Efficient Synthesis of 3-Sulfonyl-2-sulfonylmethyl-2H-chromenes via Tandem Knoevenagel Condensation/Oxa-Michael Addition Protocol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Reaction Conditions
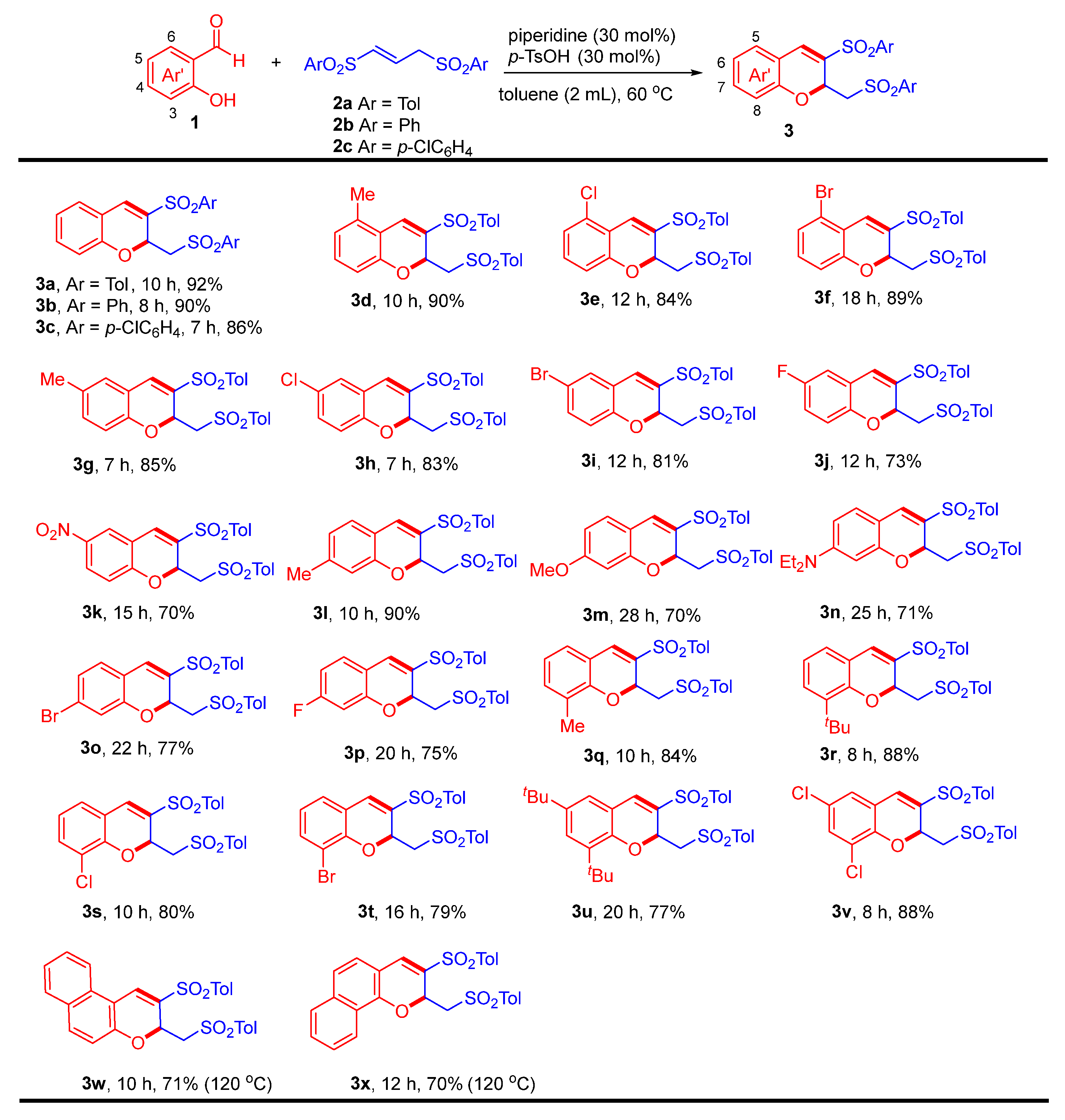
2.2. Substrate Scope of the [4 + 2] Annulation Reaction
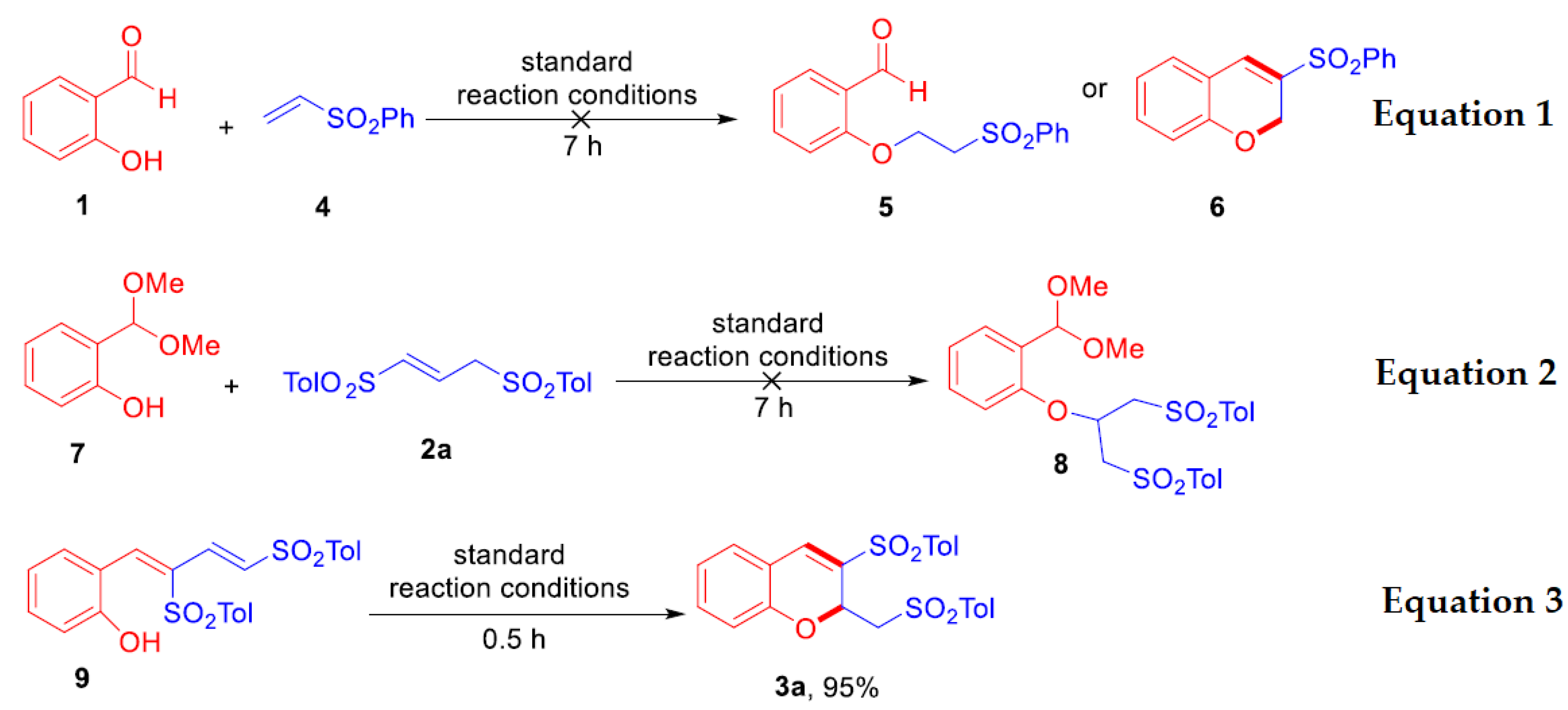
2.3. Study on Reaction Mechanism
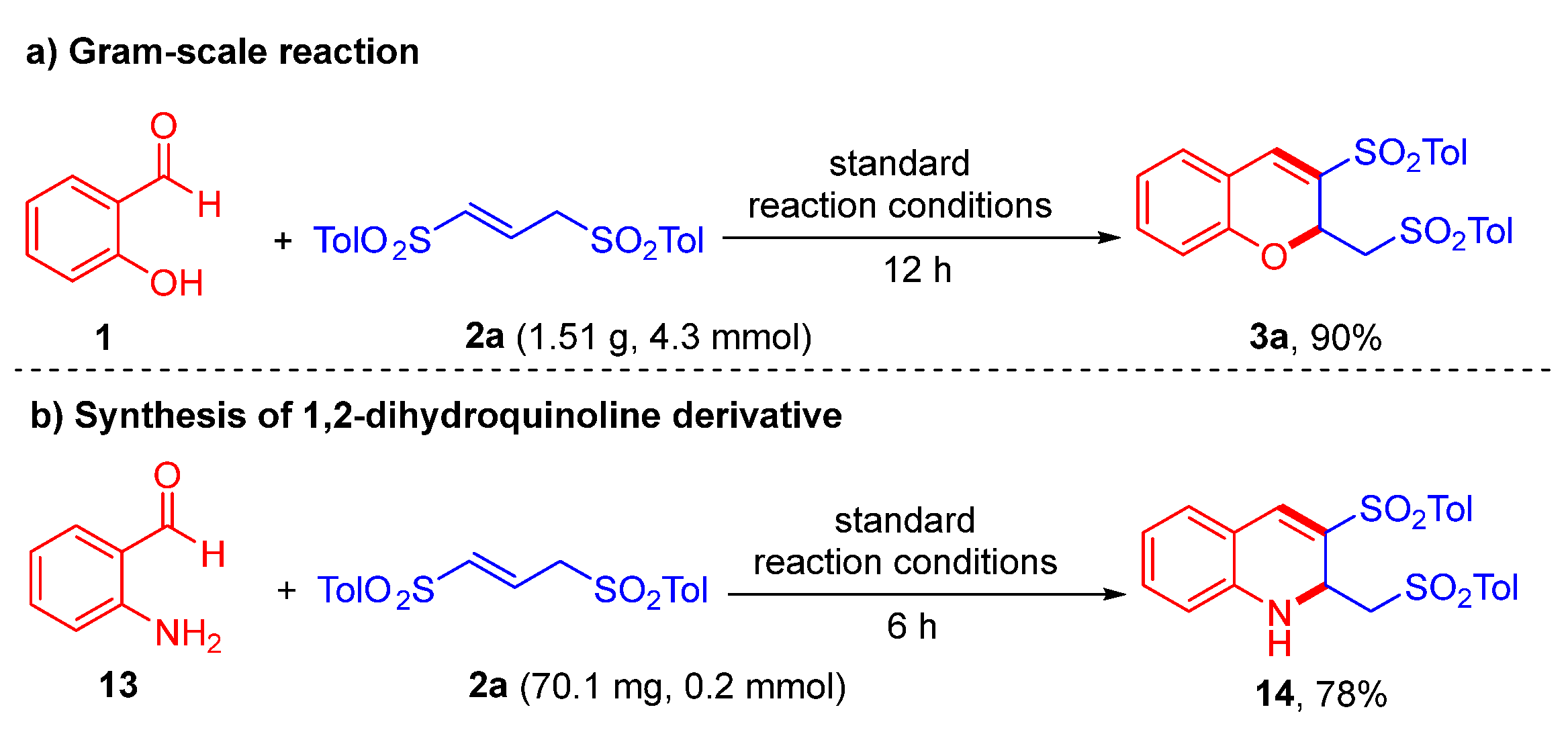
2.4. Derivation of 2H-Chromene Products
3. Materials and Methods
3.1. Instruments and Reagents
3.2. Experimental Method
3.2.1. General Procedure of the [4 + 2] Annulation Reaction−Synthesis of 2H-chromene 3
3.2.2. Synthesis of 2-(sulfonylmethyl)-2H-chromene 10
3.2.3. Synthesis of 2-(sulfonylmethyl)chromane 11
3.2.4. Synthesis of Chromane (±)−12a and (±)−12a′
3.2.5. Synthesis of 1,2-Dihydroquinoline 14
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pratap, R.; Ram, V. Natural and synthetic chromenes, fused chromenes, and versatility of dihydrobenzo[h]chromenes in organic synthesis. J. Chem. Rev. 2014, 114, 10476–10526. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, N.; Paul, N.D.; Mandal, S.; de Bruin, B.; Wulff, W.D. Catalytic synthesis of 2H-chromenes. ACS Catal. 2015, 5, 2329–2366. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, R.; Wang, J. Catalytic asymmetric syntheses of 2-aryl chromenes. Asian J. Org. Chem. 2019, 8, 1742–1765. [Google Scholar] [CrossRef]
- Schneider, P.; Hawser, S.; Islam, K. Iclaprim, a novel diaminopyrimidine with potent activity on trimethoprim sensitive and resistant bacteria. Bioorg. Med. Chem. Lett. 2003, 13, 4217–4221. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, Y.; Yamazaki, K.; Ikeshiro, Y.; Yamagishi, T.; Fujioka, T.; Mihashi, K.; Mizuki, K.; Cosentino, L.M.; Fowke, K.; Morris-Natschke, S.L.; et al. Isolation of rhododaurichromanic acid B and the anti-HIV principles rhododaurichromanic acid A and rhododaurichromenic acid from Rhododendr. dauricum. Tetrahedron 2001, 57, 1559–1563. [Google Scholar] [CrossRef]
- Gauthier, S.; Caron, B.; Cloutier, J.; Dory, Y.L.; Favre, A.; Larouche, D.; Mailhot, J.; Ouellet, C.; Schwerdtfeger, A.; Leblanc, G.; et al. (S)-(+)-4-[7-(2,2-dimethyl-1-oxopropoxy)-4-methyl-2-[4-[2-(1-piperidinyl)-ethoxy]phenyl]-2H-1-benzopyran-3-yl]-phenyl 2,2-dimethylpropanoate (EM-800): A highly potent, specific, and orally active nonsteroidal antiestrogen. J. Med. Chem. 1997, 40, 2117–2122. [Google Scholar] [CrossRef]
- Benslimane, A.F.; Pouchus, Y.F.; Verbist, J.-F.; Petit, J.-Y.; Brion, J.D.; Welin, L. Antiinflammatory mechanism of cordiachromene A action. J. Clin. Pharmacol. 1995, 35, 298–301. [Google Scholar] [CrossRef]
- Batista, J.M., Jr.; Lopes, A.A.; Ambrósio, D.L.; Regasini, L.O.; Kato, M.J.; Bolzani, V.D.S.; Cicarelli, R.M.B.; Furlan, M. Natural chromenes and chromene derivatives as potential anti-trypanosomal agents. Biol. Pharm. Bull. 2008, 31, 538–540. [Google Scholar] [CrossRef] [Green Version]
- Damodar, K.; Kim, J.-K.; Jun, J.-G. Synthesis and pharmacological properties of naturally occurring prenylated and pyranochalcones as potent anti-inflammatory agents. Chin. Chem. Lett. 2016, 27, 698–702. [Google Scholar] [CrossRef]
- Pina, F.; Melo, M.J.; Laia, C.A.T.; Parola, A.J.; Lima, J.C. Chemistry and applications of flavylium compounds: A handful of colours. Chem. Soc. Rev. 2012, 41, 869–908. [Google Scholar] [CrossRef]
- Tao, M.; Liang, X.; Guo, J.; Zheng, S.; Qi, Q.; Cao, Z.; Mi, Y.; Zhao, Z. Dynamic photochromic polymer nanoparticles based on matrix-dependent förster resonance energy transfer and aggregation-induced emission properties. ACS Appl. Mater. Interfaces 2021, 13, 33574–33583. [Google Scholar] [CrossRef] [PubMed]
- Cervi, A.; Vo, Y.; Chai, C.L.L.; Banwell, M.G.; Lan, P.; Wills, A.C. Gold(I)-catalyzed intramolecular hydroarylation of phenol-derived propiolates and certain related ethers as a route to selectively functionalized coumarins and 2H-chromenes. J. Org. Chem. 2021, 86, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Sakata, Y.; Hosoya, T.; Yoshida, S. Synthesis of functionalized benzopyran/coumarin-derived aryne precursors and their applications. Org. Lett. 2020, 22, 8505–8510. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, W. Formal carbene insertion into C-O or C-N bond: An efficient strategy for the synthesis of 2-substituted 2H-chromene derivatives from chromene acetals or hemiaminal ethers. Adv. Synth. Catal. 2018, 360, 2446–2452. [Google Scholar] [CrossRef]
- Song, L.; Su, Q.; Lin, X.; Du, Z.; Xu, H.; Ouyang, M.-A.; Yao, H.; Tong, R. Cascade claisen and meinwald rearrangement for one-pot divergent synthesis of benzofurans and 2H-chromenes. Org. Lett. 2020, 22, 3004–3009. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Chen, Y.; Zhang, H.; Tan, J.-P.; Wang, T. Catalyst-free synthesis of α-functionalized 2H-chromenes in water: A tandem self-promoted pseudo-substitution and decarboxylation process. Asian J. Org. Chem. 2019, 14, 2938–2944. [Google Scholar]
- Song, L.; Huang, F.; Guo, L.; Ouyang, M.-A.; Tong, R. A cascade Claisen rearrangement/o-quinone methide formation/electrocyclization approach to 2H-chromenes. Chem. Commun. 2017, 53, 6021–6024. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, R.; Choy, P.Y.; Liu, T.; Wang, J.; Yuen, O.Y.; Leung, M.P.; Shang, Y.; Kwong, F.Y. DMAP-catalyzed annulation approach for modular assembly of furan-fused chromenes. Org. Lett. 2020, 22, 9444–9449. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, Y.; Zhang, B.; Huang, Z.; He, Z. P(NMe2)3-mediated reductive intramolecular annulation of benzoylformates tethered with a trisubstituted alkene unit and synthesis of 2,2-disubstituted 2H-chromenes. Org. Lett. 2021, 23, 1880–1885. [Google Scholar] [CrossRef]
- Bu, H.-Z.; Li, H.-H.; Luo, W.-F.; Luo, C.; Qian, P.-C.; Ye, L.-W. Synthesis of 2H-chromenes via unexpected [4 + 2] annulation of alkynyl thioethers with o-hydroxybenzyl alcohols. Org. Lett. 2020, 22, 648–652. [Google Scholar] [CrossRef]
- Kawase, Y.; Yamaguchi, S.; Horita, H. A new preparative method of 2,2-dimethyl-2H-chromenes. Bull. Chem. Soc. Jpn. 1982, 55, 1153–1155. [Google Scholar] [CrossRef] [Green Version]
- Petasis, N.A.; Butkevich, A.N. Synthesis of 2H-chromenes and 1,2-dihydroquinolines from aryl aldehydes, amines, and alkenylboron compounds. J. Organomet. Chem. 2009, 694, 1747–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisag, G.D.; Ruggieri, S.; Fochi, M.; Bernardi, L. Catalyst- and substrate-dependent chemodivergent reactivity of stabilised sulfur ylides with salicylaldehydes. Adv. Synth. Catal. 2021, 363, 3053–3061. [Google Scholar] [CrossRef]
- Olomola, F.T.O.; Tukulula, M.; Klein, R.; Kaye, P.T. Strong base- or acid-mediated chemoselectivity shifts in the synthesis of 2H-chromene or coumarin derivatives from common Baylis-Hillman adducts. Tetrahedron 2015, 71, 4868–4873. [Google Scholar]
- Rao, L.C.; Kumar, N.S. A simple and efficient one-pot synthesis of 2-alkyl/aryl/pyridyl substituted 2H-chromenes. New J. Chem. 2015, 39, 7164–7171. [Google Scholar]
- Somprasong, S.; Prasitwatcharakorn, W.; Luanphaisarnnont, T. Efficient synthesis of 2H-chromene derivatives via a dual-organocatalytic reaction. Tetrahedron Lett. 2020, 61, 152402. [Google Scholar] [CrossRef]
- Mohanta, R.; Bez, G. Augmentation of enantioselectivity by spatial tuning of aminocatalyst: Synthesis of 2-alkyl/aryl-3-nitro-2H-chromenes by tandem oxa-Michael–Henry reaction J. Org. Chem. 2020, 85, 4627–4636. [Google Scholar] [CrossRef]
- Tanaka, K.; Sukekawa, M.; Shigematsu, Y.; Hoshino, Y.; Honda, K. Highly regioselective synthesis of 2,3-disubstituted 2H-1-benzopyrans: Brønsted acid catalyzed [4 + 2] cycloaddition reaction with a variety of arylalkynes via ortho-quinone methides. Tetrahedron 2017, 73, 6456–6464. [Google Scholar] [CrossRef]
- Zhu, C.; Cai, Y.; Jiang, H. Recent advances for the synthesis of chiral sulfones with the sulfone moiety directly connected to the chiral center. Org. Chem. Front. 2021, 8, 5574–5589. [Google Scholar] [CrossRef]
- El-Awa, A.; Noshi, M.N.; du Jourdin, X.M.; Fuchs, P.L. Evolving organic synthesis fostered by the pluripotent phenylsulfone moiety. Chem. Rev. 2009, 109, 2315–2349. [Google Scholar] [CrossRef]
- Scott, K.A.; Njardarson, J.T. Analysis of US FDA-approved drugs containing sulfur atoms. Top. Curr. Chem. 2018, 376, 5. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-Y.; Chen, H.-Y.; Chen, Y.-H. Synthesis of 2-aryl-3-sulfonylchromans via Knoevenagel condensation and reduction protocol. J. Org. Chem. 2017, 82, 12631–12639. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Thadkapally, S.; Menon, R.S. Base-mediated cyclocondensation of salicylaldehydes and 2-bromoallyl sulfones for the synthesis of 3-sulfonylchromene derivatives and their regioselective Friedel-Crafts heteroarylation reactions. J. Org. Chem. 2015, 80, 11048–11056. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Z.; Liang, J.; Zhang, X.-J.; Sun, R.W.-Y.; Yan, M.; Chan, A.S.C. Synthesis of multisubstituted arylsulfones via one-pot, three-component [3+3] benzannulation reaction. Org. Biomol. Chem. 2019, 17, 4753–4760. [Google Scholar] [CrossRef]
- Jiang, L.; Li, H.; Zhou, J.-F.; Yuan, M.-W.; Li, H.-L.; Chuan, Y.-M.; Yuan, M.-L. Secondary amine-caialyzed [3+3] benzanulation to aces polysubstituted benzenes through iminium activation. Synth. Commun. 2018, 48, 336–343. [Google Scholar] [CrossRef]
- Yadav, D.; Sharma, S.K.; Menon, R.S. Facile synthesis of biarylmethanes and tetrasubstituted arenes via a base-mediated [3+3] benzannulation reaction of Morita-Baylis-Hillman adducts and unsaturated sulfones. Org. Biomol. Chem. 2019, 17, 4073–4076. [Google Scholar] [CrossRef]
- Joshi, P.R.; Undeela, S.; Reddy, D.D.; Singarapu, K.K.; Menon, R.S. Regioselective synthesis of substituted arenes via aerobic oxidative [3+3] benzannulation reactions of α,β-unsaturated aldehydes and ketones. Org. Lett. 2015, 17, 1449–1452. [Google Scholar] [CrossRef]
- Du, Y.; Yu, A.; Jia, J.; Zhang, Y.; Meng, X. Direct N–H/α,α,β,β-C(sp3)–H functionalization of piperidine via an azomethine ylide route: Synthesis of spirooxindoles bearing 3-substituted oxindoles. Chem. Commun. 2017, 53, 1684–1687. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Du, Y.; Zhang, Q.; Yu, A.; Zhang, Y.; Jia, J.; Liu, X. Direct functionalization of azepane via azomethine ylides: A highly efficient synthesis of spirooxindoles bearing a 1-azabicyclo [5.3.0]decane moiety. Asian J. Org. Chem. 2017, 6, 1719–1723. [Google Scholar] [CrossRef]




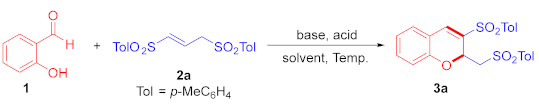
| Entry | Base (Mol%) | Brønsted Acid (Mol%) | Solvent | Temp. (°C) | Time (h) | Yield of 3a (%) b |
|---|---|---|---|---|---|---|
| 1 | DBU (30) | / | toluene | 90 | 8 | 22 |
| 2 | Cs2CO3 | / | toluene | 90 | 12 | 40 (45) c |
| 3 | piperidine (30) | / | toluene | 90 | 7 | 45 |
| 4 | piperidine (30) | AcOH (30) | toluene | 90 | 6 | 77 |
| 5 | / | AcOH (30) | toluene | 90 | 24 | N.R. |
| 6 | piperidine (30) | PhCOOH (30) | toluene | 90 | 6 | 66 |
| 7 | piperidine (30) | TFA (30) | toluene | 90 | 8 | trace |
| 8 | piperidine (30) | p-TsOH (30) | toluene | 90 | 3 | 85 |
| 9 | / | p-TsOH (30) | toluene | 90 | 24 | N.R. |
| 10 | pyrrolidine | p-TsOH (30) | toluene | 90 | 3 | 81 |
| 11 | DABCO | p-TsOH (30) | toluene | 90 | 24 | 53 |
| 12 | NH4OAc (30) | p-TsOH (30) | toluene | 90 | 24 | trace |
| 13 | Pyridine (30) | p-TsOH (30) | toluene | 90 | 24 | trace |
| 14 d | piperidine (30) | p-TsOH (30) | toluene | 60 | 10 | 92 |
| 15 e | piperidine (30) | p-TsOH (30) | toluene | 60 | 10 | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Peng, P.; Li, M.; Li, L.; Zhao, M.; Yuan, M.; Yuan, M. Efficient Synthesis of 3-Sulfonyl-2-sulfonylmethyl-2H-chromenes via Tandem Knoevenagel Condensation/Oxa-Michael Addition Protocol. Catalysts 2022, 12, 491. https://doi.org/10.3390/catal12050491
Jiang L, Peng P, Li M, Li L, Zhao M, Yuan M, Yuan M. Efficient Synthesis of 3-Sulfonyl-2-sulfonylmethyl-2H-chromenes via Tandem Knoevenagel Condensation/Oxa-Michael Addition Protocol. Catalysts. 2022; 12(5):491. https://doi.org/10.3390/catal12050491
Chicago/Turabian StyleJiang, Lin, Peiying Peng, Min Li, Lu Li, Menglin Zhao, Minglong Yuan, and Mingwei Yuan. 2022. "Efficient Synthesis of 3-Sulfonyl-2-sulfonylmethyl-2H-chromenes via Tandem Knoevenagel Condensation/Oxa-Michael Addition Protocol" Catalysts 12, no. 5: 491. https://doi.org/10.3390/catal12050491
APA StyleJiang, L., Peng, P., Li, M., Li, L., Zhao, M., Yuan, M., & Yuan, M. (2022). Efficient Synthesis of 3-Sulfonyl-2-sulfonylmethyl-2H-chromenes via Tandem Knoevenagel Condensation/Oxa-Michael Addition Protocol. Catalysts, 12(5), 491. https://doi.org/10.3390/catal12050491








