Changes in Heavy Oil Saturates and Aromatics in the Presence of Microwave Radiation and Iron-Based Nanoparticles
Abstract
:1. Introduction
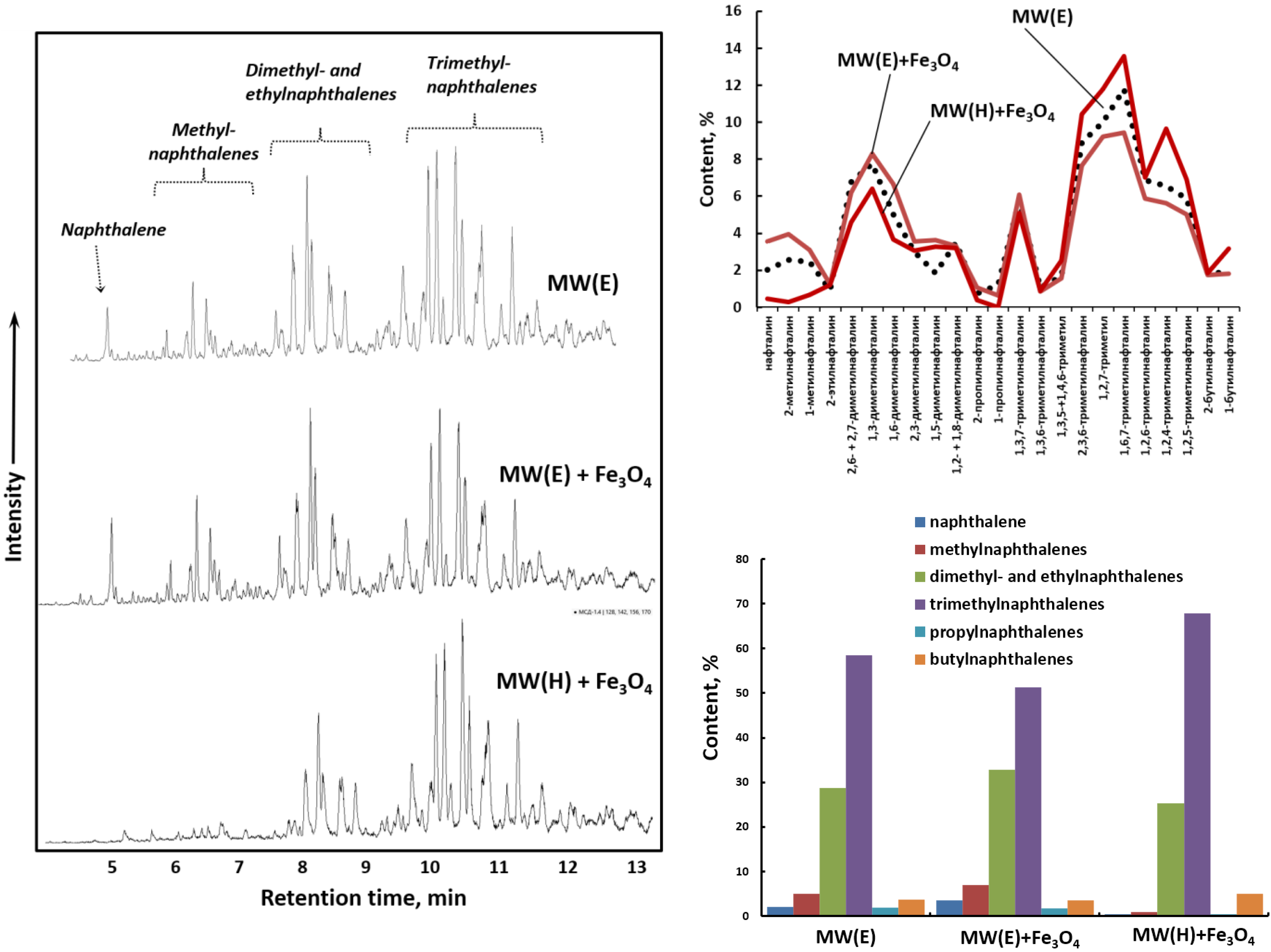
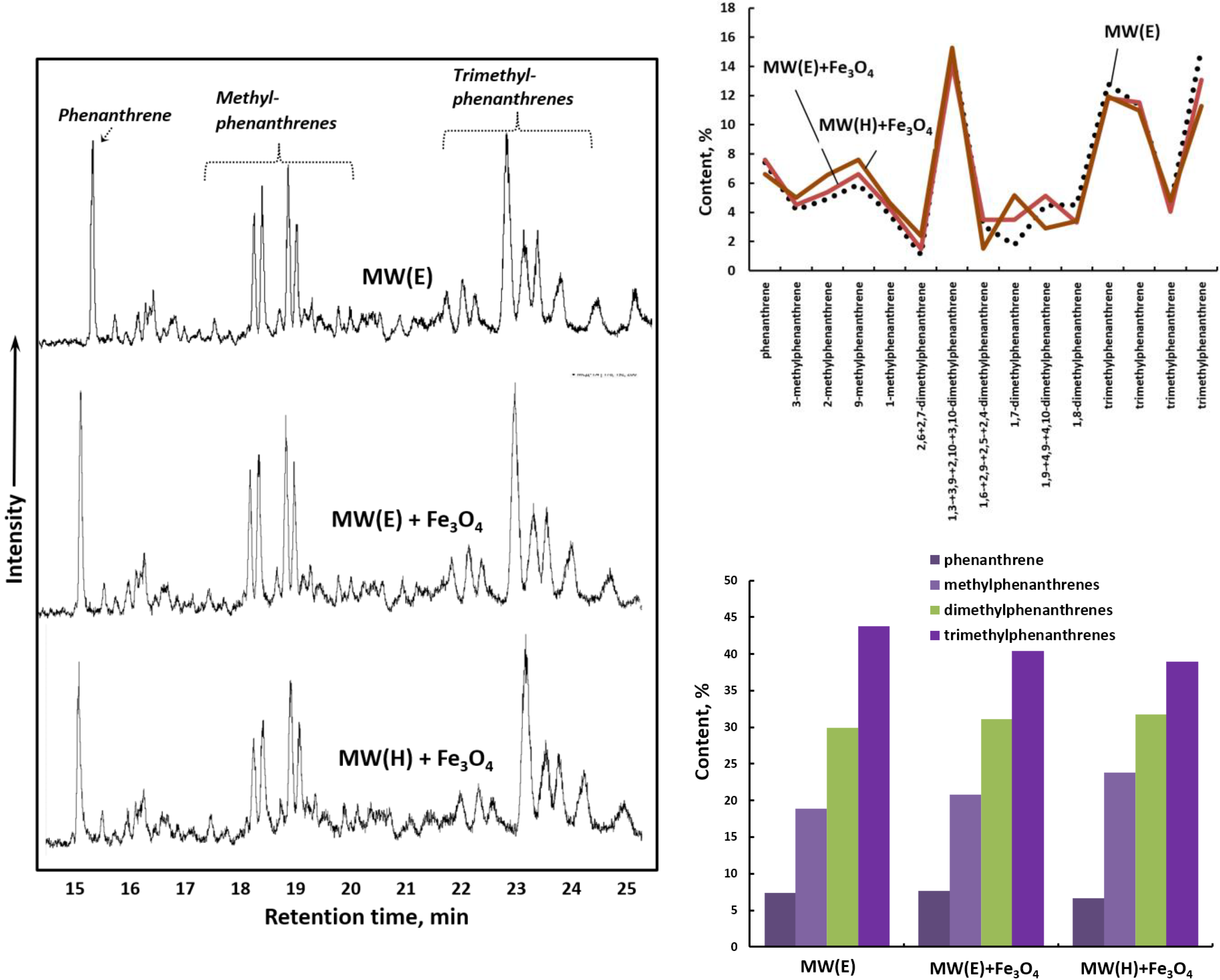
2. Results and Discussions
3. Experimental Section
3.1. Object of Research
3.2. Catalytic Agent
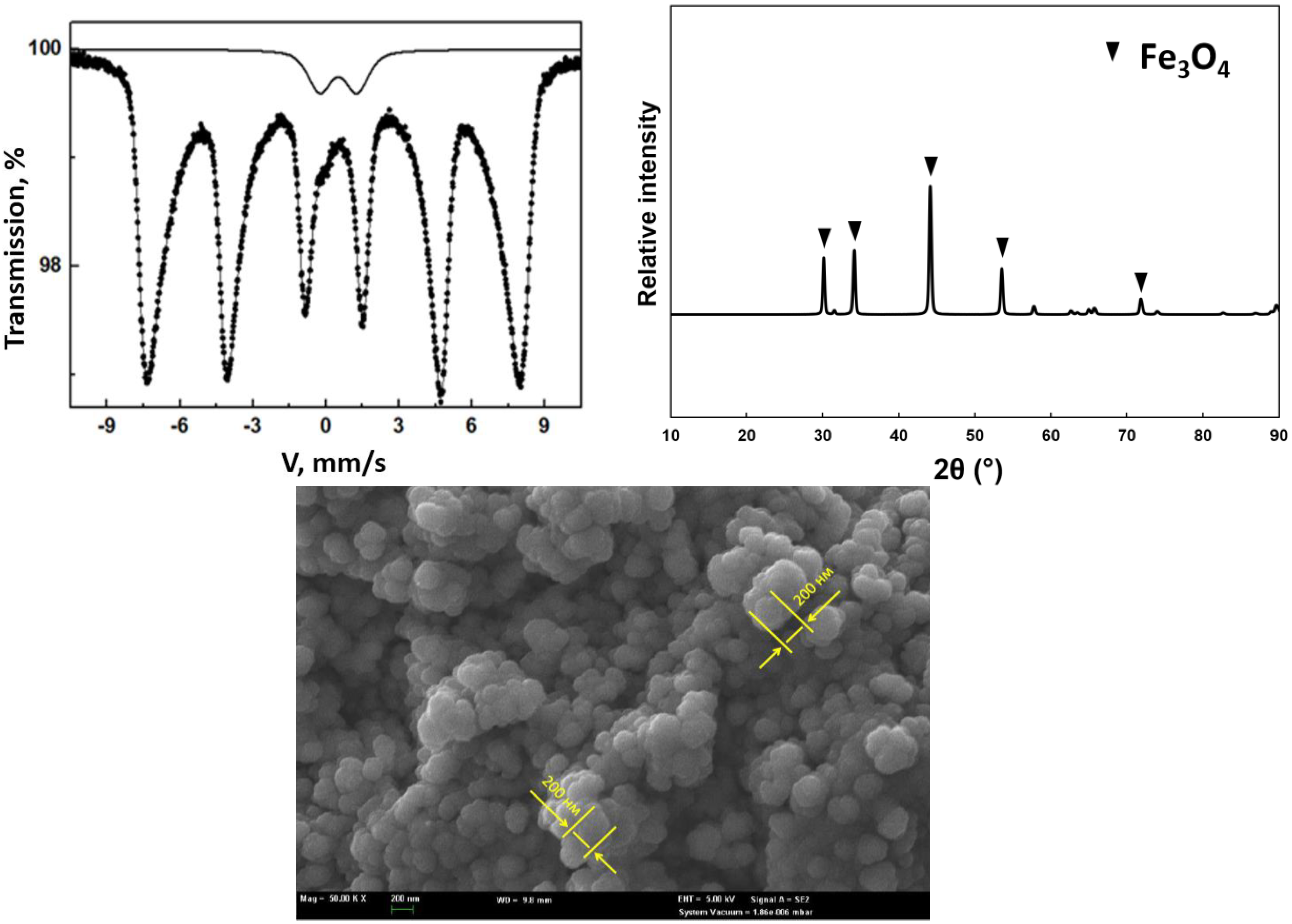
3.2.1. X-ray Diffraction Analysis
3.2.2. Scanning Electron Microscope (SEM) Analysis
3.2.3. Mössbauer Spectroscopy
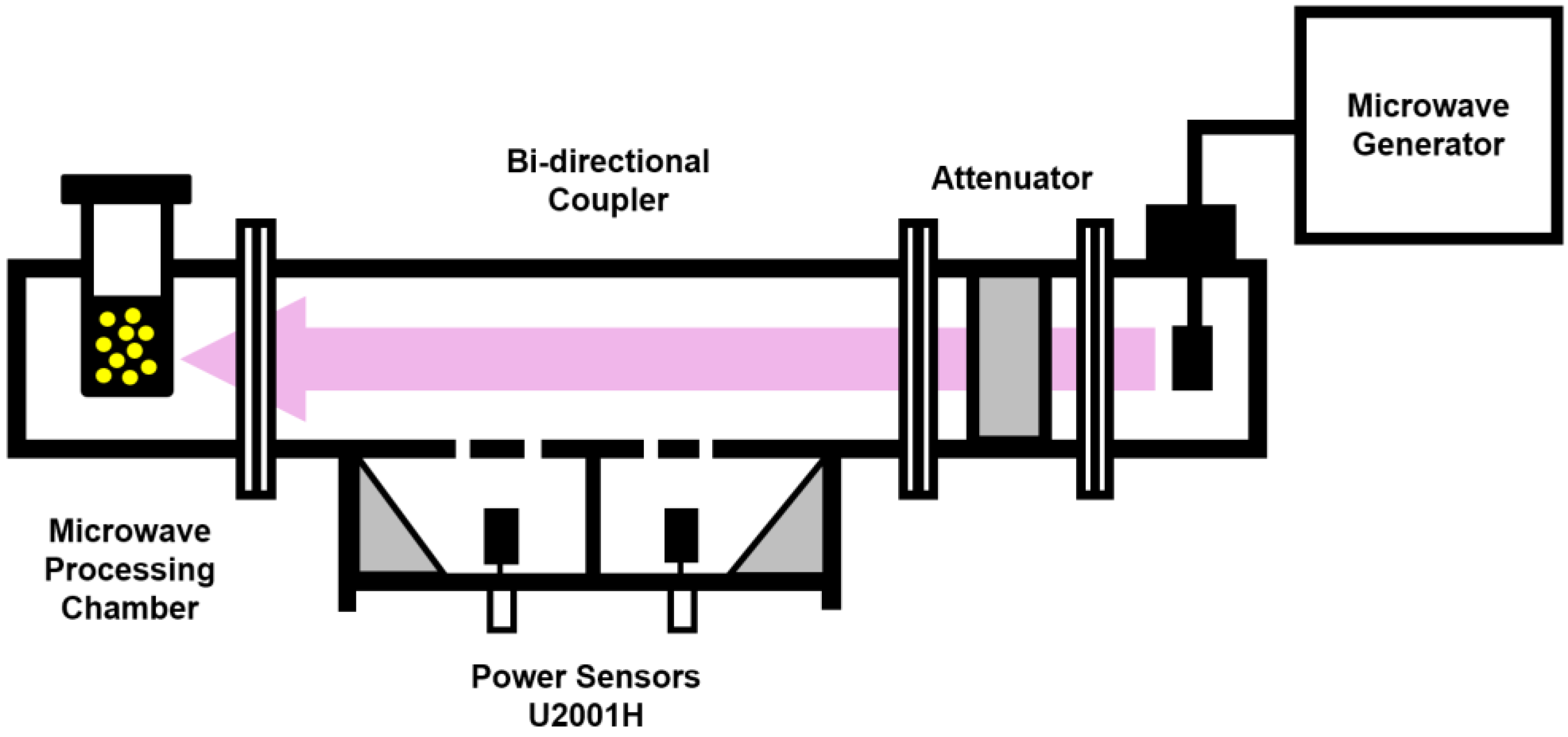
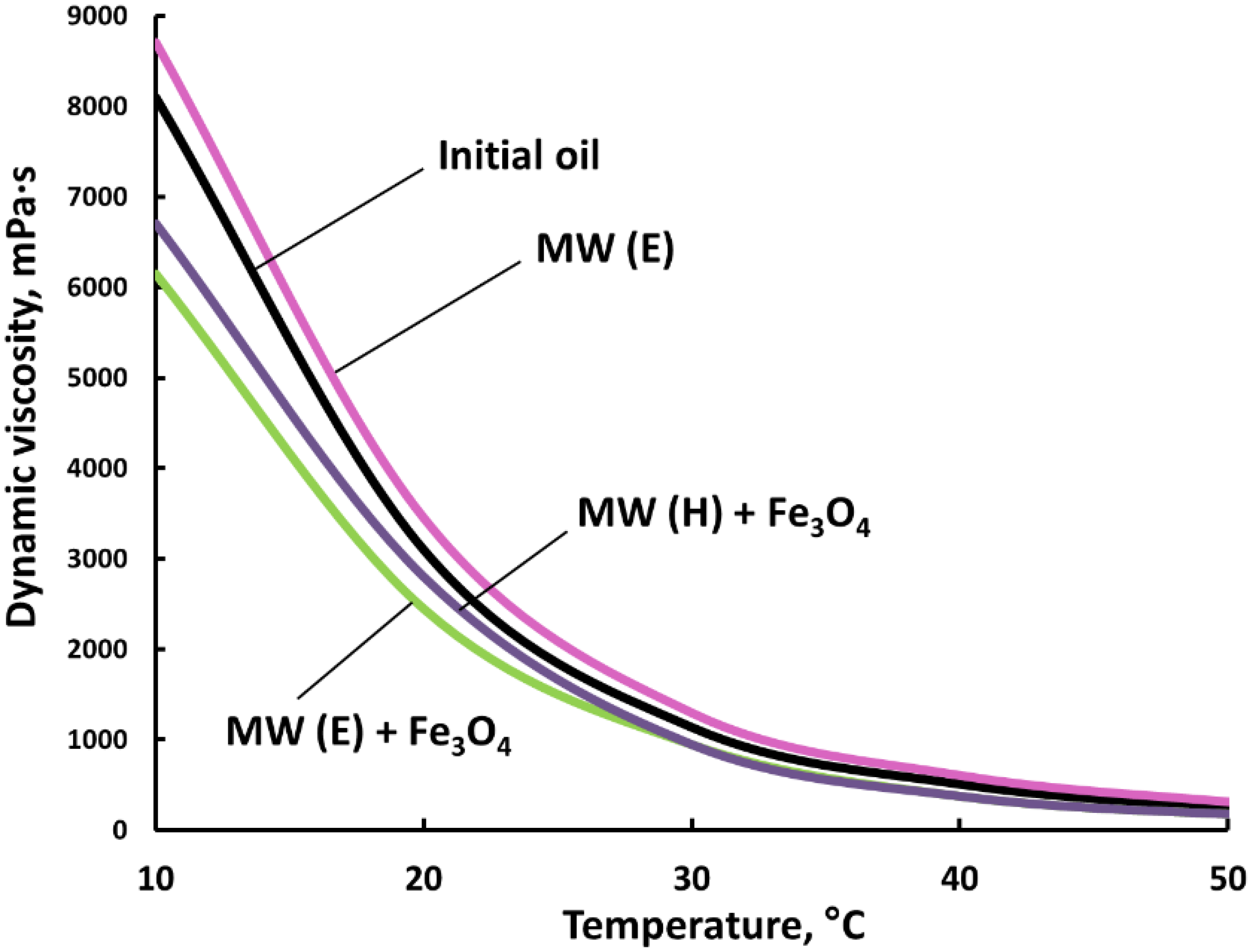
3.3. Dynamic Viscosity Measurements
3.4. SARA Analysis
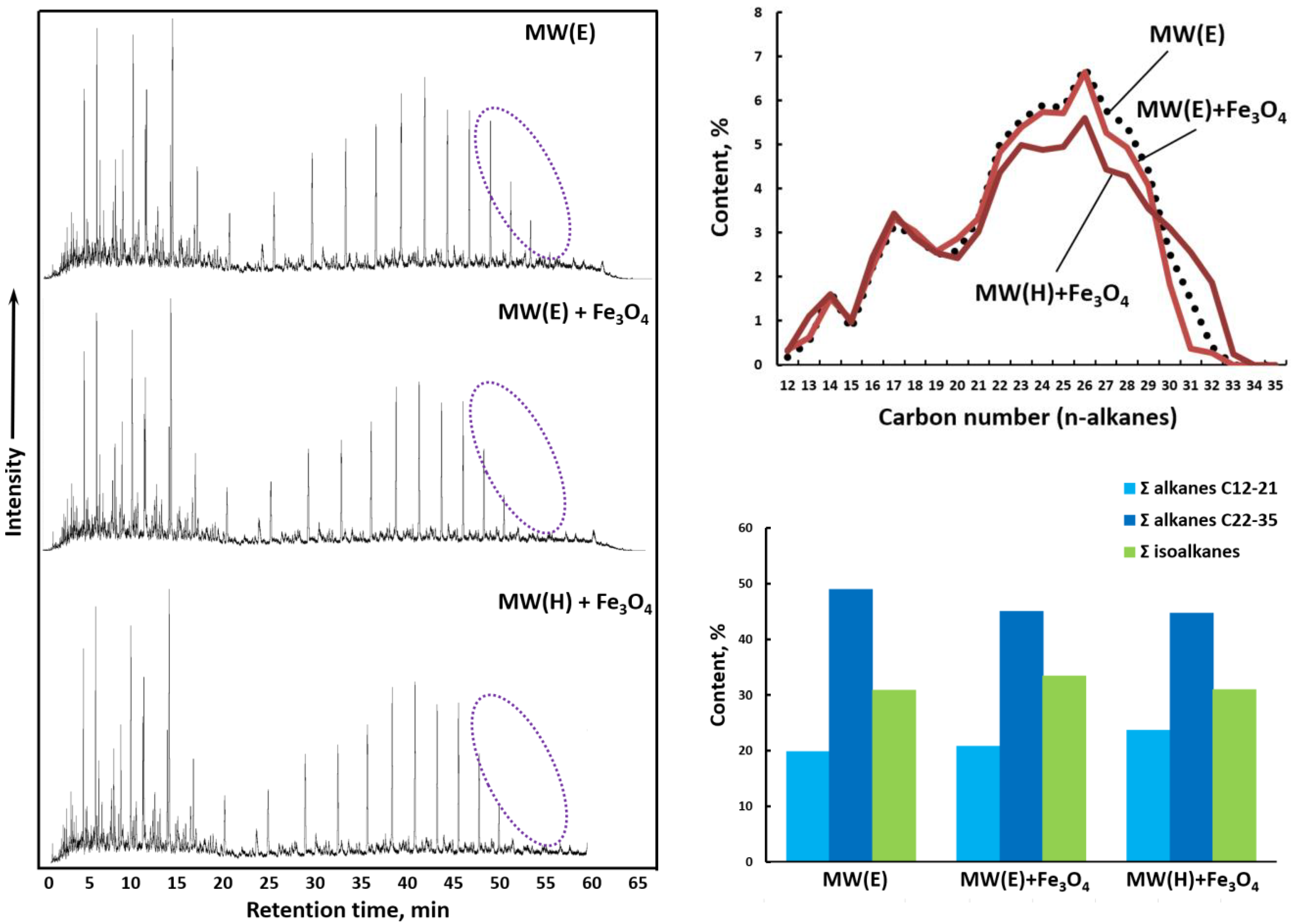
3.5. Gas Chromatography–Mass Spectroscopy (GC-MS)
3.6. Elemental Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toma, S.H.; Santos, J.J.; da Silva, D.G.; Huila, M.F.G.; Toma, H.E.; Araki, K. Improving stability of iron oxide nanofluids for enhanced oil recovery: Exploiting wettability modifications in carbonaceous rocks. J. Pet. Sci. Eng. 2022, 212, 110311. [Google Scholar] [CrossRef]
- Board, N.E. Canada’s Oil Sands Opportunities and Challenges To 2015: An Update; National Energy Board: Calgary, AB, Canada, 2006; ISBN 066243353X. [Google Scholar]
- Jia, C. Breakthrough and significance of unconventional oil and gas to classical petroleum geological theory. Shiyou Kantan Yu Kaifa/Petroleum Explor. Dev. 2017, 44, 1–11. [Google Scholar] [CrossRef]
- Zheng, R.; Liu, D.; Tang, J.; Song, Q.; Yao, Q. Analysis of montmorillonite affecting coke formation during the thermal conversion of heavy oil. Fuel 2021, 288, 119687. [Google Scholar] [CrossRef]
- Alvarado, V.; Manrique, E. Enhanced oil recovery: An update review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Panait-Patica, A.; Serban, D.; Ilie, N.; Pavel, L.; Barsan, N. Suplacu de Barcau Field-A Case History of a Successful In-Situ Combustion Exploitation. In Proceedings of the SPE Europec/EAGE Annual Conference and Exhibition, Society of Petroleum Engineers, Vienna, Austria, 12–15 June 2006; p. 10. [Google Scholar]
- Gholami, Z.; Gholami, F.; Tišler, Z.; Vakili, M. A Review on the Production of Light Olefins Using Steam Cracking of Hydrocarbons. Energies 2021, 14, 8190. [Google Scholar] [CrossRef]
- Aycaguer, A.-C.; Lev-On, M.; Winer, A.M. Reducing carbon dioxide emissions with enhanced oil recovery projects: A life cycle assessment approach. Energy Fuels 2001, 15, 303–308. [Google Scholar] [CrossRef]
- Mehlman, S. Carbon Capture and Sequestration (via Enhanced Oil Recovery) from a Hydrogen Production Facility in an Oil Refinery; Praxair, Incorporated: Danbury, CT, USA, 2010. [Google Scholar]
- Tirado, A.; Yuan, C.; Varfolomeev, M.A.; Ancheyta, J. Kinetic modeling of aquathermolysis for upgrading of heavy oils. Fuel 2022, 310, 122286. [Google Scholar] [CrossRef]
- Rehman, M.M.; Meribout, M. Conventional versus electrical enhanced oil recovery: A review. J. Pet. Explor. Prod. Technol. 2012, 2, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S. Enhanced oil recovery-an overview. Oil Gas Sci. Technol. l’IFP 2008, 63, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery; Gulf Professional Publishing: Burlington, VT, USA, 2011; ISBN 9781856177450. [Google Scholar]
- Brandt, A.R.; Unnasch, S. Energy intensity and greenhouse gas emissions from thermal enhanced oil recovery. Energy Fuels 2010, 24, 4581–4589. [Google Scholar] [CrossRef]
- Al-Muntaser, A.A.; Varfolomeev, M.A.; Suwaid, M.A.; Feoktistov, D.A.; Yuan, C.; Klimovitskii, A.E.; Gareev, B.I.; Djimasbe, R.; Nurgaliev, D.K.; Kudryashov, S.I.; et al. Hydrogen donating capacity of water in catalytic and non-catalytic aquathermolysis of extra-heavy oil: Deuterium tracing study. Fuel 2021, 283, 118957. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Eskin, A.A.; Sharifullin, A.V.; Vakhin, A.V. Differential scanning calorimetric study of heavy oil catalytic oxidation in the presence of manganese tallates. Pet. Sci. Technol. 2019, 37, 1194–1200. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Salih, I.S.S.; Khelkhal, M.A.; Vakhin, A.V. Application of Aromatic and Industrial Solvents for Enhancing Heavy Oil Recovery from the Ashalcha Field. Energy Fuels 2020, 35, 374–385. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Khelkhal, M.A.; Tajik, A.; Gafurov, M.R.; Morozov, O.G.; Nasybullin, A.R.; Karandashov, S.A.; Ponomarev, A.A.; Krapivnitskaia, T.O.; Glyavin, M.Y. The Role of Nanodispersed Catalysts in Microwave Application during the Development of Unconventional Hydrocarbon Reserves: A Review of Potential Applications. Processes 2021, 9, 420. [Google Scholar] [CrossRef]
- Hascakir, B.; Babadagli, T.; Akin, S. Experimental and Numerical Modeling of Heavy-Oil Recovery by Electrical Heating. In Proceedings of the International Thermal Operations and Heavy Oil Symposium, Society of Petroleum Engineers, Calgary, AB, Canada, 20–23 October 2008. [Google Scholar]
- Baptist, O.C. Oil Recovery and Formation Damage in Permafrost, Umiat Field, Alaska; U.S. Department of the Interior, Bureau of Mines: Washington, DC, USA, 1960; Volume 5642.
- Zhou, Z.; Gunter, W.D.; Kadatz, B.; Cameron, S. Effect of clay swelling on reservoir quality. J. Can. Pet. Technol. 1996, 35, 07. [Google Scholar] [CrossRef]
- Bailey, S.A.; Kenney, T.M.; Schneider, D.R. Microbial Enhanced Oil Recovery: Diverse Successful Applications of Biotechnology in the Oil Field. In Proceedings of the SPE Asia Pacific Improved Oil Recovery Conference, Society of Petroleum Engineers, Kuala Lumpur, Malaysia, 8–9 October 2001. [Google Scholar]
- Taheri-Shakib, J.; Shekarifard, A.; Naderi, H. Experimental investigation of comparing electromagnetic and conventional heating effects on the unconventional oil (heavy oil) properties: Based on heating time and upgrading. Fuel 2018, 228, 243–253. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Khelkhal, M.A.; Tajik, A.; Ignashev, N.E.; Krapivnitskaya, T.O.; Peskov, N.Y.; Glyavin, M.Y.; Bulanova, S.A.; Slavkina, O.V.; Schekoldin, K.A. Microwave Radiation Impact on Heavy Oil Upgrading from Carbonate Deposits in the Presence of Nano-Sized Magnetite. Processes 2021, 9, 2021. [Google Scholar] [CrossRef]
- Sierra, R.; Tripathy, B.; Bridges, J.E.; Ali, S.M. Promising progress in field application of reservoir electrical heating methods. In Proceedings of the SPE International Thermal Operations and Heavy Oil Symposium, Society of Petroleum Engineers, Porlamar, Venezuela, 12–14 March 2001. [Google Scholar]
- Li, H.; Gao, H.; Zhao, X.; Xia, Z.; Yu, B.; Sun, D. Experimental study on viscosity reduction of heavy oil with water content by synergistic effect of microwave and nano-catalyst. J. Pet. Sci. Eng. 2022, 208, 109271. [Google Scholar] [CrossRef]
- Boukherissa, M.; Mutelet, F.; Modarressi, A.; Dicko, A.; Dafri, D.; Rogalski, M. Ionic liquids as dispersants of petroleum asphaltenes. Energy Fuels 2009, 23, 2557–2564. [Google Scholar] [CrossRef]
- Li, Y.-B.; Gao, H.; Pu, W.-F.; Li, L.; Chen, Y.; Bai, B. Study of the catalytic effect of copper oxide on the low-temperature oxidation of Tahe ultra-heavy oil. J. Therm. Anal. Calorim. 2019, 135, 3353–3362. [Google Scholar] [CrossRef]
- Kniazeva, M.; Maximov, A. Effect of Additives on the Activity of Nickel–Tungsten Sulfide Hydroconversion Catalysts Prepared In Situ from Oil-Soluble Precursors. Catalysts 2018, 8, 644. [Google Scholar] [CrossRef] [Green Version]
- Kok, M.V. The thermal characterization of crude oils in a limestone matrix of different particle size. Energy Sources Part A Recover. Util. Environ. Eff. 2014, 36, 923–928. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Salih, I.S.; Vakhin, A.V. Changes in the subfractional composition of heavy oil asphaltenes under aquathermolysis with oil-soluble CO-based catalyst. Pet. Sci. Technol. 2019, 37, 1589–1595. [Google Scholar] [CrossRef]
- Salih, I.S.S.; Mukhamatdinov, I.I.; Vahin, A.V.; Garifullina, E.I. Fractional composition of heavy oil asphaltenes of volga-ural petroleum province. Int. Multidiscip. Sci. GeoConf. SGEM Surv. Geol. Min. Ecol. Manag. 2017, 17, 485–491. [Google Scholar]
- Khelkhal, M.A.; Eskin, A.A.; Nurgaliev, D.K.; Vakhin, A.V. Thermal study on stabilizing combustion front via bimetallic Mn@Cu tallates during heavy oil oxidation. Energy Fuels 2019, 34, 5121–5127. [Google Scholar] [CrossRef]
- Farhadian, A.; Khelkhal, M.A.; Tajik, A.; Lapuk, S.E.; Rezaeisadat, M.; Eskin, A.A.; Rodionov, N.O.; Vakhin, A.V. Effect of Ligand Structure on the Kinetics of Heavy Oil Oxidation: Toward Biobased Oil-Soluble Catalytic Systems for Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2021, 60, 14713–14727. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Lapuk, S.E.; Ignashev, N.E.; Eskin, A.A.; Glyavin, M.Y.; Peskov, N.Y.; Krapivnitskaia, T.O.; Vakhin, A.V. A Thermal Study on Peat Oxidation Behavior in the Presence of an Iron-Based Catalyst. Catalysts 2021, 11, 1344. [Google Scholar] [CrossRef]
- Sitnov, S.; Mukhamatdinov, I.; Aliev, F.; Khelkhal, M.A.; Slavkina, O.; Bugaev, K. Heavy oil aquathermolysis in the presence of rock-forming minerals and iron oxide (II, III) nanoparticles. Pet. Sci. Technol. 2020, 38, 574–579. [Google Scholar] [CrossRef]
- Qi, H.; Jiang, H.; You, Y.; Hu, J.; Wang, Y.; Wu, Z.; Qi, H. Mechanism of Magnetic Nanoparticle Enhanced Microwave Pyrolysis for Oily Sludge. Energies 2022, 15, 1254. [Google Scholar] [CrossRef]
- Korneev, D.S.; Pevneva, G.S.; Golovko, A.K. Thermal transformations of asphaltenes in heavy oils at 120 °C. J. Sib. Fed. Univ. Chem. 2019, 12, 101–117. [Google Scholar]
- Golovko, A.K.; Grin’ko, A.A. Structural Transformations of Petroleum Resins and Their Fractions by Thermolysis. Pet. Chem. 2018, 58, 599–606. [Google Scholar] [CrossRef]
- Korneev, D.S.; Pevneva, G.S.; Voronetskaya, N.G. Effects of the Composition and Molecular Structure of Heavy Oil Asphaltenes on Their Reactivity in Thermal Decomposition Processes. Pet. Chem. 2021, 61, 152–161. [Google Scholar] [CrossRef]
- Voronetskaya, N.G.; Pevneva, G.S.; Korneev, D.S.; Golovko, A.K. Influence of asphaltenes on the direction of thermal transformations of heavy oil hydrocarbons. Pet. Chem. 2020, 60, 166–173. [Google Scholar] [CrossRef]
- Montgomery, W.; Court, R.W.; Rees, A.C.; Sephton, M.A. High temperature reactions of water with heavy oil and bitumen: Insights into aquathermolysis chemistry during steam-assisted recovery. Fuel 2013, 113, 426–434. [Google Scholar] [CrossRef] [Green Version]

| Sample | Abbreviation | SARA Fractions, wt.% | Elemental Compositions, wt.% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saturates | Aromatics | Resins | Asphaltenes | C | H | N | S | O | ||||
| Initial heavy oil | 23.9 | 39.2 | 29.2 | 7.9 | 82.1 | 10.1 | 0.6 | 2.6 | 4.6 | |||
| MW- Processed heavy oil | Non-catalytic MW-processed heavy oil | MW(E) | 25.7 | 36.4 | 30.7 | 7.2 | 82.4 | 9.9 | 0.6 | 2.4 | 4.7 | |
| Catalytic MW-processed heavy oil | Place for Electric field | MW(E) + Fe3O4 | 30.7 | 43.8 | 19.4 | 6.1 | 82.8 | 10.2 | 0.6 | 2.0 | 4.4 | |
| Place for Magnetic field | MW(H) + Fe3O4 | 30.3 | 37.2 | 25.5 | 7.0 | 82.3 | 10.1 | 0.6 | 2.1 | 4.9 | ||
| Geochemical Coefficients | Without Catalyst. Place for Electric Field Processing | With Catalyst | |
|---|---|---|---|
| Place for Electric Field Processing | Place for Magnetic Field Processing | ||
| MW(E) | MW(E) + Fe3O4 | MW(H) + Fe3O4 | |
| Pristane/Phytane | 0.52 | 0.52 | 0.68 |
| Pr/C17 | 1.57 | 1.55 | 1.72 |
| Ph/C18 | 3.22 | 3.28 | 3.02 |
| C27/C17 | 1.82 | 1.58 | 1.29 |
| Σ(C27−C31)/Σ(C15−C19) | 1.69 | 1.35 | 1.46 |
| Σ(C12−C21)/Σ(C22−C30) | 0.41 | 0.54 | 0.52 |
| 2nC29/(C28 + C30) | 1.11 | 1.21 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakhin, A.V.; Khelkhal, M.A.; Mukhamatdinov, I.I.; Mukhamatdinova, R.E.; Tajik, A.; Slavkina, O.V.; Malaniy, S.Y.; Gafurov, M.R.; Nasybullin, A.R.; Morozov, O.G. Changes in Heavy Oil Saturates and Aromatics in the Presence of Microwave Radiation and Iron-Based Nanoparticles. Catalysts 2022, 12, 514. https://doi.org/10.3390/catal12050514
Vakhin AV, Khelkhal MA, Mukhamatdinov II, Mukhamatdinova RE, Tajik A, Slavkina OV, Malaniy SY, Gafurov MR, Nasybullin AR, Morozov OG. Changes in Heavy Oil Saturates and Aromatics in the Presence of Microwave Radiation and Iron-Based Nanoparticles. Catalysts. 2022; 12(5):514. https://doi.org/10.3390/catal12050514
Chicago/Turabian StyleVakhin, Alexey V., Mohammed A. Khelkhal, Irek I. Mukhamatdinov, Rezeda E. Mukhamatdinova, Arash Tajik, Olga V. Slavkina, Sergey Y. Malaniy, Marat R. Gafurov, Aydar R. Nasybullin, and Oleg G. Morozov. 2022. "Changes in Heavy Oil Saturates and Aromatics in the Presence of Microwave Radiation and Iron-Based Nanoparticles" Catalysts 12, no. 5: 514. https://doi.org/10.3390/catal12050514
APA StyleVakhin, A. V., Khelkhal, M. A., Mukhamatdinov, I. I., Mukhamatdinova, R. E., Tajik, A., Slavkina, O. V., Malaniy, S. Y., Gafurov, M. R., Nasybullin, A. R., & Morozov, O. G. (2022). Changes in Heavy Oil Saturates and Aromatics in the Presence of Microwave Radiation and Iron-Based Nanoparticles. Catalysts, 12(5), 514. https://doi.org/10.3390/catal12050514












