Effect of Different Carbon Supports on the Activity of PtNi Bimetallic Catalysts toward the Oxygen Reduction
Abstract
:1. Introduction
2. Results and Discussion
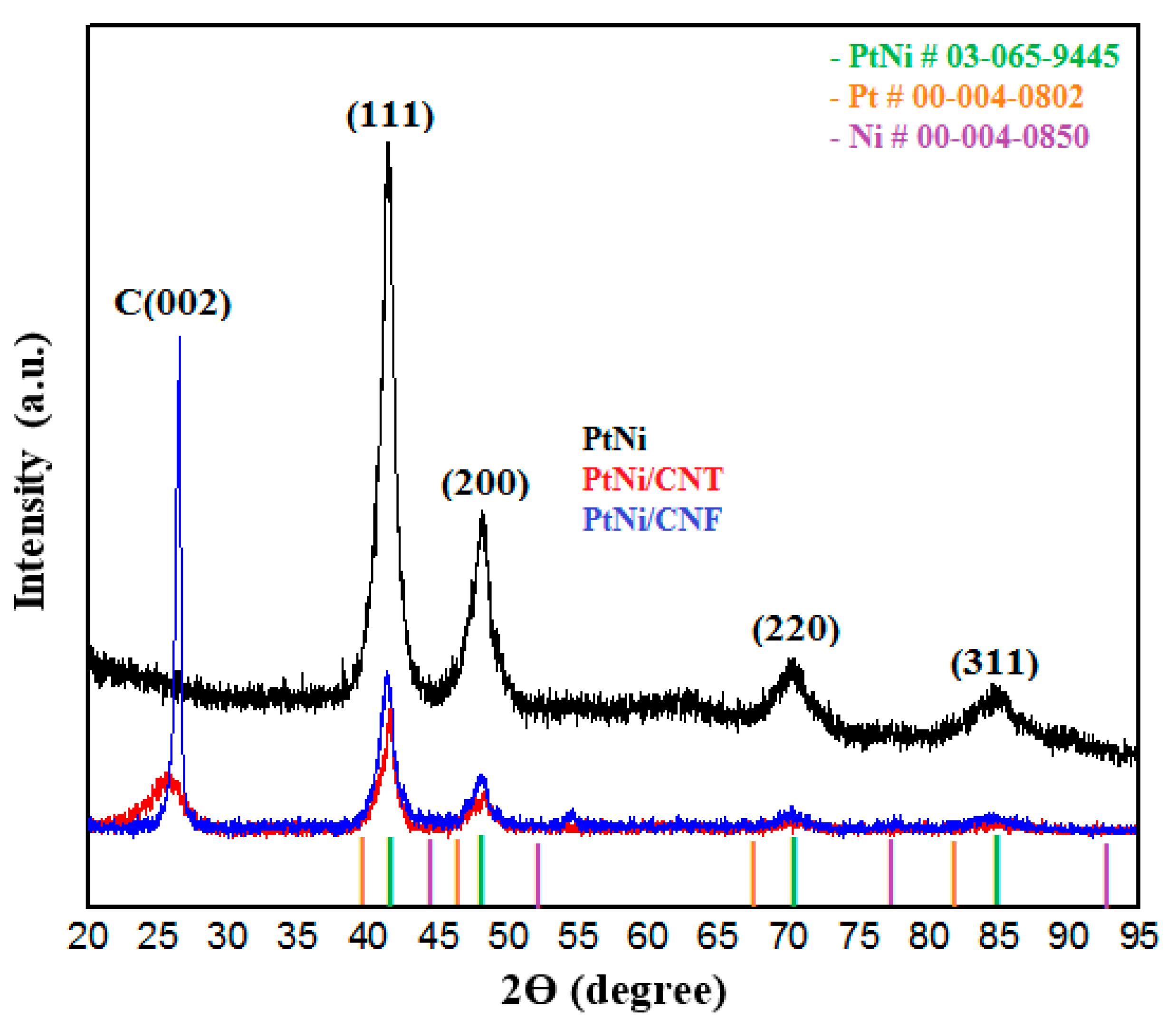
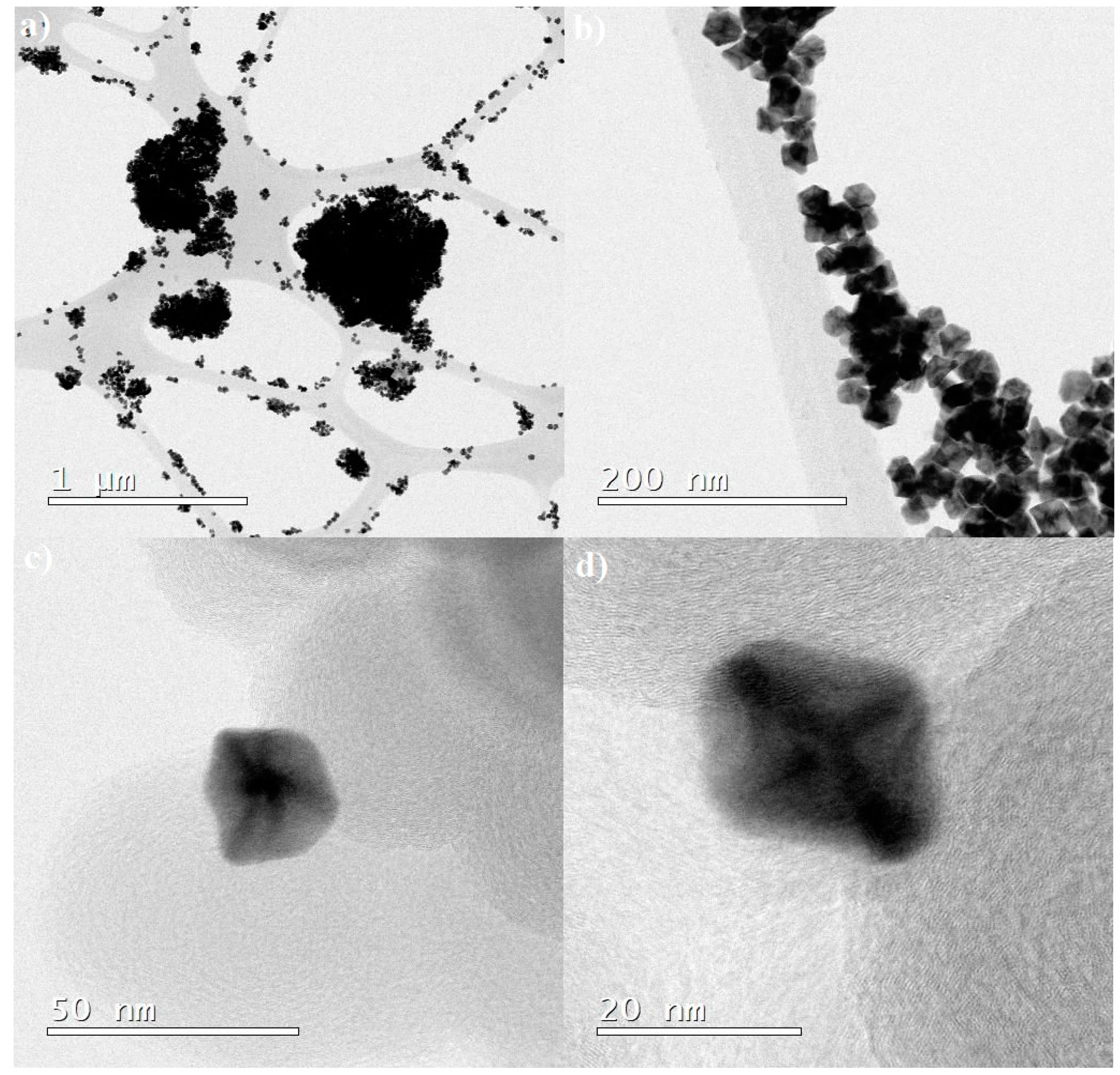
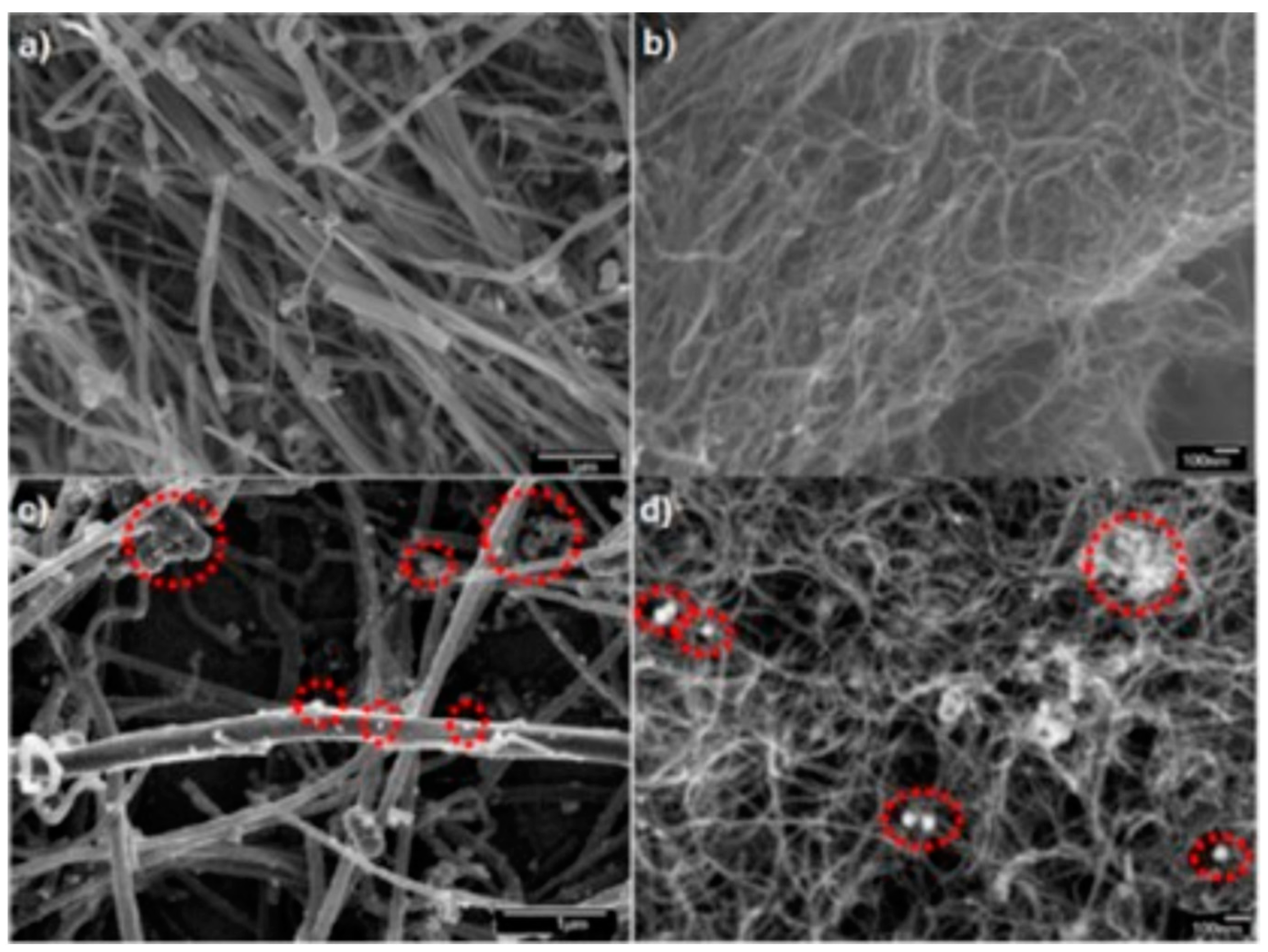
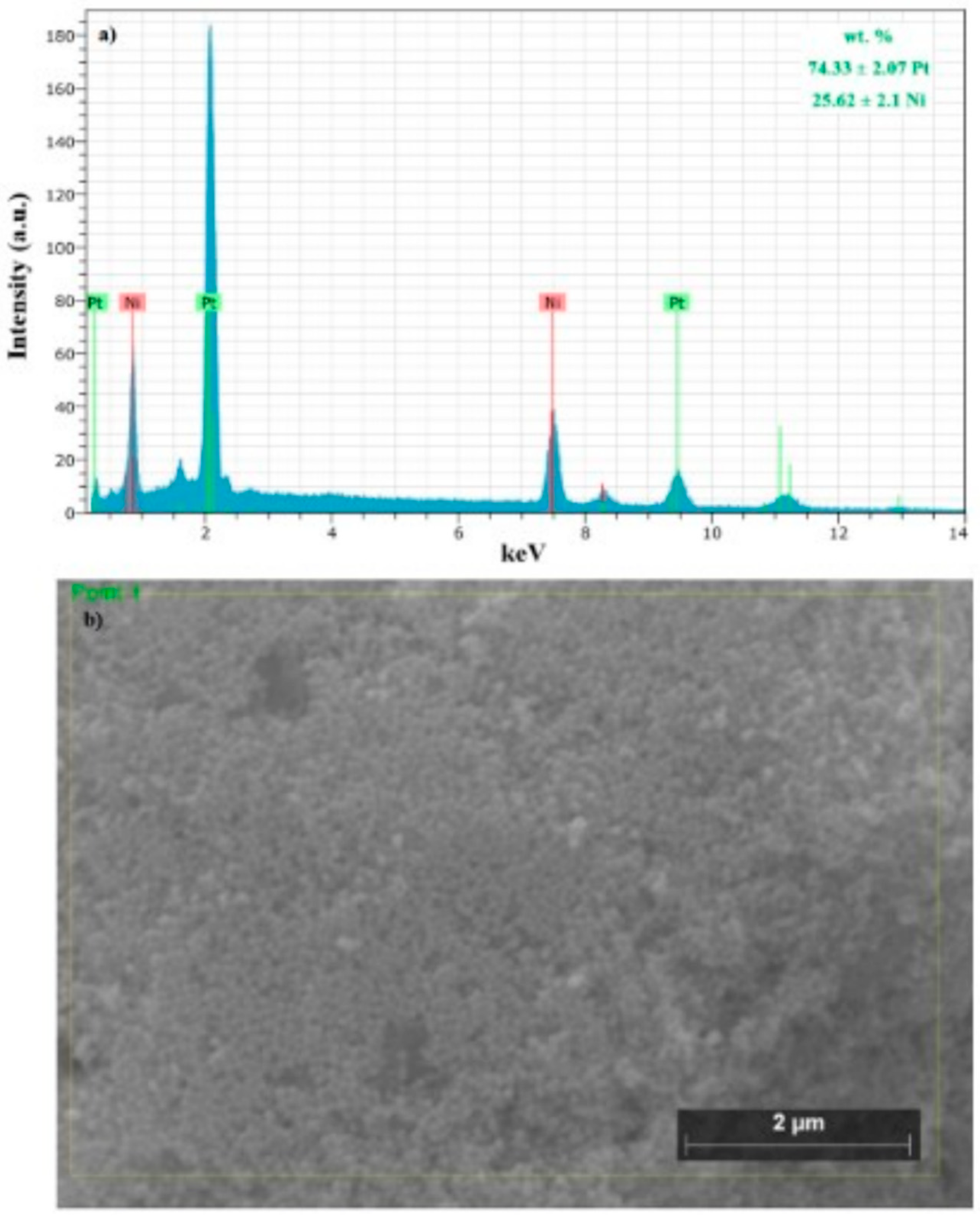
2.1. Physical Characterization of the PtNi Nanoparticles
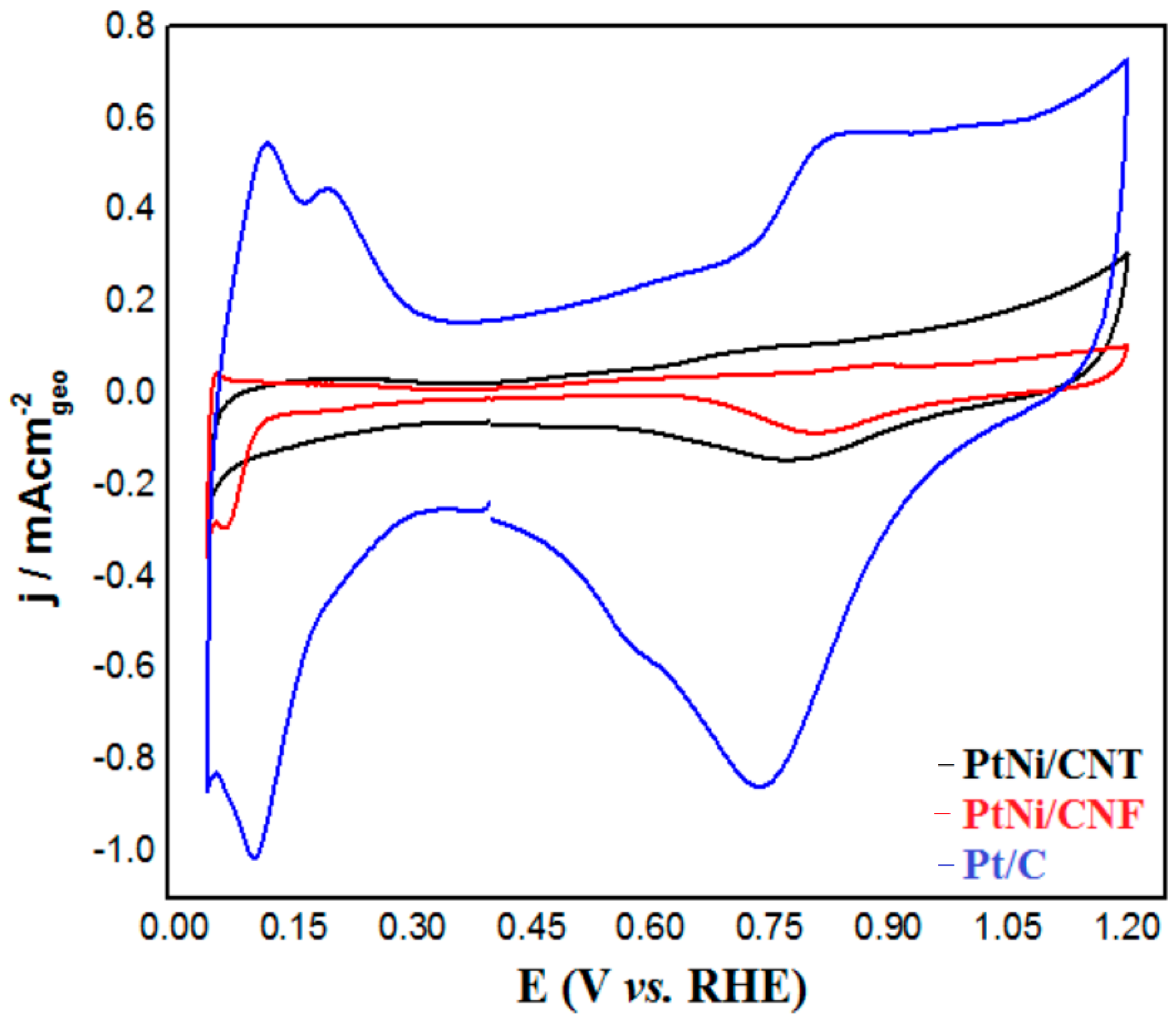
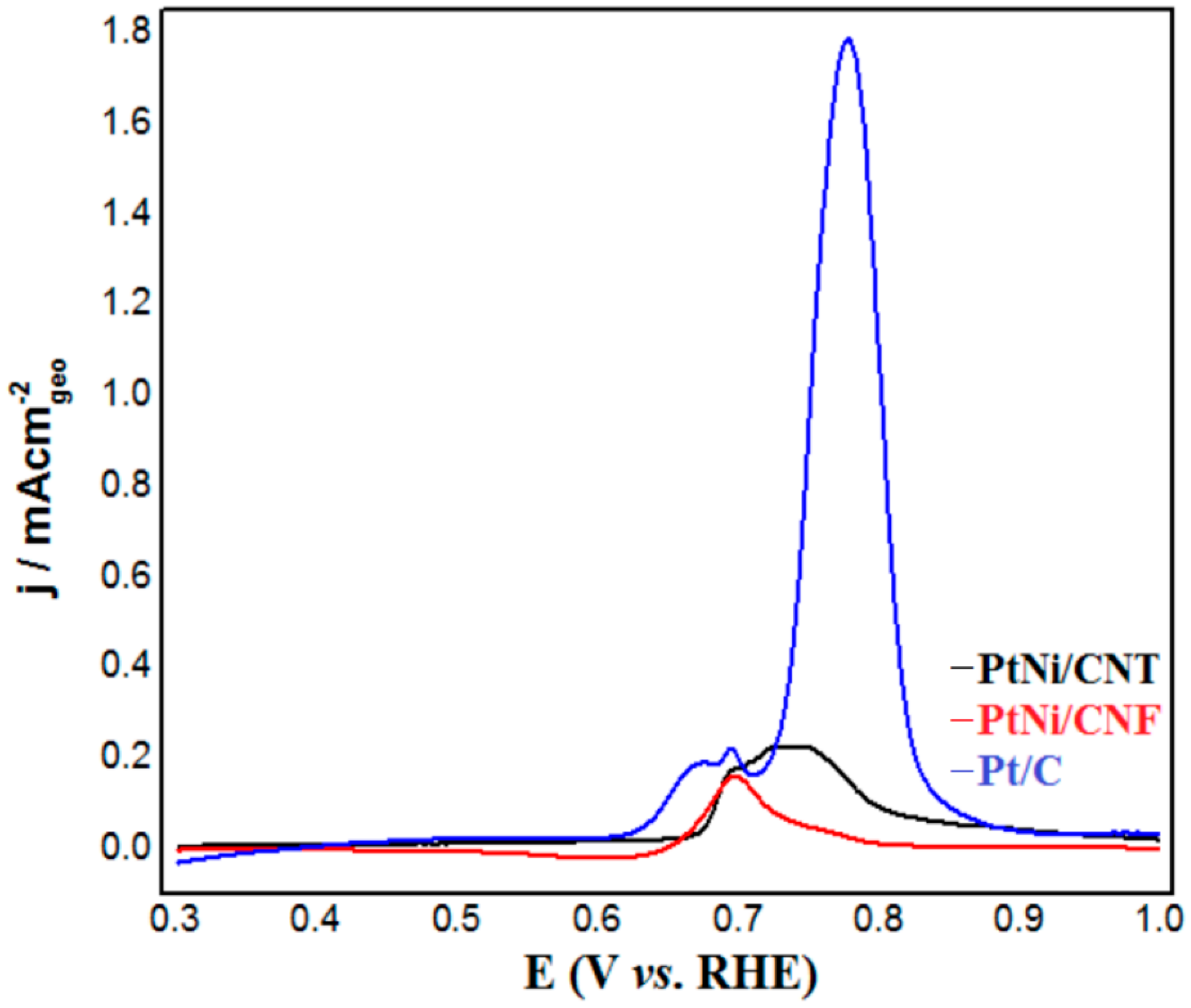
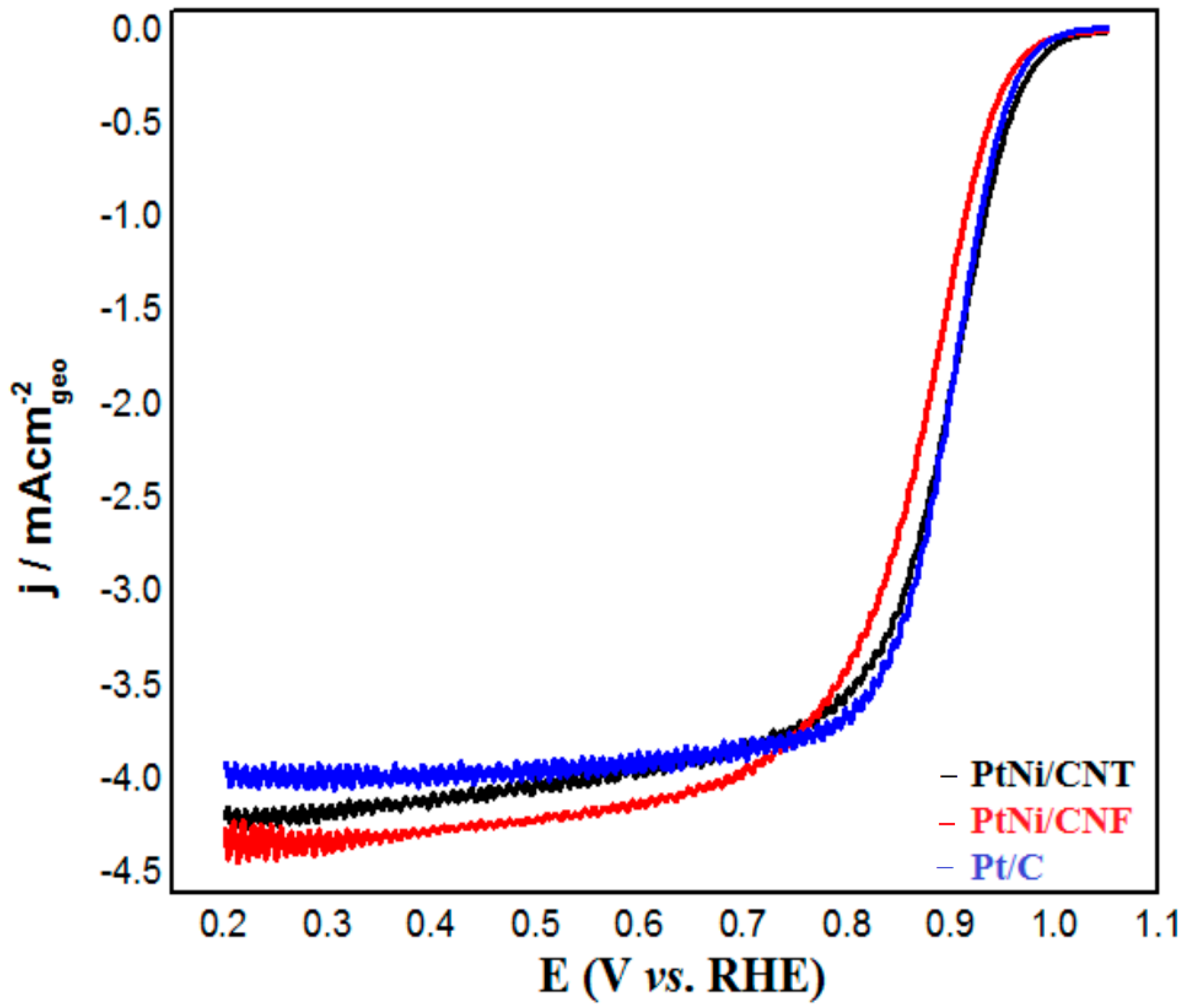
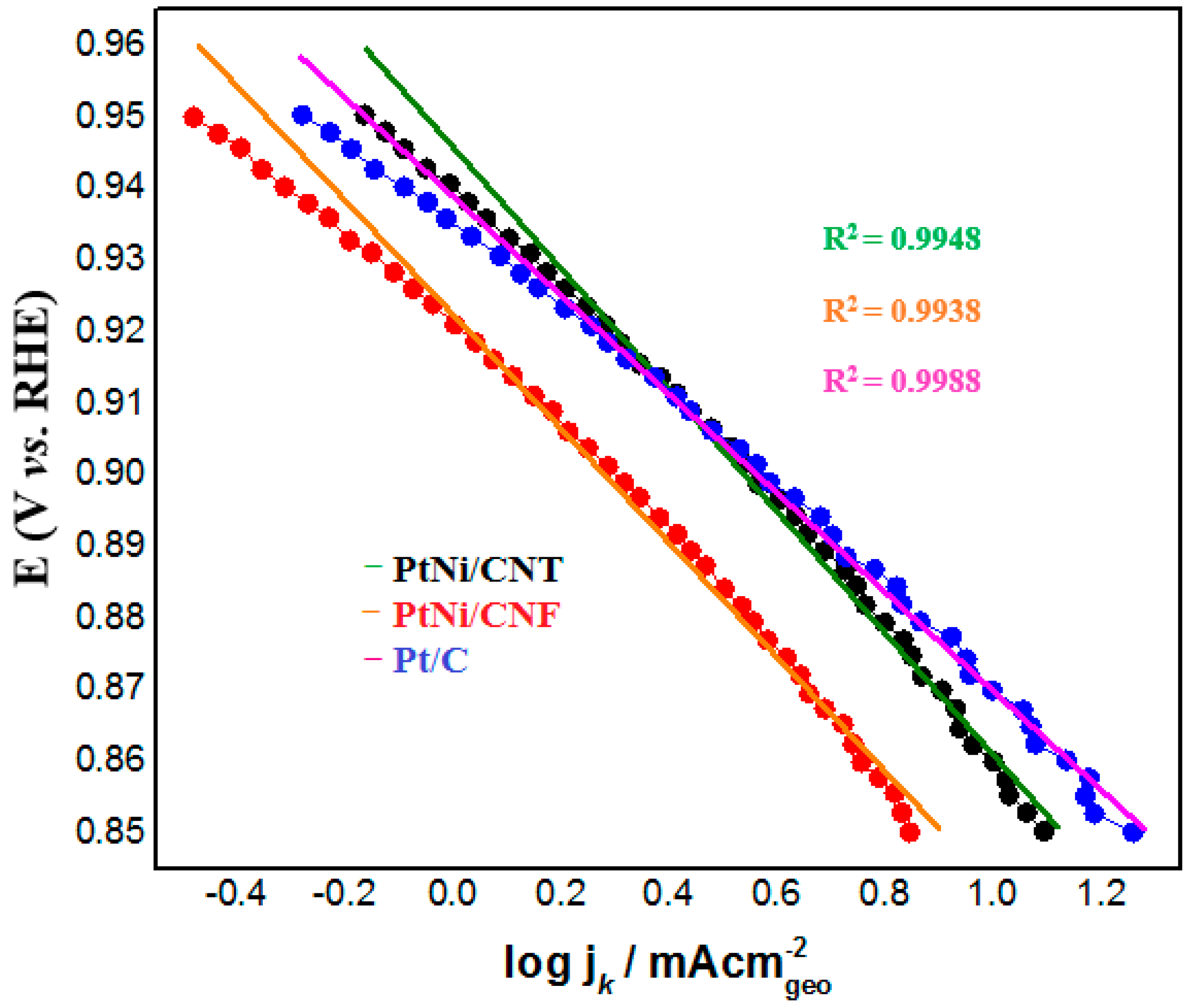
2.2. Electrochemical Characterization of PtNi/CNT and PtNi/CNF
3. Materials and Methods
3.1. Reagents
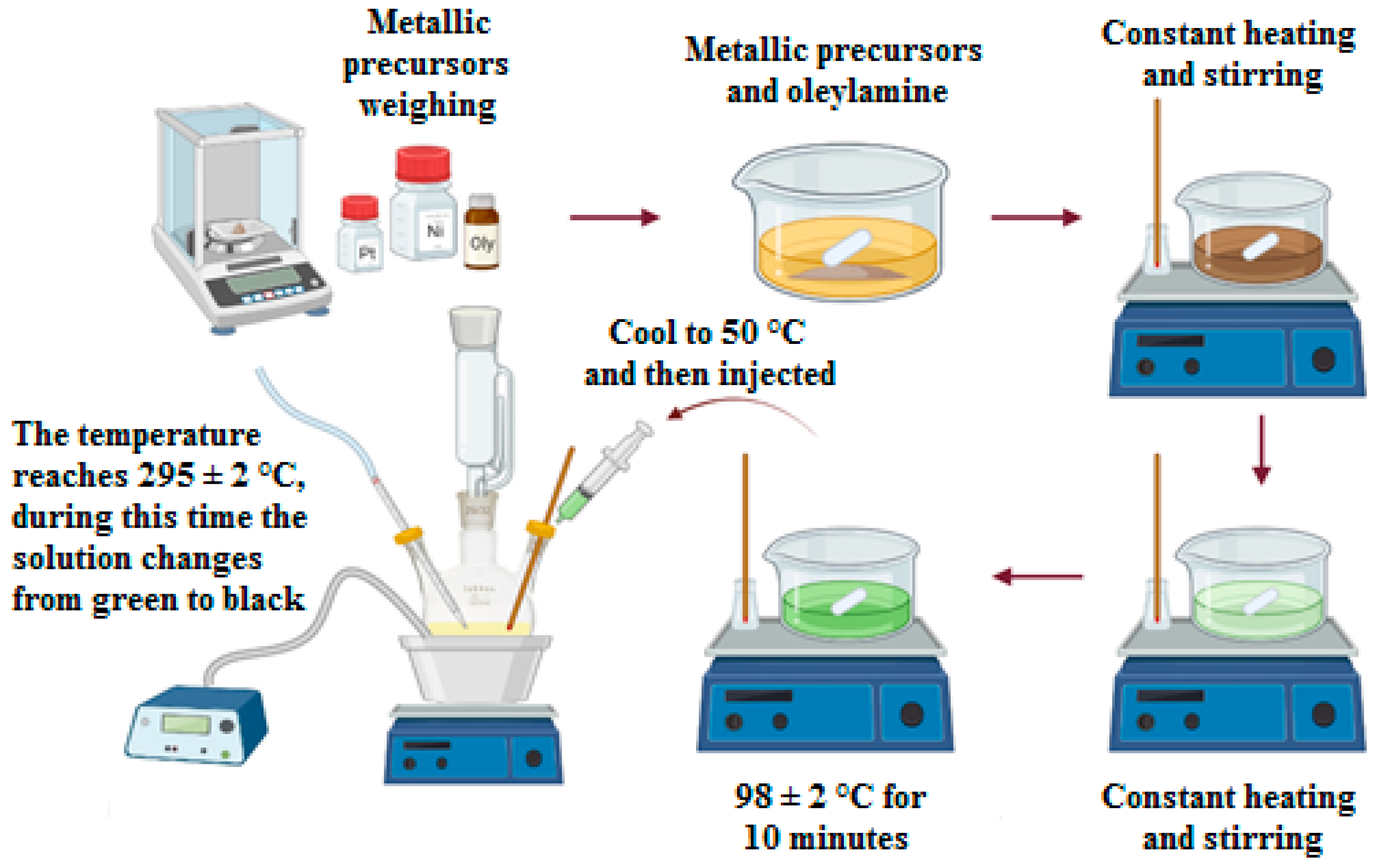
3.2. Synthesis of PtNi Alloy Nanoparticles
3.3. Preparation of PtNi/CNT and PtNi/CNF Catalysts
3.4. Physical Characterization
3.5. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Yang, H. Platinum-based oxygen reduction electrocatalysts. Acc. Chem. Res. 2013, 46, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Martínez, H.; Tellez-Cruz, M.M.; Guerrero-Gutiérrez, O.X.; Ramírez-Herrera, C.A.; Salinas-Juárez, M.G.; Velázquez-Osorio, A.; Solorza-Feria, O. Mexican contributions for the improvement of electrocatalytic properties for the oxygen reduction reaction in PEM fuel cells. Int. J. Hydrogen Energy 2019, 44, 12477–12491. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Matadamas-Ortiz, P.T.; Ortiz-Herrera, J.C.; López-Chávez, E.; Solorza-Feria, O.; Medina, D.I. Current progress of Pt-based ORR electrocatalysts for PEMFCs: An integrated view combining theory and experiment. Mater. Today Phys. 2021, 19, 100406. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Takeichi, M.; Uchida, H.; Watanabe, M. Temperature dependence of oxygen reduction activity at Pt−Fe, Pt−Co, and Pt−Ni alloy electrodes. J. Phys. Chem. B 2005, 109, 5836–5841. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Tellez-Cruz, M.M.; Rojas-Chávez, H.; Ramírez-Herrera, C.A.; Calaminici, P.; Solorza-Feria, O. NiPdPt trimetallic nanoparticles as efficient electrocatalysts towards the oxygen reduction reaction. Int. J. Hydrogen Energy 2019, 44, 12463–12469. [Google Scholar] [CrossRef]
- Flores-Rojas, E.; Cruz-Martínez, H.; Tellez-Cruz, M.M.; Pérez-Robles, J.F.; Leyva-Ramírez, M.A.; Calaminici, P.; Solorza-Feria, O. Electrocatalysis of oxygen reduction on CoNi-decorated-Pt nanoparticles: A theoretical and experimental study. Int. J. Hydrogen Energy 2016, 41, 23301–23311. [Google Scholar] [CrossRef]
- Flores-Rojas, E.; Cruz-Martínez, H.; Rojas-Chávez, H.; Tellez-Cruz, M.M.; Reyes-Rodríguez, J.L.; Cabañas-Moreno, J.G.; Calaminici, P.; Solorza-Feria, O. A combined DFT and experimental investigation of Pt-wrapped CoNi nanoparticles for the oxygen reduction reaction. Electrocatalysis 2018, 9, 662–672. [Google Scholar] [CrossRef]
- Cui, C.; Gan, L.; Li, H.-H.; Yu, S.-H.; Heggen, M.; Strasser, P. Octahedral PtNi nanoparticle catalysts: Exceptional oxygen reduction activity by tuning the alloy particle surface composition. Nano Lett. 2012, 12, 5885–5889. [Google Scholar] [CrossRef]
- Wu, J.; Gross, A.; Yang, H. Shape and composition-controlled platinum alloy nanocrystals using carbon monoxide as reducing agent. Nano Lett. 2011, 11, 798–802. [Google Scholar] [CrossRef]
- Jayabal, S.; Saranya, G.; Geng, D.; Lin, L.-Y.; Meng, X. Insight into the correlation of Pt–support interactions with electrocatalytic activity and durability in fuel cells. J. Mater. Chem. A 2020, 8, 9420–9446. [Google Scholar] [CrossRef]
- Gerber, I.C.; Serp, P. A theory/experience description of support effects in carbon-supported catalysts. Chem. Rev. 2019, 120, 1250–1349. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.T.; Yan, X.; Asghar, M.R.; Husnain, N.; Shen, S.; Luo, L.; Zhang, J. Recent advances in hybrid support material for Pt-based electrocatalysts of proton exchange membrane fuel cells. Int. J. Energy Res. 2019, 43, 2694–2721. [Google Scholar] [CrossRef]
- Samad, S.; Loh, K.S.; Wong, W.Y.; Lee, T.K.; Sunarso, J.; Chong, S.T.; Wan-Daud, W.R. Carbon and non-carbon support materials for platinum-based catalysts in fuel cells. Int. J. Hydrogen Energy 2018, 43, 7823–7854. [Google Scholar] [CrossRef]
- Soni, S.K.; Thomas, B.; Kar, V.R. A comprehensive review on CNTs and CNT-reinforced composites: Syntheses, characteristics and applications. Mat. Today Commun. 2020, 25, 101546. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Zhang, J.; Gao, Y. Comparative investigation of the resistance to electrochemical oxidation of carbon black and carbon nanotubes in aqueous sulfuric acid solution. Electrochim. Acta 2006, 51, 5853–5857. [Google Scholar] [CrossRef]
- Lin, Z.; Ji, L.; Krause, W.E.; Zhang, X. Synthesis and electrocatalysis of 1-aminopyrene-functionalized carbon nanofiber-supported platinum–ruthenium nanoparticles. J. Power Sources 2010, 195, 5520–5526. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Hsin, Y.L.; Hwang, K.C.; Yeh, C.-T. Poly(vinylpyrrolidone)-modified graphite carbon nanofibers as promising supports for PtRu catalysts in direct methanol fuel cells. J. Am. Chem. Soc. 2007, 129, 9999–10010. [Google Scholar] [CrossRef]
- Maiyalagan, T. Pt–Ru nanoparticles supported PAMAM dendrimer functionalized carbon nanofiber composite catalysts and their application to methanol oxidation. J. Solid State Electrochem. 2009, 13, 1561–1566. [Google Scholar] [CrossRef]
- Matsumoto, T.; Komatsu, T.; Arai, K.; Yamazaki, T.; Kijima, M.; Shimizu, H.; Takasawa, Y.; Nakamura, J. Reduction of Pt usage in fuel cell electrocatalysts with carbon nanotube electrodes. Chem. Commun. 2004, 7, 840–841. [Google Scholar] [CrossRef]
- Li, X.; Hsing, I.-M. The effect of the Pt deposition method and the support on Pt dispersion on carbon nanotubes. Electrochim. Acta 2006, 51, 5250–5258. [Google Scholar] [CrossRef]
- Nagao, D.; Shimazaki, Y.; Saeki, S.; Kobayashi, Y.; Konno, M. Effect of ultrasonic irradiation on carbon-supported Pt–Ru nanoparticles prepared at high metal concentration. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 623–627. [Google Scholar] [CrossRef]
- Satishkumar, B.; Vogl, E.M.; Govindaraj, A.; Rao, C.N.R. The decoration of carbon nanotubes by metal nanoparticles. J. Phys. D Appl. Phys. 1996, 29, 3173. [Google Scholar] [CrossRef]
- Asset, T.; Job, N.; Busby, Y.; Crisci, A.; Martin, V.; Stergiopoulos, V.; Bonaud, C.; Serov, A.; Atanassov, P.; Chattot, R.; et al. Porous hollow PtNi/C electrocatalysts: Carbon support considerations to meet performance and stability requirements. ACS Catal. 2018, 8, 893–903. [Google Scholar] [CrossRef]
- Du, S.; Lu, Y.; Malladi, S.K.; Xu, Q.; Steinberger-Wilckens, R. A simple approach for PtNi–MWCNT hybrid nanostructures as high performance electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 692–698. [Google Scholar] [CrossRef]
- Guo, D.-J.; Cui, S.-K.; Cheng, D.; Zhang, P.; Jiang, L.; Zhang, C.-C. One-pot synthesis of PtNi alloy nanoflowers supported on multi-walled carbon nanotubes with superior electrocatalytic activity for the oxygen reductio. J. Power Sources 2014, 255, 157–162. [Google Scholar] [CrossRef]
- Litkohi, H.R.; Bahari, A.; Gatabi, M.P. Improved oxygen reduction reaction in PEMFCs by functionalized CNTs supported Pt–M (M=Fe, Ni, Fe–Ni) bi-and tri-metallic nanoparticles as efficient electrocatalyst. Int. J. Hydrogen Energy 2020, 45, 23543–23556. [Google Scholar] [CrossRef]
- Qiao, L.; Liao, M.; Chen, S.; Wei, Z.; Zhang, S. Synthesis of Pt3Ni-based functionalized MWCNTs to enhance electrocatalysis for PEM fuel cells. J. Solid State Electrochem. 2014, 18, 1893–1898. [Google Scholar] [CrossRef]
- Rosado, G.; Verde, Y.; Valenzuela-Muñiz, A.M.; Barbosa, R.; Yoshida, M.M.; Escobar, B. Catalytic activity of Pt-Ni nanoparticles supported on multi-walled carbon nanotubes for the oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 23260–23271. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Jin, J.; Yang, S. Hollow porous carbon nanofibers as novel support for platinum-based oxygen reduction reaction electrocatalysts. Int. J. Hydrogen Energy 2017, 42, 5938–5947. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Yang, D.; Lv, H.; Zhang, C. Preparation of an octahedral PtNi/CNT catalyst and its application in high durability PEMFC cathodes. RSC Adv. 2018, 8, 18381–18387. [Google Scholar] [CrossRef] [Green Version]
- Becknell, N.; Son, Y.; Kim, D.; Li, D.; Yu, Y.; Niu, Z.; Lei, T.; Sneed, B.T.; More, K.L.; Markovic, N.M.; et al. Control of architecture in rhombic dodecahedral Pt–Ni nanoframe electrocatalysts. J. Am. Chem. Soc. 2017, 139, 11678–11681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Niu, Z.; Xie, C.; Gao, M.; Lai, M.; Li, M.; Yang, P. Effects of catalyst processing on the activity and stability of Pt–Ni nanoframe electrocatalysts. ACS Nano 2018, 12, 8697–8705. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Rodríguez, J.L.; Velázquez-Osorio, A.; Solorza-Feria, O.; Bahena-Uribe, D.; Roque, J. Influence of the injection temperature on the size of Ni–Pt polyhedral nanoparticles synthesized by the hot-injection method. MRS Commun. 2017, 7, 947–952. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Liz-Marzán, L.M. Oleylamine in nanoparticle synthesis. Chem. Mater. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Tian, C.; Geng, D.; Wang, D.; Bai, S. One-Pot Synthesis of Highly Efficient Carbon-Supported Polyhedral Pt 3 Ni Alloy Nanoparticles for Oxygen Reduction Reaction. Electrocatalysis 2019, 10, 613–620. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Zhao, N.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: Particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef] [Green Version]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Choi, S.-I.; Xie, S.; Shao, M.; Lu, N.; Guerrero, S.; Odell, J.H.; Park, J.; Wang, J.; Kim, M.J.; Xia, Y. Controlling the size and composition of nanosized Pt–Ni octahedra to optimize their catalytic activities toward the oxygen reduction reaction. ChemSusChem 2014, 7, 1476–1483. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Shao, Z.-G.; Lu, W.; Xie, F.; Xiao, H.; Qin, X.; Yi, B. Core–shell Pt modified Pd/C as an active and durable electrocatalyst for the oxygen reduction reaction in PEMFCs. Appl. Catal. B Environ. 2013, 183–194. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Cao, L.; Chen, Y.; Zhu, E.; Lin, Z.; Li, M.; Yan, A.; Zettl, A.; Wang, Y.M.; et al. High-performance transition metal–doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidaković, T.; Christov, M.; Sundmacher, K. The use of CO stripping for in situ fuel cell catalyst characterization. Electrochim. Acta 2007, 52, 5606–5613. [Google Scholar] [CrossRef]
- Mayrhofer, K.J.J.; Arenz, M.; Blizanac, B.B.; Stamenkovic, V.; Ross, P.; Markovic, N.M. CO surface electrochemistry on Pt-nanoparticles: A selective review. Electrochim. Acta 2005, 50, 5144–5154. [Google Scholar] [CrossRef]
- Ciapina, E.G.; Santos, S.F.; Gonzalez, E.R. Electrochemical CO stripping on nanosized Pt surfaces in acid media: A review on the issue of peak multiplicity. J. Electroanal. Chem. 2018, 815, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Tellez-Cruz, M.M.; Padilla-Islas, M.A.; Godínez-Salomón, J.F.; Lartundo-Rojas, L.; Solorza-Feria, O. Y-OH-decorated-Pt/C electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 23318–23328. [Google Scholar] [CrossRef]
- Garsany, Y.; Baturina, O.A.; Swider-Lyons, K.E.; Kocha, S.S. Experimental methods for quantifying the activity of platinum electrocatalysts for the oxygen reduction reaction. Anal. Chem. 2010, 82, 6321–6328. [Google Scholar] [CrossRef]
- Alfaro-López, H.M.; Valdés-Madrigal, M.A.; Rojas-Chávez, H.; Cruz-Martínez, H.; Padilla-Islas, M.A.; Tellez-Cruz, M.M.; Solorza-Feria, O. A Trimetallic Pt2NiCo/C Electrocatalyst with Enhanced Activity and Durability for Oxygen Reduction Reaction. Catalysts 2020, 10, 170. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Erikson, H.; Kongi, N.; Merisalu, M.; Ritslaid, P.; Sammelselg, V.; Tammeveski, K. Heat-treatment effects on the ORR activity of Pt nanoparticles deposited on multi-walled carbon nanotubes using magnetron sputtering technique. Int. J. Hydrogen Energy 2017, 42, 5958–5970. [Google Scholar] [CrossRef]
- Kong, J.; Qin, Y.-H.; Wang, T.-L.; Wang, C.-W. Photodeposition of Pt nanoparticles onto TiO2@CNT as high-performance electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2020, 45, 1991–1997. [Google Scholar] [CrossRef]
- Louisia, S.; Thomas, Y.R.J.; Lecante, P.; Heitzmann, M.; Axet, M.R.; Jacques, P.-A.; Serp, P. Alloyed Pt3M (M = Co, Ni) nanoparticles supported on S-and N-doped carbon nanotubes for the oxygen reduction reaction. Beilstein J. Nanotechnol. 2019, 10, 1251–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garapati, M.S.; Sundara, R. Highly efficient and ORR active platinum-scandium alloy-partially exfoliated carbon nanotubes electrocatalyst for Proton Exchange Membrane Fuel Cell. Int. J. Hydrogen Energy 2019, 44, 10951–10963. [Google Scholar] [CrossRef]
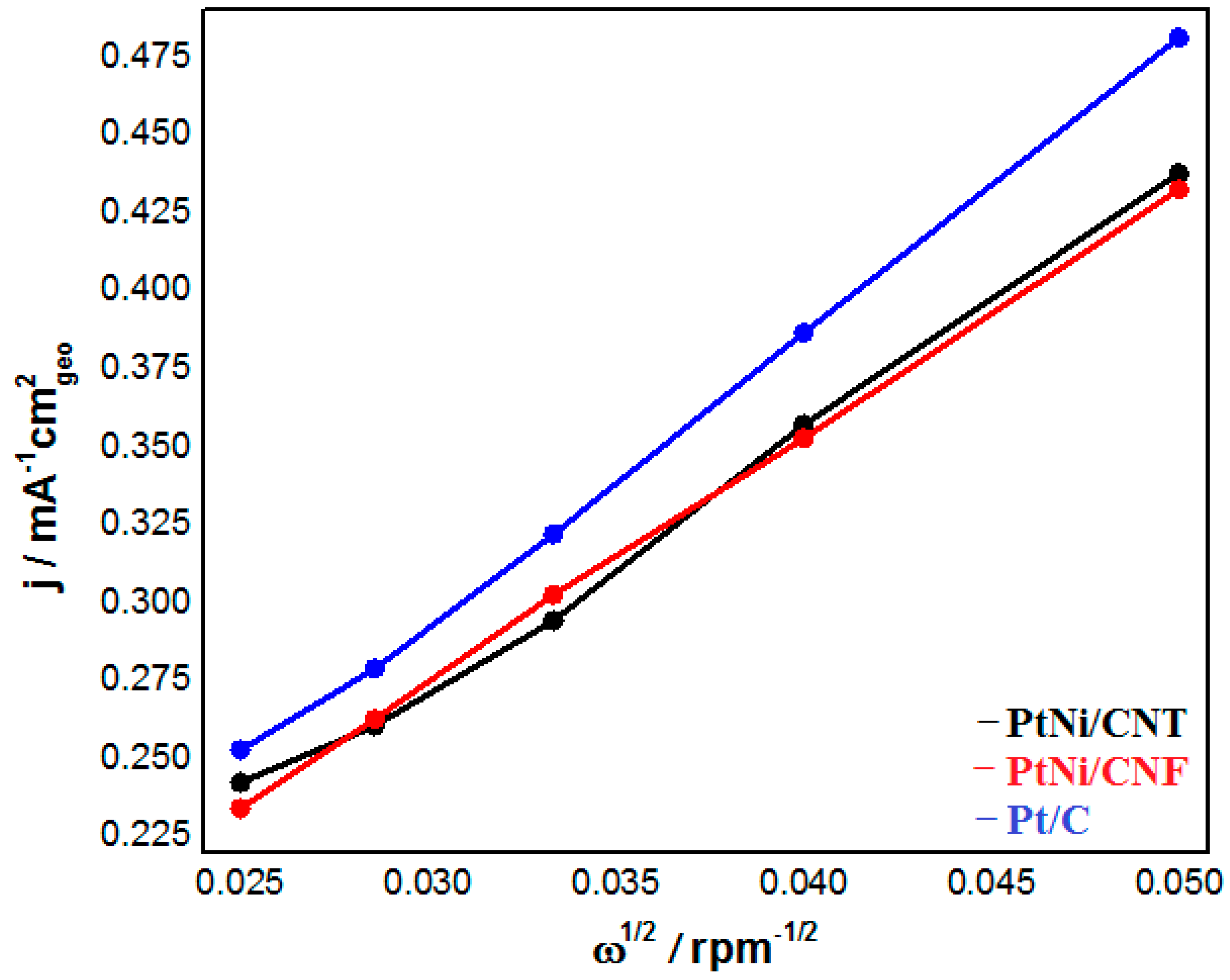
| Catalysts | ECSA (m2 g−1 Pt) | SA @ 0.9 V (mA cm−2 Pt) | MA @ 0.9 V (A mg−1 Pt) | Tafel Slope (mV dec−1) | B0 (mA cm−2 rpm−1/2) |
|---|---|---|---|---|---|
| PtNi/CNT | 12.15 | 1.45 | 0.18 | 84.9 | 0.1245 |
| PtNi/CNF | 6.72 | 1.47 | 0.10 | 79.8 | 0.1263 |
| Pt/C | 53.22 | 0.35 | 0.19 | 69.1 | 0.1080 |
| PtNi/CNT a [24] | - | 0.98 | 0.28 | - | - |
| PtNi/C [24] | - | 1.29 | 0.59 | - | - |
| PtNi/CNT b [31] | - | 1.38 | 0.48 | - | - |
| Pt/C [31] | - | 0.16 | 0.09 | - | - |
| Catalyst | ECSA (m2 g−1 Pt) | SA @ 0.9 V (mA cm−2 Pt) | MA @ 0.9 V (A mg−1 Pt) | Reference |
|---|---|---|---|---|
| PtNi/CNT | 12.15 | 1.45 | 0.180 | This work |
| Pt/MWCNT-RT a | Not given | 0.26 | 0.017 | [49] |
| Pt/MWCNT-300 b | Not given | 0.32 | 0.020 | [49] |
| Pt/MWCNT-400 b | Not given | 0.29 | 0.006 | [49] |
| Pt/MWCNT-500 b | Not given | 0.15 | 0.007 | [49] |
| Pt/MWCNT-600 b | Not given | 0.15 | 0.007 | [49] |
| Pt/MWCNT-700 b | Not given | 0.15 | 0.003 | [49] |
| Pt/TiO2@CNT | 99.3 | 0.36 | 0.358 | [50] |
| PtNi-MWCNT | 47.9 | 1.07 | 0.510 | [25] |
| PtNi/CNT | 34.8 | 1.38 | 0.479 | [31] |
| Pt3Co/N-CNT | 565 | 0.09 | 0.050 | [51] |
| Pt3Co/N-CNTHT c | 34.9 | 0.06 | 0.019 | [51] |
| Pt3Co/S-CNT | 23.1 | 0.11 | 0.025 | [51] |
| Pt3Ni/N-CNT | 34.7 | 0.01 | 0.033 | [51] |
| Pt3Ni/N-CNTHTc | 46.4 | 0.06 | 0.029 | [51] |
| Pt3Ni/S-CNT | 5.6 | 0.14 | 0.001 | [51] |
| Pt3 Sc/PE d-CNT | 102.1 | Not given | 0.080 | [52] |
| Pt/PE d-CNT | 95.7 | Not given | 0.070 | [52] |
| Pt3 Sc/CNT | 62.4 | Not given | 0.060 | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortíz-Herrera, J.C.; Tellez-Cruz, M.M.; Solorza-Feria, O.; Medina, D.I. Effect of Different Carbon Supports on the Activity of PtNi Bimetallic Catalysts toward the Oxygen Reduction. Catalysts 2022, 12, 477. https://doi.org/10.3390/catal12050477
Ortíz-Herrera JC, Tellez-Cruz MM, Solorza-Feria O, Medina DI. Effect of Different Carbon Supports on the Activity of PtNi Bimetallic Catalysts toward the Oxygen Reduction. Catalysts. 2022; 12(5):477. https://doi.org/10.3390/catal12050477
Chicago/Turabian StyleOrtíz-Herrera, Juan C., Miriam M. Tellez-Cruz, Omar Solorza-Feria, and Dora I. Medina. 2022. "Effect of Different Carbon Supports on the Activity of PtNi Bimetallic Catalysts toward the Oxygen Reduction" Catalysts 12, no. 5: 477. https://doi.org/10.3390/catal12050477
APA StyleOrtíz-Herrera, J. C., Tellez-Cruz, M. M., Solorza-Feria, O., & Medina, D. I. (2022). Effect of Different Carbon Supports on the Activity of PtNi Bimetallic Catalysts toward the Oxygen Reduction. Catalysts, 12(5), 477. https://doi.org/10.3390/catal12050477






