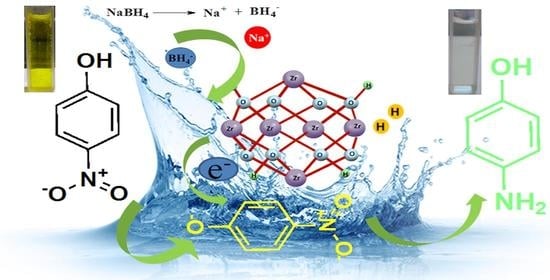
Role of Lewis Acid Metal Centers in Metal–Organic Frameworks for Ultrafast Reduction of 4-Nitrophenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the UiO-66 MOF
2.2. Characterization of the UiO-66 MOF
2.3. Reduction of 4-Nitrophenol
3. Results and Discussion
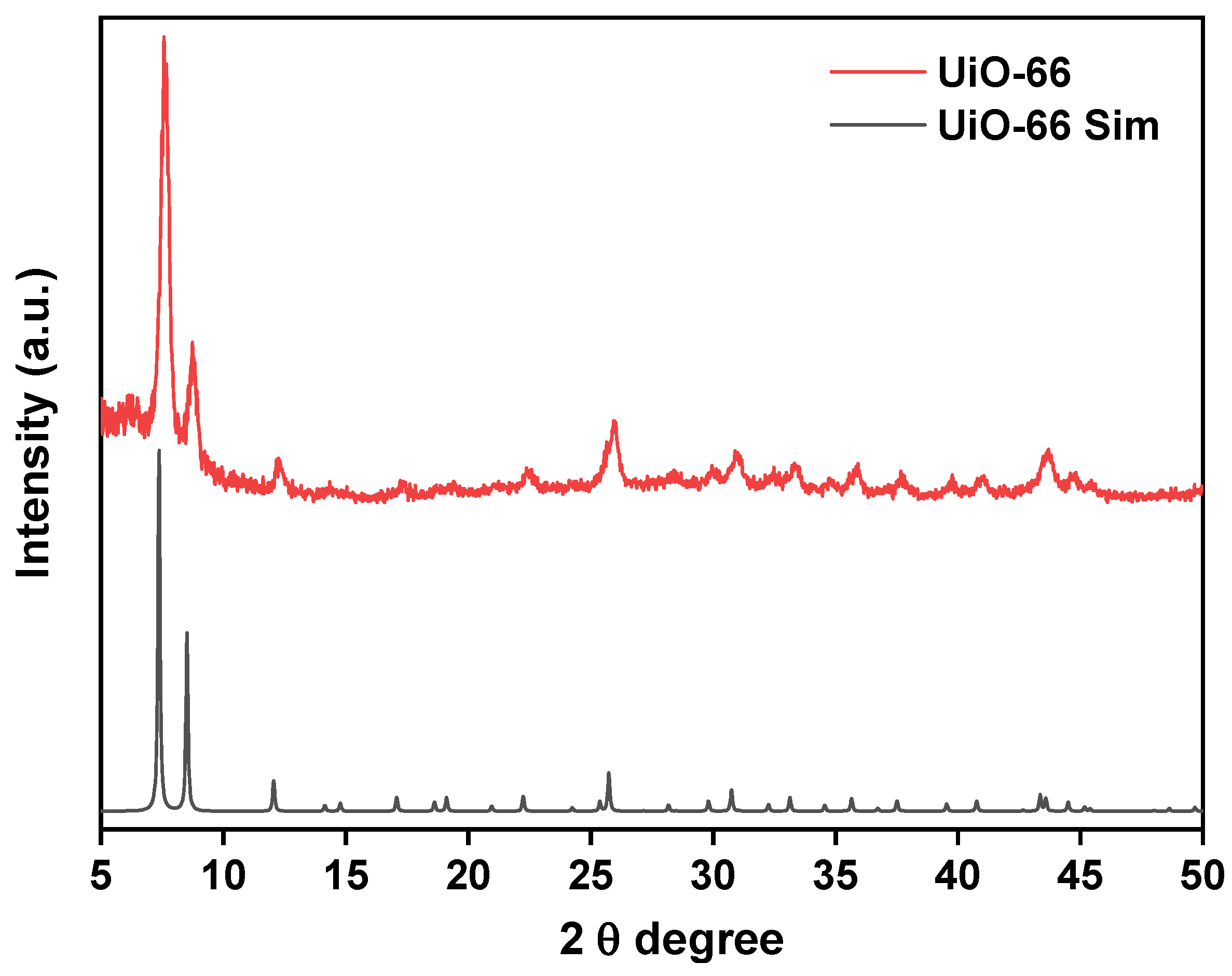
3.1. Powder X-ray Diffraction Study
3.2. Diffused Reflectance UV-Visible Spectra
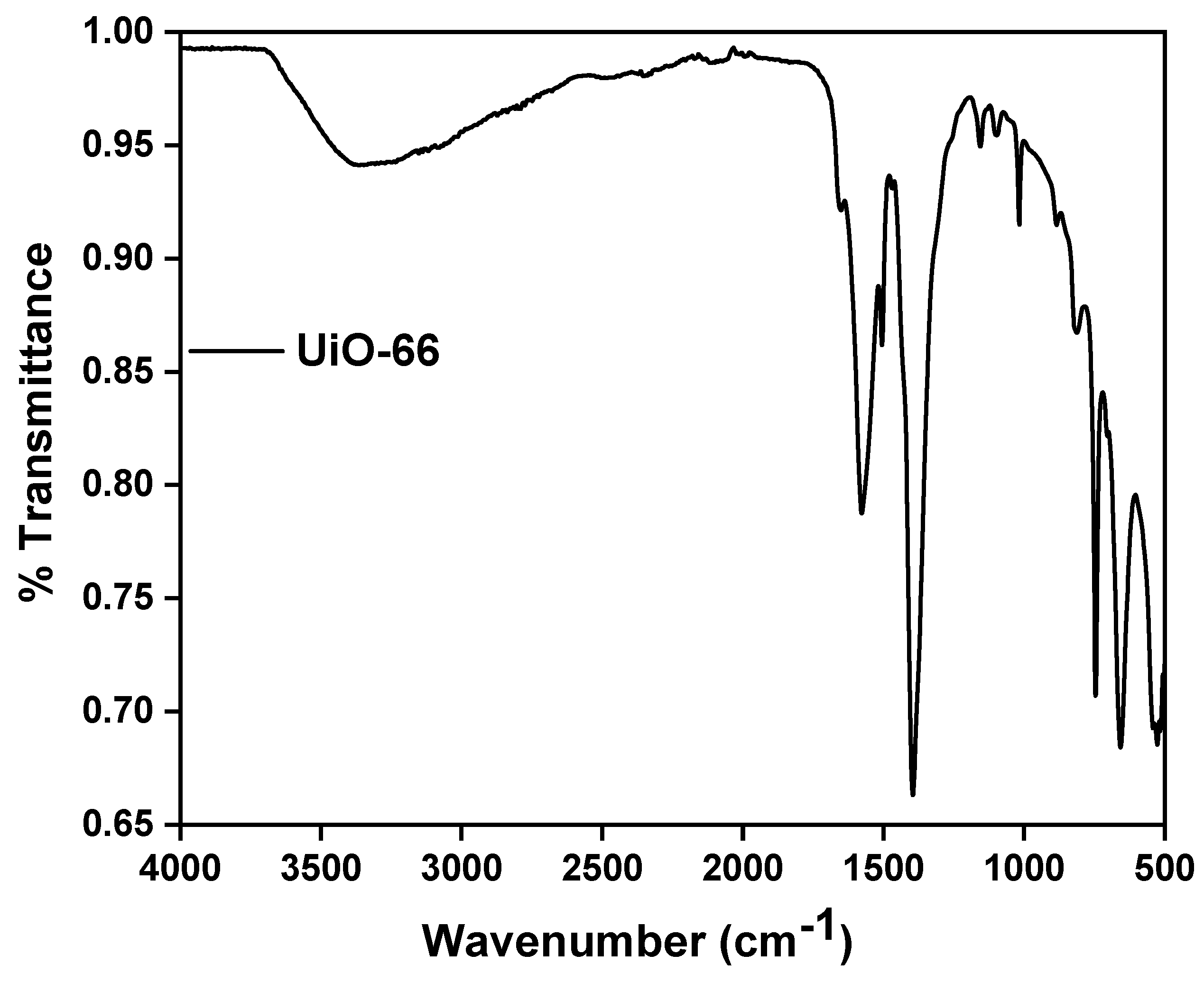
3.3. Fourier Transform Infrared Spectra (FT-IR)
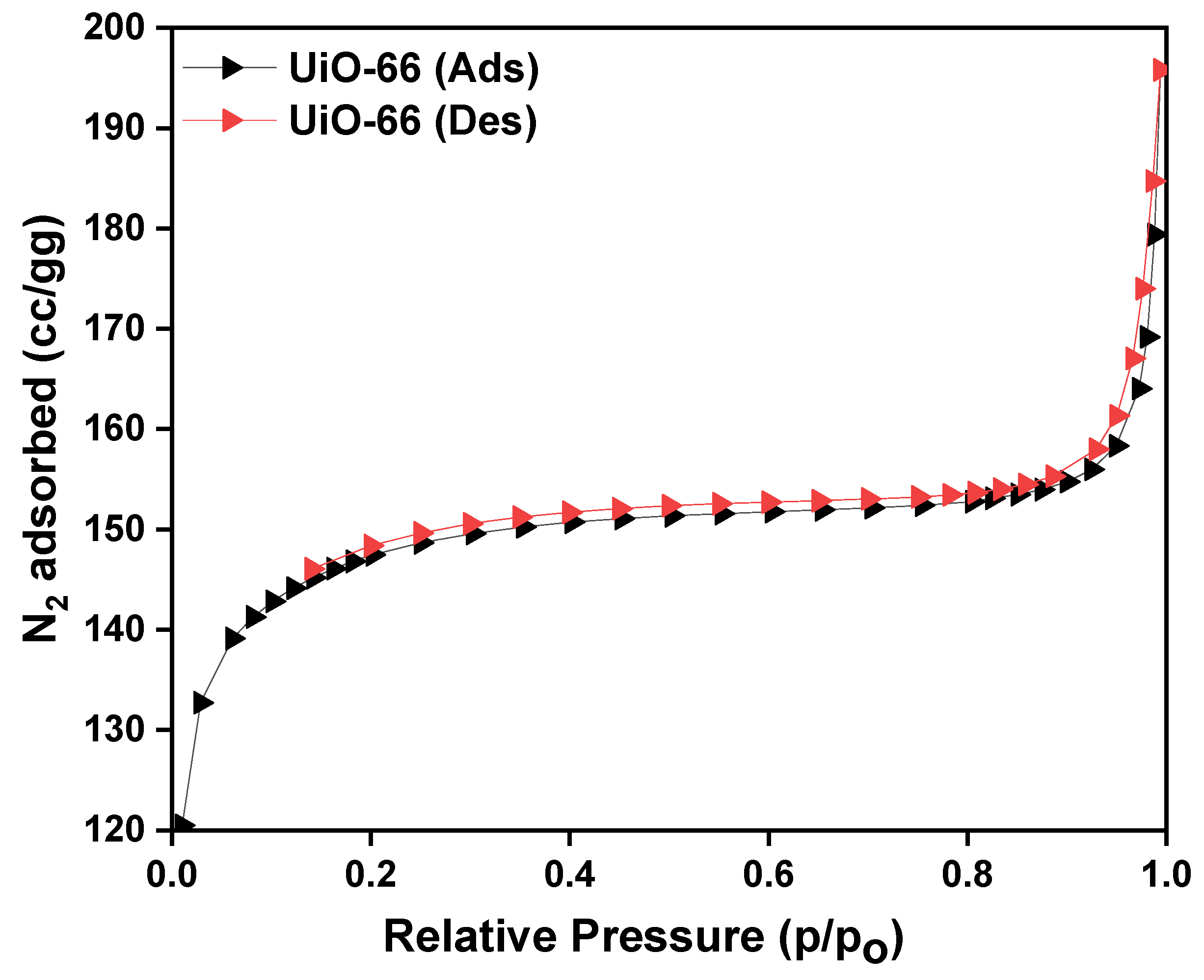
3.4. Specific Surface Area Analysis
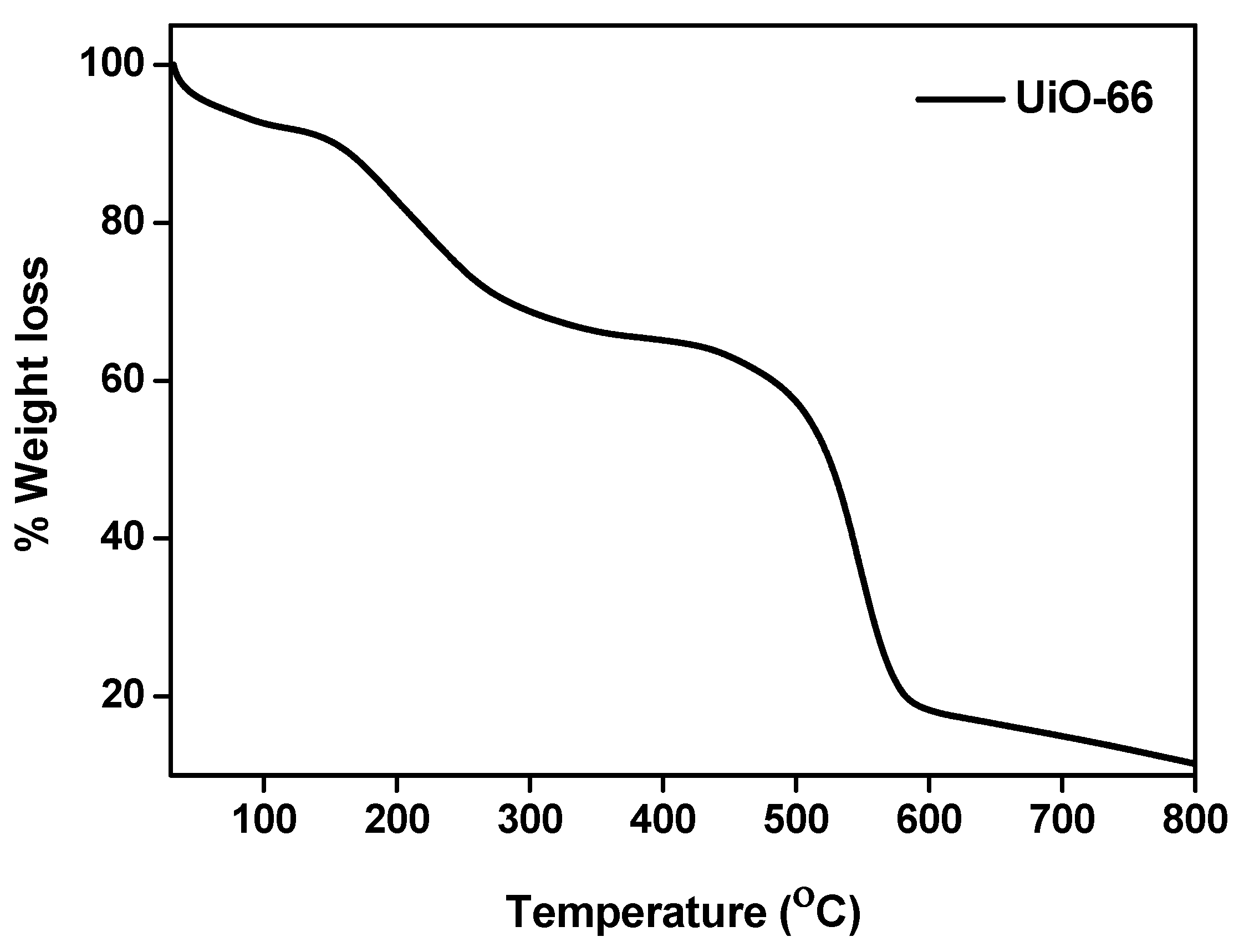
3.5. Thermo Gravimetric Analysis (TGA)
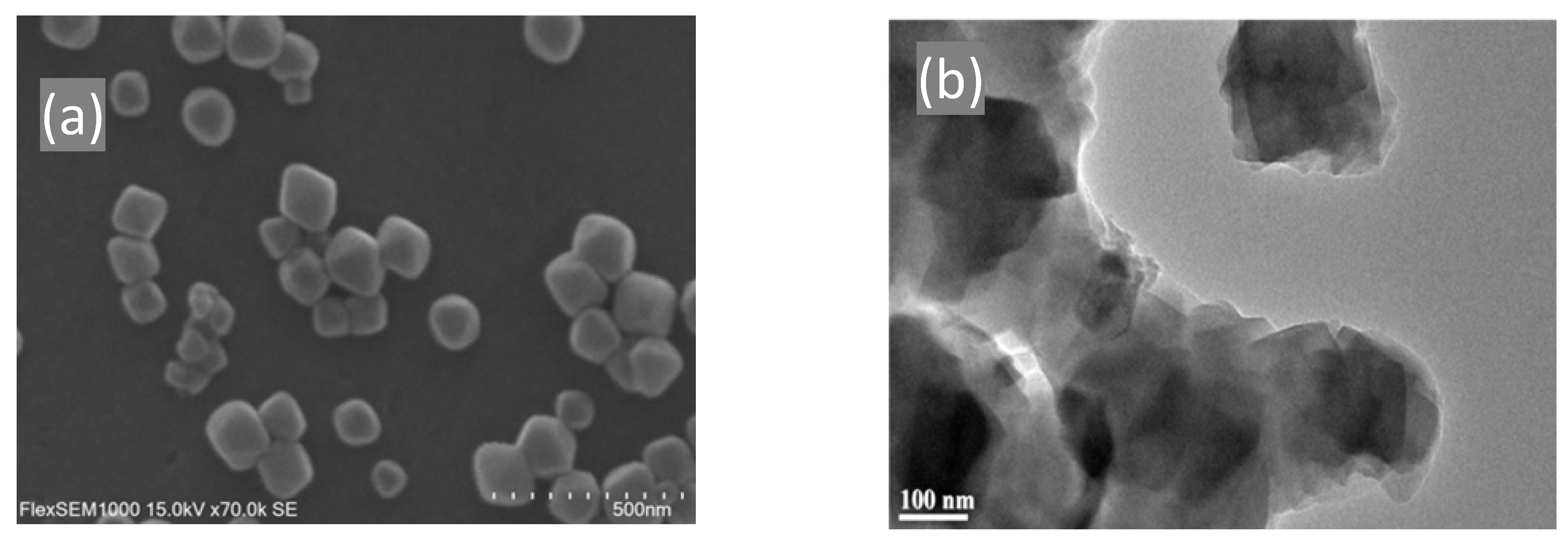
3.6. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
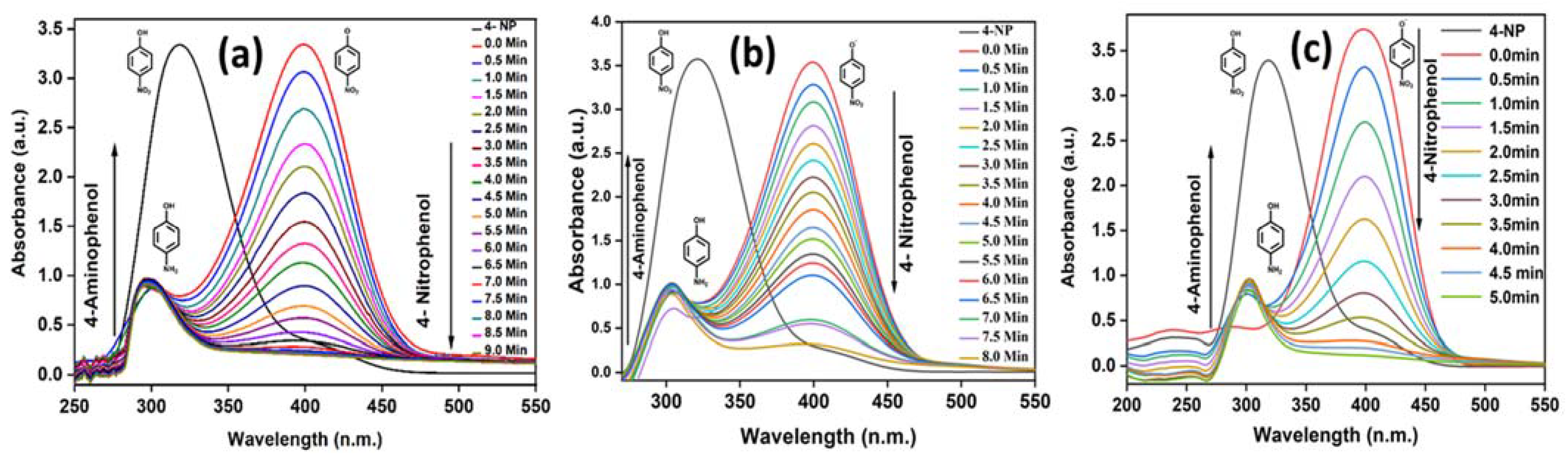
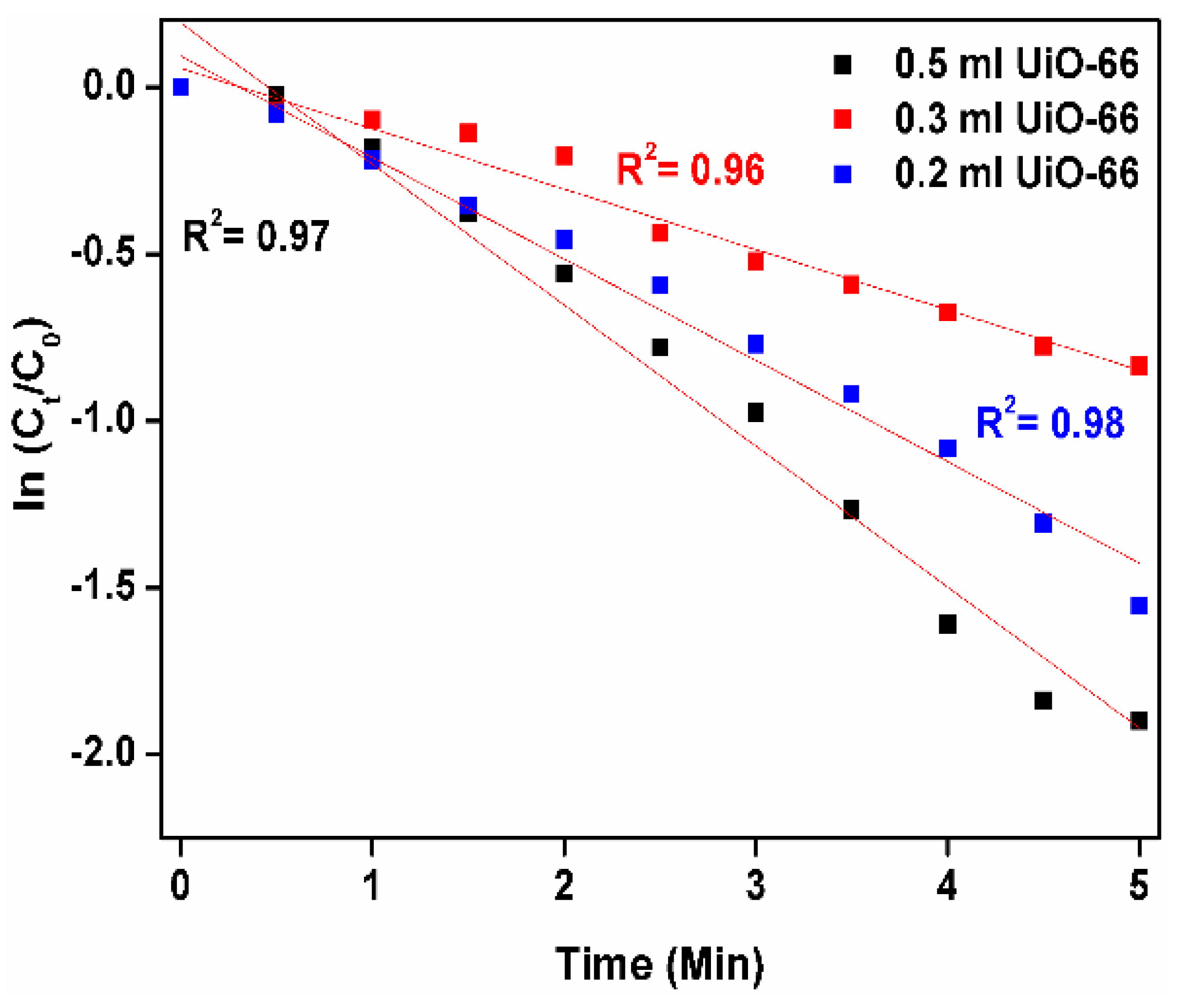
3.7. Catalytic and Reduction Reaction Kinetics Activity Study of Nano UiO-66 MOF
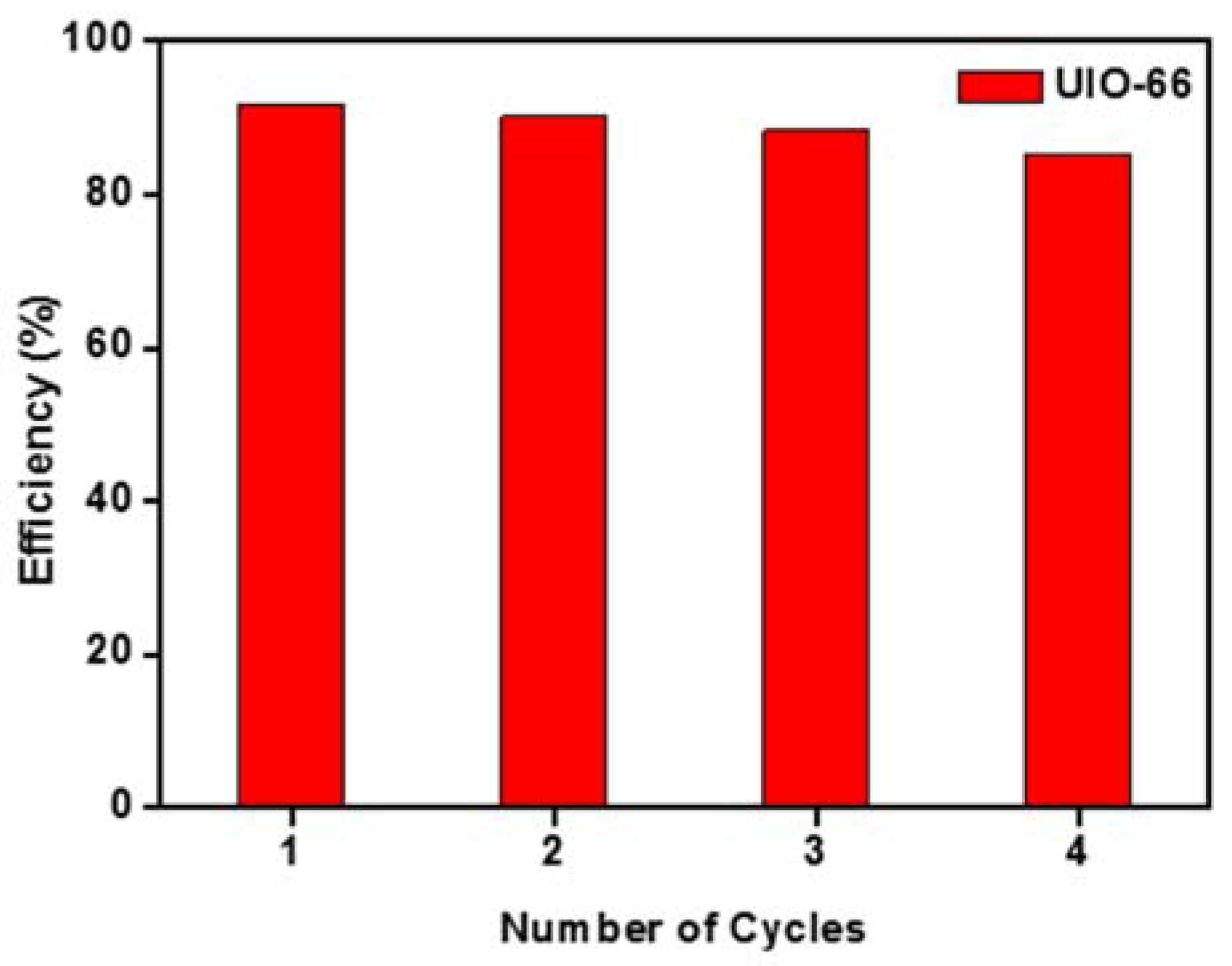
3.8. Reusability Study of the UiO-66 Nano MOF
3.9. Proposed Mechanism of the Reduction Reaction by the UiO-66 Nano MOF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mallick, A.; El-Zohry, A.M.; Shekhah, O.; Yin, J.; Jia, J.; Aggarwal, H.; Emwas, A.-H.; Mohammed, O.F.; Eddaoudi, M. Unprecedented Ultralow Detection Limit of Amines using a Thiadiazole-Functionalized Zr(IV)-Based Metal-Organic Framework. J. Am. Chem. Soc. 2019, 141, 7245–7249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.G.T.; Schweitzer, N.M.; Chang, C.-Y.; Drake, T.L.; So, M.C.; Stair, P.C.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. Vanadium-Node-Functionalized UiO-66: A Thermally Stable MOF-Supported Catalyst for the Gas-Phase Oxidative Dehydrogenation of Cyclohexene. ACS Catal. 2014, 4, 2496–2500. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.-L.; Xu, Q. Metal-Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2018, 30, e1703663. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Keser Demir, N.; Chen, J.P.; Li, K. Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Y.; Li, D.-S.; Bu, X.; Feng, P. Metal-Organic Frameworks for Separation. Adv. Mater. 2018, 30, e1705189. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.; Khutia, A.; Janiak, C. Brønsted instead of Lewis acidity in functionalized MIL-101Cr MOFs for efficient heterogeneous (nano-MOF) catalysis in the condensation reaction of aldehydes with alcohols. Inorg. Chem. 2014, 53, 7319–7333. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Santiago-Portillo, A.; Asiri, A.M.; Garcia, H. Engineering UiO-66 Metal Organic Framework for Heterogeneous Catalysis. ChemCatChem 2019, 11, 899–923. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.-R.; Li, J.-R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.-C. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.O.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Vermeulen, N.A.; Malliakas, C.D.; Gómez-Gualdrón, D.A.; Howarth, A.J.; Mehdi, B.L.; Dohnalkova, A.; Browning, N.D.; O’Keeffe, M.; Farha, O.K. Bottom-up construction of a superstructure in a porous uranium-organic crystal. Science 2017, 356, 624–627. [Google Scholar] [CrossRef] [Green Version]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal-organic framework materials with ultrahigh surface areas: Is the sky the limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grünker, R.; Bon, V.; Müller, P.; Stoeck, U.; Krause, S.; Mueller, U.; Senkovska, I.; Kaskel, S. A new metal-organic framework with ultra-high surface area. Chem. Commun. 2014, 50, 3450–3452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhakshinamoorthy, A.; Garcia, H. Catalysis by metal nanoparticles embedded on metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 5262–5284. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-T.; Liu, Y.-M.; Wang, T.; Wu, Y.-L.; He, Y.-L.; Yang, R.; Zheng, S.-R. Regulation of the surface area and surface charge property of MOFs by multivariate strategy: Synthesis, characterization, selective dye adsorption and separation. Microporous Mesoporous Mater. 2018, 272, 101–108. [Google Scholar] [CrossRef]
- Zong, M.-Y.; Fan, C.-Z.; Yang, X.-F.; Wang, D.-H. Promoting Ni-MOF with metallic Ni for highly-efficient p-nitrophenol hydrogenation. Mol. Catal. 2021, 509, 111609. [Google Scholar] [CrossRef]
- Agarwal, R.A.; Gupta, A.K.; De, D. Flexible Zn-MOF Exhibiting Selective CO2 Adsorption and Efficient Lewis Acidic Catalytic Activity. Cryst. Growth Des. 2019, 19, 2010–2018. [Google Scholar] [CrossRef]
- Sadeghzadeh, S.M.; Zhiani, R.; Emrani, S. The reduction of 4-nitrophenol and 2-nitroaniline by the incorporation of Ni@Pd MNPs into modified UiO-66-NH2 metal–organic frameworks (MOFs) with tetrathia-azacyclopentadecane. New J. Chem. 2018, 42, 988–994. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Xie, H.; Ma, Y.; Chen, T.; Wang, J. Zinc cobalt bimetallic nanoparticles embedded in porous nitrogen-doped carbon frameworks for the reduction of nitro compounds. J. Mater. Res. 2017, 32, 1777–1786. [Google Scholar] [CrossRef]
- Rani, P.; Srivastava, R. Nucleophilic addition of amines, alcohols, and thiophenol with epoxide/olefin using highly efficient zirconium metal organic framework heterogeneous catalyst. RSC Adv. 2015, 5, 28270–28280. [Google Scholar] [CrossRef]
- Xie, K.; He, Y.; Zhao, Q.; Shang, J.; Gu, Q.; Qiao, G.G.; Webley, P.A. Pd(0) loaded Zn2(azoBDC)2(dabco) as a heterogeneous catalyst. CrystEngComm 2017, 19, 4182–4186. [Google Scholar] [CrossRef]
- Hinde, C.S.; Webb, W.R.; Chew, B.K.J.; Tan, H.R.; Zhang, W.-H.; Hor, T.S.A.; Raja, R. Utilisation of gold nanoparticles on amine-functionalised UiO-66 (NH2-UiO-66) nanocrystals for selective tandem catalytic reactions. Chem. Commun. 2016, 52, 6557–6560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.; Shang, N.; Zhou, X.; Feng, C.; Gao, S.; Wang, C.; Wang, Z. High catalytic activity of a bimetallic AgPd alloy supported on UiO-66 derived porous carbon for transfer hydrogenation of nitroarenes using formic acid-formate as the hydrogen source. New J. Chem. 2017, 41, 9857–9865. [Google Scholar] [CrossRef]
- Yin, D.; Li, C.; Ren, H.; Liu, J.; Liang, C. Gold-Palladium-Alloy-Catalyst Loaded UiO-66-NH2 for Reductive Amination with Nitroarenes Exhibiting High Selectivity. ChemistrySelect 2018, 3, 5092–5097. [Google Scholar] [CrossRef]
- Gole, B.; Sanyal, U.; Mukherjee, P.S. A smart approach to achieve an exceptionally high loading of metal nanoparticles supported by functionalized extended frameworks for efficient catalysis. Chem. Commun. 2015, 51, 4872–4875. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Xi, Q.; Zhang, Y.; Jin, M.; Zhao, Y.; Wu, C.; Zhou, H.; Guo, P.; Xu, J. Atomic-scale engineering of MOF array confined Au nanoclusters for enhanced heterogeneous catalysis. Nanoscale 2019, 11, 1169–1176. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, S.; Niu, X.; Hu, J.; Ren, C.; Chen, H.; Chen, X. Metallic nanoparticles immobilized in magnetic metal–organic frameworks: Preparation and application as highly active, magnetically isolable and reusable catalysts. Catal. Sci. Technol. 2014, 4, 3013–3024. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. High performance and ultrafast reduction of 4-nitrophenol using metal-organic frameworks. J. Environ. Chem. Eng. 2021, 9, 104404. [Google Scholar] [CrossRef]
- Panda, J.; Sahu, S.N.; Sahoo, J.K.; Biswal, S.P.; Pattanayak, S.K.; Samantaray, R.; Sahu, R. Efficient removal of two anionic dyes by a highly robust zirconium based metal organic framework from aqueous medium: Experimental findings with molecular docking study. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100340. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Hendrickx, K.; Vanpoucke, D.E.P.; Leus, K.; Lejaeghere, K.; van Yperen-De Deyne, A.; van Speybroeck, V.; van der Voort, P.; Hemelsoet, K. Understanding Intrinsic Light Absorption Properties of UiO-66 Frameworks: A Combined Theoretical and Experimental Study. Inorg. Chem. 2015, 54, 10701–10710. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Massoudinejad, M.; Ghaderpoori, M.; Shahsavani, A.; Amini, M.M. Adsorption of fluoride over a metal organic framework Uio-66 functionalized with amine groups and optimization with response surface methodology. J. Mol. Liq. 2016, 221, 279–286. [Google Scholar] [CrossRef]
- Shen, L.; Liang, S.; Wu, W.; Liang, R.; Wu, L. Multifunctional NH2-mediated zirconium metal-organic framework as an efficient visible-light-driven photocatalyst for selective oxidation of alcohols and reduction of aqueous Cr(VI). Dalton Trans. 2013, 42, 13649–13657. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liang, R.; Luo, M.; Jing, F.; Wu, L. Electronic effects of ligand substitution on metal-organic framework photocatalysts: The case study of UiO-66. Phys. Chem. Chem. Phys. 2015, 17, 117–121. [Google Scholar] [CrossRef]
- Ghani, M.; Font Picó, M.F.; Salehinia, S.; Palomino Cabello, C.; Maya, F.; Berlier, G.; Saraji, M.; Cerdà, V.; Turnes Palomino, G. Metal-organic framework mixed-matrix disks: Versatile supports for automated solid-phase extraction prior to chromatographic separation. J. Chromatogr. A 2017, 1488, 1–9. [Google Scholar] [CrossRef]
- Zhu, X.; Gu, J.; Wang, Y.; Li, B.; Li, Y.; Zhao, W.; Shi, J. Inherent anchorages in UiO-66 nanoparticles for efficient capture of alendronate and its mediated release. Chem. Commun. 2014, 50, 8779–8782. [Google Scholar] [CrossRef] [PubMed]
- Navalón, S.; Álvaro, M.; Dhakshinamoorthy, A.; García, H. Encapsulation of Metal Nanoparticles within Metal-Organic Frameworks for the Reduction of Nitro Compounds. Molecules 2019, 24, 3050. [Google Scholar] [CrossRef] [Green Version]
- Satapathy, S.; Mohanta, J.; Si, S. Modulating the Catalytic Activity of Gold Nanoparticles through Surface Tailoring. ChemistrySelect 2016, 1, 4940–4948. [Google Scholar] [CrossRef]
- Krishna, R.; Fernandes, D.M.; Ventura, J.; Freire, C.; Titus, E. Facile synthesis of reduced graphene oxide supported Pd@NixB/RGO nanocomposite: Novel electrocatalyst for ethanol oxidation in alkaline media. Int. J. Hydrogen Energy 2016, 41, 11811–11822. [Google Scholar] [CrossRef]
- Yusran, Y.; Xu, D.; Fang, Q.; Zhang, D.; Qiu, S. MOF-derived Co@N-C nanocatalyst for catalytic reduction of 4-nitrophenol to 4-aminophenol. Microporous Mesoporous Mater. 2017, 241, 346–354. [Google Scholar] [CrossRef]
- El-Bahy, Z.M. Preparation and characterization of Pt-promoted NiY and CoY catalysts employed for 4-nitrophenol reduction. Appl. Catal. A Gen. 2013, 468, 175–183. [Google Scholar] [CrossRef]
- Praus, P.; Turicová, M.; Karlíková, M.; Kvítek, L.; Dvorský, R. Nanocomposite of montmorillonite and silver nanoparticles: Characterization and application in catalytic reduction of 4-nitrophenol. Mater. Chem. Phys. 2013, 140, 493–498. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Huang, D.-D.; Zhou, Z.-H.; Dong, W.-W.; Wu, Y.-P.; Zhao, J.; Li, D.-S.; Zhang, Q.; Bu, X. Ag-NPs embedded in two novel Zn3/Zn5-cluster-based metal-organic frameworks for catalytic reduction of 2/3/4-nitrophenol. Dalton Trans. 2017, 46, 2430–2438. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.L.; Scola, B.; Li, Y.; Lim, C.-K.; Liu, Y.; Prasad, P.N.; Swihart, M.T.; Knecht, M.R. Remote Optically Controlled Modulation of Catalytic Properties of Nanoparticles through Reconfiguration of the Inorganic/Organic Interface. ACS Nano 2016, 10, 9470–9477. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-J.; Sun, M.; Zang, H.-Y.; Ma, Y.-Y.; Feng, X.-J.; Tan, H.-Q.; Wang, Y.-H.; Li, Y.-G. Hybrid Coordination Networks Constructed from ε-Keggin-Type Polyoxometalates and Rigid Imidazole-Based Bridging Ligands as New Carriers for Noble-Metal Catalysts. Chem. Asian J. 2016, 11, 858–867. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhao, Y.H.; Zhou, Y.; Guo, X.L.; Chen, Z.T.; Zhang, W.J.; Zhang, Y.; Chen, J.; Wang, Z.M.; Sun, L.T.; et al. High-efficient catalytic reduction of 4-nitrophenol based on reusable Ag nanoparticles/graphene-loading loofah sponge hybrid. Nanotechnology 2018, 29, 315702. [Google Scholar] [CrossRef] [Green Version]
- Strachan, J.; Barnett, C.; Masters, A.F.; Maschmeyer, T. 4-Nitrophenol Reduction: Probing the Putative Mechanism of the Model Reaction. ACS Catal. 2020, 10, 5516–5521. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, B.; Chai, X.; Liu, J.; Gu, J.; Ning, P. Comparison of Pd-UiO-66 and Pd-UiO-66-NH2 catalysts performance for phenol hydrogenation in aqueous medium. Fuel 2017, 205, 130–141. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, H.-Y.; Wang, L.; Zhang, Y.; Zhao, J. Ru/UiO-66 Catalyst for the Reduction of Nitroarenes and Tandem Reaction of Alcohol Oxidation/Knoevenagel Condensation. ACS Omega 2018, 3, 4199–4212. [Google Scholar] [CrossRef]



| Catalyst | Quantity (mg) | First-Order, k1 (S−1) | Time (min) |
|---|---|---|---|
| UIO-66 | 0.5 | 0.23 | 5 |
| 0.3 | 0.303 | 8 | |
| 0.2 | 0.367 | 9 |
| Catalyst | Catalyst Used | Reaction Rate Constants Per Unit | Reference |
|---|---|---|---|
| PtCoY Composite | 20 mg | 3.89 | [41] |
| Ag-MMT | 10 mg | 3.33 | [42] |
| Ag@CTGU-3 | 1 mg | 8.64 | [43] |
| Au NPs | 1 mL | 0.51 | [44] |
| Ag/POM-1 | 2 mg | 3.65 | [45] |
| Ag NPs/RGO-LShybrid | 76 mg | 1.893 | [46] |
| Co@N-C from ZIF-67 | 0.1 mg | 1.25 | [40] |
| UiO-66-NH2/TTACP/Ni@Pd | 1.0 mg | 1.42 × 10−2 | [17] |
| UiO-66 | 0.2 | 0.364 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, J.; Biswal, S.P.; Jena, H.S.; Mitra, A.; Samantray, R.; Sahu, R. Role of Lewis Acid Metal Centers in Metal–Organic Frameworks for Ultrafast Reduction of 4-Nitrophenol. Catalysts 2022, 12, 494. https://doi.org/10.3390/catal12050494
Panda J, Biswal SP, Jena HS, Mitra A, Samantray R, Sahu R. Role of Lewis Acid Metal Centers in Metal–Organic Frameworks for Ultrafast Reduction of 4-Nitrophenol. Catalysts. 2022; 12(5):494. https://doi.org/10.3390/catal12050494
Chicago/Turabian StylePanda, Jagannath, Soumya Prakash Biswal, Himanshu Sekhar Jena, Arijit Mitra, Raghabendra Samantray, and Rojalin Sahu. 2022. "Role of Lewis Acid Metal Centers in Metal–Organic Frameworks for Ultrafast Reduction of 4-Nitrophenol" Catalysts 12, no. 5: 494. https://doi.org/10.3390/catal12050494
APA StylePanda, J., Biswal, S. P., Jena, H. S., Mitra, A., Samantray, R., & Sahu, R. (2022). Role of Lewis Acid Metal Centers in Metal–Organic Frameworks for Ultrafast Reduction of 4-Nitrophenol. Catalysts, 12(5), 494. https://doi.org/10.3390/catal12050494