Photocatalytic Degradation of Recalcitrant Pollutants of Greywater
Abstract
:1. Introduction
2. Characteristics and Compositions of Greywater
2.1. Greywater Quality
2.2. Standard of Greywater
| Location | Generation Rate (Litre/Person/Day) | References |
|---|---|---|
| Asia | 72–225 | [10] |
| Malaysia | 225 | [16] |
| Africa and Middle East | 14–161 | [10,17,18] |
| Muscat, Oman | 151 | [19] |
| Tucson Arizona, USA | 123 | [20] |
| Australia | 113 | [10] |
| Switzerland | 110 | [10] |
| Vietnam | 80–110 | [21] |
| Israel | 98 | [22] |
| Nepal | 72 | [23] |
| Stockholm | 65 | [24] |
| Jordan | 50 | [25] |
| Mali | 30 | [26] |
| Gauteng, South Africa | 20 | [27] |
2.3. Greywater Compositions
2.3.1. Physical Parameters
Temperature
Suspended Solids
Turbidity
2.3.2. Chemical Parameters
pH
Ionic Conductivity
Biological Oxygen Demand (BOD)
Chemical Oxygen Demand (COD)
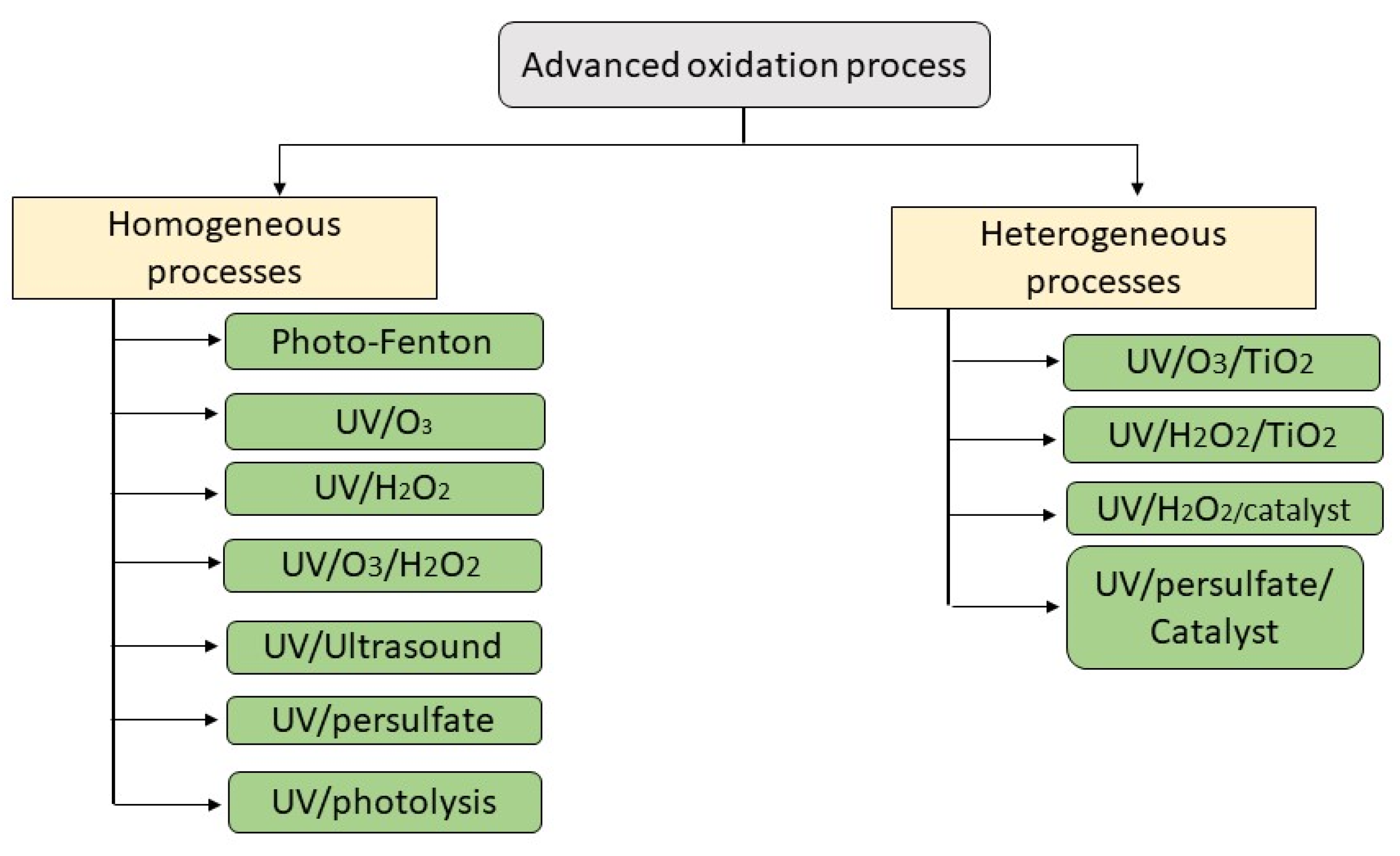
3. Classification of Advanced Oxidation Processes (AOPs)
3.1. Homogeneous AOP
3.2. Heterogeneous AOP
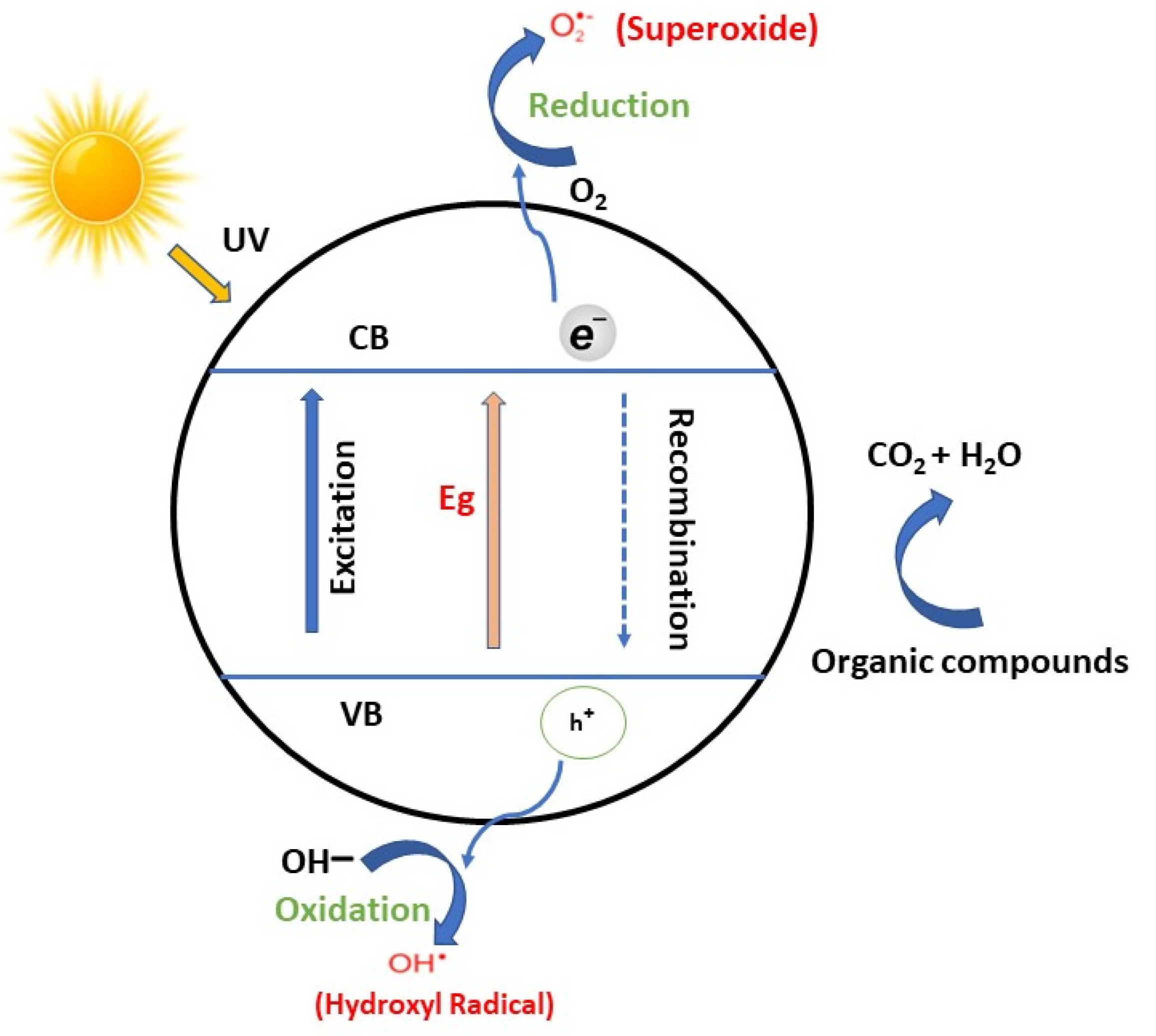
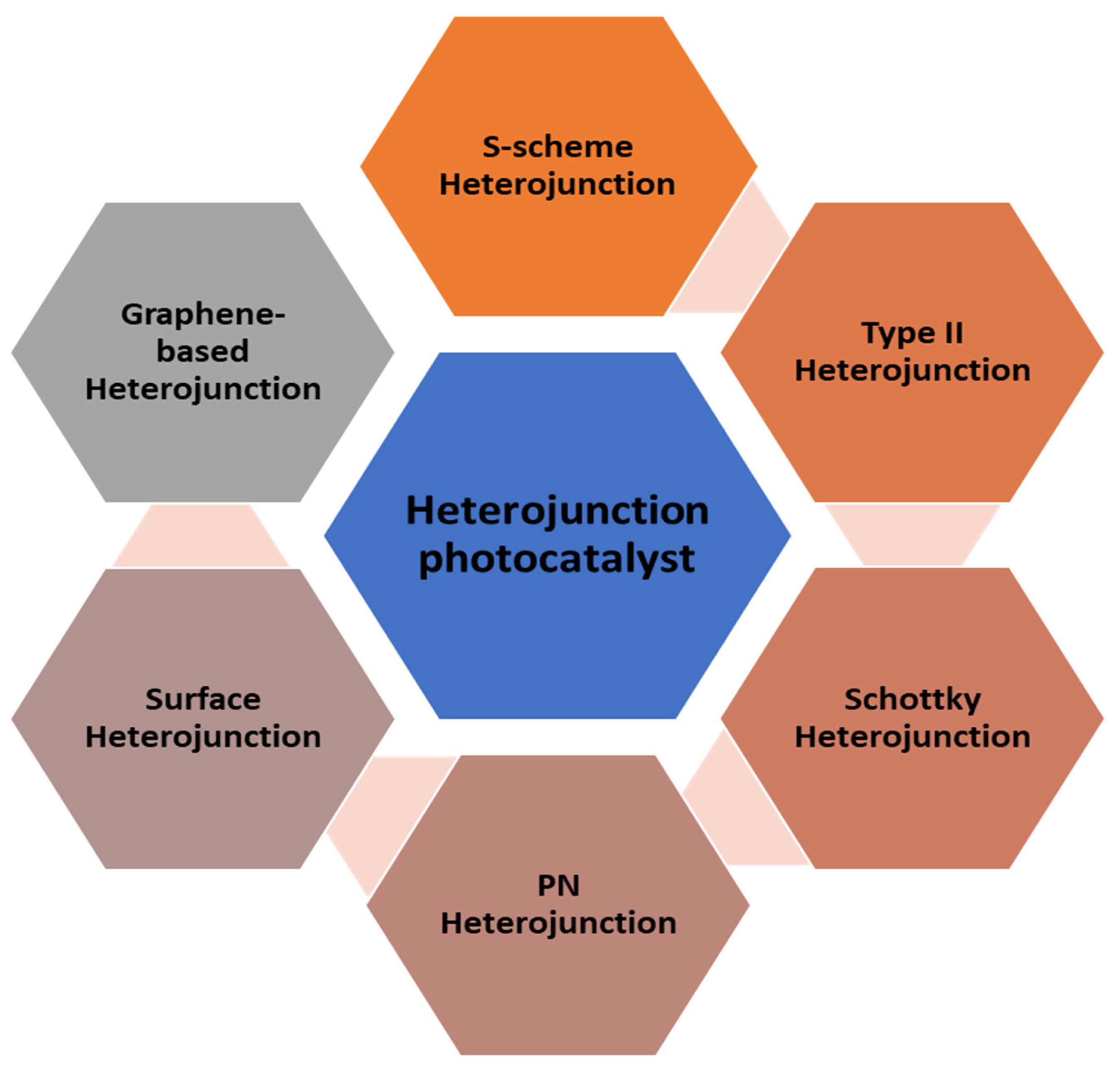
4. Heterogeneous Photocatalyst
5. TiO2 and ZnO Photocatalyst
5.1. TiO2 Catalyst
5.2. Modification of TiO2 Catalyst
5.3. ZnO Photocatalyst Degradation of Organic Compounds
6. Factors Controlling Photocatalytic Reaction
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chong, M.N.; Cho, Y.J.; Poh, P.E.; Jin, B. Evaluation of Titanium dioxide photocatalytic technology for the treatment of reactive Black 5 dye in synthetic and real greywater effluents. J. Clean. Prod. 2015, 89, 196–202. [Google Scholar] [CrossRef]
- Hariharan, C. Photocatalytic degradation of organic contaminants in water by ZnO nanoparticles: Revisited. Appl. Catal. A Gen. 2006, 304, 55–61. [Google Scholar] [CrossRef]
- Bhadiyadra, J.G.; Vaghani, M.V. A review on applicability of photocatalyst titanium dioxide for treatment of greywater. Int. J. Eng. Res. Appl. 2015, 5, 102–105. [Google Scholar]
- Sanchez, M.; Rivero, M.J.; Ortiz, I. Photocatalytic oxidation of grey water over titanium dioxide suspensions. Desalination 2010, 262, 141–146. [Google Scholar] [CrossRef]
- Gulyas, H.; Argáez, Á.S.O.; Kong, F.; Jorge, C.L.; Eggers, S.; Otterpohl, R. Combining activated carbon adsorption with heterogeneous photocatalytic oxidation: Lack of synergy for biologically treated greywater and tetraethylene glycol dimethyl ether. Environ. Technol. 2013, 34, 1393–1403. [Google Scholar] [CrossRef] [Green Version]
- Tsoumachidou, S.; Velegraki, T.; Antoniadis, A.; Poulios, I. Greywater as a sustainable water source: A photocatalytic treatment technology under artificial and solar illumination. J. Environ. Manag. 2017, 195, 232–241. [Google Scholar] [CrossRef]
- Bodzek, M.; Rajca, M. Photocatalysis in the treatment and disinfection of water. Part I. Theoretical backgrounds. Ecol. Chem. Eng. S 2012, 19, 489–512. [Google Scholar] [CrossRef]
- Rakesh, S.S.; Ramesh, P.T.; Murugaragavan, R.; Avudainayagam, S.; Karthikeyan, S. Characterization and treatment of grey water: A review. IJCS 2020, 8, 34–40. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Pareek, V.K.; Ang, M. A review of greywater characteristics and treatment processes. Water Sci. Technol. 2013, 67, 1403–1424. [Google Scholar] [CrossRef]
- Morel, A. Greywater Management in Low and Middle-Income Countries; (No. 628.2 G842g); Swiss Federal Institute of Aquatic Science and Technology: Dubenforf, Switzerland, 2006. [Google Scholar]
- Oh, K.S.; Leong, J.Y.C.; Poh, P.E.; Chong, M.N.; Von Lau, E. A review of greywater recycling related issues: Challenges and future prospects in Malaysia. J. Clean. Prod. 2018, 171, 17–29. [Google Scholar] [CrossRef]
- Eriksson, E. Potential and Problems Related to Reuse of Water in Households. Ph.D. Thesis, Environment & Resources DTU, Technical University of Denmark, Lyngby, Denmark, 2002. [Google Scholar]
- Birks, R.; Hills, S. Characterisation of indicator organisms and pathogens in domestic greywater for recycling. Environ. Monit. Assess. 2007, 129, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Sushmitha, M.B.; Chanakya, H.N.; Khuntia, H.K. Efficient grey water treatment and reuse options for India—A Review. In Waste Water Recycling and Management; Springer: Singapore, 2019; pp. 143–149. [Google Scholar] [CrossRef]
- Li, F. Treatment of Household Grey Water for Non-Potable Reuses. Ph.D. Thesis, Hamburg University of Technology, Hamburg, Germany, 2009. [Google Scholar]
- Martin, C. Ecological Sanitation Greywater Demonstration Project at Hui Sing Garden; Urban Environmental Management System Project Report; Natural Resources and Environment Board: Kuching, Malaysia, 2005.
- Halalsheh, M.; Dalahmeh, S.; Sayed, M.; Suleiman, W.; Shareef, M.; Mansour, M.; Safi, M. Grey water characteristics and treatment options for rural areas in Jordan. Bioresour. Technol. 2008, 99, 6635–6641. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamaiedeh, H.; Bino, M. Effect of treated grey water reuse in irrigation on soil and plants. Desalination 2010, 256, 115–119. [Google Scholar] [CrossRef]
- Jamrah, A.; Al-Futaisi, A.; Prathapar, S.; Harrasi, A.A. Evaluating greywater reuse potential for sustainable water resources management in Oman. Environ. Monit. Assess. 2008, 137, 315–327. [Google Scholar] [CrossRef]
- Casanova, L.M.; Gerba, C.P.; Karpiscak, M. Chemical and microbial characterization of household greywater. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2001, 34, 395–401. [Google Scholar] [CrossRef]
- Büsser, S.; Pham, T.N.; Antoine, M.; Nguyen, V.A. Characteristics and Quantities of Domestic Wastewater in Urban and Peri-Urban Households in Hanoi; Annual Report of FY 2006, The Core University Program between Japan Society for the Promotion of Science (JSPS) and Vietnamese Academy of Science and Technology (VAST); Core University Program Office, Ike Laboratory, Div. of Sustainable Energy and Environmental Engineering, Osaka University: Osaka, Japan, 2007; pp. 395–397. [Google Scholar]
- Friedler, E. Quality of individual domestic greywater streams and its implication for on-site treatment and reuse possibilities. Environ. Technol. 2004, 25, 997–1008. [Google Scholar] [CrossRef]
- Shrestha, R.R.; Haberl, R.; Laber, J. Constructed wetland technology transfer to Nepal. Water Sci. Technol. 2001, 43, 345–360. [Google Scholar] [CrossRef]
- Ottoson, J.; Stenstrom, T.A. Faecal contamination of greywater and associated microbial risks. Water Res. 2003, 37, 645–655. [Google Scholar] [CrossRef]
- Faraqui, N.; Al-Jayyousi, O. Greywater reuse in urban agriculture for poverty alleviation. A case study in Jordan. Water Int. 2002, 27, 387–394. [Google Scholar] [CrossRef]
- Alderlieste, M.C.; Langeveld, J.G. Wastewater planning in Djenne, Mali. A pilot project for the local infiltration of domestic wastewater. Water Sci. Technol. 2005, 51, 57–64. [Google Scholar] [CrossRef]
- Adendorff, J.; Stimie, C. Food from used water-making the previously impossible happen. Water Wheel. S. Afr. Water Res. Comm. (WRC) 2005, 4, 26–29. [Google Scholar]
- Metcalf and Eddy, Inc. Wastewater Engineering—Treatment, Disposal and Reuse, 3rd ed.; Tchobanoglous, G., Burton, F.L., Eds.; Series in Water Resources and Environmental Engineering; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Knerr, H.; Engelhart Hansen, J.; Sagawe, G. Separated grey- and blackwater treatment by the KOMPLETT water recycling system—A possibility to close domestic water cycle. In Proceedings of the Sanitation Challenge: New Sanitation Concepts and Models of Governance, Wageningen, The Netherlands, 19–21 May 2008; pp. 260–269. [Google Scholar]
- Jefferson, B.; Burgess, J.E.; Pichon, A.; Harkness, J.; Judd, S.J. Nutrient addition to enhance biological treatment of greywater. Water Res. 2001, 35, 2702–2710. [Google Scholar] [CrossRef]
- Hernandez Leal, L.; Zeeman, G.; Temmink, H.; Buisman, C. Characterisation and biological treatment of greywater. Water Sci. Technol. 2007, 56, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, H.; Hanæus, J. Hazardous substances in separately collected grey-and blackwater from ordinary Swedish households. Sci. Total Environ. 2005, 348, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.E.; Quarmby, J.; Stephenson, T. Role of micronutrients in activated sludge-based biotreatment of industrial effluents. Biotechnol. Adv. 1999, 17, 49–70. [Google Scholar] [CrossRef]
- Fowdar, H.S.; Hatt, B.E.; Breen, P.; Cook, P.L.; Deletic, A. Designing living walls for greywater treatment. Water Res. 2017, 110, 218–232. [Google Scholar] [CrossRef]
- Finley, S.; Barrington, S.; Lyew, D. Reuse of domestic greywater for the irrigation of food crops. Water Air Soil Pollut. 2009, 199, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Shafy, H.I.; Al-Sulaiman, A.M. Assessment of physico-chemical processes for treatment and reuse of greywater. Egypt. J. Chem. 2014, 57, 215–231. [Google Scholar] [CrossRef] [Green Version]
- Hourlier, F.; Masse, A.; Jaouen, P.; Lakel, A.; Gerente, C.; Faur, C.; Le Cloirec, P. Formulation of synthetic greywater as an evaluation tool for wastewater recycling technologies. Environ. Technol. 2010, 31, 215–223. [Google Scholar] [CrossRef]
- Ghaitidak, D.M.; Yadav, K.D. Characteristics and treatment of greywater—A review. Environ. Sci. Pollut. Res. 2013, 20, 2795–2809. [Google Scholar] [CrossRef]
- Ramprasad, C.; Smith, C.S.; Memon, F.A.; Philip, L. Removal of chemical and microbial contaminants from greywater using a novel constructed wetland: GROW. Ecol. Eng. 2017, 106, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Wurochekke, A.A.; Harun, N.A.; Mohamed, R.M.S.R.; Kassim, A.H.B.M. Constructed wetland of Lepironiaarticulata for household greywater treatment. APCBEE Procedia 2014, 10, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Al-Atawneh, N.; Mahmoud, N.; van der Steen, P.; Lens, P.N. Wastewater characteristics in partially sealed cesspit: Case study from Beit Dajan, Palestine. Linnaeus Eco-Tech 2014, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.F.; Avery, L.; Winward, G.; Jeffrey, P.; Smith, C.S.; Liu, S.; Memon, F.A.; Jefferson, B. Constructed wetlands for urban grey water recycling. Int. J. Environ. Pollut. 2008, 33, 93. [Google Scholar] [CrossRef]
- Oteng-Peprah, M.; Acheampong, M.A.; DeVries, N.K. Greywater characteristics, treatment systems, reuse strategies and user perception—A review. Water Air Soil Pollut. 2018, 229, 255. [Google Scholar] [CrossRef] [Green Version]
- Poyatos, J.M.; Muñio, M.M.; Almecija, M.C.; Torres, J.C.; Hontoria, E.; Osorio, F. Advanced oxidation processes for wastewater treatment: State of the art. Water Air Soil Pollut. 2010, 205, 187–204. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Kosma, C.; Albanis, T.; Konstantinou, I. An overview of homogeneous and heterogeneous photocatalysis applications for the removal of pharmaceutical compounds from real or synthetic hospital wastewaters under lab or pilot scale. Sci. Total Environ. 2021, 765, 144163. [Google Scholar] [CrossRef]
- Zhan, M.; Yang, X.; Xian, Q.; Kong, L. Photosensitized degradation of bisphenol A involving reactive oxygen species in the presence of humic substances. Chemosphere 2006, 63, 378–386. [Google Scholar] [CrossRef]
- Carlos, L.; Martire, D.O.; Gonzalez, M.C.; Gomis, J.; Bernabeu, A.; Amat, A.M.; Arques, A. Photochemical fate of a mixture of emerging pollutants in the presence of humic substances. Water Res. 2012, 46, 4732–4740. [Google Scholar] [CrossRef]
- Marin, M.L.; Santos-Juanes, L.; Arques, A.; Amat, A.M.; Miranda, M.A. Organic photocatalysts for the oxidation of pollutants and model compounds. Chem. Rev. 2012, 112, 1710–1750. [Google Scholar] [CrossRef]
- Barbieri, Y.; Massad, W.A.; Díaz, D.J.; Sanz, J.; Amat-Guerri, F.; García, N.A. Photodegradation of bisphenol A and related compounds under natural-like conditions in the presence of riboflavin: Kinetics, mechanism and photoproducts. Chemosphere 2008, 73, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Escalada, J.P.; Pajares, A.; Gianotti, J.; Massad, W.A.; Bertolotti, S.; Amat-Guerri, F.; García, N.A. Dye-sensitized photodegradation of the fungicide carbendazim and related benzimidazoles. Chemosphere 2006, 65, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Haya, R.; Miranda, M.A.; Marin, M.L. Type I vs. Type II photodegradation of pollutants. Catal. Today 2018, 313, 161–166. [Google Scholar] [CrossRef]
- Martinez-Haya, R.; Gomis, J.; Arques, A.; Marin, M.L.; Amat, A.M.; Miranda, M.A. Time-resolved kinetic assessment of the role of singlet and triplet excited states in the photocatalytic treatment of pollutants at different concentrations. Appl. Catal. B Environ. 2017, 203, 381–388. [Google Scholar] [CrossRef]
- Martinez-Haya, R.; Gomis, J.; Arques, A.; Amat, A.M.; Miranda, M.A.; Marin, M.L. Direct detection of the triphenylpyrylium-derived short-lived intermediates in the photocatalyzed degradation of acetaminophen, acetamiprid, caffeine and carbamazepine. J. Hazard. Mater. 2018, 356, 91–97. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ollis, D.F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Zhang, X.; Tryk, D.A. Heterogeneous photocatalysis: From water photolysis to applications in environmental cleanup. Int. J. Hydrogen Energy 2007, 32, 2664–2672. [Google Scholar] [CrossRef]
- Fu, L.; Wu, C.; Zhou, Y.; Zuo, J.; Song, G.; Tan, Y. Ozonation reactivity characteristics of dissolved organic matter in secondary petrochemical wastewater by single ozone, ozone/H2O2, and ozone/catalyst. Chemosphere 2019, 233, 34–43. [Google Scholar] [CrossRef]
- Ahmad, M.; Chen, S.; Ye, F.; Quan, X.; Afzal, S.; Yu, H.; Zhao, X. Efficient photo-Fenton activity in mesoporous MIL-100 (Fe) decorated with ZnO nanosphere for pollutants degradation. Appl. Catal. B Environ. 2019, 245, 428–438. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S. Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A review. J. Environ. Chem. Eng. 2018, 6, 6088–6107. [Google Scholar] [CrossRef]
- Lee, C.M.; Palaniandy, P.; Dahlan, I. Pharmaceutical residues in aquatic environment and water remediation by TiO2 heterogeneous photocatalysis: A review. Environ. Earth Sci. 2017, 76, 611. [Google Scholar] [CrossRef]
- Ye, S.; Yan, M.; Tan, X.; Liang, J.; Zeng, G.; Wu, H.; Wang, H. Facile assembled biochar-based nanocomposite with improved graphitization for efficient photocatalytic activity driven by visible light. Appl. Catal. B Environ. 2019, 250, 78–88. [Google Scholar] [CrossRef]
- Akerdi, A.G.; Bahrami, S.H. Application of heterogeneous nano-semiconductors for photocatalytic advanced oxidation of organic compounds: A review. J. Environ. Chem. Eng. 2019, 7, 103283. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Nottrott, A.; Kleissl, J.; Washom, B. Energy dispatch schedule optimization and cost benefit analysis for grid-connected, photovoltaic-battery storage systems. Renew. Energy 2013, 55, 230–240. [Google Scholar] [CrossRef]
- Lewis, N.S. Toward cost-effective solar energy use. Science 2007, 315, 798–801. [Google Scholar] [CrossRef] [Green Version]
- Bouadila, S.; Kooli, S.; Lazaar, M.; Skouri, S.; Farhat, A. Performance of a new solar air heater with packed-bed latent storage energy for nocturnal use. Appl. Energy 2013, 110, 267–275. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Corrigan, N.; Shanmugam, S.; Xu, J.; Boyer, C. Photocatalysis in organic and polymer synthesis. Chem. Soc. Rev. 2016, 45, 6165–6212. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ojha, A.; Borthakur, A.; Singh, R.; Lahiry, D.; Tiwary, D.; Mishra, P.K. Emerging trends in photodegradation of petrochemical wastes: A review. Environ. Sci. Pollut. Control Ser. 2016, 23, 22340–22364. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Vishnu, M.C.; Sharma, K.K.; Borthakur, A.; Srivastava, P.; Pal, D.B.; Tiwary, D.; Mishra, P.K. Photocatalytic degradation of Acid Red dye stuff in the presence of activated carbon-TiO2 composite and its kinetic enumeration. J. Water Process Eng. 2016, 12, 20–31. [Google Scholar] [CrossRef]
- Zhou, C.; Luo, J.; Chen, Q.; Jiang, Y.; Dong, X.; Cui, F. Titanate nanosheets as highly efficient non-light-driven catalysts for degradation of organic dyes. Chem. Commun. 2015, 51, 10847–10849. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Mehta, S.K.; Kansal, S.K. Highly effective Fe-doped TiO2 nanoparticles photocatalysts for visible-light driven photocatalytic degradation of toxic organic compounds. J. Colloid Interface Sci. 2015, 450, e213–e223. [Google Scholar] [CrossRef]
- Lee, K.M. Degradation of Dyes Using Zinc Oxide as The Photocatalyst. Ph.D. Thesis, Universiti Putra Malaysia, Selangor, Malaysia, 2008. [Google Scholar]
- Meephon, S.; Rungrotmongkol, T.; Puttamat, S.; Praserthdam, S.; Pavarajarn, V. Heterogeneous photocatalytic degradation of diuron on zinc oxide: Influence of surface-dependent adsorption on kinetics, degradation pathway, and toxicity of intermediates. J. Environ. Sci. 2019, 84, 97–111. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Degradation of wastewaters containing organic dyes photocatalysed by zinc oxide: A review. Desalination Water Treat. 2012, 41, 131–169. [Google Scholar] [CrossRef]
- Ghosh, M.; Manoli, K.; Shen, X.; Wang, J.; Ray, A.K. Solar photocatalytic degradation of caffeine with titanium dioxide and zinc oxide nanoparticles. J. Photochem. Photobiol. A Chem. 2019, 377, 1–7. [Google Scholar] [CrossRef]
- Saray, A.M.; Zare-Dehnavi, N.; Jamali-Sheini, F.; Yousefi, R. Type-II p (SnSe)-n(g-C3N4) heterostructure as a fast visible-light photocatalytic material: Boosted by an efficient interfacial charge transfer of p-n heterojunction. J. Alloys Compd. 2020, 829, 154436. [Google Scholar] [CrossRef]
- Nagaraja, R.; Kottam, N.; Girija, C.R.; Nagabhushana, B.M. Photocatalytic degradation of Rhodamine B dye under UV/solar light using ZnOnanopowder synthesized by solution combustion route. Powder Technol. 2012, 215–216, 91–97. [Google Scholar] [CrossRef]
- Birben, N.C.; Uyguner-Demirel, C.S.; Bekbolet, M. Photocatalytic removal of microbiological consortium and organic matter in greywater. Catalysts 2016, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Boyjoo, Y.; Ang, M.; Pareek, V. Photocatalytic treatment of shower water using a pilot scale reactor. Int. J. Photoenergy 2012, 2012, 578916. [Google Scholar] [CrossRef]
- Priyanka, K.; Remya, N.; Behera, M. Greywater treatment using modified solar photocatalyst- degradation, kinetics, pathway and toxicity analysis. Sep. Purif. Technol. 2020, 251, 117319. [Google Scholar] [CrossRef]
- Priyanka, K.; Remya, N.; Behera, M. Comparison of titanium dioxide based catalysts preparation methods in the mineralisation and nutrients removal from greywater by solar photocatalysis. J. Clean. Prod. 2019, 235, 1–10. [Google Scholar] [CrossRef]
- Liu, C.; Lei, Z.; Yang, Y.; Zhang, Z. Preliminary trial on degradation of waste activated sludge and simultaneous hydrogen production in a newly-developed solar photocatalytic reactor with AgX/TiO2-coated glass tubes. Water Res. 2013, 47, 4986–4992. [Google Scholar] [CrossRef]
- Saran, S.; Arunkumar, P.; Manjari, G.; Devipriya, S.P. Reclamation of grey water for non-potable purposes using pilot-scale solar photocatalytic tubular reactors. Environ. Technol. 2019, 40, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A.; Varghese, S.; Nair, S.S. Photocatalytic water treatment by titanium dioxide: Recent updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, R.P.; Dantas, R.F.; Wender, H.; Bayarri, B.; González, O.; Giménez, J.; Machulek, A., Jr. Photocatalytic treatment of metoprolol with B-doped TiO2: Effect of water matrix, toxicological evaluation and identification of intermediates. Appl. Catal. B Environ. 2015, 176, 173–182. [Google Scholar] [CrossRef]
- Duarah, R.; Karak, N. Hyperbranched polyurethane/reduced carbon dot-zinc oxide nanocomposite-mediated solar-assisted photocatalytic degradation of organic contaminant: An approach towards environmental remediation. Chem. Eng. J. 2019, 370, 716–728. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Cho, E.; Sreedhar, A.; Noh, J.S. Mixed-dimensional, three-level hierarchical nanostructures of silver and zinc oxide for fast photocatalytic degradation of multiple dyes. J. Catal. 2019, 371, 1–9. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.H.; Holmes, A.; Haridass, I.N.; Sanchez, W.Y.; Studier, H.; Grice, J.E.; Roberts, M.S. Support for the safe use of zinc oxide nanoparticle sunscreens: Lack of skin penetration or cellular toxicity after repeated application in volunteers. J. Investig. Dermatol. 2019, 139, 308–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Poulios, I.; Kositzi, M.; Pitarakis, K.; Beltsios, S.; Oikonomou, I. Photocatalytic oxidation of Auramine O in the presence of semiconducting oxides. Int. J. Environ. Pollut. 2000, 28, 33–44. [Google Scholar] [CrossRef]
- Kaneva, N.V.; Dimitrov, D.T.; Dushkin, C.D. Effect of nickel doping on the photocatalytic activity of ZnO thin films under UV and visible light. Appl. Surf. Sci. 2011, 257, 8113–8120. [Google Scholar] [CrossRef]
- Pannee, A.; Chradda, B.; Wichien, S. Photocatalytic Activity under Solar Irradiation of Silver and Copper Doped Zincoxide: Photodeposition Versus Liquid Impregnation Methods. J. Appl. Sci. 2012, 12, 1809–1816. [Google Scholar] [CrossRef]
- Yashni, G.; Al Gheethi, A.; Mohamed, R.M.S.R.; Arifin, S.N.H.; Shanmugan, V.A.; Kassim, A.H.M. Photocatalytic degradation of basic red 51 dye in artificial bathroom greywater using zinc oxide nanoparticles. Mater. Today Proc. 2020, 31, 136–139. [Google Scholar] [CrossRef]
- Lam, S.M.; Kee, M.W.; Sin, J.C. Influence of PVP surfactant on the morphology and properties of ZnO micro/nanoflowers for dye mixtures and textile wastewater degradation. Mater. Chem. Phys. 2018, 212, 35–43. [Google Scholar] [CrossRef]
- Boussatha, N.; Gilliot, M.; Ghoualem, H.; Martin, J. Formation of nanogranular ZnO ultrathin films and estimation of their performance for photocatalytic degradation of amoxicillin antibiotic. Mater. Res. Bull. 2017, 99, 485–490. [Google Scholar] [CrossRef]
- Chin, Y.H.; Sin, J.C.; Lam, S.M. A facile route for fabrication of hierarchical porous Nb2O5/ZnO composites with enhanced photocatalytic degradation of palm oil mill effluent. Mater. Lett. 2018, 216, 8–11. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Matarangolo, M. ZnO supported on zeolite pellets as efficient catalytic system for the removal of caffeine by adsorption and photocatalysis. Sep. Purif. Technol. 2017, 193, 303–310. [Google Scholar] [CrossRef]
- Shinde, D.R.; Tambade, P.S.; Chaskar, M.G.; Gadave, K.M. Photocatalytic degradation of Dyes in Water by Analytical Reagent Grade Photocatalysts—A comparative study. Drink. Water Eng. Sci. Discuss. 2017, 10, 1–16. [Google Scholar] [CrossRef]
- Mohamed, S.K.; Hegazy, S.H.; Abdelwahab, N.A.; Ramadan, A.M. Coupled adsorption-photocatalytic degradation of crystal violet under sunlight using chemically synthesized grafted sodium alginate/ZnO/graphene oxide composite. Int. J. Biol. Macromol. 2017, 108, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Zarei, N.; Behnajady, M.A. Synthesis, characterization, and photocatalytic activity of sol–gel prepared Mg/ZnO nanoparticles. Desalination Water Treat. 2016, 57, 16855–16861. [Google Scholar] [CrossRef]
- Assi, N.; Mohammadi, A.; Manuchehri, Q.S.; Walker, R.B. Synthesis and characterization of ZnO nanoparticle synthesized by a microwave-assisted combustion method and catalytic activity for the removal of ortho-nitrophenol. Desalination Water Treat. 2015, 54, 1939–1948. [Google Scholar] [CrossRef]
- Faisal, M.; Ibrahim, A.A.; Harraz, F.A.; Bouzid, H.; Al-Assiri, M.S.A.A. Ismail, SnO2 doped ZnO nanostructures for highly efficient photocatalyst. J. Mol. Catal. A Chem. 2015, 397, 19–25. [Google Scholar] [CrossRef]
- Hamdia, A.; Boufib, S.; Bouattour, S. Phthalocyanine/chitosan-TiO2 photocatalysts: Characterization and photocatalytic activity. Appl. Surf. Sci. 2015, 339, 128–136. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; el Azab, W.I.M.; Ali, H.R.; Mansour, M.S.M. Green synthesis and characterization of ZnO nanoparticles for photocatalytic degradation of anthracene. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 045012. [Google Scholar] [CrossRef]
- Abdiryim, T.; Ali, A.; Jamal, R.; Osman, Y.; Zhang, Y. A facile solid-state heating method for preparation of poly (3,4-ethelenedioxythiophene)/ZnO nanocomposite and photocatalytic activity. Nanoscale 2014, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Ghaedi, M.; Ansari, A.; Habibi, M.H.; Asghari, A.R. Removal of malachite green from aqueous solution by zinc oxide nanoparticle loaded on activated carbon: Kinetics and isotherm study. J. Ind. Eng. Chem. 2014, 20, 17–28. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Aazam, E. Synthesis and characterization of Pt-ZnO-hydroxyapatite nanoparticles for photocatalytic degradation of benzene under visible light. Desalination Water Treat. 2013, 51, 6082–6090. [Google Scholar] [CrossRef]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Enhanced photocatalytic activity of iron doped zinc oxide nanowires for water decontamination. Surf. Coat. Technol. 2013, 217, 119–123. [Google Scholar] [CrossRef]
- Rezaee, A.; Masoumbeigi, H.; Soltani, R.D.C.; Khataee, A.R.; Hashemiyan, S. Photocatalytic decolorization of methylene blue using immobilized ZnO nanoparticles prepared by solution combustion method. Desalination Water Treat. 2012, 44, 174–179. [Google Scholar] [CrossRef]
- Whang, T.-J.; Hsieh, M.-T.; Chen, H.-H. Visible-light photocatalytic degradation of methylene blue with laserinduced Ag/ZnO nanoparticles. Appl. Surf. Sci. 2012, 258, 2796–2801. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, T.; Zhou, H. Applied surface science hdrothermal preparation of ZnO-reduced graphene oxide hybrid with high performance in photocatalytic degradation. Appl. Surf. Sci. 2012, 258, 6204–6211. [Google Scholar] [CrossRef]
- Wang, L.S.; Xiao, M.W.; Huang, X.J.; Wu, Y.D. synthesis, characterization, and photocatalytic activities of titanate nanotubes surface-decorated by zinc oxide nanoparticles. J. Hazard. Mater. 2009, 161, 49–54. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Diyauddeen, B.H.; Daud, W.M.A.W.; Abdul Aziz, A.R. Treatment technologies for petroleum refinery effluents: A review. Process Saf. Environ. Protect. 2011, 89, 95–105. [Google Scholar] [CrossRef]
- Gao, H.T.; Si, C.D.; Zhou, J.; Liu, G.J. Sound assisted photocatalytic degradation of formaldehyde in fluidized bed reactor. J. Taiwan Inst. Chem. Eng. 2011, 42, 108–113. [Google Scholar] [CrossRef]
- Gayaa, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Turolla, A.; Santoro, D.; de Bruyn, J.R.; Crapulli, F.; Antonelli, M. Nanoparticle scattering characterization and mechanistic modelling of UVeTiO2 photocatalytic reactors using computational fluid dynamics. Water Res. 2016, 88, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Saggioro, E.M.; Oliveira, A.S.; Pavesi, T.; Maia, C.G.; Ferreira, L.F.V.; Moreira, J.C. Use of titanium dioxide photocatalysis on the remediation of model textile wastewaters containing azo dyes. Molecules 2011, 16, 10370–10386. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kansal, S.K. Bi2WO6 nanocuboids: An efficient visible light active photocatalyst for the degradation of levofloxacin drug in aqueous phase. Chem. Eng. J. 2016, 302, 194–203. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Jallouli, N.; Elghniji, K.; Trabelsi, H.; Ksibi, M. Photocatalytic degradation of paracetamol on TiO2 nanoparticles and TiO2/cellulosic fiber under UV and sunlight irradiation. Arab. J. Chem. 2017, 10, S3640–S3645. [Google Scholar] [CrossRef] [Green Version]
- Cavigli, L.; Bogani, F.; Vinattieri, A.; Faso, V.; Baldi, G. Volume versus surface-mediated recombination in anatase TiO2 nanoparticles. J. Appl. Phys. 2009, 106, 053516. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G. A review on the factors affecting the photocatalytic degradation of hazardous materials. Mater. Sci. Eng. Int. J. 2017, 3, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Gogniat, G.; Thyssen, M.; Denis, M.; Pulgarin, C.; Dukan, S. The bactericidal effect of TiO2 photocatalysis involves adsorption onto catalyst and the loss of membrane integrity. FEMS Microbiol. Lett. 2006, 258, 18–24. [Google Scholar] [CrossRef] [Green Version]

| Parameters | Kitchen | Bathroom | Laundry | Light Greywater | D Ark Greywater |
|---|---|---|---|---|---|
| Temperature | 24.4–30.9 °C | 25.8–29.0 °C | 22.4–35.0 °C | 13.4–29.0 °C | 22.4–35.0 °C |
| pH | 5.9–7.4 | 6.4–8.1 | 7.1–10 | 4.90–8.53 | 5.00–10.33 |
| Total Suspended Solids (mg/L) | 134–1300 | 7–505 | 68–465 | 7–793 | 11–4564 |
| Chemical Oxygen Demand (mg/L) | 26–2050 | 100–633 | 231–2950 | 23–1489 | 58–8071 |
| Biological Oxygen Demand (mg/L) | 536–1460 | 50–300 | 48–472 | 20–673 | 44–3330 |
| Total Nitrogen (mg/L) | 11.4–74 | 3.6–19.4 | 1.1–40.3 | 1.3–148.0 | 0.5–65.0 |
| Total Phosphorus (mg/L) | 2.9–>74 | 0.11–>48.8 | ND–>171 | 0.1–60.0 | 0.2–187.0 |
| Turbidity (NTU) | 210–357 | 19–375 | 34–510 | 13–375 | 34–510 |
| Parameters | Country | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Australia [34] | Canada [35] | Egypt [36] | France [37] | Germany [38] | India [38,39] | Israel [38] | Jordan [38] | Malaysia [40] | Palestine [41] | Sweden [38] | UK [42] | USA [38] | Yemen [38] | |
| Total Suspended Solids (mg/L) | 74 | - | 70–202 | 23–80 | - | 53.80–788.00 | 30–298 | 23–845 | 19–175 | 304–4952 | - | 37–153 | 17 | 511 |
| BOD5 (mg/L) | 104 | - | 220–375 | 85–155 | 59 | 17.10–290.00 | 74–890 | 36–1240 | 1.1–309 | 407–512 | 425 | 8.7–155 | 86 | 518 |
| COD (mg/L) | - | 278–435 | 301–557 | 176–323 | 109 | 43.90–733.00 | 840–1340 | 58–2263 | 16–1103 | 863–1240 | 890 | 33–587 | - | 2000 |
| pH | - | 6.7–7.6 | 6.05–7.96 | 6.46–7.48 | 7.6 | 5.90–8.34 | 6.3–8.2 | 6.4–9 | 6.5–7.2 | 5.8–8.26 | 7.8 | 6.6–7.8 | 6.4 | 6 |
| Total Phosporus (mg/L) | 3 | 0.24–1.02 | 8.4–12.1 | - | 1.6 | 0.01–3.84 | 1.9–48 | 0.69–51.58 | 4.5 | 5.8–15.16 | 4.2 | 0.4–0.9 | 4 | - |
| Total Nitrogen (mg/L) | 5.3 | - | - | - | 15.2 | 17.00–28.82 | 10–34.3 | 6.44–61 | - | 111–322 | 75 | 4.6–10.4 | 13.5 | - |
| Total Coliforms (MPN/100 mL) | - | - | - | 1.7 × 108–1.4 × 109 | - | - | - | 250–1.0 × 107 | - | - | - | 1.8 × 103–2.2 × 107 | - | - |
| Faecal Coliforms (MPN/100 mL) | - | 4.7 × 104– 8.3 × 105 | - | 4.0 × 103–5.7 × 106 | 1.4 × 105 | 5.0 × 101–1.2 × 102 | 3.5 × 104–4.0 × 106 | 1.3 × 101–3.0 × 105 | 0–1.9 × 106 | - | 1.7 × 105 | 1.0 × 101–2.2 × 105 | - | 1.9 × 107 |
| E. coli (MPN/100 mL) | - | - | - | - | - | - | 5.0 × 104 | 2.0 × 105 | 0–6.7 × 103 | - | - | 1.0 × 101–3.9 × 105 | 5.4 × 102 | - |
| Source of Greywater | Type of Catalyst | Method /Supplier | Nature of Lamp | Power (W) | Intensity (kilo lux)/(W/m2) | Time (h)/(min), Reactor Design with Dimensions (L) | Findings | References | |
|---|---|---|---|---|---|---|---|---|---|
| Laundry water | TiO2 | Sigma-Aldrich | - | - | - | 150 min, UV-photoreactor | - | The optimum conditions were = pH = 5, photocatalyst amount = 0.1 gL−1 without compressed air sparging and initial Reactive Black 5 (RB5) concentration of a 1 ppm. Reaction time = 150 min, RB5 removal = 97%. Lesser removal (76%) of Reactive Black 5 from real greywater was observed after 330 min. The monitoring findings revealed 60% O&G, 54% COD, 69% BOD5, and 41% removal of TN. | [1] |
| Simulated greywater (Shower, hand basin, washing machine) | TiO2 P-25 | Evonik | UV-A lamp | 9 Watt/78 | Natural Sunlight | 250–300 min, Thermostated pyrex cell (0.5 L) | 350–400 nm | In photo-Fenton-mediated titania photocatalytic process, ~72% DOC removal was observed in the bench-scale treatment after 210 min, whereas under the same photocatalytic conditions but under solar light in pilot reactor, the DOC removal reached decreased to ~64%. | [6] |
| Hotel greywater Laundry greywater | TiO2 P-25 | Evonik Degussa (Aeroxide® P 25) | Hg lamp TQ 150Z1 (Heraeus Nobelight) | - | - | 160–400 min, Batch cylindrical glass photoreactor (1.0 L) | 200–700 nm | Treatment of greywater showed removal of 65% DOC after 150 min. Anionic surfactants were completely removed. | [4] |
| Synthetic greywater | TiO2 P-25 | Evonik P-25 | Black light fluorescent lamp (BLF) | 125 W | - | 0–180 min | 300–420 nm | The best dissolved organic carbon removal rates were obtained from greywater containing low OM and low anion content (L1). | [80] |
| Shower water | TiO2 | Sigma-Aldrich | UV mercury lamp | Primarc Ltd. (PM 3426, 800 W) | - | 6.5 h, Pilot-scale reactor (31 L) | Under optimum conditions, approximately 57% removal of total organic carbon (TOC) was achieved after 6 h: initial solution pH = 3, photocatalyst amount = 0.07 gL−1, flow rate of air = 1.8 Lmin−1, circulation rate of solution = 4.4 Lmin−1. | [81] |
| Source of Greywater | Type of Catalyst | Material/Method | Nature of Lamp | Power (W) | Intensity (kilo lux)/ (W/m2) | Time (h)/(min) | Reactor Design with Dimensions | Findings | References | |
|---|---|---|---|---|---|---|---|---|---|---|
| Residential apartment | Gravel-NP-TiO2 | Sol-gel method | Solar light | - | 18.3, 45.7 kilo lux | 6 h | - | Tray-type reactor | Significant removal was achieved by solar photocatalytic process: TOC removal was 93.7 % with 0.393 h−1 removal rate. Approximately 50% TKN was removed with photocatalytic oxidation to nitrate. 43% removal of nitrate was observed due to photocatalytic reduction. Toxicity of the treated greywater(for 30 min incubation) was reduced from the 13.6 to 4%. | [82] |
| Simulated greywater | M-TiO2 U-TiO2 NP-TiO2 NT-TiO2 | Microwave Ultrasonication Modified sol-gel | Visible light | Tungsten-halogen lamp 150 W | - | 3 h 6 h | 400–700 nm | Pencil-type immersion photo-reactor Solar photocatalytic reactor | The performance of different photocatalysts for the mineralisation was found to be NT-TiO2 > UeTiO2 > NP-TiO2M-TiO2 > P-25. The maximum mineralisation (~75%) was observed after 3 h in presence of NT-TiO2. Then activity for nitrate degradation was determined to beNT-TiO2 > UeTiO2~NP-TiO2 > M-TiO2 > P-25. The nitrate degradation was ~92.5 %. As compared to other photocatalytic systems, the NT-TiO2 was found to be most energy-efficient (~31.86 kWh (kgCOD)1). | [83] |
| Source | Catalyst | Reactor | Light Source | Findings | Reference |
|---|---|---|---|---|---|
| Bathroom and kitchen Greywater | Ag deposited TiO2 catalyst | tubular reactors | sunlight | In presence of bare and Ag-modified TiO2, the COD removal was ~32% and ~69%, respectively. The experiments were conducted under sunlight at neutral pH for 3 h. The findings suggest that silver deposition boosted the effectiveness of TiO2 photocatalysis by serving as electron sink and facilitating interfacial electron transport, minimizing the charge carrier recombination- and producing more ROS in Ag coated titanium dioxide as compared to bare titanium-dioxide. | [85] |
| Greywater | Silver and silver compounds (AgX) doped TiO2 film | fluidized tubular photocatalytic reactor (SFTPR) | sunlight | The AgX/TiO2 filmcoated reactor had a substantially higher rate of waste activated sludge (WAS) degradation, as measured by COD elimination, than the titanium-dioxide filmcoated reactor, with 69.1 percent and 45.3 percent respectively, in 72 h. | [84] |
| Greywater | TiO2 (coated on α-alumina) | Photoreactor | sunlight | Chen et al. used TiO2-coated –Al2O3 to treat wastewater originated from domestic and agricultural uses (COD was 36.27.4) before transferring them to a bench-scale wetland system. | [86] |
| Greywater | Boron-modified TiO2 | solar simulator | Sunlight | A total of 35% COD reduction was achieved in the UW trials. The consequent mineralization, on the other hand, was lower [12%], indicating that chemicals in the effluent are resistant to mineralization. | [87] |
| Organic Pollutant in Greywater | Type of Catalyst | Amount of Catalyst (g)/Time of Irradiation (h) | Catalyst Synthesis Method | Findings | References |
|---|---|---|---|---|---|
| MB | CdS/ZnO composites | 3/3 | Adsorption, interaction of successive ionic layers. Deposition in a chemical bath | Methylene blue degradation is estimated to be around 91%. | [95] |
| MO, Methyl green | ZnO / PVP composites | 0.1/4 | Coprecipitation | Both MO and methyl-green degraded at a rate of 82.7 percent and 99.5 percent. | [97] |
| Amoxycillin | ZnO ultrathin layers | 0.2/2 | Deposition of Sol-gel, and spin-coatings | The efficacy of degradation increased by about 65%. | [98] |
| Color | Nb2O5-ZnO-composite | -/4 | Chemical solution method with no smooth surfactants | Nb2O5/ZnO had ability to elutriate POME with a colour-removal rate of 100%. | [99] |
| Caffeine | ZnO/ZEO-composite | 25/2 | Impregnation method | UV light was able to remove nearly all of the caffeine. | [100] |
| Crystal violet, Methyl red and Basic blue | ZnO, SnO2 and TiO2 | -/- | NA | When compared to TiO2 and SnO2, ZnO had highest photocatalytic activity. | [101] |
| Crystal violet | ZnO-modified polymer nanocomposite | 0.1/5 | Chemical precipitation, free radical polymerization | The elimination efficiency was 94 percent in presence of sunlight and 84 percent in the absence of the sunlight, respectively. | [102] |
| RhB | Mg/ZnO nano-particles | -/2 | Sol-gel | The Mg/ZnO nano-particles photocatalytic activitywas controlled by their maximum content, with best removal attained at 2 weight percent Mg. | [103] |
| Orthonitrophenol | ZnO nano-particles | 0.05/5 | Microwave assisted combustion | 98 percent of the orthonitrophenol was removed. | [104] |
| MB | SnO2 doped ZnO nano-particles | 1/2 | Smooth chemical method | SnO2 doping boosts the photocatalytic activity of ZnO. | [105] |
| Aniline | Hybrid chitosanphthalocyanine- TiO2 | 0.04/1 | SolGel | Using chitosan-phthalocyanine-TiO2 as a hybrid photocatalyst resulted in more degradation. | [106] |
| Anthracene | ZnO nano-particles | 1/5 | Corriandrum Sativum by green synthesis method | Anthracene was photocatalytically degraded at a rate of 96%. | [107] |
| MB | Poly(Ethylenedioxy-Terthiophene)/ZnO Composites | -/5 | Ball mill | Using Poly(Ethylenedioxy-Terthiophene)/ZnO Composite produced by ball-milling and exposed to UV radiation, nearly 100 percent elimination was achieved. | [108] |
| Malachite green | ZnO nano-particles with activated carbon | 0.005/1 | Sol-gel method | The high-adsorption capacity of ZnO NPs loaded on activated carbon (322.58 mg g-1) enabled the removal of malachite green within twenty min of adsorption. | [109] |
| Benzene | Pt-ZnO hydroxyapatite nano-particles | -/- | Template and ultrasonication | The photocatalytic activity of Pt–ZnO NPs altered with hydroxyapatite for benzene removal from aqueous solution was significantly improved. | [110] |
| Dichloro-benzene Methyl orange | ZnO and Fe-doped ZnO (ZnO/Fe) nanowires | 0.1/4 | Hydro-thermal | ZnO/Fe nano-wires outperform ZnO in terms of photocatalytic activity. | [111] |
| RhB | ZnO nano-powder | 0.02/0.13 | Solution-combustion | Application of the ZnO catalyst resulted in the most dye decolorization (more than 95%). | [79] |
| MB | Surface-decorated ZnO nano-particles | -/0.8 | Solution-combustion | ZnO NPs-mediated colour removal from waste-water while lowering chemical oxygen requirement by 62 percent. | [112] |
| MB | Ag/ZnO nano-particles | 0.15/8 | Laser induction | MB has been degraded by 92 percent. | [113] |
| MB | ZnO/RGO | -/0.6 | Hydro-thermal | RGO was mixed with ZnO nano-particles to increase colour removal. | [114] |
| RhB | Zinc-oxide modified Titanate nanotubes (ZnO–TNTs) | 0.2/0.8 | Smooth chemical method | ZnO-TNTs nanocomposite outperformed both pure TNTs and ZnO for removal of RhB to. | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, M.; Fazal, D.B.; Ahmad, F.; Fazal, A.B.; Abdullah, A.Z.; Ahmed, M.; Qamar, M.; Rafatullah, M. Photocatalytic Degradation of Recalcitrant Pollutants of Greywater. Catalysts 2022, 12, 557. https://doi.org/10.3390/catal12050557
Aslam M, Fazal DB, Ahmad F, Fazal AB, Abdullah AZ, Ahmed M, Qamar M, Rafatullah M. Photocatalytic Degradation of Recalcitrant Pollutants of Greywater. Catalysts. 2022; 12(5):557. https://doi.org/10.3390/catal12050557
Chicago/Turabian StyleAslam, Mohammad, Dawood Bin Fazal, Faizan Ahmad, Abdullah Bin Fazal, Ahmad Zuhairi Abdullah, Mukhtar Ahmed, Mohammad Qamar, and Mohd Rafatullah. 2022. "Photocatalytic Degradation of Recalcitrant Pollutants of Greywater" Catalysts 12, no. 5: 557. https://doi.org/10.3390/catal12050557
APA StyleAslam, M., Fazal, D. B., Ahmad, F., Fazal, A. B., Abdullah, A. Z., Ahmed, M., Qamar, M., & Rafatullah, M. (2022). Photocatalytic Degradation of Recalcitrant Pollutants of Greywater. Catalysts, 12(5), 557. https://doi.org/10.3390/catal12050557







