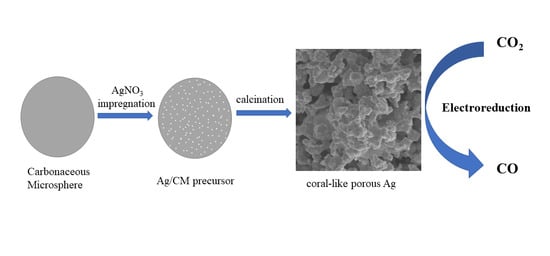
Silver-Carbonaceous Microsphere Precursor-Derived Nano-Coral Ag Catalyst for Electrochemical Carbon Dioxide Reduction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Electrocatalysts
2.1.1. Phase Structure
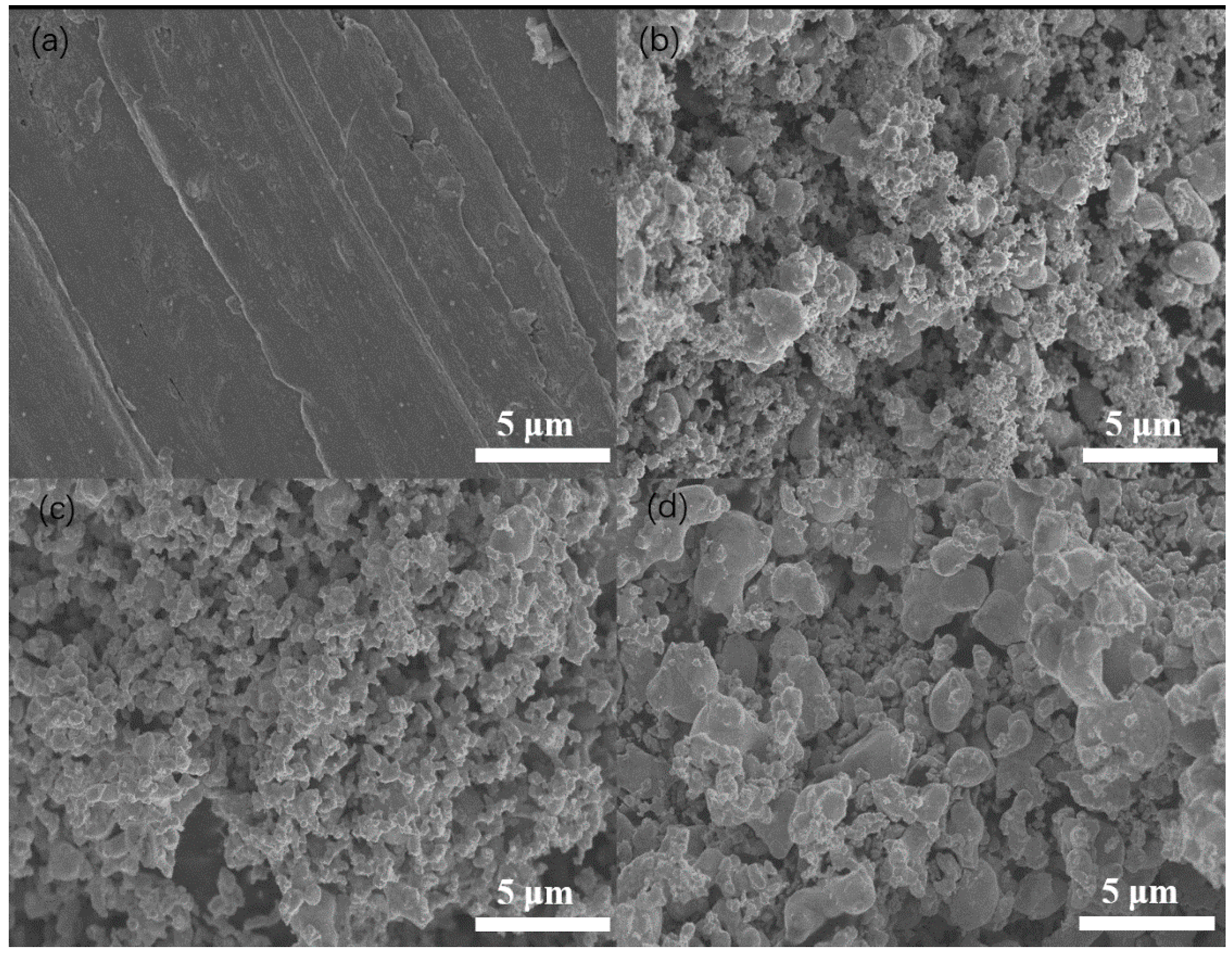
2.1.2. Surface Morphology and Composition
2.1.3. Surface Chemical States of Elements
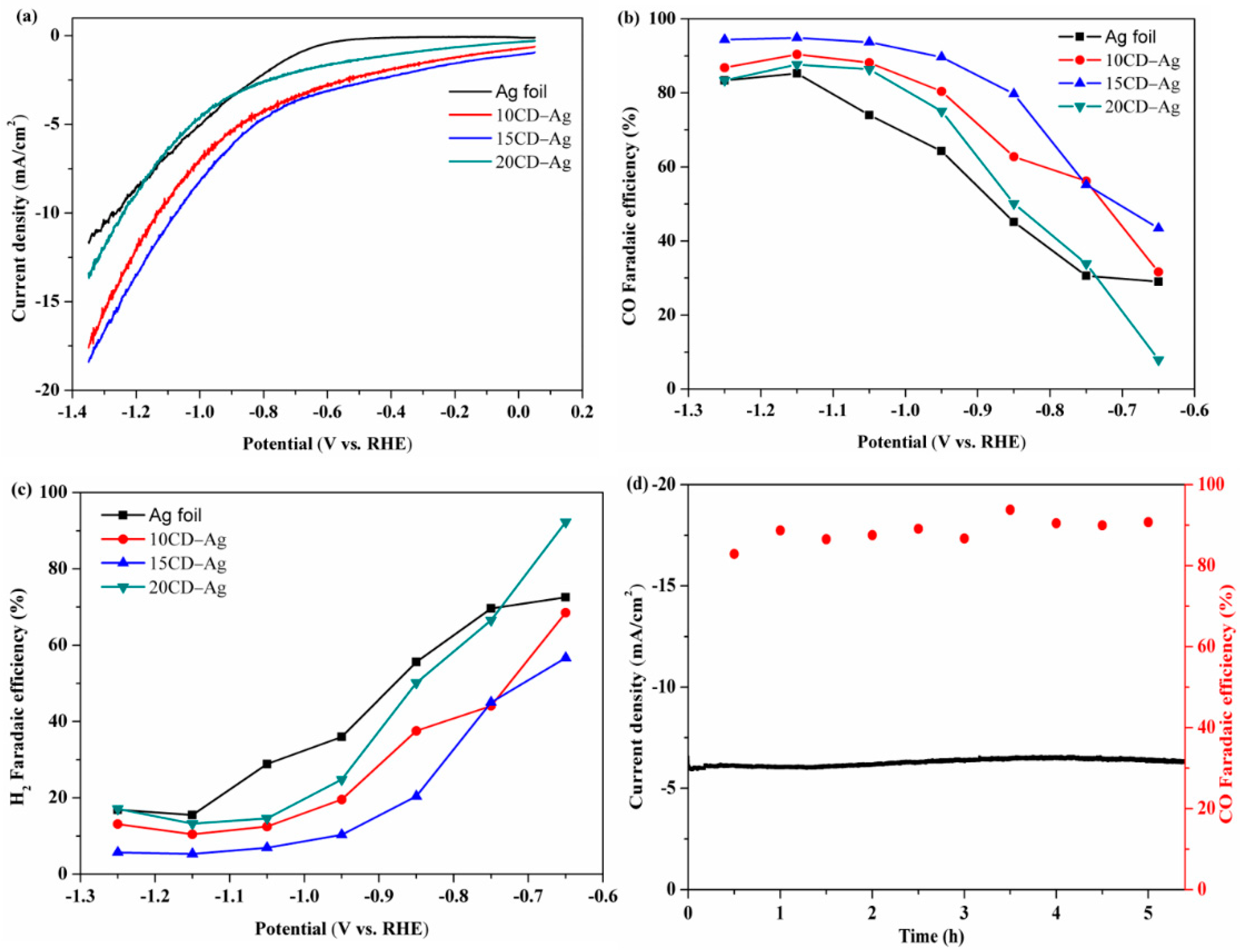
2.2. Electrocatalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Electrode Preparation
3.4. Electrochemical Methods
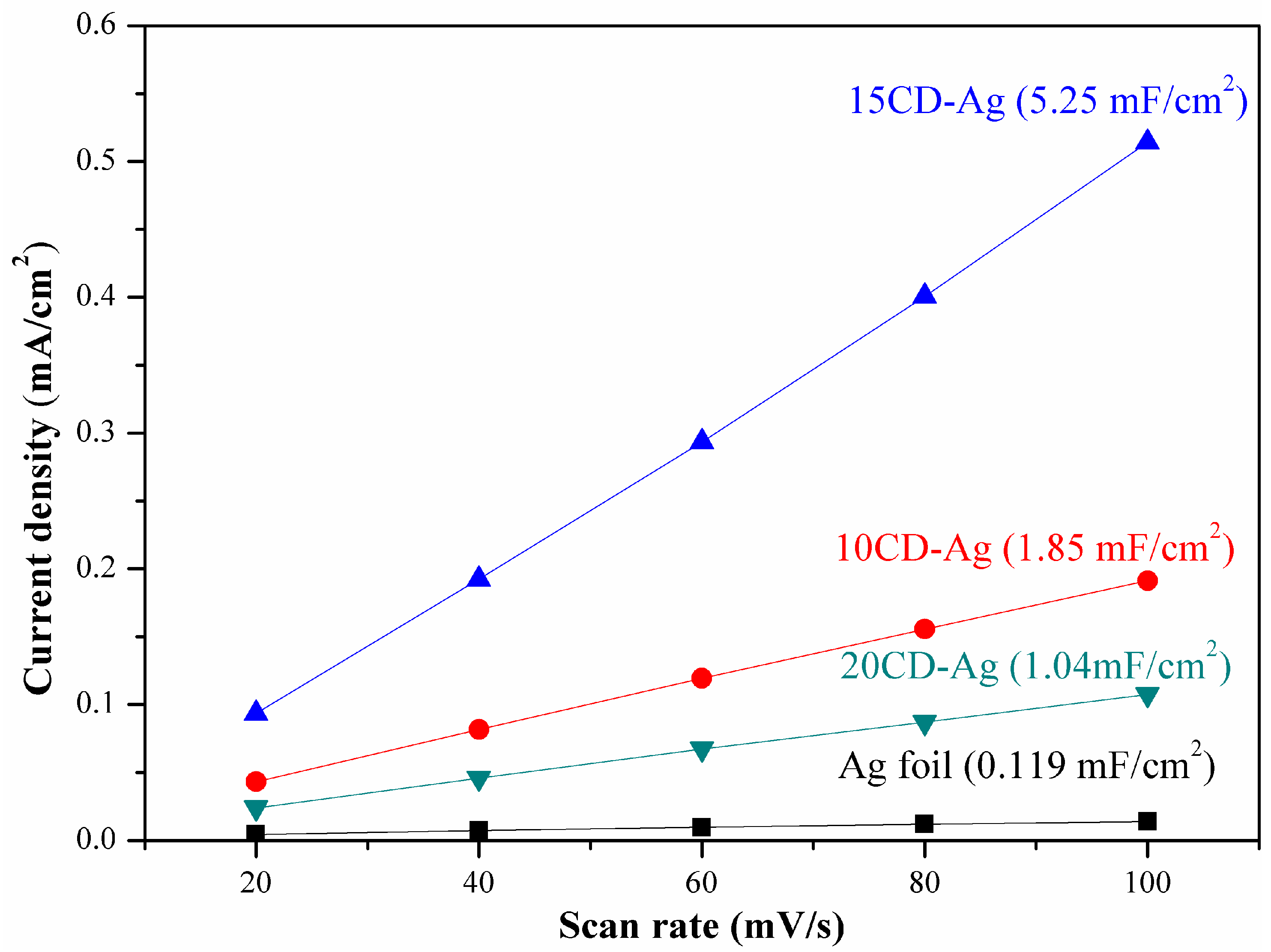
3.5. Electrochemical Active Surface Area
3.6. Physical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.A.; Salehi-Khojin, A.; Thorson, M.R.; Zhu, W.; Whipple, D.T.; Kenis, P.J.; Masel, R.I. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science 2011, 334, 643–644. [Google Scholar] [CrossRef]
- Benson, E.E.; Kubiak, C.P.; Sathrum, A.J.; Smieja, J.M. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem. Soc. Rev. 2009, 38, 89–99. [Google Scholar] [CrossRef]
- Peterson, A.A.; Norskov, J.K. Activity Descriptors for CO2 Electroreduction to Methane on Transition-Metal Catalysts. J. Phys.Chem. Lett. 2012, 3, 251–258. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic Conversion of Carbon Dioxide to Methane and Methanol on Transition Metal Surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, T.; Liu, B.; Cheng, D.; Hu, C.; Zhang, G.; Zhu, W.; Wang, H.; Zhao, Z.J.; Gong, J. Grain-Boundary-Rich Copper for Efficient Solar-Driven Electrochemical CO2 Reduction to Ethylene and Ethanol. J. Am. Chem. Soc. 2020, 142, 6878–6883. [Google Scholar] [CrossRef]
- Song, R.B.; Zhu, W.; Fu, J.; Chen, Y.; Liu, L.; Zhang, J.R.; Lin, Y.; Zhu, J.J. Electrode Materials Engineering in Electrocatalytic CO2 Reduction: Energy Input and Conversion Efficiency. Adv. Mater. 2019, 32, 1903796. [Google Scholar] [CrossRef] [PubMed]
- Ham, Y.S.; Kim, M.J.; Choi, J.; Choe, S.; Lim, T.; Kim, S.-K.; Kim, J.J. Fabrication of Au Catalysts for Electrochemical Reduction of CO2 to Syngas. J. Nanosci. Nanotechnol. 2016, 16, 10846–10852. [Google Scholar] [CrossRef]
- Kim, K.-S.; Kim, W.J.; Lim, H.-K.; Lee, E.K.; Kim, H. Tuned Chemical Bonding Ability of Au at Grain Boundaries for Enhanced Electrochemical CO2 Reduction. ACS Catal. 2016, 6, 4443–4448. [Google Scholar] [CrossRef]
- Cave, E.R.; Montoya, J.H.; Kuhl, K.P.; Abram, D.N.; Hatsukade, T.; Shi, C.; Hahn, C.; Norskov, J.K.; Jaramillo, T.F. Electrochemical CO2 reduction on Au surfaces: Mechanistic aspects regarding the formation of major and minor products. Phys. Chem. Chem. Phys. 2017, 19, 15856–15863. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Michalsky, R.; Metin, O.; Lv, H.; Guo, S.; Wright, C.; Sun, X.; Peterson, A.A.; Sun, S. Monodisperse Au Nanoparticles for Selective Electrocatalytic Reduction of CO2 to CO. J. Am. Chem. Soc. 2017, 139, 9408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi-Khojin, A.; Jhong, H.-R.M.; Rosen, B.A.; Zhu, W.; Ma, S.; Kenis, P.J.A.; Masel, R.I. Nanoparticle Silver Catalysts That Show Enhanced Activity for Carbon Dioxide Electrolysis. J. Phys. Chem. C 2013, 117, 1627–1632. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Lu, Q.; Rivera, S.; Zhou, Y.; Vlachos, D.G.; Jiao, F. Mechanistic Insights into the Electrochemical Reduction of CO2 to CO on Nanostructured Ag Surfaces. ACS Catal. 2015, 5, 4293–4299. [Google Scholar] [CrossRef]
- Kim, C.; Eom, T.; Jee, M.S.; Jung, H.; Kim, H.; Min, B.K.; Hwang, Y.J. Insight into Electrochemical CO2 Reduction on Surface-Molecule-Mediated Ag Nanoparticles. ACS Catal. 2016, 7, 779–785. [Google Scholar] [CrossRef]
- Qi, K.; Zhang, Y.; Li, J.; Charmette, C.; Ramonda, M.; Cui, X.; Wang, Y.; Zhang, Y.; Wu, H.; Wang, W.; et al. Enhancing the CO2-to-CO Conversion from 2D Silver Nanoprisms via Superstructure Assembly. ACS Nano 2021, 15, 7682–7693. [Google Scholar] [CrossRef]
- Won, D.H.; Shin, H.; Koh, J.; Chung, J.; Lee, H.S.; Kim, H.; Woo, S.I. Highly Efficient, Selective, and Stable CO2 Electroreduction on a Hexagonal Zn Catalyst. Angew. Chem. Int. Ed. 2016, 55, 9297–9300. [Google Scholar] [CrossRef]
- Zhang, T.; Zhong, H.; Qiu, Y.; Li, X.; Zhang, H. Zn electrode with a layer of nanoparticles for selective electroreduction of CO2 to formate in aqueous solutions. J. Mater. Chem. A 2016, 4, 16670–16676. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Jee, M.S.; Won, D.H.; Jung, H.; Oh, H.-S.; Min, B.K.; Hwang, Y.J. Selective CO2 Reduction on Zinc Electrocatalyst: The Effect of Zinc Oxidation State Induced by Pretreatment Environment. ACS Sustain. Chem. Eng. 2017, 5, 11377–11386. [Google Scholar] [CrossRef]
- Guo, M.; Li, X.; Huang, Y.; Li, L.; Li, J.; Lu, Y.; Xu, Y.; Zhang, L. CO2-Induced Fibrous Zn Catalyst Promotes Electrochemical Reduction of CO2 to CO. Catalysts 2021, 11, 477. [Google Scholar] [CrossRef]
- Yu, Q.; Meng, X.; Shi, L.; Liu, H.; Ye, J. Superfine Ag nanoparticle decorated Zn nanoplates for the active and selective electrocatalytic reduction of CO2 to CO. Chem. Commun. 2016, 52, 14105–14108. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Xu, X.; Qin, Y.; Jiang, S.P.; Shao, Z. Rational Design of Ag-Based Catalysts for the Electrochemical CO2 Reduction to CO: A Review. ChemSusChem 2020, 13, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Mahyoub, S.A.; Qaraah, F.A.; Chen, C.; Zhang, F.; Yan, S.; Cheng, Z. An overview on the recent developments of Ag-based electrodes in the electrochemical reduction of CO2 to CO. Sustain. Energy Fuels 2020, 4, 50–67. [Google Scholar] [CrossRef]
- Lu, Q.; Rosen, J.; Zhou, Y.; Hutchings, G.S.; Kimmel, Y.C.; Chen, J.G.; Jiao, F. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 2014, 5, 3242. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Trzesniewski, B.J.; Xie, J.; Smith, W.A. Selective and Efficient Reduction of Carbon Dioxide to Carbon Monoxide on Oxide-Derived Nanostructured Silver Electrocatalysts. Angew. Chem. Int. Ed. 2016, 55, 9748–9752. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-C.; Senanayake, S.D.; Zhang, Y.; Xu, W.; Polyansky, D.E. Effect of Chloride Anions on the Synthesis and Enhanced Catalytic Activity of Silver Nanocoral Electrodes for CO2 Electroreduction. ACS Catal. 2015, 5, 5349–5356. [Google Scholar] [CrossRef]
- Yang, J.M.; Du, H.S.; Yu, Q.; Zhang, W.; Zhang, Y.; Ge, J.Y.; Li, H.; Liu, J.Y.; Li, H.M.; Xu, H. Porous silver microrods by plasma vulcanization activation for enhanced electrocatalytic carbon dioxide reduction. J. Colloid Interface Sci. 2022, 606, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, K.; Shen, J.; Kas, R.; Smith, W.A. In Situ Fabrication and Reactivation of Highly Selective and Stable Ag Catalysts for Electrochemical CO2 Conversion. ACS Energy Lett. 2018, 3, 1301–1306. [Google Scholar] [CrossRef]
- Yu, Y.; Zhong, N.; Fang, J.H.; Tang, S.S.; Ye, X.C.; He, Z.Q.; Song, S. Comparative Study between Pristine Ag and Ag Foam for Electrochemical Synthesis of Syngas with Carbon Dioxide and Water. Catalysts 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Zhang, J.; Wang, P.; Sun, Q.; Zheng, J.; Zhu, Y. Light scattering enhanced photoanodes for dye-sensitized solar cells prepared by carbon spheres/TiO2 nanoparticle composites. Curr. Appl. Phys. 2011, 11, 376–381. [Google Scholar] [CrossRef]
- Zhang, S.; Mo, Z.Z.; Wang, J.; Liu, H.L.; Liu, P.; Hu, D.; Tan, T.X.; Wang, C. Ultra-stable oxygen species in Ag nanoparticles anchored on g-C3N4 for enhanced electrochemical reduction of CO2. Electrochim. Acta 2021, 390, 138831. [Google Scholar] [CrossRef]
- Costa, R.S.; Aranha, B.S.R.; Ghosh, A.; Lobo, A.O.; da Silva, E.T.S.G.; Alves, D.C.B.; Viana, B.C. Production of oxalic acid by electrochemical reduction of CO2 using silver-carbon material from babassu coconut mesocarp. J. Phys. Chem. Solids 2020, 147, 109678. [Google Scholar] [CrossRef]
- Rocha, T.C.; Oestereich, A.; Demidov, D.V.; Havecker, M.; Zafeiratos, S.; Weinberg, G.; Bukhtiyarov, V.I.; Knop-Gericke, A.; Schlogl, R. The silver-oxygen system in catalysis: New insights by near ambient pressure X-ray photoelectron spectroscopy. Phys. Chem. Chem. Phys. 2012, 14, 4554–4564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, K.; Kharel, P.; Peng, Y.; Gangishetty, M.K.; Lin, H.-Y.G.; Stavitski, E.; Attenkofer, K.; Wang, H. Silver Nanoparticles with Surface-Bonded Oxygen for Highly Selective CO2 Reduction. ACS Sustain. Chem. Eng. 2017, 5, 8529–8534. [Google Scholar] [CrossRef]
- Jalil, P.A.; Faiz, M.; Tabet, N.; Hamdan, N.M.; Hussain, Z. A study of the stability of tungstophosphoric acid, H3PW12O40, using synchrotron XPS, XANES, hexane cracking, XRD, and IR spectroscopy. J. Catal. 2003, 217, 292–297. [Google Scholar] [CrossRef]
- Prieto, P.; Nistor, V.; Nouneh, K.; Oyama, M.; Abd-Lefdil, M.; Díaz, R. XPS study of silver, nickel and bimetallic silver–nickel nanoparticles prepared by seed-mediated growth. Appl. Surf. Sci. 2012, 258, 8807–8813. [Google Scholar] [CrossRef]
- Jee, M.S.; Kim, H.; Jeon, H.S.; Chae, K.H.; Cho, J.; Min, B.K.; Hwang, Y.J. Stable surface oxygen on nanostructured silver for efficient CO2 electroreduction. Catal. Today 2017, 288, 48–53. [Google Scholar] [CrossRef]
- Hoshi, N.; Kato, M.; Hori, Y. Electrochemical reduction of CO, on single crystal electrodes of silver Ag(111), Ag(100) and Agi(110). J. Electroan. Chem. 1997, 440, 283–286. [Google Scholar] [CrossRef]
- Ham, Y.S.; Choe, S.; Kim, M.J.; Lim, T.; Kim, S.-K.; Kim, J.J. Electrodeposited Ag catalysts for the electrochemical reduction of CO2 to CO. Appl. Catal. B Environ. 2017, 208, 35–43. [Google Scholar] [CrossRef]
- Pan, F.P.; Yang, Y. Designing CO2 reduction electrode materials by morphology and interface engineering. Energy Environ. Sci. 2020, 13, 2275–2309. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Wu, S.-W.; Gao, M.-R.; Li, M.-S.; Fu, X.-Z.; Luo, J.-L. Hollow Porous Ag Spherical Catalysts for Highly Efficient and Selective Electrocatalytic Reduction of CO2 to CO. ACS Sustain. Chem. Eng. 2019, 7, 14443–14450. [Google Scholar] [CrossRef]
- He, Z.; Liu, T.; Tang, J.; Zhou, C.; Wen, L.; Chen, J.; Song, S. Highly active, selective and stable electroreduction of carbon dioxide to carbon monoxide on a silver catalyst with truncated hexagonal bipyramidal shape. Electrochim. Acta 2016, 222, 1234–1242. [Google Scholar] [CrossRef]
- Qiu, J.P.; Tang, J.T.; Shen, J.; Wu, C.W.; Qian, M.Q.; He, Z.Q.; Chen, J.M.; Song, S. Preparation of a silver electrode with a three-dimensional surface and its performance in the electrochemical reduction of carbon dioxide. Electrochim. Acta 2016, 203, 99–108. [Google Scholar]
- Sun, X.M.; Li, Y.D. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Ma, R.; Wang, H.; Gao, Z.; Zhang, W.; Zhao, Y. Ultrathin Ag Nanowires Electrode for Electrochemical Syngas Pro-duction from Carbon Dioxide. ACS Sustain. Chem. Eng. 2018, 6, 7687–7694. [Google Scholar] [CrossRef]
- Liu, S.; Tao, H.; Zeng, L.; Liu, Q.; Xu, Z.; Liu, Q.; Luo, J.L. Shape-Dependent Electrocatalytic Reduction of CO2 to CO on Triangular Silver Nanoplates. J. Am. Chem. Soc. 2017, 139, 2160–2163. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chang, S.; Wang, Y.; Zhang, L. Silver-Carbonaceous Microsphere Precursor-Derived Nano-Coral Ag Catalyst for Electrochemical Carbon Dioxide Reduction. Catalysts 2022, 12, 479. https://doi.org/10.3390/catal12050479
Li X, Chang S, Wang Y, Zhang L. Silver-Carbonaceous Microsphere Precursor-Derived Nano-Coral Ag Catalyst for Electrochemical Carbon Dioxide Reduction. Catalysts. 2022; 12(5):479. https://doi.org/10.3390/catal12050479
Chicago/Turabian StyleLi, Xiangxiang, Shuling Chang, Yanting Wang, and Lihong Zhang. 2022. "Silver-Carbonaceous Microsphere Precursor-Derived Nano-Coral Ag Catalyst for Electrochemical Carbon Dioxide Reduction" Catalysts 12, no. 5: 479. https://doi.org/10.3390/catal12050479
APA StyleLi, X., Chang, S., Wang, Y., & Zhang, L. (2022). Silver-Carbonaceous Microsphere Precursor-Derived Nano-Coral Ag Catalyst for Electrochemical Carbon Dioxide Reduction. Catalysts, 12(5), 479. https://doi.org/10.3390/catal12050479