Effect of Potassium Promoter on the Performance of Nickel-Based Catalysts Supported on MnOx in Steam Reforming of Ethanol
Abstract
1. Introduction
2. Experimental Procedure
2.1. Catalyst Synthesis
2.2. Catalysts Characterization
2.3. Catalyst Evaluation in the SRE Process
3. Results and Discussion
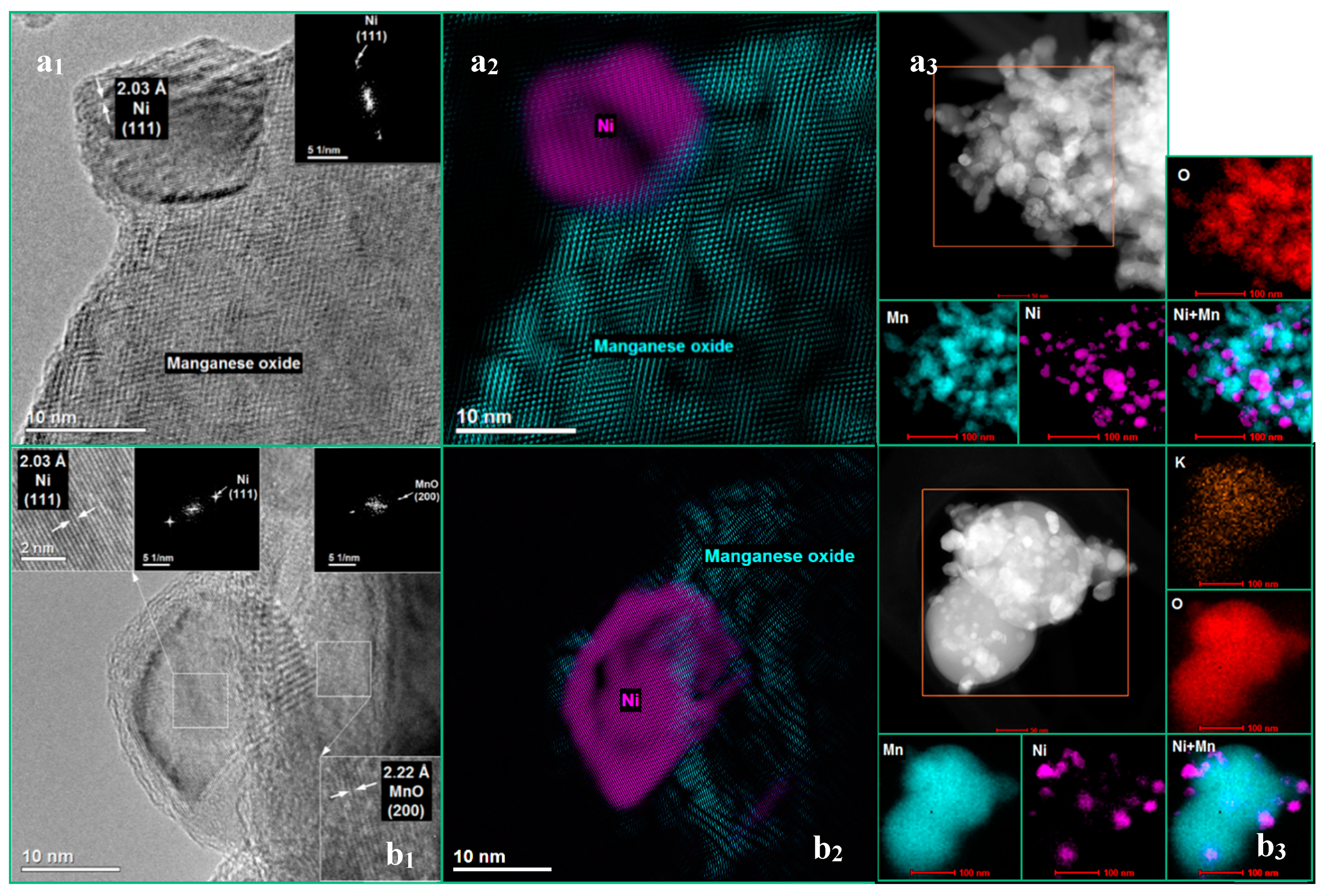
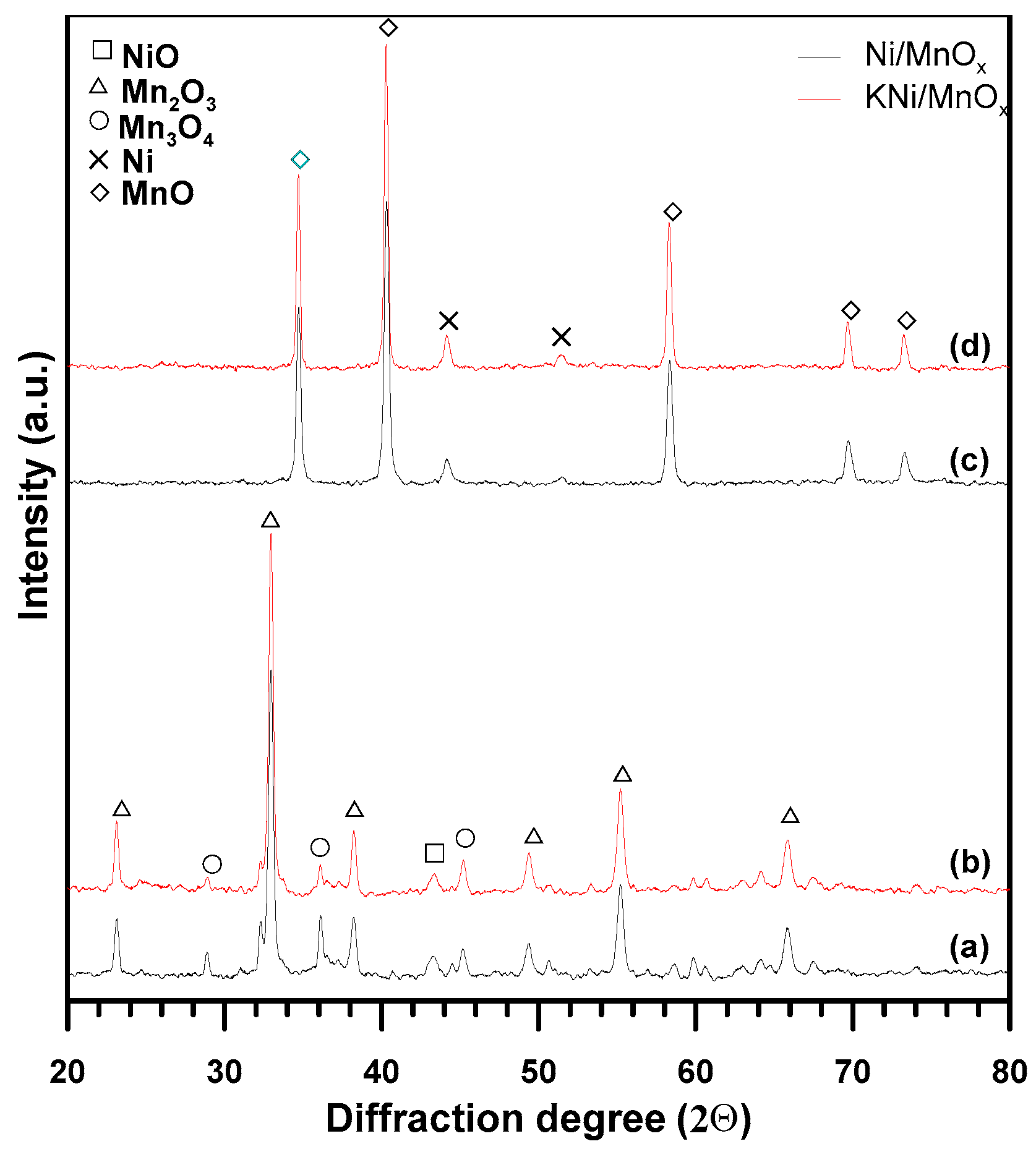
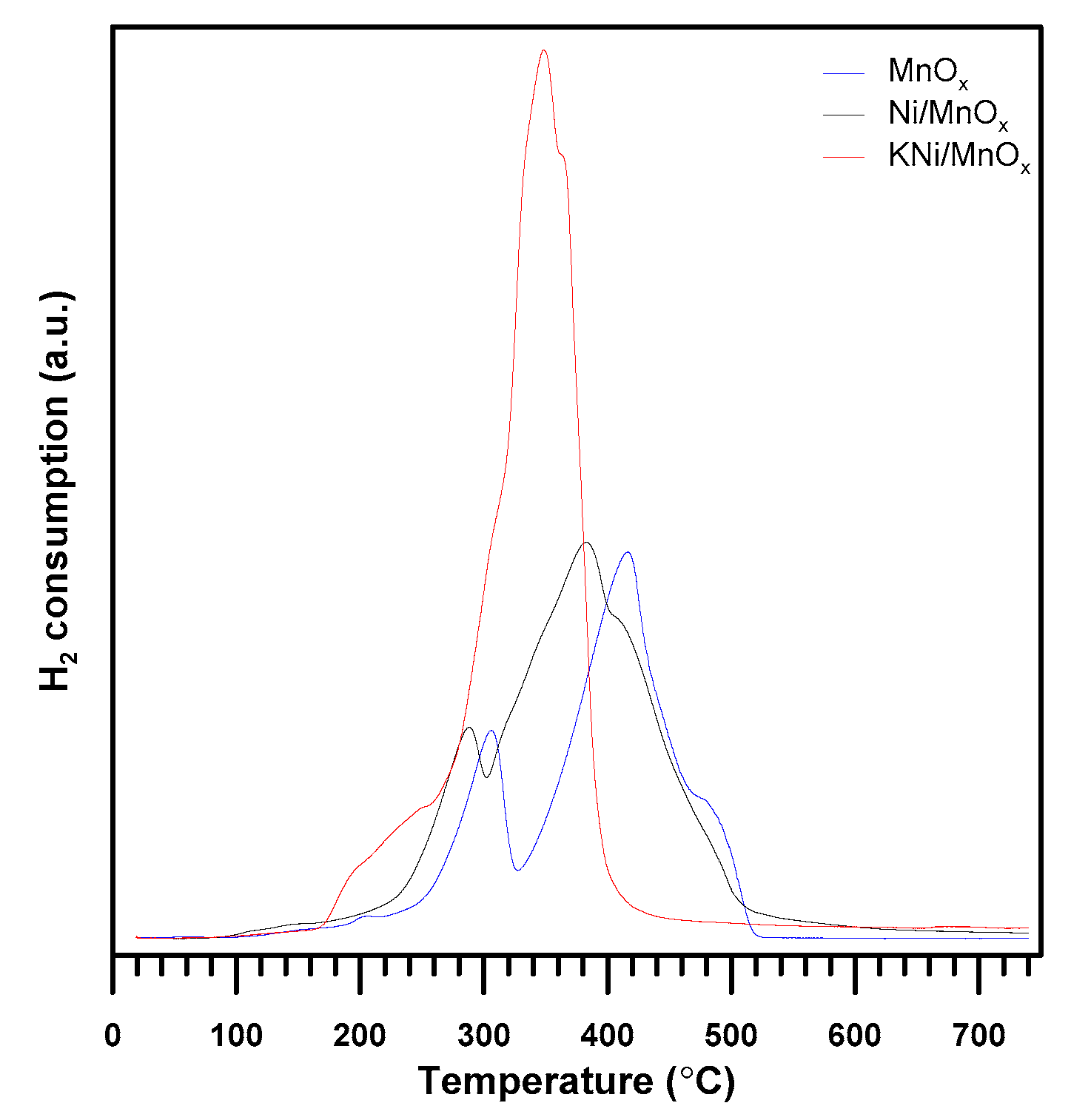
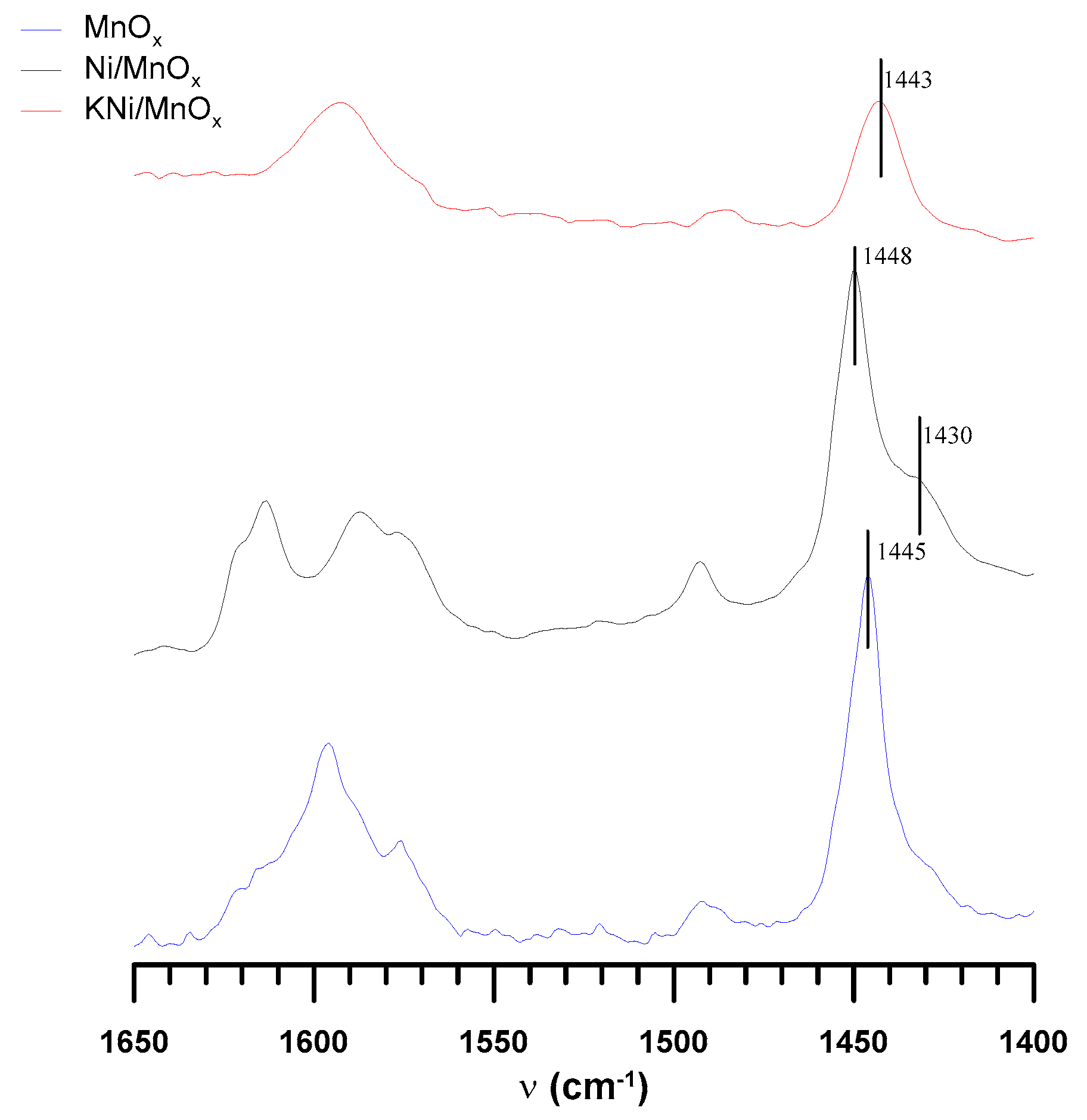
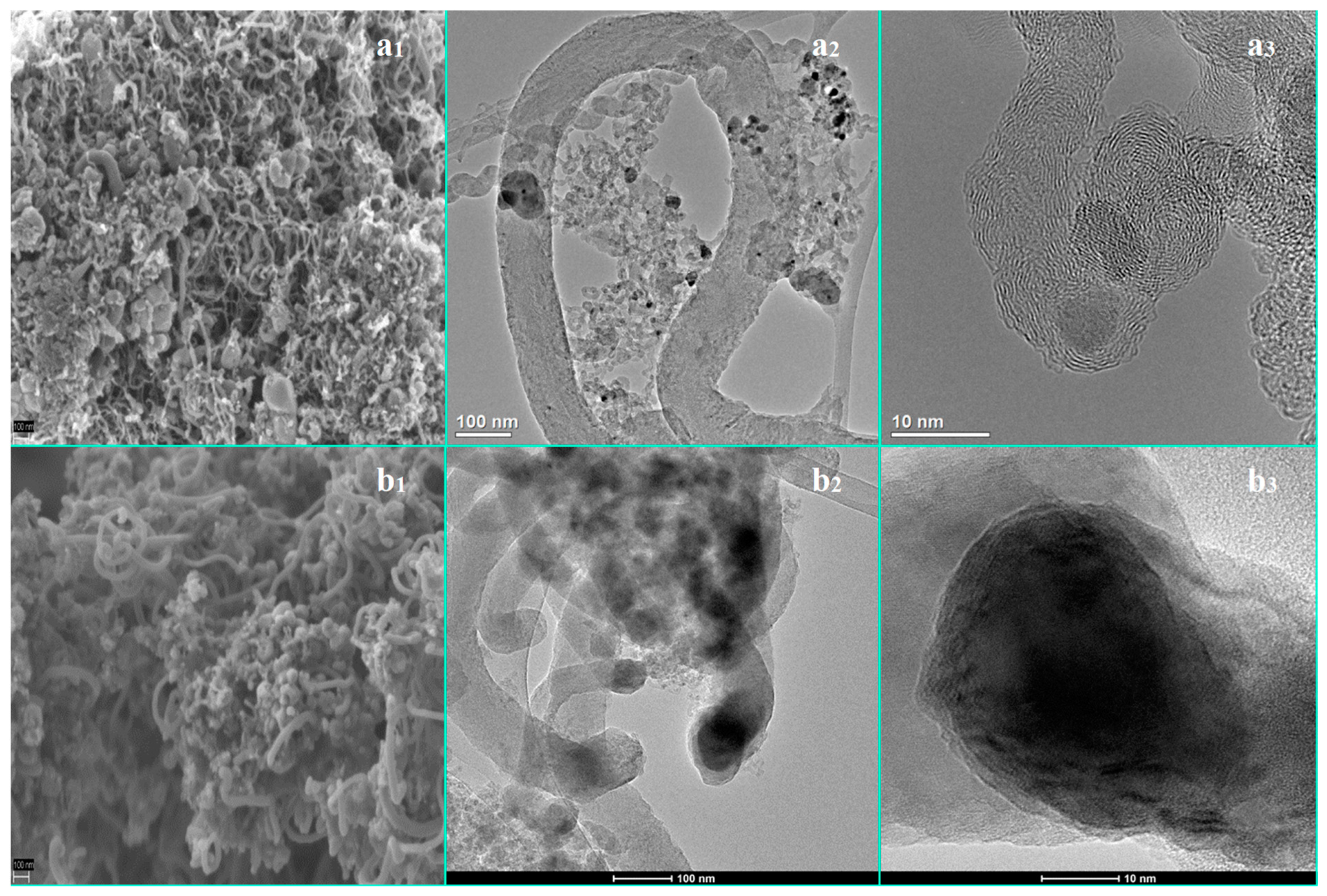
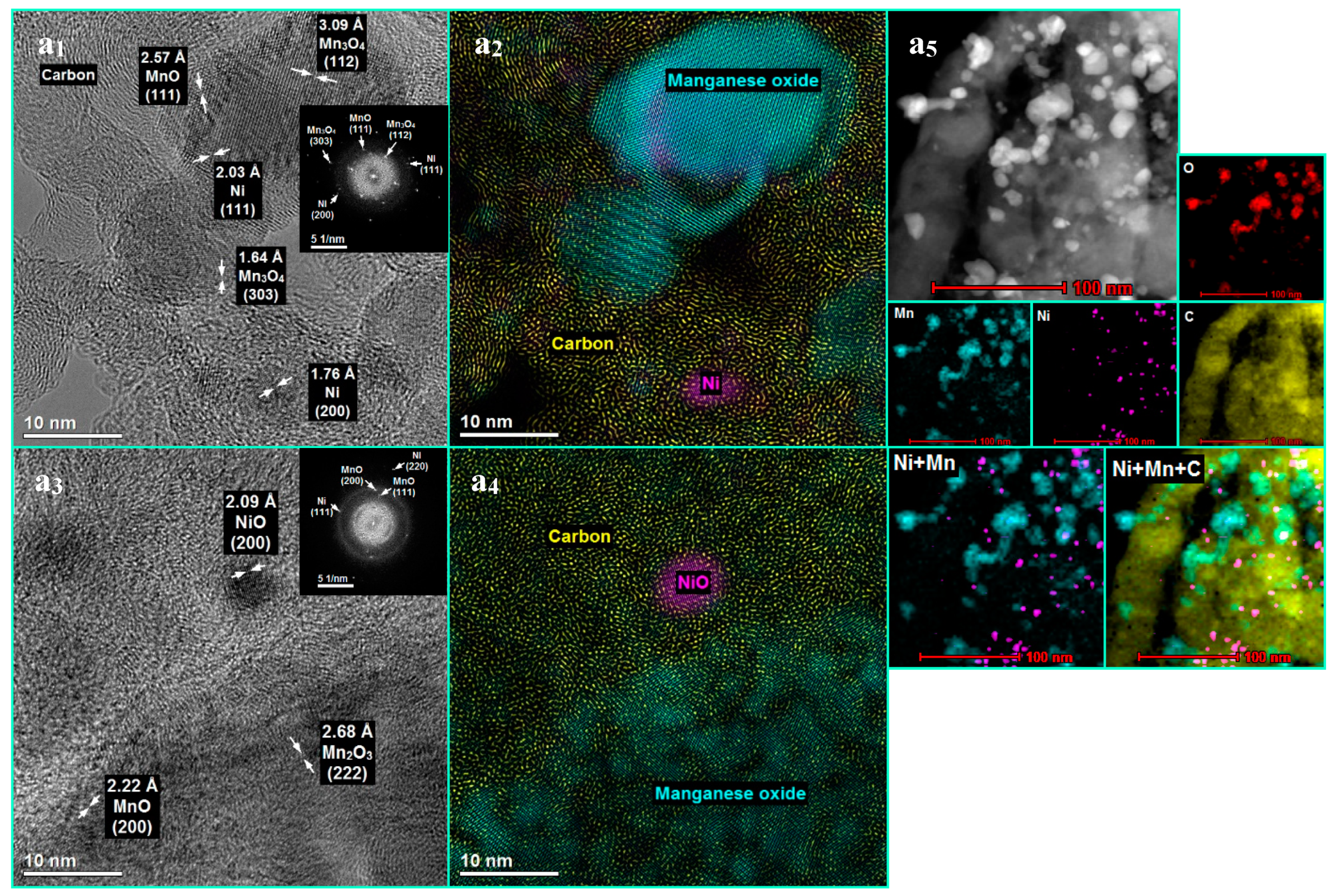
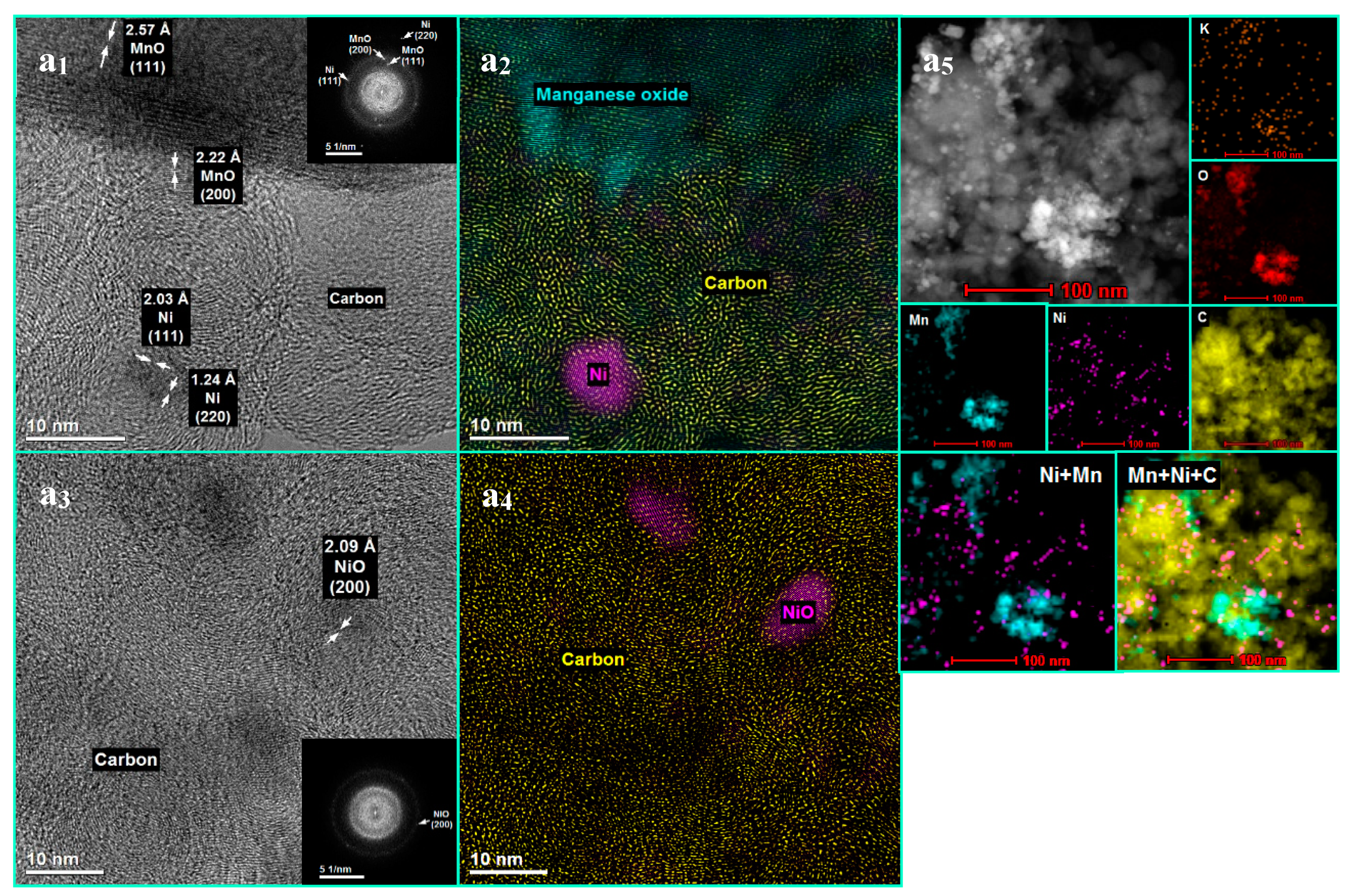
3.1. The Influence of a Potassium Promoter on the Catalysts’ Physicochemical Properties
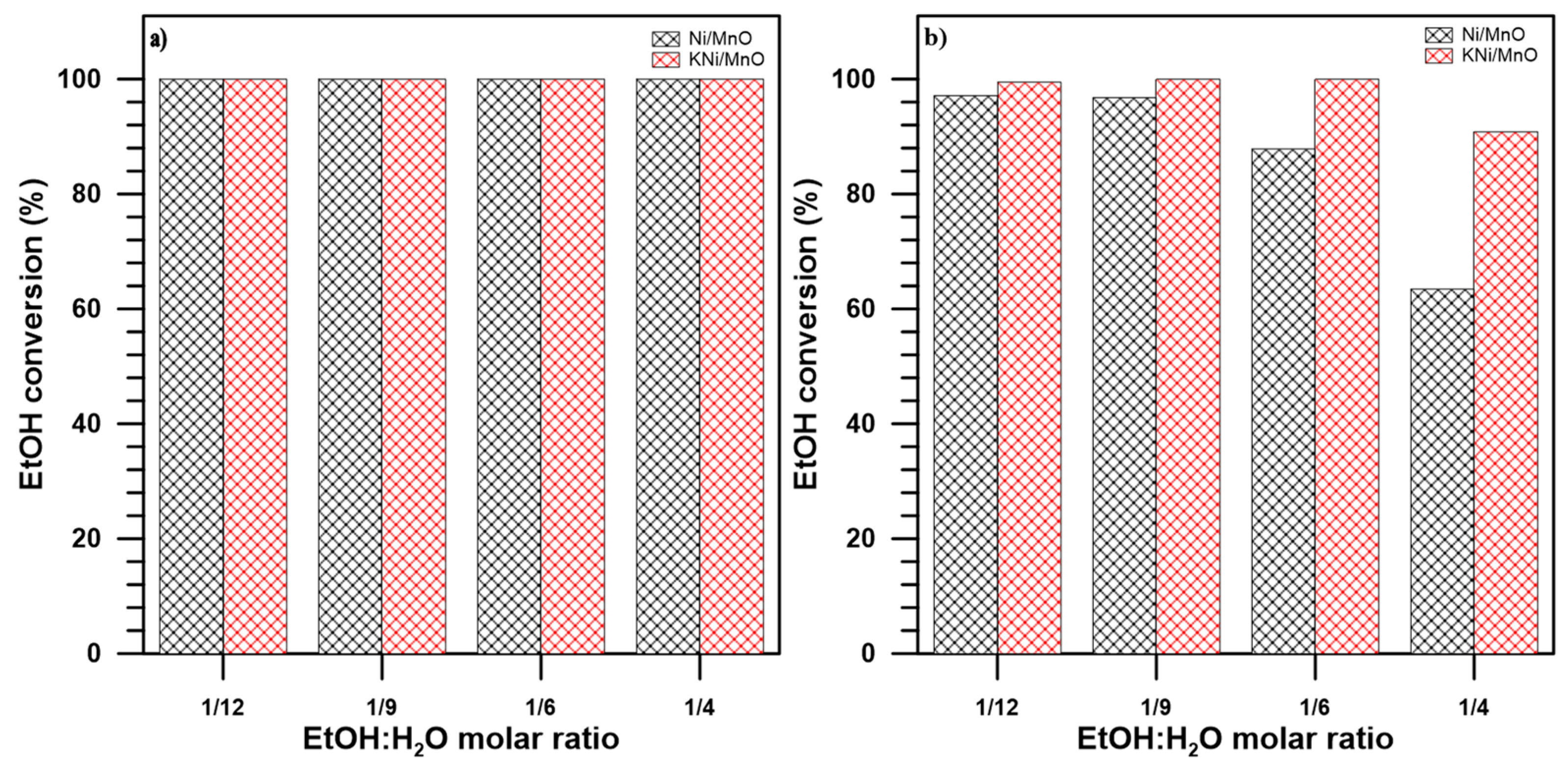
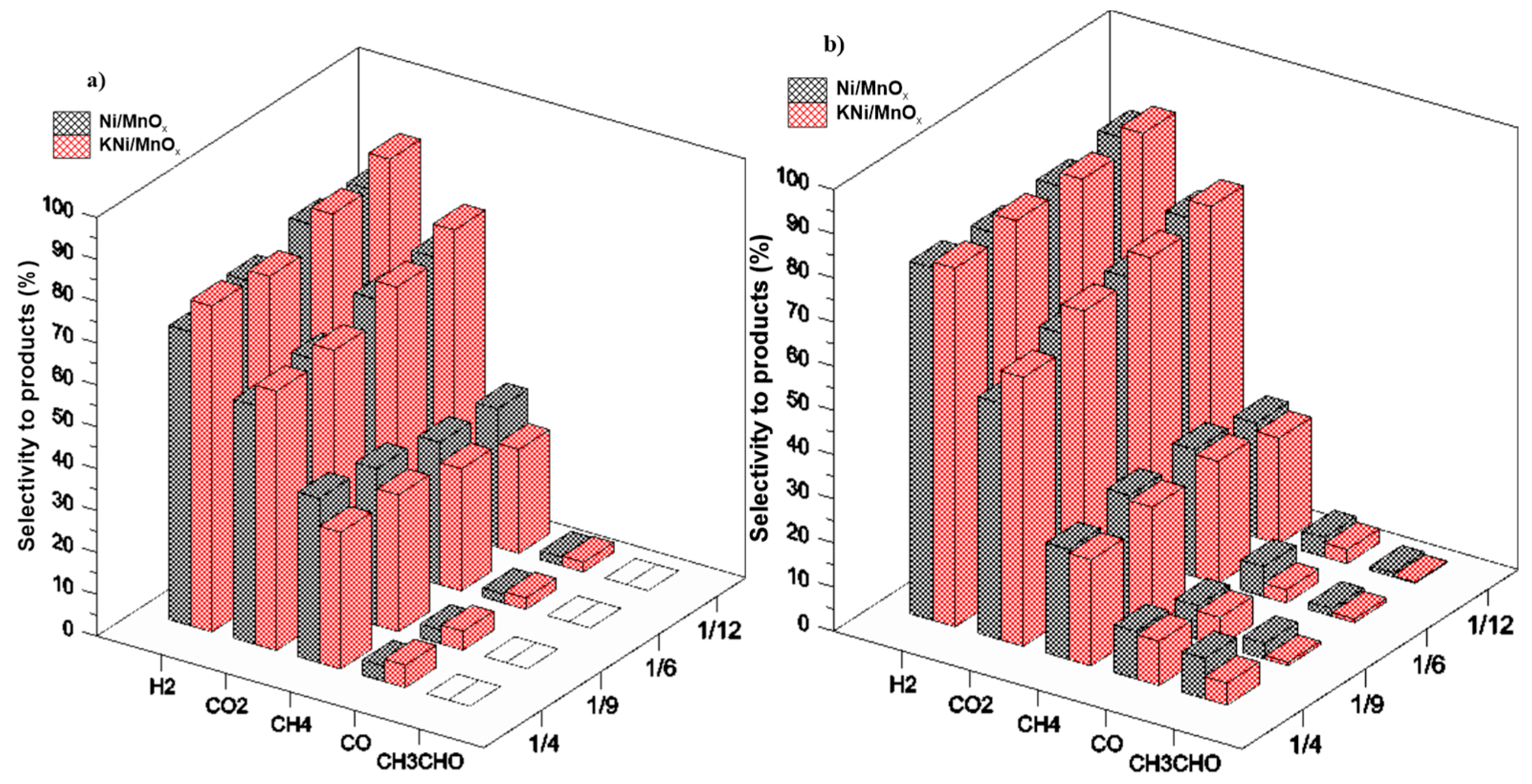
3.2. The Influence of a Potassium Promoter on the Performance of the Catalysts in Ethanol Steam Reforming Process
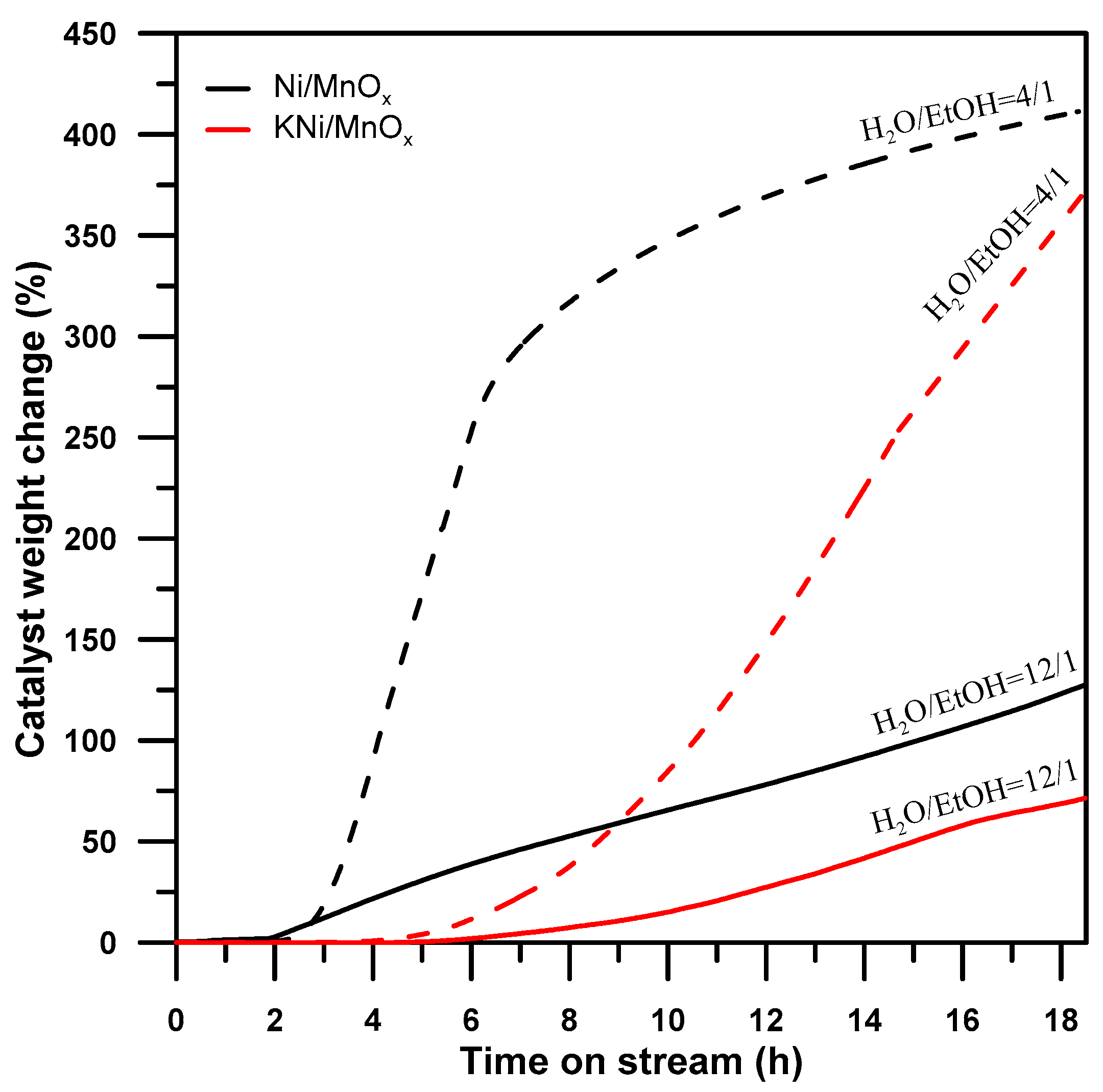
3.3. The Influence of a Potassium Promoter on Prevention of the Nickel-Based Catalyst Deactivation under SRE Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jo, W.J.; Im, Y.; Do, J.Y.; Park, N.-K.; Lee, T.J.; Lee, S.T.; Cha, M.S.; Jeon, M.-K.; Kang, M. Synergies between Ni, Co, and Mn ions in trimetallic Ni1-xCoxMnO4 catalysts for effective hydrogen production from propane steam reforming. Renew. Energy 2017, 113, 248–256. [Google Scholar] [CrossRef]
- Da Costa-Serra, J.F.; Chica, A. Catalysts based on Co-Birnessite and Co-Todorokite for the efficient production of hydrogen by ethanol steam reforming. Int. J. Hydrogen Energy 2018, 43, 16859–16865. [Google Scholar] [CrossRef]
- Manfro, R.L.; Ribeiro, N.F.P.; Souza, M.M.V.M. Production of hydrogen from steam reforming of grycerol using nickel catalysts supported on Al2O3, CeO2 and ZrO2. Catal. Sustain. Energy 2013, 1, 60–70. [Google Scholar]
- Marcos, F.C.F.; Lucrédio, A.F.; Assaf, E.M. Effects of adding basic oxides of La and/or Ce to SiO2-supported Co catalysts for ethanol steam reforming. RSC Adv. 2014, 4, 43839–43849. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Jia, P.; Dong, D.; Wang, Y.; Song, H.; Xiang, J.; Liu, Q.; Hu, X. Ethanol steam reforming over cobalt catalysts: Effect of range of additives on the catalytic behaviors. J. Energy Inst. 2020, 93, 165–184. [Google Scholar] [CrossRef]
- Riani, P.; Garbarino, G.; Canepa, F.; Busca, G. Cobalt nanoparticles mechanically deposited on α-Al2O3: A competitive catalyst for the production of hydrogen through ethanol steam reforming. J. Chem. Technol. Biotechnol. 2019, 94, 538–546. [Google Scholar] [CrossRef]
- Yu, S.-W.; Huang, H.-H.; Tang, C.-W.; Wang, C.-B. The effect of accessible oxygen over Co3O4-CeO2 catalysts on the steam reforming of ethanol. Int. J. Hydrogen Energy 2014, 39, 20700–20711. [Google Scholar] [CrossRef]
- Ibrahim, S.h.M.; El-Shobaky, G.A.; Mohamed, G.M.; Hassan, N.A. Effects of ZnO and MoO3 Doping on surface and catalytic properties of manganese oxide supported on alumina system. Open Catal. J. 2011, 4, 27–35. [Google Scholar] [CrossRef][Green Version]
- Comas, J.; Mariňo, F.; Laborde, M.; Amadeo, N. Bio-ethanol steam reforming on Ni/Al2O3 catalyst. Chem. Eng. J. 2004, 98, 61–68. [Google Scholar] [CrossRef]
- Bshish, A.; Yaakob, A.; Ebshish, A.; Alhasan, F.H. Hydrogen production via ethanol steam reforming over Ni/Al2O3 catalysts: Effect of Ni loading. J. Energy Resour. Technol. 2014, 136, 012601. [Google Scholar] [CrossRef]
- Alberton, A.L.; Souza, M.M.V.M.; Schmal, M. Carbon formation and its influence on ethanol steam reforming over Ni/Al2O3 catalysts. Catal. Today 2007, 123, 257–264. [Google Scholar] [CrossRef]
- Garcia, S.R.; Assaf, J.M. Effect of the preparation method on Co/Al2O3 catalyst applied to ethanol steam reforming reaction production of hydrogen. Mod. Res. Catal. 2012, 1, 52–57. [Google Scholar] [CrossRef][Green Version]
- Lucredio, A.F.; Bellido, J.D.A.; Zawadzki, A.; Assaf, E.M. Co catalysts supported on SiO2 and γ-Al2O3 applied to ethanol steam reforming: Effect of the solvent used in the catalyst preparation method. Fuel 2011, 90, 1424–1430. [Google Scholar] [CrossRef]
- Kaddouri, A.; Mazzocchia, C. A study of the influence of the synthesis conditions upon the catalytic properties of Co/SiO2 or Co/Al2O3 catalysts used for ethanol steam reforming. Catal. Commun. 2014, 5, 339–345. [Google Scholar] [CrossRef]
- Carvalho, F.L.S.; Asencios, Y.J.O.; Rego, A.M.B.; Assaf, E.M. Hydrogen production by steam reforming of ethanol over Co3O4/La2O3/CeO2 catalysts synthesized by one-step polymerization method. Appl. Catal. A Gen. 2014, 483, 52–62. [Google Scholar] [CrossRef]
- Arena, F.; Torre, T.; Raimondo, C.; Parmaliana, A. Structure and redox properties of bulk and supported manganese oxide catalysts. Phys. Chem. Chem. Phys. 2001, 3, 1911–1917. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Cheng, W.; Wu, F.; Lu, X.; Li, Z. Controlled synthesis of diverse manganese based catalysts for complete oxidation of toluene and carbon monoxide. Chem. Eng. J. 2014, 22, 59–67. [Google Scholar] [CrossRef]
- Lee, G.; Kim, D.; Kwak, B.S.; Kang, M. Hydrogen rich production by ethanol steam reforming reaction over Mn/Co10Si90MCM-48 catalysts. Catal. Today 2014, 232, 139–150. [Google Scholar] [CrossRef]
- Kwak, B.S.; Lee, G.; Park, S.-M.; Kang, M. Effect of MnOx in the catalytic stabilization of Co2MnO4 spinel during the ethanol steam reforming reaction. Appl. Catal. A Gen. 2015, 503, 165–175. [Google Scholar] [CrossRef]
- Fuertes, A.; Da Costa-Serra, J.F.; Chica, A. New catalysts based on Ni-Birnessite and Ni-Todorokite for the efficient production of hydrogen by bioethanol steam reforming. Energy Procedia 2012, 29, 181–191. [Google Scholar] [CrossRef][Green Version]
- Gac, W.; Greluk, M.; Słowik, G.; Turczyniak-Surdacka, S. Structural and surface changes of cobalt modified manganese oxide during activation and ethanol steam reforming reaction. Appl. Surf. Sci. 2018, 440, 1047–1062. [Google Scholar] [CrossRef]
- Sohrabi, S.; Irankhah, A. Synthesis, characterization, and catalytic activity of Ni/CeMnO2 catalysts promoted by copper, cobalt, potassium and iron for ethanol steam reforming. Int. J. Hydrogen Energy 2021, 46, 12846–12856. [Google Scholar] [CrossRef]
- Greluk, M.; Rybak, P.; Słowik, G.; Rotko, M.; Machocki, A. Comparative study on steam and oxidative steam reforming of ethanol over 2KCo/ZrO2 catalyst. Catal. Today 2015, 242, 50–59. [Google Scholar] [CrossRef]
- Banach, B.; Machocki, A. Effect of potassium addition on a long term performance of Co-ZnO-Al2O3 catalysts in the low-temperature steam reforming of ethanol: Co-precipitation vs citrate method of catalysts synthesis. Appl. Catal. A Gen. 2015, 505, 173–182. [Google Scholar] [CrossRef]
- Greluk, M.; Rotko, M.; Machocki, A. Conversion of ethanol over Co/CeO2 and KCo/CeO2 catalysts for hydrogen production. Catal. Lett. 2016, 146, 163–173. [Google Scholar] [CrossRef]
- Słowik, G.; Greluk, M.; Machocki, A. Microscopic characterization of changes in the structure of KCo/CeO2 catalyst used in the steam reforming of ethanol. Mater. Chem. Phys. 2016, 173, 219–237. [Google Scholar] [CrossRef]
- Greluk, M.; Słowik, G.; Rotko, M.; Machocki, A. Steam reforming and oxidative steam reforming of ethanol over PtKCo/CeO2 catalyst. Fuel 2016, 183, 518–530. [Google Scholar] [CrossRef]
- Słowik, G.; Greluk, M.; Rotko, M.; Machocki, A. Evolution of the structure of unpromoted and potassium-promoted ceria-supported nickel catalysts in the steam reforming of ethanol. Appl. Catal. B Environ. 2018, 221, 490–509. [Google Scholar] [CrossRef]
- Grzybek, G.; Greluk, M.; Indyka, P.; Góra-Marek, K.; Legutko, P.; Słowik, G.; Turczyniak-Surdacka, S.; Rotko, M.; Sojka, Z.; Kotarba, A. Cobalt catalyst for steam reforming of ethanol–Insights into the promotional role of potassium. Int. J. Hydrogen Energy 2020, 45, 22658–22673. [Google Scholar] [CrossRef]
- Grzybek, G.; Góra-Marek, K.; Patulski, P.; Greluk, M.; Rotko, M.; Słowik, G.; Kotarba, A. Optimization of the potassium promotion of the Co|α-Al2O3 catalyst for the effective hydrogen production via ethanol steam reforming. Appl. Catal. A Gen. 2021, 614, 1188051. [Google Scholar] [CrossRef]
- Llorca, J.; Homs, N.; Sales, J.; Fierro, J.L.G.; Ramirez de la Piscina, P. Effect of sodium addition on the performance of Co–ZnO-based catalysts for hydrogen production from bioethanol. J. Catal. 2004, 222, 470–480. [Google Scholar] [CrossRef]
- Oktaviano, H.S.; Trisunaryanti, W. Sol-gel derived Co and Ni based catalysts: Application for steam reforming of ethanol. Indones. J. Chem. 2008, 8, 47–53. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Carrero, A.; Calles, J.A. Ethanol steam reforming on Mg- and Ca-modified Cu–Ni/SBA-15 catalysts. Catal. Today 2009, 146, 63–70. [Google Scholar] [CrossRef]
- Ogo, S.; Shimizu, T.; Nakazawa, Y.; Mukawa, K.; Mukai, D.; Sekine, Y. Steam reforming of ethanol over K promoted Co catalyst. Appl. Catal. A Gen. 2015, 495, 30–38. [Google Scholar] [CrossRef]
- Barrientos, J.; Gonzalez, N.; Boutonnet, M.; Järås, S. Deactivation of Ni/γ-Al2O3 catalysts in CO methanation: Effect of Zr, Mg, Ba and Ca oxide promoters. Top. Catal. 2017, 60, 1276–1284. [Google Scholar] [CrossRef]
- Wang, A.; Tian, Z.; Liu, Q.; Qiao, Y.; Tian, Y. Facile preparation of a Ni/MgAl2O4 catalyst with high surface area: Enhancement in activity and stability for CO methanation. Main Group Met. Chem. 2018, 41, 73–89. [Google Scholar] [CrossRef]
- Richardson, J.T.; Scates, R.; Twigg, M.V. X-ray diffraction study of nickel oxide reduction by hydrogen. Appl. Catal. A Gen. 2003, 246, 137–150. [Google Scholar] [CrossRef]
- Sharrouf, M.; Awad, R.; Roumié, M.; Marhaba, S. Structural, optical and room temperature magnetic study of Mn2O3 nanoparticles. Mater. Sci. Appl. 2015, 6, 850–859. [Google Scholar]
- Khalaji, A.D.; Soleymanifard, M.; Jarosova, M.; Machek, P. Facile synthesis and characterization of Mn3O4, Co3O4, and NiO. Acta Phys. Pol. 2002, 137, 1043–1045. [Google Scholar] [CrossRef]
- Zhang, P.; Zhan, Y.; Cai, B.; Hao, C.; Wang, J.; Liu, C.; Meng, Z.; Yin, Z.; Chen, Q. Shape-controlled synthesis of Mn3O4 nanocrystals and their catalysis of the degradation of Methylene Blue. Nano Res. 2010, 3, 235–243. [Google Scholar] [CrossRef]
- Martinez de la Torre, C.; Bennewitz, M.F. Manganese oxide nanoparticle synthesis by thermal decomposition of manganese(II) acetylacetonate. J. Vis. Exp. 2020, 160, e61572. [Google Scholar] [CrossRef] [PubMed]
- Indra, A.; Menezes, P.W.; Schuster, F.; Driess, M. Significant role of Mn(III) sites in eg1 configuration in manganese oxide catalysts for efficient artificial water oxidation. J. Photochem. Photobiol. B Biol. 2015, 152, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Bayón, A.; de la Peña O’Shea, V.; Coronado, J.M.; Serrano, D.P. Role of the physicochemical properties of hausmannite on the hydrogen production via the Mn3O4-NaOH thermochemical cycle. Int. J. Hydrogen Energy 2016, 41, 113–122. [Google Scholar] [CrossRef]
- Shu, S.; Guo, J.; Li, J.; Fang, N.; Li, J.; Yuan, S. Effect of post-treatment on the selective catalytic reduction of NO with NH3 over Mn3O4. Mater. Chem. Phys. 2019, 237, 121845. [Google Scholar] [CrossRef]
- Xu, X.; Li, L.; Yu, F.; Peng, H.; Fang, X.; Wang, X. Mesoporous high surface area NiO synthesized with soft templates: Remarkable for catalytic CH4 deep oxidation. Mol. Catal. 2017, 441, 81–91. [Google Scholar] [CrossRef]
- Gil, A.; Gandía, L.M.; Korili, S.A. Effect of the temperature of calcination on the catalytic performance of manganese- and samarium-manganese-based oxides in the complete oxidation of acetone. Appl. Catal. A Gen. 2004, 274, 229–235. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.; Li, H.; Li, X.; Wang, L.; Tsang, S.C. Cr–MnOx mixed-oxide catalysts for selective catalytic reduction of NOx with NH3 at low temperature. J. Catal. 2010, 276, 56–65. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, Y.; Zhang, J.-Y.; Chen, Y.; Yang, X.; Song, W.; Wei, L.; Li, W. Synergy of Mn and Ni enhanced catalytic performance for toluene combustion over Ni-doped α-MnO2 catalysts. Chem. Eng. J. 2020, 388, 124244. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Z.; Wang, G.; Zhu, H.; Dong, M.; Li, S.; Wu, Z.; Li, Z.; Wu, Z.; Zhang, J.; et al. Catalytic performance of MnOx–NiO composite oxide in lean methane combustion at low temperature. Appl. Catal. B Environ. 2013, 129, 172–181. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Zhu, Q.; Ma, M.; Jiang, Z.; Liao, X.; He, C. Unraveling the effects of potassium incorporation routes and positions on toluene oxidation over α-MnO2 nanorods: Based on experimental and density functional theory (DFT) studies. J. Colloid Interface Sci. 2021, 598, 324–338. [Google Scholar] [CrossRef]
- Greluk, M.; Rotko, M.; Turczyniak-Surdacka, S. Comparison of catalytic performance and coking resistant behaviors of cobalt- and nickel based catalyst with different Co/Ce and Ni/Ce molar ratio under SRE conditions. Appl. Catal. A Gen. 2021, 590, 117334. [Google Scholar] [CrossRef]
- Grzona, C.B.; Lick, I.D.; Rodriguez Castellón, E.; Ponzi, M.I.; Ponzi, E.N. Cobalt and KNO3 supported on alumina catalysts for diesel soot combustion. Mater. Chem. Phys. 2010, 123, 557–562. [Google Scholar] [CrossRef]
- Morrow, B.A.; Cody, I.A.; Moran, L.E.; Palepu, R. An infrared study of the adsorption of pyridine on platinum and nickel. J. Catal. 1976, 44, 467–476. [Google Scholar] [CrossRef]
- Tarach, K.A.; Śrębowata, A.; Kowalewski, E.; Gołąbek, K.; Kostuch, A.; Kruczała, K.; Girman, V.; Góra-Marek, K. Nickel loaded zeolites FAU and MFI: Characterization and activity in water-phase hydrodehalogenation of TCE. Appl. Catal. A Gen. 2018, 568, 64–75. [Google Scholar] [CrossRef]
- Verhoef, R.W.; Asscherm, M. The work function of adsorbed alkalis on metals revisited: A coverage-dependent polarizability approach. Surf. Sci. 1997, 391, 11–18. [Google Scholar] [CrossRef]
- Ramírez de la Piscina, P.; Homs, N. Use of biofuels to produce hydrogen (reformation processes). Chem. Soc. Rev. 2008, 37, 2459–2467. [Google Scholar] [CrossRef]
- Espinal, R.; Taboada, E.; Molins, E.; Chimentao, R.J.; Medinac, F.; Llorca, J. Cobalt hydrotalcites as catalysts for bioethanol steam reforming. The promoting effect of potassium on catalyst activity and long-term stability. Appl. Catal. B Environ. 2012, 127, 59–67. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Akiyama, M.; Nagai, M. Steam reforming of ethanol over nickel molybdenum carbides for hydrogen production. Catal. Today 2009, 146, 87–95. [Google Scholar] [CrossRef]
- Subramani, V.; Song, C. Advances in catalysis and processes for hydrogen production from ethanol reforming. Catalysis 2007, 20, 65–106. [Google Scholar]
- Greluk, M.; Gac, W.; Rotko, M.; Słowik, G.; Turczyniak-Surdacka, S. Co/CeO2 and Ni/CeO2 catalysts for ethanol steam reforming: Effect of the cobalt/nickel dispersion on catalysts properties. J. Catal. 2021, 393, 159–178. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Azmi, R.; Trouillet, V.; Strafela, M.; Ulrich, S.; Ehrenberg, H.; Bruns, M. Surface analytical approaches to reliably characterize lithium ion battery electrodes. Surf. Interface Anal. 2018, 50, 43–51. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Bengaard, H.S.; Alstrup, I.; Chorkendorff, I.; Ullmann, S.; Rostrup-Nielsen, J.R.; Nørskov, J.K. Chemisorption of methane on Ni(100) and Ni(111) surfaces with preadsorbed potassium. J. Catal. 1999, 187, 238–244. [Google Scholar] [CrossRef]
- Snoeck, J.-W.; Froment, G.F. Steam/CO2 reforming of methane. Carbon formation and gasification on catalysts with various potassium contents. Ind. Eng. Chem. Res. 2002, 41, 3548–3556. [Google Scholar] [CrossRef]
- Juan-Juan, J.; Román-Martínez, M.C.; Illán-Gómez, M.J. Effect of potassium contenet in the activity of K-promotes Ni/Al2O3 catalysts for the dry reforming of methane. Appl. Catal A Gen. 2006, 301, 9–15. [Google Scholar] [CrossRef]


| Sample | Metal Content (wt.%) | SBET (m2 g−1) | Ni0 Particle Size (nm) * | ||
|---|---|---|---|---|---|
| Ni | K | By XRD | By TEM | ||
| Ni/MnO | 9.7 | - | 16.7 | 19 | 14 |
| KNi/MnO | 9.1 | 1.6 | 10.1 | 23 | 20 |
| MnOx | - | - | 12.0 | - | - |
| Sample | Lewis Acid Sites Concentration (mmol g−1) |
|---|---|
| MnOx | 54 |
| Ni/MnOx | 50 |
| KNi/MnOx | 13 |
| Sample | Ni Particle Size (nm) | |
|---|---|---|
| H2O:EtOH = 12/1 | H2O:EtOH = 4/1 | |
| Ni/MnO | 8 | 18 |
| KNi/MnO | 10 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greluk, M.; Rotko, M.; Słowik, G.; Turczyniak-Surdacka, S.; Grzybek, G.; Góra-Marek, K.; Kotarba, A. Effect of Potassium Promoter on the Performance of Nickel-Based Catalysts Supported on MnOx in Steam Reforming of Ethanol. Catalysts 2022, 12, 600. https://doi.org/10.3390/catal12060600
Greluk M, Rotko M, Słowik G, Turczyniak-Surdacka S, Grzybek G, Góra-Marek K, Kotarba A. Effect of Potassium Promoter on the Performance of Nickel-Based Catalysts Supported on MnOx in Steam Reforming of Ethanol. Catalysts. 2022; 12(6):600. https://doi.org/10.3390/catal12060600
Chicago/Turabian StyleGreluk, Magdalena, Marek Rotko, Grzegorz Słowik, Sylwia Turczyniak-Surdacka, Gabriela Grzybek, Kinga Góra-Marek, and Andrzej Kotarba. 2022. "Effect of Potassium Promoter on the Performance of Nickel-Based Catalysts Supported on MnOx in Steam Reforming of Ethanol" Catalysts 12, no. 6: 600. https://doi.org/10.3390/catal12060600
APA StyleGreluk, M., Rotko, M., Słowik, G., Turczyniak-Surdacka, S., Grzybek, G., Góra-Marek, K., & Kotarba, A. (2022). Effect of Potassium Promoter on the Performance of Nickel-Based Catalysts Supported on MnOx in Steam Reforming of Ethanol. Catalysts, 12(6), 600. https://doi.org/10.3390/catal12060600







