou, J.; Song, H.; Li, Z. Effects of complexation between organic matter (OM) and clay mineral on OM pyrolysis. Geochim. Cosmochim. Acta 2017, 212, 1–15. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Gokhale, D.V. Lignocellulosic biomass: Hurdles and challenges in its valorization. Appl. Microbiol. Biotechnol. 2019, 103, 9305–9320. [Google Scholar] [CrossRef] [PubMed]
- Işıtan, S.; Ceylan, S.; Topcu, Y.; Hintz, C.; Tefft, J.; Chellappa, T.; Guo, J.; Goldfarb, J.L.; Istan, S.; Ceylan, S.; et al. Product quality optimization in an integrated biorefinery: Conversion of pistachio nutshell biomass to biofuels and activated biochars via pyrolysis. Energy Convers. Manag. 2016, 127, 576–588. [Google Scholar] [CrossRef] [Green Version]
- Dehkhoda, A.M.; Gyenge, E.; Ellis, N. A novel method to tailor the porous structure of KOH-activated biochar and its application in capacitive deionization and energy storage. Biomass Bioenergy 2016, 87, 107–121. [Google Scholar] [CrossRef] [Green Version]
- Linares-Solano, A.; Lillo-Ródenas, M. NaOH and KOH for preparing activated carbons used in energy and environmental applications. J. Energy 2012, 20, 59–91. [Google Scholar]
- Gao, Y.; Yue, Q.; Gao, B.; Sun, Y.; Wang, W.; Li, Q.; Wang, Y. Comparisons of porous, surface chemistry and adsorption properties of carbon derived from Enteromorpha prolifera activated by H4P2O7 and KOH. Chem. Eng. J. 2013, 232, 582–590. [Google Scholar] [CrossRef]
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Hu, X.; Wang, X.; Liu, S.; Jiang, L. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Goldfarb, J.L.; Buessing, L.; Gunn, E.; Lever, M.; Billias, A.; Casoliba, E.; Schievano, A.; Adani, F. Novel Integrated Biorefinery for Olive Mill Waste Management: Utilization of Secondary Waste for Water Treatment. ACS Sustain. Chem. Eng. 2017, 5, 876–884. [Google Scholar] [CrossRef]
- Ramola, S.; Belwal, T.; Li, C.J.; Wang, Y.Y.; Lu, H.H.; Yang, S.M.; Zhou, C.H. Improved lead removal from aqueous solution using novel porous bentonite - and calcite-biochar composite. Sci. Total Environ. 2020, 709, 136171. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.L.; Zhou, C.H.; Yang, H.M.; Ji, S.F.; Tong, D.S.; Zhong, Z.K.; Yu, W.H.; Chu, M.Q. Environmental-friendly montmorillonite-biochar composites: Facile production and tunable adsorption-release of ammonium and phosphate. J. Clean. Prod. 2017, 156, 648–659. [Google Scholar] [CrossRef]
- Zelaya Soulé, M.E.; Fernández, M.A.; Montes, M.L.; Suárez-García, F.; Torres Sánchez, R.M.; Tascón, J.M.D. Montmorillonite- hydrothermal carbon nanocomposites: Synthesis, characterization and evaluation of pesticides retention for potential treatment of agricultural wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124192. [Google Scholar] [CrossRef]
- Wu, X.; Gao, P.; Zhang, X.; Jin, G.; Xu, Y.; Wu, Y. Synthesis of clay/carbon adsorbent through hydrothermal carbonization of cellulose on palygorskite. Appl. Clay Sci. 2014, 95, 60–66. [Google Scholar] [CrossRef]
- Sarkar, B.; Liu, E.; McClure, S.; Sundaramurthy, J.; Srinivasan, M.; Naidu, R. Biomass derived palygorskite-carbon nanocomposites: Synthesis, characterisation and affinity to dye compounds. Appl. Clay Sci. 2015, 114, 617–626. [Google Scholar] [CrossRef]
- “Avocados”. Agricultural Marketing Resource Center, Iowa State University. Available online: https://www.agmrc.org/commodities-products/fruits/avocados (accessed on 7 June 2022).
- Alvarez, F. “Here’s the Scoop on Guacamole”. Los Angeles Times. Available online: https://www.latimes.com/archives/la-xpm-2001-dec-06-me-12000-story.html (accessed on 7 June 2022).
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Sangaré, D.; Moscosa-Santillan, M.; Antonio, P.A.; Bostyn, S.; Belandria, V.; Gökalp, I. Hydrothermal carbonization of biomass: Experimental study, energy balance, process simulation, design, and techno-economic analysis. Biomass Convers. Biorefin. 2022, 1–16. [Google Scholar] [CrossRef]
- Wüst, D.; Correa, C.R.; Jung, D.; Zimmermann, M.; Kruse, A.; Fiori, L. Understanding the influence of biomass particle size and reaction medium on the formation pathways of hydrochar. Biomass Convers. Biorefin. 2020, 10, 1357–1380. [Google Scholar] [CrossRef]
- Hubble, A.H.; Goldfarb, J.L. Synergistic effects of biomass building blocks on pyrolysis gas and bio-oil formation. J. Anal. Appl. Pyrolysis 2021, 156, 105100. [Google Scholar] [CrossRef]
- Louden, M. Reduction of Aldehydes and Ketones to Alcohols. In Organic Chemistry; Pearson: London, UK, 2015; p. 926. ISBN 1936221349. [Google Scholar]
- Carriazo, J.G.; Centeno, M.A.; Odriozola, J.A.; Moreno, S.; Molina, R. Effect of Fe and Ce on Al-pillared bentonite and their performance in catalytic oxidation reactions. Appl. Catal. A Gen. 2007, 317, 120–128. [Google Scholar] [CrossRef]
- Hubble, A.H.; Ryan, E.M.; Goldfarb, J.L. Enhancing pyrolysis gas and bio-oil formation through transition metals as in situ catalysts. Fuel 2022, 308, 121900. [Google Scholar] [CrossRef]
- Negahdar, L.; Gonzalez-Quiroga, A.; Otyuskaya, D.; Toraman, H.E.; Liu, L.; Jastrzebski, J.T.B.H.; Van Geem, K.M.; Marin, G.B.; Thybaut, J.W.; Weckhuysen, B.M. Characterization and Comparison of Fast Pyrolysis Bio-oils from Pinewood, Rapeseed Cake, and Wheat Straw Using 13C NMR and Comprehensive GC × GC. ACS Sustain. Chem. Eng. 2016, 4, 4974–4985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacio Lozano, D.C.; Jones, H.E.; Ramirez Reina, T.; Volpe, R.; Barrow, M.P. Unlocking the potential of biofuels via reaction pathways in van Krevelen diagrams. Green Chem. 2021, 23, 8949–8963. [Google Scholar] [CrossRef]
- Komadel, P.; Anastácio, A.S.; Andrejkovičová, S.; Stucki, J.W. Iron phases identified in bentonite from the Lieskovec deposit (Slovakia) by variable-temperature Mössbauer spectroscopy. Clay Miner. 2008, 43, 107–115. [Google Scholar] [CrossRef]
- Sulman, M.; Kosivtsov, Y.; Sulman, E.; Alfyorov, V.; Lugovoy, Y.; Molchanov, V.; Tyamina, I.; Misnikov, O.; Afanasjev, A.; Kumar, N.; et al. Influence of aluminosilicate materials on the peat low-temperature pyrolysis and gas formation. Chem. Eng. J. 2009, 154, 355–360. [Google Scholar] [CrossRef]
- Ro, D.; Shafaghat, H.; Jang, S.H.; Lee, H.W.; Jung, S.C.; Jae, J.; Cha, J.S.; Park, Y.K. Production of an upgraded lignin-derived bio-oil using the clay catalysts of bentonite and olivine and the spent FCC in a bench-scale fixed bed pyrolyzer. Environ. Res. 2019, 172, 658–664. [Google Scholar] [CrossRef]
- Rahman, M.M.; Liu, R.; Cai, J. Catalytic fast pyrolysis of biomass over zeolites for high quality bio-oil—A review. Fuel Process. Technol. 2018, 180, 32–46. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, Q.; Deng, W.; Zhang, Q.; Wang, Y. Catalytic valorization of biomass and bioplatforms to chemicals through deoxygenation. Adv. Catal. 2020, 66, 1–108. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, S.; Li, Z.; Yi, W.; Zhang, A.; Li, Y.; Zhang, P. Co-pyrolysis of lignin and spent bleaching clay: Insight into the catalytic characteristic and hydrogen supply of spent bleaching clay. J. Anal. Appl. Pyrolysis 2022, 163, 105491. [Google Scholar] [CrossRef]
- Barbosa-Martín, E.; Chel-Guerrero, L.; González-Mondragón, E.; Betancur-Ancona, D. Chemical and technological properties of avocado (Persea americana Mill.) seed fibrous residues. Food Bioprod. Process. 2016, 100, 457–463. [Google Scholar] [CrossRef]
- Zuo, W.; Wong, H.-W. Green synthesis of linear alkylbenzenes via Diels−Alder cycloaddition between furan and linear alkenes over niobic acid catalyst. Green Chem. Lett. Rev. 2017, 10, 393–403. [Google Scholar] [CrossRef]
- Cara, S.; Carcangiu, G.; Padalino, G.; Palomba, M.; Tamanini, M. The bentonites in pelotherapy: Thermal properties of ž/clay pastes from Sardinia Italy. Appl. Clay Sci. 2000, 16, 125–132. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Curran, H.J. Detailed kinetics of fossil and renewable fuel combustion. Comput. Aided Chem. Eng. 2019, 45, 363–443. [Google Scholar] [CrossRef]
- Bello, S.; Méndez-Trelles, P.; Rodil, E.; Feijoo, G.; Moreira, M.T. Towards improving the sustainability of bioplastics: Process modelling and life cycle assessment of two separation routes for 2,5-furandicarboxylic acid. Sep. Purif. Technol. 2020, 233, 116056. [Google Scholar] [CrossRef]
- Evans, R.J.; Milne, T.A. Molecular Characterization of the Pyrolysis of Biomass. 2. Applications. Energy Fuels 1987, 1, 311–319. [Google Scholar] [CrossRef]
- Huang, Y.F.; Kuan, W.H.; Chiueh, P.T.; Lo, S.L. Pyrolysis of biomass by thermal analysis–mass spectrometry (TA–MS). Bioresour. Technol. 2011, 102, 3527–3534. [Google Scholar] [CrossRef]
- Volpe, M.; Fiori, L. From olive waste to solid biofuel through hydrothermal carbonisation: The role of temperature and solid load on secondary char formation and hydrochar energy properties. J. Anal. Appl. Pyrolysis 2017, 124, 63–72. [Google Scholar] [CrossRef]



| Pyrolysis @ 600 °C | HTC @ 250 °C | |||
|---|---|---|---|---|
| Compound | Avocado | Avocado + Bentonite Clay | Avocado | Avocado + Bentonite Clay |
| Percent (%) Compound Type | ||||
| Alcohols Data Range (%) | 7.63% | 8.86% | ||
| 5.27–9.99 | 7.23–10.48 | |||
| Aldehydes Data Range (%) | 3.75% | |||
| 1.92–5.58 | ||||
| Alkanes Data Range (%) | 0.96% | 0.47% | ||
| 0.00–1.92 | 0.28–0.66 | |||
| Alkenes Data Range (%) | 0.52% | |||
| 0.35–0.68 | ||||
| Esters Data Range (%) | 0.29% | 0.39% | ||
| 0.26–0.32 | 0.30–0.49 | |||
| Furans Data Range (%) | 28.35% | 19.74% | 100.00% | 33.74% |
| 25.56–31.13 | 13.45–26.03 | - | 31.56–35.92 | |
| Ketones Data Range (%) | 20.09% | 18.88% | 66.26% | |
| 17.86–22.32 | 15.83–21.93 | 64.08–68.44 | ||
| Phenols Data Range (%) | 29.84% | 45.52% | ||
| 21.36–38.31 | 31.27–59.78 | |||
| Oxygenated Aromatics Data Range (%) | 6.56% | 1.63% | ||
| 6.47–6.65 | 0.00–3.27 | |||
| Others Data Range (%) | 0.24% | |||
| 0.00–0.48 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karod, M.; Pollard, Z.A.; Ahmad, M.T.; Dou, G.; Gao, L.; Goldfarb, J.L. Impact of Bentonite Clay on In Situ Pyrolysis vs. Hydrothermal Carbonization of Avocado Pit Biomass. Catalysts 2022, 12, 655. https://doi.org/10.3390/catal12060655
Karod M, Pollard ZA, Ahmad MT, Dou G, Gao L, Goldfarb JL. Impact of Bentonite Clay on In Situ Pyrolysis vs. Hydrothermal Carbonization of Avocado Pit Biomass. Catalysts. 2022; 12(6):655. https://doi.org/10.3390/catal12060655
Chicago/Turabian StyleKarod, Madeline, Zoe A. Pollard, Maisha T. Ahmad, Guolan Dou, Lihui Gao, and Jillian L. Goldfarb. 2022. "Impact of Bentonite Clay on In Situ Pyrolysis vs. Hydrothermal Carbonization of Avocado Pit Biomass" Catalysts 12, no. 6: 655. https://doi.org/10.3390/catal12060655
APA StyleKarod, M., Pollard, Z. A., Ahmad, M. T., Dou, G., Gao, L., & Goldfarb, J. L. (2022). Impact of Bentonite Clay on In Situ Pyrolysis vs. Hydrothermal Carbonization of Avocado Pit Biomass. Catalysts, 12(6), 655. https://doi.org/10.3390/catal12060655