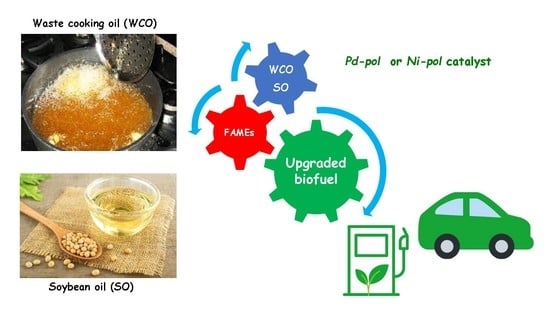
Partial Hydrogenation of Soybean and Waste Cooking Oil Biodiesel over Recyclable-Polymer-Supported Pd and Ni Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
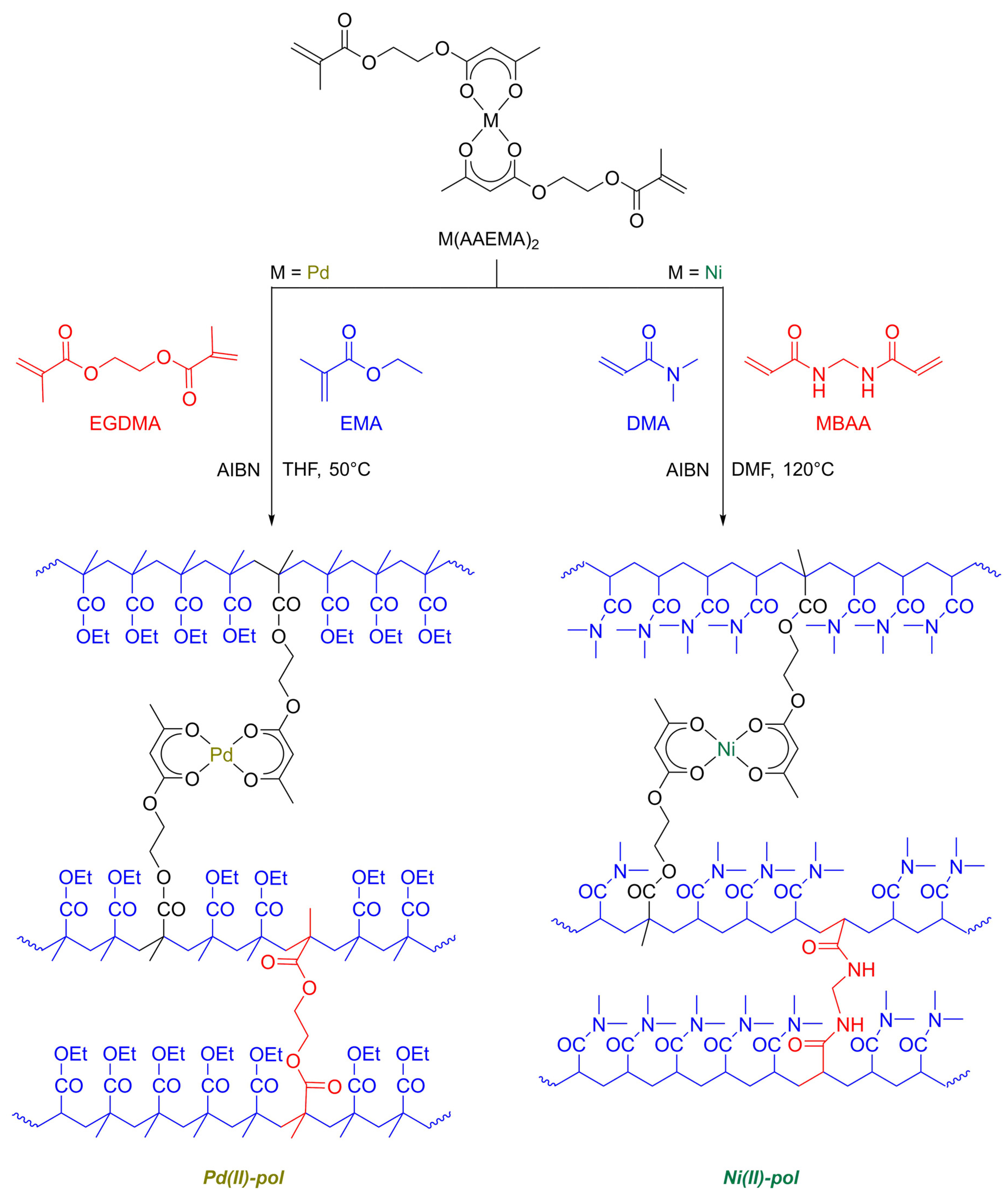
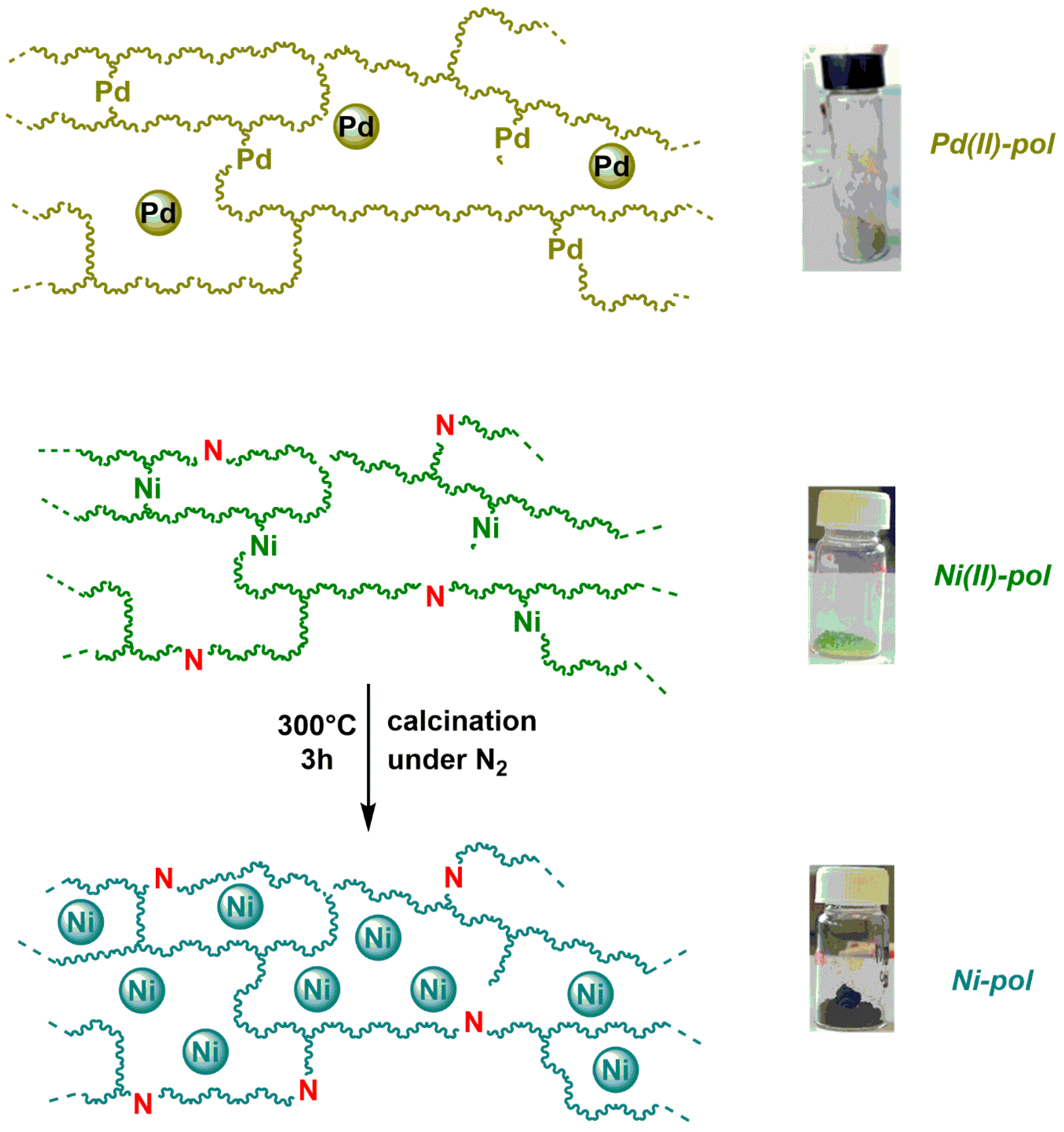
2.1. Catalysts
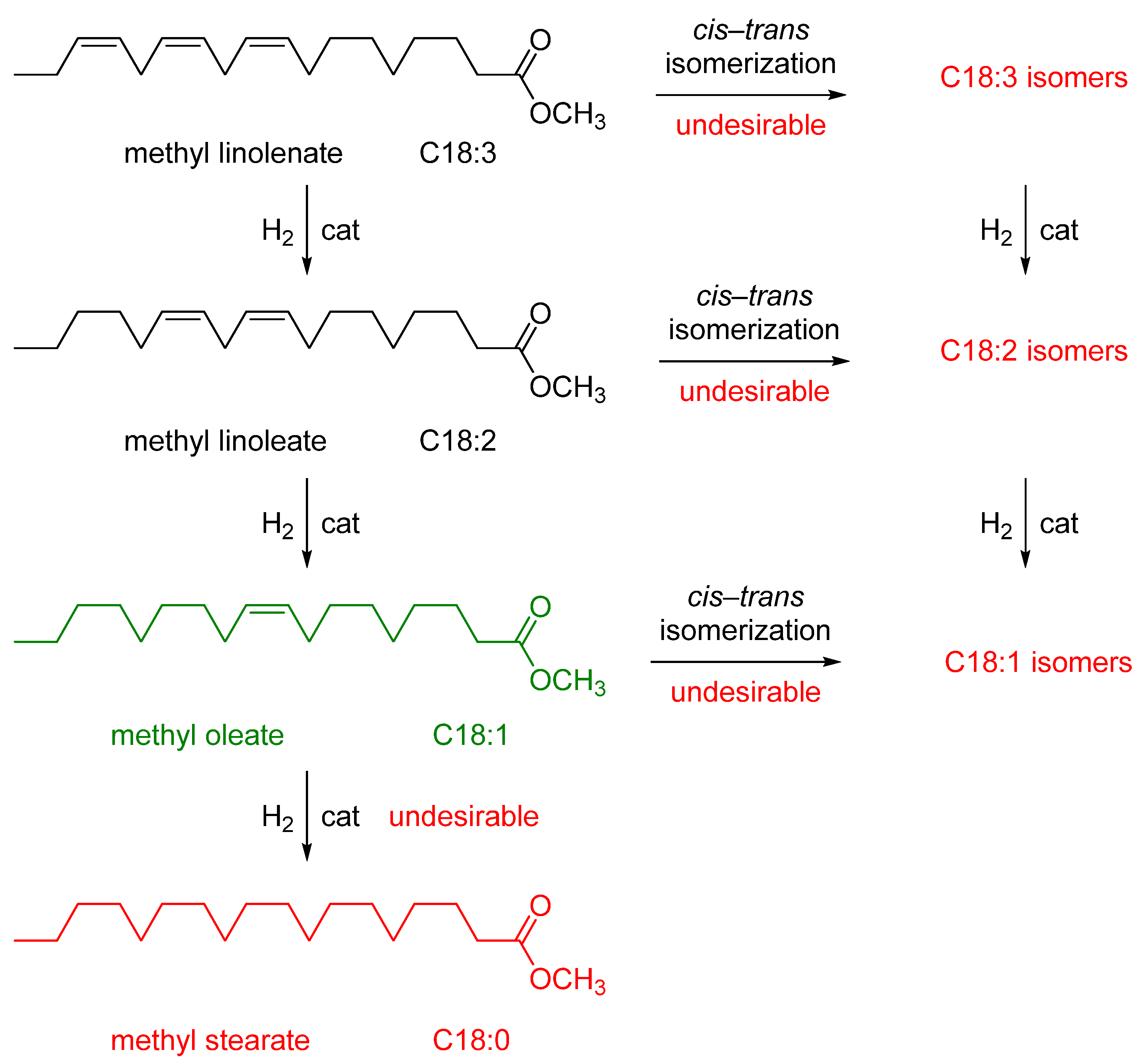
2.2. Fatty Acid Methyl Esters (FAMEs)
2.3. Catalytic Tests
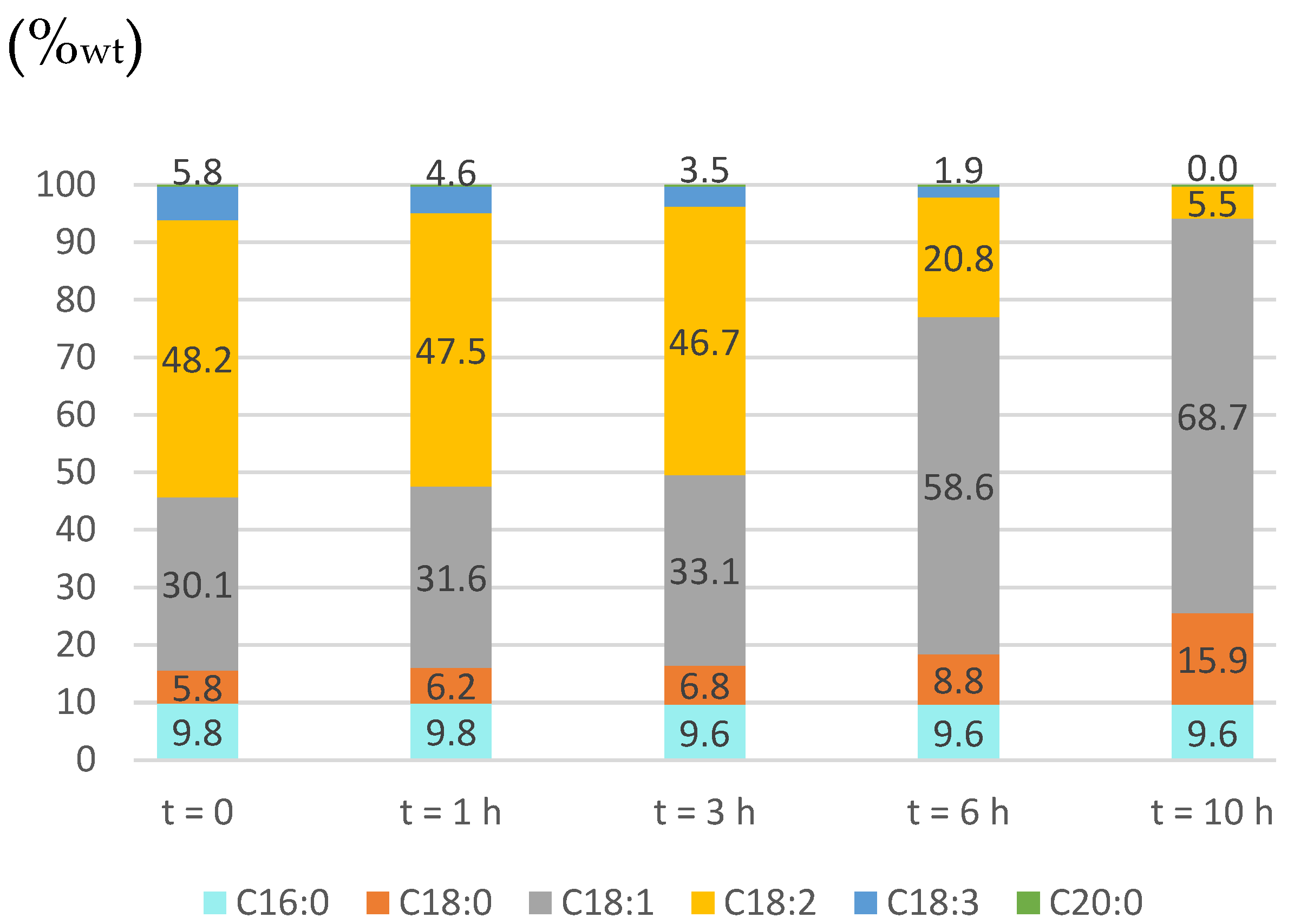
2.3.1. Pd Catalyst
2.3.2. Ni Catalyst
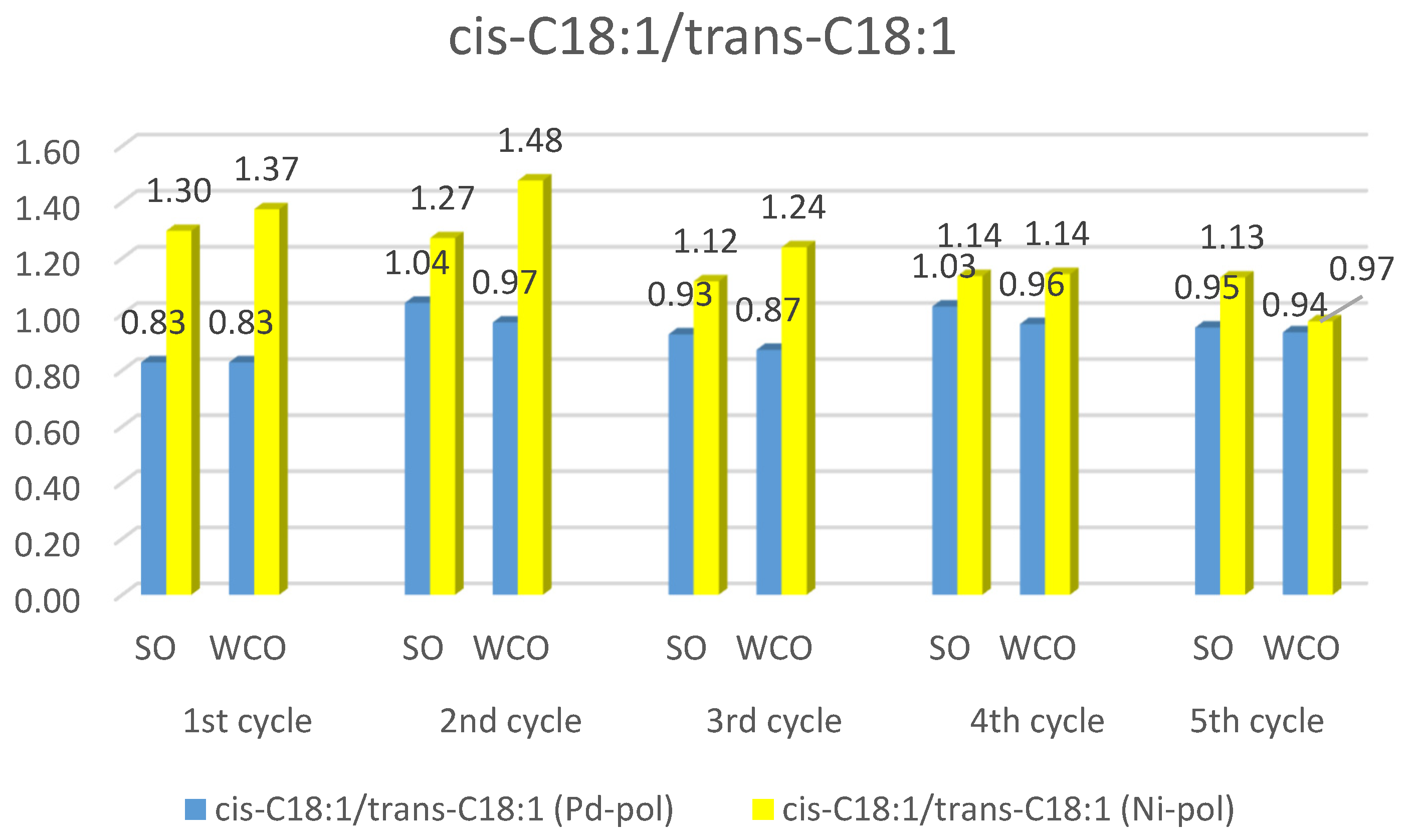
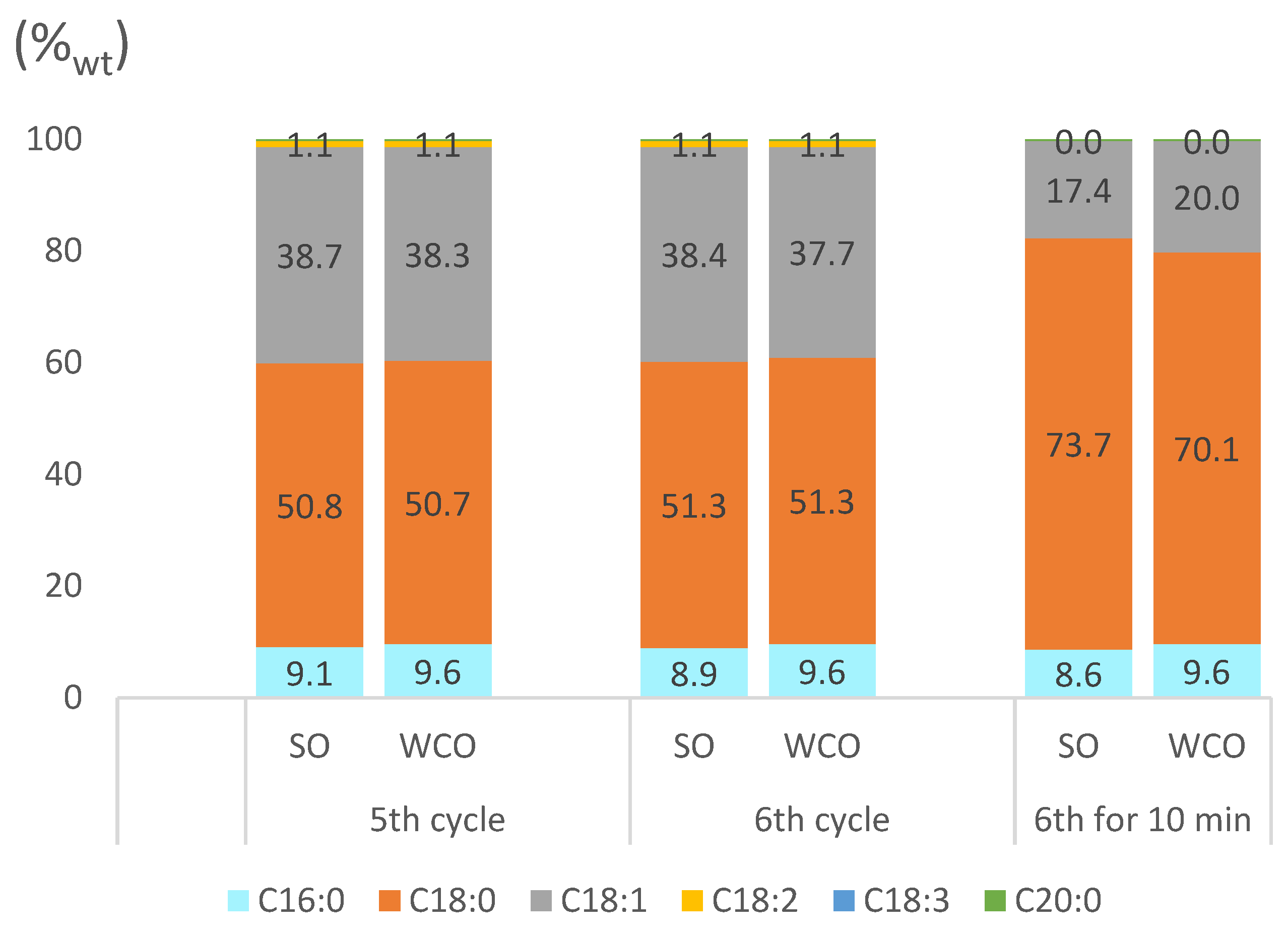
2.3.3. Partially Hydrogenated Biodiesel Features
2.4. Possible Explanation of the Different Performances of Pd-pol and Ni-pol Systems
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis of FAMEs
3.3. Catalytic Tests
3.3.1. Upgrading with Pd-pol
3.3.2. Upgrading with Ni-pol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeyaseean, T.; Ekambaram, P.; Subramanian, J.; Shamim, A. A comprehensive review on the current trends, challenges and future prospects for sustainable mobility. Renew. Sust. Energy Rev. 2022, 157, 112073. [Google Scholar] [CrossRef]
- Munyentwali, A.; Li, H. Review of advances in bifunctional solid acid/base catalysts for sustainable biodiesel production. Appl. Catal. A Gen. 2022, 633, 118525. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of biodiesel production from waste cooking oil and its use as fuel in compression ignition engines: 3rd generation cleaner feedstock. J. Clean. Prod. 2021, 307, 127299. [Google Scholar] [CrossRef]
- Lucas Nascimento, L.; Ribeiro, A.; Ferreira, A.; Valério, N.; Pinheiro, V.; Araújo, J.; Vilarinho, C.; Carvalho, J. Turning waste cooking oils into biofuels—Valorization Technologies: A review. Energies 2022, 15, 116. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Li, C.; Chen, X.; Peng, W.; Aghbashlo, M.; Lam, S.S.; Tabatabaei, M. Managing the hazardous waste cooking oil by conversion into bioenergy through the application of waste-derived green catalysts: A review. J. Hazard. Mater. 2022, 424, 127636. [Google Scholar] [CrossRef]
- Tayari, S.; Abedi, R.; Rahi, A. Comparative assessment of engine performance and emissions fueled with three different biodiesel generations. Renew. Energy 2020, 147, 1058–1069. [Google Scholar] [CrossRef]
- Casiello, M.; Catucci, L.; Fracassi, F.; Fusco, C.; Laurenza, G.; di Bitonto, L.; Pastore, C.; D’Accolti, L.; Nacci, A. ZnO/Ionic liquid catalyzed biodiesel production from renewable and waste lipids as feedstocks. Catalysts 2019, 9, 71. [Google Scholar] [CrossRef] [Green Version]
- McCormick, R.L.; Ratcliff, M.; Moens, L.; Lawrence, R. Several factors affecting the stability of biodiesel in standard accelerated tests. Fuel Process. Technol. 2007, 88, 651–657. [Google Scholar] [CrossRef]
- Weidong, Z.; Jiangwei, Y.; Xiaoyin, Z.; Xiaolong, Q. Review on the progress of the first-generation biodiesel hydrogenation and upgrading. Energy Sources A Recovery Util. Environ. Eff. 2020, 42, 2704–2714. [Google Scholar] [CrossRef]
- Adu-Mensah, D.; Mei, D.; Zuo, L.; Zhang, Q.; Wang, J. A review on partial hydrogenation of biodiesel and its influence on fuel properties. Fuel 2019, 251, 660–668. [Google Scholar] [CrossRef]
- Wongjaikham, W.; Kongprawes, G.; Wongsawaeng, D.; Ngaosuwan, R.; Kiatkittipong, W.; Hosemann, P.; Assabumrungrat, S. Highly effective microwave plasma application for catalyst-free and low temperature hydrogenation of biodiesel. Fuel 2021, 305, 121524. [Google Scholar] [CrossRef]
- Kongprawes, G.; Wongsawaeng, D.; Hosemann, P.; Ngaosuwan, K.; Kiatkittipong, W.; Assabumrungrat, S. Improvement of oxidation stability of fatty acid methyl esters derived from soybean oil via partial hydrogenation using dielectric barrier discharge plasma. Int. J. Energy Res 2021, 45, 4519–4533. [Google Scholar] [CrossRef]
- Laverdura, U.P.; Rossi, L.; Ferella, F.; Courson, C.; Zarli, A.; Alhajyoussef, R.; Gallucci, K. Selective catalytic hydrogenation of vegetable oils on lindlar catalyst. ACS Omega 2020, 5, 22901–22913. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Attanatho, L.; Chang, A.; Laosombut, T.; Nishi, M.; Mochizuki, T.; Takagi, H.; Yang, C.-M.; Abe, H.; Toba, M.; et al. Profiling and catalytic upgrading of commercial palm oil-derived biodiesel fuels for high-blend fuels. Catal. Today 2019, 332, 122–131. [Google Scholar] [CrossRef]
- Gao, L.; Liu, K.; Zhang, L.; Xin, Z.; Yang, Y.; Wei, G.; Yuan, T. Microwave-assisted catalytic transfer hydrogenation of fatty acid methyl esters using metal-doped nickelboride-cetyltrimethylammonium bromide amorphous alloy catalyst. Int. J. Energy Res. 2021, 45, 13098–13116. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lee, J.; Seo, H.; Kang, H.; Kim, D.-H.; Lee, Y.-W. Evaluation of Pd/ZSM-5 catalyst for simultaneous reaction of transesterification and partial catalytic transfer hydrogenation of soybean oil under supercritical methanol. Fuel Process. Technol. 2021, 218, 106870. [Google Scholar] [CrossRef]
- Xin, Z.; Wei, G.; Zhang, L.; Gao, L.; Li, G.; Zhao, W. Partial hydrogenation of fatty acid methyl esters under mild conditions using sodium borohydride as hydrogen donor. Fuel 2021, 299, 120877. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, L.; Li, Z.; Wei, G.; Xin, Z.; Xiong, D.; Ou, Z. Partial Hydrogenation of Jatropha Oil Biodiesel Catalyzed by Nickel/Bentonite Catalyst. Waste Biomass Valorization 2021, 12, 465–474. [Google Scholar] [CrossRef]
- Wei, G.; Liu, Z.; Zhang, L.; Li, Z. Catalytic upgrading of Jatropha oil biodiesel by partial hydrogenation using Raney-Ni as catalyst under microwave heating. Energy Convers. Manag. 2018, 163, 208–218. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Attanatho, L.; Mochizuki, T.; Abe, Y.; Toba, M.; Yoshimura, Y.; Kumpidet, C.; Somwonhsa, P.; Laoubol, S. Upgrading of palm biodiesel fuel over supported palladium catalysts. Comptes Rendus Chim. 2016, 19, 1166–1173. [Google Scholar] [CrossRef]
- Numwong, N.; Prabnasak, P.; Prayoonpunratn, P.; Triphatthanaphong, P.; Thunyaratchatanon, C.; Mochizuki, T.; Chen, S.-Y.; Luengnaruemitchai, A.; Sookno, T. Effect of Pd particle size on activity and cis-trans selectivity in partial hydrogenation of soybean oil-derived FAMEs over Pd/SiO2 catalysts. Fuel Process. Technol. 2020, 203, 106393. [Google Scholar] [CrossRef]
- Thunyaratchatanon, C.; Luengnaruemitchai, A.; Chollacoop, N.; Yoshimura, Y. Catalytic upgrading of soybean oil methyl esters by partial hydrogenation using Pd catalysts. Fuel 2016, 163, 8–16. [Google Scholar] [CrossRef]
- Troncoso, F.D.; Tonetto, G.M. Highly stable platinum monolith catalyst for the hydrogenation of vegetable oil. Chem. Eng. Process. 2022, 170, 108669. [Google Scholar] [CrossRef]
- Lu, Z.; Cherepakhin, V.; Kapenstein, T.; Williams, T.J. Upgrading Biodiesel from Vegetable Oils by Hydrogen Transfer to Its Fatty Esters. ACS Sustain. Chem. Eng. 2018, 6, 5749–5753. [Google Scholar] [CrossRef]
- Dell’Anna, M.M.; Capodiferro, V.F.; Mali, M.; Manno, D.; Cotugno, P.; Monopoli, A.; Mastrorilli, P. Highly selective hydrogenation of quinolines promoted by recyclable polymer supported palladium nanoparticles under mild conditions in aqueous medium. Appl. Catal. A Gen. 2014, 481, 89–95. [Google Scholar] [CrossRef]
- Dell’Anna, M.M.; Capodiferro, V.F.; Mali, M.; Mastrorilli, P. Esterification, transesterification and hydrogenation reactions of polyunsaturated compounds catalyzed by a recyclable polymer supported palladium catalyst. J. Organomet. Chem. 2016, 818, 106–114. [Google Scholar] [CrossRef]
- Dell’Anna, M.M.; Romanazzi, G.; Intini, S.; Rizzuti, A.; Leonelli, C.; Piccinni, A.F.; Mastrorilli, P. A polymer supported palladium (II) b--ketoesterate complex as active and recyclable pre-catalyst for selective reduction of quinolines in water with sodium borohydride. J. Mol. Catal. A Chem. 2015, 402, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Fiore, A.M.; Romanazzi, G.; Dell’Anna, M.M.; Latronico, M.; Leonelli, C.; Mali, M.; Rizzuti, A.; Mastrorilli, P. Mild and efficient synthesis of secondary aromatic amines by one-pot stepwise reductive amination of arylaldehydes with nitroarenes promoted by reusable nickel nanoparticles. Mol. Catal. 2019, 476, 110507. [Google Scholar] [CrossRef]
- Mastrorilli, P.; Dell’Anna, M.M.; Rizzuti, A.; Mali, M.; Zapparoli, M.; Leonelli, C. Resin-immobilized palladium nanoparticle catalysts for organic reactions in aqueous media: Morphological aspects. Molecules 2015, 20, 18661–18684. [Google Scholar] [CrossRef] [Green Version]
- Dell’Anna, M.M.; Romanazzi, G.; Mastrorilli, P. Polymer Supported Catalysts Obtained from Metal-Containing Monomers. Curr. Org. Chem. 2013, 17, 1236–1273. [Google Scholar]
- Dell’Anna, M.M.; Mastrorilli, P.; Nobile, C.F. Solid-Phase Catalytic Activity of a Polymer-Supported Palladium Complex. In Solid-Phase Organic Syntheses; Scott, P.J.H., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 79–86. [Google Scholar]
- Romanazzi, G.; Fiore, A.M.; Mali, M.; Rizzuti, A.; Leonelli, C.; Nacci, A.; Mastrorilli, P.; Dell’Anna, M.M. Polymer supported Nickel nanoparticles as recyclable catalyst for the reduction of nitroarenes to anilines in aqueous medium. Mol. Catal. 2018, 446, 31–38. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Surkus, A.-E.; Junge, H.; Pohl, M.-M.; Radnik, J.; Rabeah, J.; Huan, H.; Schünemann, V.; Brückner, A.; Beller, M. Nanoscale Fe2O3-based catalysts for selective hydrogenation of nitroarenes to anilines. Science 2013, 342, 1073–1076. [Google Scholar] [CrossRef]
- Murugesan, K.; Senthamarai, T.; Chandrashekhar, V.G.; Natte, K.; Kamer, P.C.J.; Beller, M.; Jagadeesh, R.V. Catalytic reductive aminations using molecular hydrogen for synthesis of different kinds of amines. Chem. Soc. Rev. 2020, 49, 6273–6328. [Google Scholar] [CrossRef]
- Fiore, A.M.; Nefedova, D.; Romanazzi, G.; Petrelli, V.; Mali, M.; Leonelli, C.; Mortalò, C.; Catauro, M.; Mastrorilli, P.; Dell’Anna, M.M. How the Calcination Procedure Affects the Morphology and the Catalytic Activity of Polymer-Supported Nickel Nanoparticles. Macromol. Symp. 2021, 395, 2000195. [Google Scholar] [CrossRef]
- Sukjit, E.; Tongroon, M.; Chollacoop, N.; Yoshimura, Y.; Poapongsakorn, P.; Lapuerta, M.; Dearn, K.D. Improvement of the tribological behaviour of palm biodiesel via partial hydrogenation of unsaturated fatty acid methyl esters. Wear 2019, 426–427, 813–818. [Google Scholar] [CrossRef]
- Bateni, H.; Saraeian, A.; Able, C. A comprehensive review on biodiesel purification and upgrading. Biofuel Res. J. 2017, 15, 668–690. [Google Scholar] [CrossRef] [Green Version]
- Talebi, A.F.; Tabatabaei, M.; Chisti, Y. BiodieselAnalyzer@: A user-friendly software for predicting the properties of prospective biodiesel. Biofuel Res. J. 2014, 2, 55–57. [Google Scholar] [CrossRef]
- Lapuerta, M.; Fernandez, J.M.; Font de Mora, E. Correlation for the estimation of the cetane number of biodiesel fuels and implications on the iodine number. Energy Policy 2009, 37, 4337–4344. [Google Scholar] [CrossRef]
- Makareviciene, V.; Kazancev, K.; Kazanceva, I. Possibilities for improving the cold flow properties of biodiesel fuel by blending with butanol. Renew. Energy 2015, 75, 805–807. [Google Scholar] [CrossRef]
- Quaranta, E.; Cornacchia, D. Partial hydrogenation of a C18:3-rich FAME mixture over Pd/C. Renew. Energy 2020, 157, 33–42. [Google Scholar] [CrossRef]
- Souza, B.S.; Pinho, D.M.M.; Leopoldino, E.C.; Suarez, P.A.Z.; Nome, F. Selective partial biodiesel hydrogenation using highly active supported palladium nanoparticles in imidazolium-based ionic liquid. Appl. Catal. A Gen. 2012, 433, 109–114. [Google Scholar] [CrossRef]
- Ramayeni, E.; Susanto, B.H.; Pratama, D.F. Palm H-FAME Production through Partially Hydrogenation using Nickel/Carbon Catalyst to Increase Oxidation Stability. In MATEC Web of Conferences; RSCE: Entebbe, Uganda, 2018; Volume 156, p. 03004. [Google Scholar]


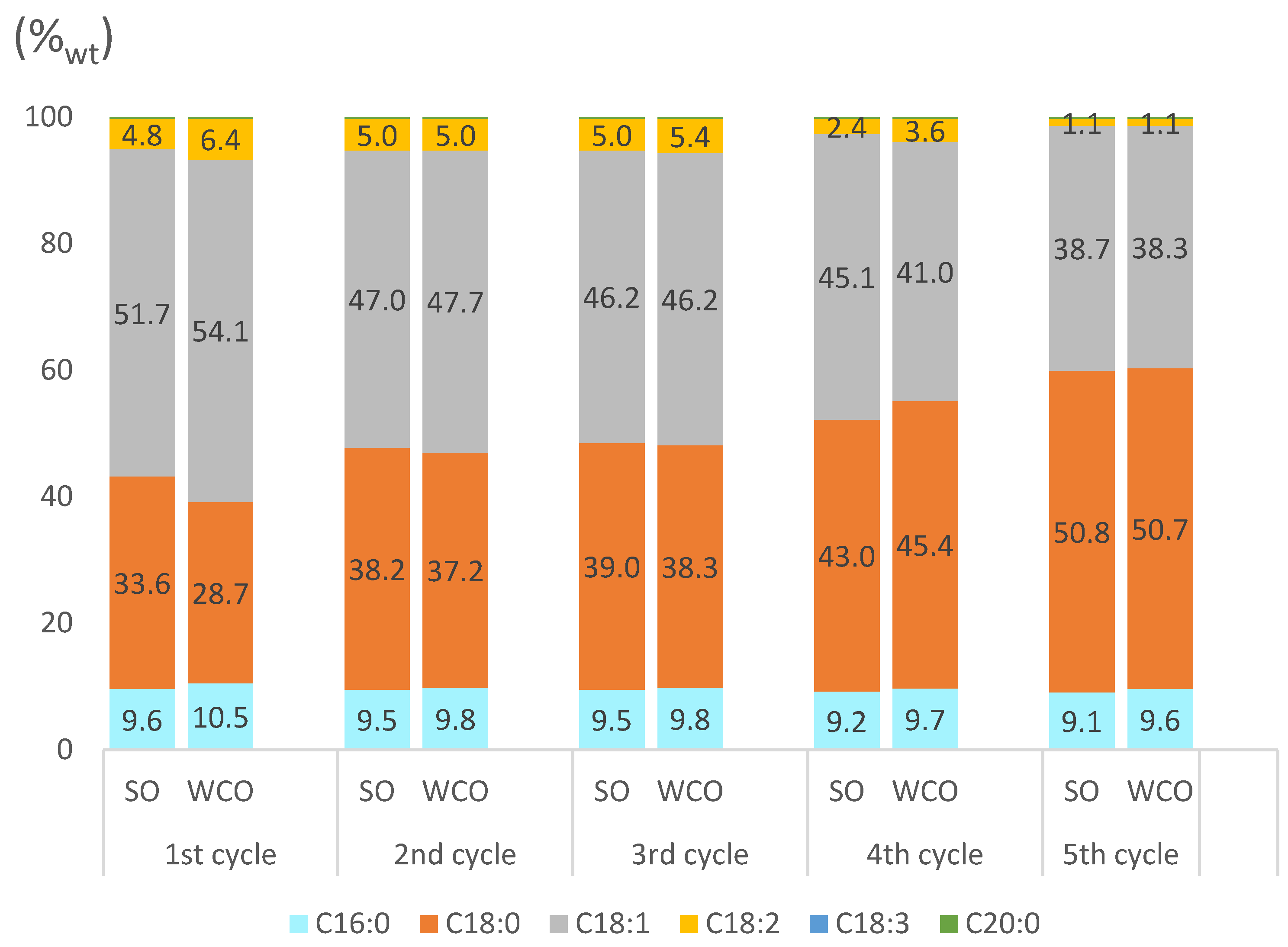


| Biodiesel | Cat | Cycle | CFPP (°C) | IV (g/100 g) | OS (h) |
|---|---|---|---|---|---|
| SO | - | - | –3.3 | 130 | 4.7 |
| Pd-pol | 1st | 40.3 | 55.2 | 27.2 | |
| Pd-pol | 2nd | 47.5 | 51.3 | 26.2 | |
| Pd-pol | 3rd | 48.7 | 50.6 | 26.2 | |
| Pd-pol | 4th | 54.9 | 44.9 | 51.7 | |
| Pd-pol | 5th | 67.1 | 36.8 | 109.8 | |
| WCO | - | - | –1.4 | 126 | 4.8 |
| Pd-pol | 1st | 32.8 | 60.2 | 21.0 | |
| Pd-pol | 2nd | 46.0 | 51.9 | 26.2 | |
| Pd-pol | 3rd | 47.7 | 51.3 | 24.4 | |
| Pd-pol | 4th | 58.8 | 43.4 | 35.3 | |
| Pd-pol | 5th | 67.1 | 36.4 | 109.8 | |
| SO | Ni-pol | 1st | 12.5 | 71.7 | 24.0 |
| Ni-pol | 2nd | 12.8 | 72.9 | 22.2 | |
| Ni-pol | 3rd | 12.4 | 72.4 | 21.9 | |
| Ni-pol | 4th | 11.0 | 73.0 | 22.2 | |
| Ni-pol | 5th | 10.6 | 73.3 | 22.6 | |
| WCO | Ni-pol | 1st | 14.0 | 69.2 | 29.4 |
| Ni-pol | 2nd | 14.6 | 69.9 | 26.1 | |
| Ni-pol | 3rd | 13.0 | 71.2 | 24.4 | |
| Ni-pol | 4th | 12.2 | 70.8 | 28.2 | |
| Ni-pol | 5th | 10.6 | 72.5 | 25.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, A.M.; Romanazzi, G.; Leonelli, C.; Mastrorilli, P.; Dell’Anna, M.M. Partial Hydrogenation of Soybean and Waste Cooking Oil Biodiesel over Recyclable-Polymer-Supported Pd and Ni Nanoparticles. Catalysts 2022, 12, 506. https://doi.org/10.3390/catal12050506
Fiore AM, Romanazzi G, Leonelli C, Mastrorilli P, Dell’Anna MM. Partial Hydrogenation of Soybean and Waste Cooking Oil Biodiesel over Recyclable-Polymer-Supported Pd and Ni Nanoparticles. Catalysts. 2022; 12(5):506. https://doi.org/10.3390/catal12050506
Chicago/Turabian StyleFiore, Ambra Maria, Giuseppe Romanazzi, Cristina Leonelli, Piero Mastrorilli, and Maria Michela Dell’Anna. 2022. "Partial Hydrogenation of Soybean and Waste Cooking Oil Biodiesel over Recyclable-Polymer-Supported Pd and Ni Nanoparticles" Catalysts 12, no. 5: 506. https://doi.org/10.3390/catal12050506
APA StyleFiore, A. M., Romanazzi, G., Leonelli, C., Mastrorilli, P., & Dell’Anna, M. M. (2022). Partial Hydrogenation of Soybean and Waste Cooking Oil Biodiesel over Recyclable-Polymer-Supported Pd and Ni Nanoparticles. Catalysts, 12(5), 506. https://doi.org/10.3390/catal12050506