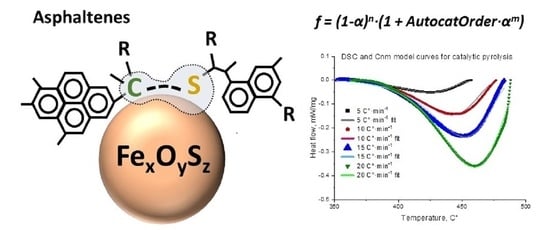
Thermal Behavior of Heavy Oil Catalytic Pyrolysis and Aquathermolysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Catalyst Synthesis
2.3. Thermal Processing of the Extra-Heavy Oil and Its SARA and Elemental Analysis
2.4. Infrared Spectrometry Essays
2.5. Sample Preparation
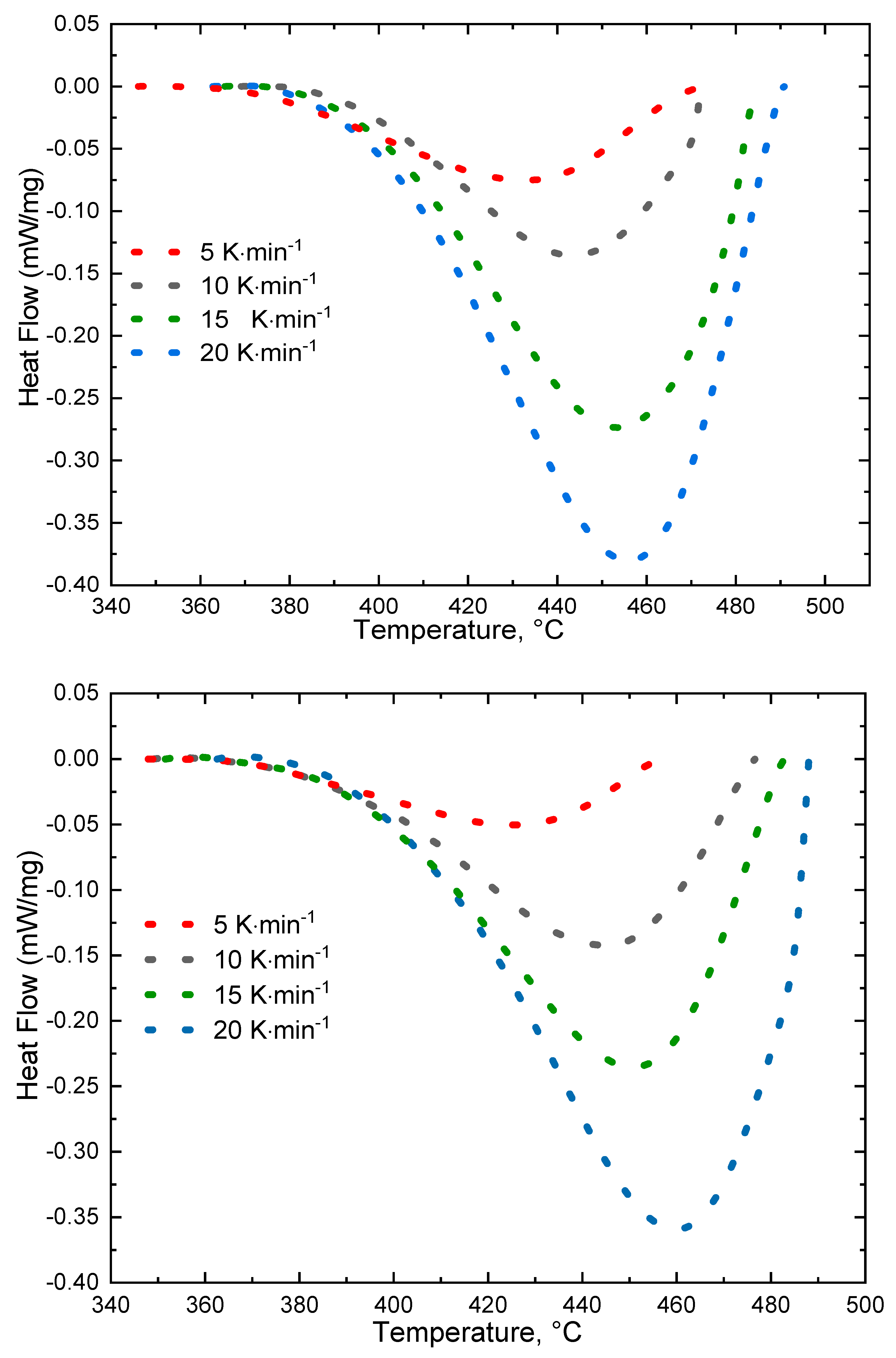
2.6. Thermal Analysis
2.7. Kinetic Theory
2.8. Isoconversional Kinetic Analysis
3. Discussion
3.1. Chemical Composition and Elemental Analysis
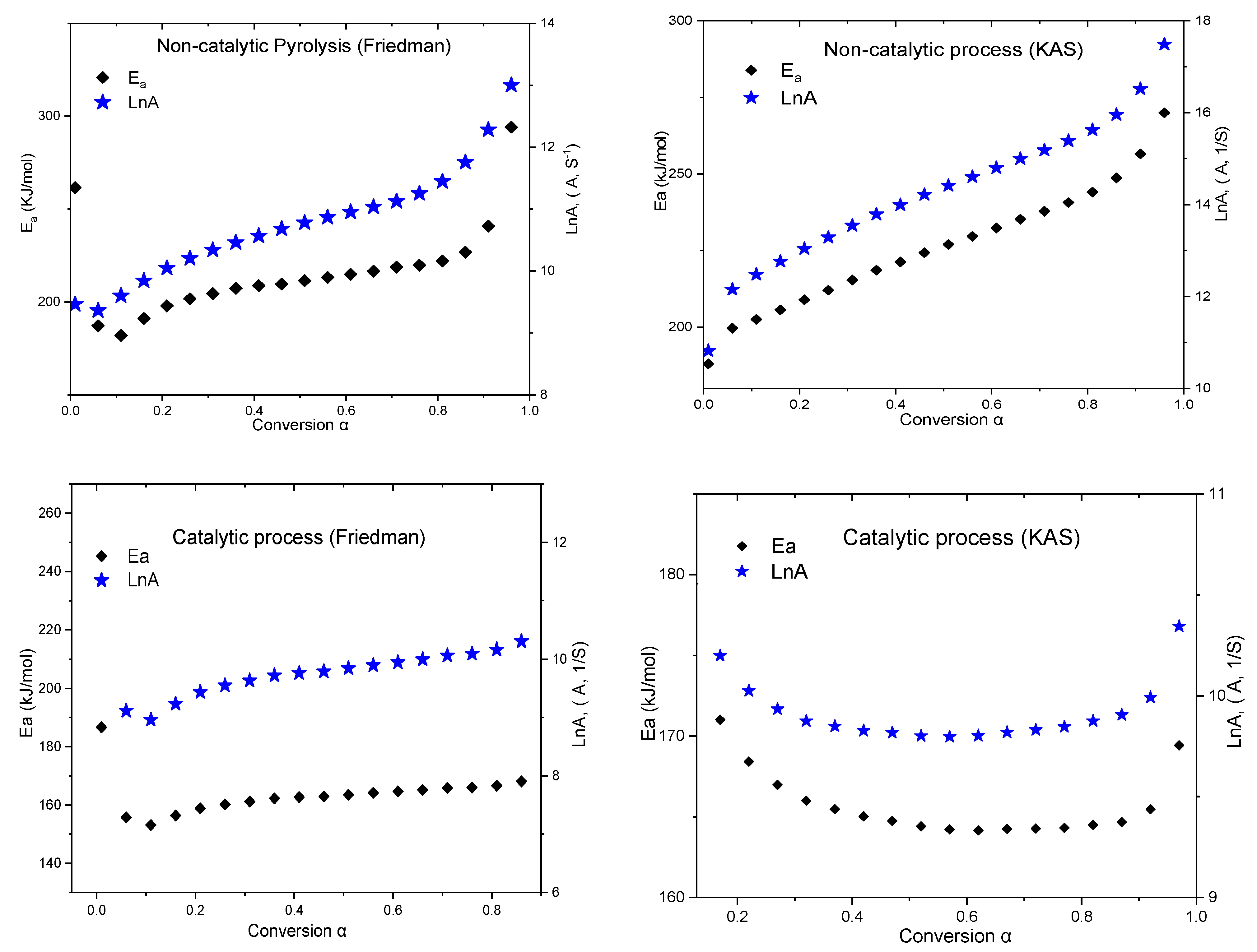
3.2. Kinetic Calculations
3.3. Thermodynamic Functions of Activated Complex Formation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, U.; Meehan, D.N. Unconventional Oil and Gas Resources: Exploitation and Development; CRC Press: Boca Raton, FL, USA, 2019; ISBN 1498759416. [Google Scholar]
- Kryukov, V.; Moe, A. Does Russian unconventional oil have a future? Energy Policy 2018, 119, 41–50. [Google Scholar] [CrossRef]
- Wang, J.; Feng, L.; Steve, M.; Tang, X.; Gail, T.E.; Mikael, H. China’s unconventional oil: A review of its resources and outlook for long-term production. Energy 2015, 82, 31–42. [Google Scholar] [CrossRef]
- Castro-Alvarez, F.; Marsters, P.; de León Barido, D.P.; Kammen, D.M. Sustainability lessons from shale development in the United States for Mexico and other emerging unconventional oil and gas developers. Renew. Sustain. Energy Rev. 2018, 82, 1320–1332. [Google Scholar] [CrossRef] [Green Version]
- Kapustin, N.O.; Grushevenko, D.A. Global prospects of unconventional oil in the turbulent market: A long term outlook to 2040. Oil Gas Sci. Technol. IFP Energies Nouv. 2018, 73, 67. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Khelkhal, M.A.; Tajik, A.; Ignashev, N.E.; Krapivnitskaya, T.O.; Peskov, N.Y.; Glyavin, M.Y.; Bulanova, S.A.; Slavkina, O.V.; Schekoldin, K.A. Microwave Radiation Impact on Heavy Oil Upgrading from Carbonate Deposits in the Presence of Nano-Sized Magnetite. Processes 2021, 9, 2021. [Google Scholar] [CrossRef]
- Meyer, R.F.; Attanasi, E.D.; Freeman, P.A. Heavy Oil and Natural Bitumen Resources in Geological Basins of the World: Map Showing Klemme Basin Classification of Sedimentary Provinces Reporting Heavy Oil or Natural Bitumen; U.S. Geological Survey Open-File Report; U.S. Geological Survey: Reston, VA, USA, 2007; Volume 2007, p. 1084.
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Alvarado, V.; Manrique, E. Enhanced oil recovery: An update review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Mokheimer, E.M.A.; Hamdy, M.; Abubakar, Z.; Shakeel, M.R.; Habib, M.A.; Mahmoud, M. A comprehensive review of thermal enhanced oil recovery: Techniques evaluation. J. Energy Resour. Technol. 2019, 141, 30801. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Gani, A.; Hourani, N.; Emwas, A.-H.; Sarathy, S.M.; Roberts, W.L. TG/DTG, FT-ICR mass spectrometry, and NMR spectroscopy study of heavy fuel oil. Energy Fuels 2015, 29, 7825–7835. [Google Scholar] [CrossRef] [Green Version]
- Jameel, A.G.A.; Khateeb, A.; Elbaz, A.M.; Emwas, A.-H.; Zhang, W.; Roberts, W.L.; Sarathy, S.M. Characterization of deasphalted heavy fuel oil using APPI (+) FT-ICR mass spectrometry and NMR spectroscopy. Fuel 2019, 253, 950–963. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Roberts, W.L. PM from the combustion of heavy fuel oils. Energy 2018, 152, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Vakhin, A.V.; Khelkhal, M.A.; Tajik, A.; Gafurov, M.R.; Morozov, O.G.; Nasybullin, A.R.; Karandashov, S.A.; Ponomarev, A.A.; Krapivnitskaia, T.O.; Glyavin, M.Y. The Role of Nanodispersed Catalysts in Microwave Application during the Development of Unconventional Hydrocarbon Reserves: A Review of Potential Applications. Processes 2021, 9, 420. [Google Scholar] [CrossRef]
- Kovscek, A.R. Emerging challenges and potential futures for thermally enhanced oil recovery. J. Pet. Sci. Eng. 2012, 98, 130–143. [Google Scholar] [CrossRef]
- Kamari, A.; Nikookar, M.; Mohammadi, A.H. Study of the Performance of Cyclic Steam Stimulation (CSS) Oil Recovery Method in a Naturally-Fractured Carbonate Reservoir. In Enhanced Oil Recovery: Methods, Economic Benefits and Impacts on the Environment; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015. [Google Scholar]
- Mukhamatdinov, I.I.; Salih, I.S.S.; Khelkhal, M.A.; Vakhin, A.V. Application of Aromatic and Industrial Solvents for Enhancing Heavy Oil Recovery from the Ashalcha Field. Energy Fuels 2020, 35, 374–385. [Google Scholar] [CrossRef]
- Shen, C. SAGD for heavy oil recovery. In Enhanced Oil Recovery Field Case Studies; Elsevier: Amsterdam, The Netherlands, 2013; pp. 413–445. [Google Scholar]
- Hamedi Shokrlu, Y. Enhancement of Heavy Oil/Bitumen Thermal Recovery Using Nano Metal Particles. Ph.D. Thesis, University of Alberta, Edmonton, AB, Canada, 2014. [Google Scholar]
- Ghashghaee, M.; Shirvani, S.; Kegnæs, S. Steam catalytic cracking of fuel oil over a novel composite nanocatalyst: Characterization, kinetics and comparative perspective. J. Anal. Appl. Pyrolysis 2019, 138, 281–293. [Google Scholar] [CrossRef]
- Farhadian, A.; Khelkhal, M.A.; Tajik, A.; Lapuk, S.E.; Rezaeisadat, M.; Eskin, A.A.; Rodionov, N.O.; Vakhin, A.V. Effect of Ligand Structure on the Kinetics of Heavy Oil Oxidation: Toward Biobased Oil-Soluble Catalytic Systems for Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2021, 60, 14713–14727. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Eskin, A.A.; Nurgaliev, D.K.; Vakhin, A.V. Thermal study on stabilizing combustion front via bimetallic Mn@Cu tallates during heavy oil oxidation. Energy Fuels 2019, 34, 5121–5127. [Google Scholar] [CrossRef]
- Jameel, A.G.A.; Han, Y.; Brignoli, O.; Telalović, S.; Elbaz, A.M.; Im, H.G.; Roberts, W.L.; Sarathy, S.M. Heavy fuel oil pyrolysis and combustion: Kinetics and evolved gases investigated by TGA-FTIR. J. Anal. Appl. Pyrolysis 2017, 127, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Amer, M.W.; Alhesan, J.S.A.; Marshall, M.; Awwad, A.M.; Al-Ayed, O.S. Characterization of Jordanian oil shale and variation in oil properties with pyrolysis temperature. J. Anal. Appl. Pyrolysis 2019, 140, 219–226. [Google Scholar] [CrossRef]
- Al-Absi, A.A.; Aitani, A.M.; Al-Khattaf, S.S. Thermal and catalytic cracking of whole crude oils at high severity. J. Anal. Appl. Pyrolysis 2020, 145, 104705. [Google Scholar] [CrossRef]
- Sitnov, S.; Mukhamatdinov, I.; Aliev, F.; Khelkhal, M.A.; Slavkina, O.; Bugaev, K. Heavy oil aquathermolysis in the presence of rock-forming minerals and iron oxide (II, III) nanoparticles. Pet. Sci. Technol. 2020, 38, 574–579. [Google Scholar] [CrossRef]
- Faillace, J.G.; de Melo, C.F.; de Souza, S.P.L.; da Costa Marques, M.R. Production of light hydrocarbons from pyrolysis of heavy gas oil and high density polyethylene using pillared clays as catalysts. J. Anal. Appl. Pyrolysis 2017, 126, 70–76. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Sharifullin, A.V.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Catalytic aquathermolysis of boca de jaruco heavy oil with nickel-based oil-soluble catalyst. Processes 2020, 8, 532. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Mukhamatdinov, I.I.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Sitnov, S.A.; Chemodanov, A.E.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part II–changes in composition of aromatic hydrocarbons. Pet. Sci. Technol. 2018, 36, 1850–1856. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Sitnov, S.A.; Mukhamatdinov, I.I.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of heavy oil in reservoir conditions with the use of oil-soluble catalysts: Part I–changes in composition of saturated hydrocarbons. Pet. Sci. Technol. 2018, 36, 1829–1836. [Google Scholar] [CrossRef]
- Feoktistov, D.A.; Kayukova, G.P.; Vakhin, A.V.; Sitnov, S.A. Catalytic Aquathermolysis of High-Viscosity Oil Using Iron, Cobalt, and Copper Tallates. Chem. Technol. Fuels Oils 2018, 53, 905–912. [Google Scholar] [CrossRef]
- Li, J.; Tang, X.; Chen, X.; Zhang, M.; Zheng, X.; Wang, C.; Deng, C. Viscosity reduction process of heavy oil by catalytic co-pyrolysis with sawdust. J. Anal. Appl. Pyrolysis 2019, 140, 444–451. [Google Scholar] [CrossRef]
- Guo, W.; Yang, Q.; Sun, Y.; Xu, S.; Kang, S.; Lai, C.; Guo, M. Characteristics of low temperature co-current oxidizing pyrolysis of Huadian oil shale. J. Anal. Appl. Pyrolysis 2020, 146, 104759. [Google Scholar] [CrossRef]
- Kapadia, P.R.; Kallos, M.S.; Gates, I.D. A comprehensive kinetic theory to model thermolysis, aquathermolysis, gasification, combustion, and oxidation of Athabasca bitumen. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 25–29 April 2010; OnePetro: Richardson, TX, USA, 2010. [Google Scholar]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Stimulated Processes; Springer: Berlin, Germany, 2015; ISBN 9783319141756. [Google Scholar]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Kok, M.V.; Gundogar, A.S. DSC study on combustion and pyrolysis behaviors of Turkish crude oils. Fuel Process. Technol. 2013, 116, 110–115. [Google Scholar] [CrossRef]
- Varfolomeev, M.A.; Rakipov, I.T.; Isakov, D.R.; Nurgaliev, D.K.; Kok, M.V. Characterization and kinetics of siberian and tatarstan regions crude oils using differential scanning calorimetry. Pet. Sci. Technol. 2015, 33, 865–871. [Google Scholar] [CrossRef]

| Physical Properties | Values |
|---|---|
| Oil density in reservoir conditions, kg/m3 | 1029 |
| Oil dynamic viscosity at 20 °C, mPa·s | 271,000 |
Oil elemental composition, wt.%
| 75.47 10.12 8.40 5.60 0.41 76, 26, 24 |
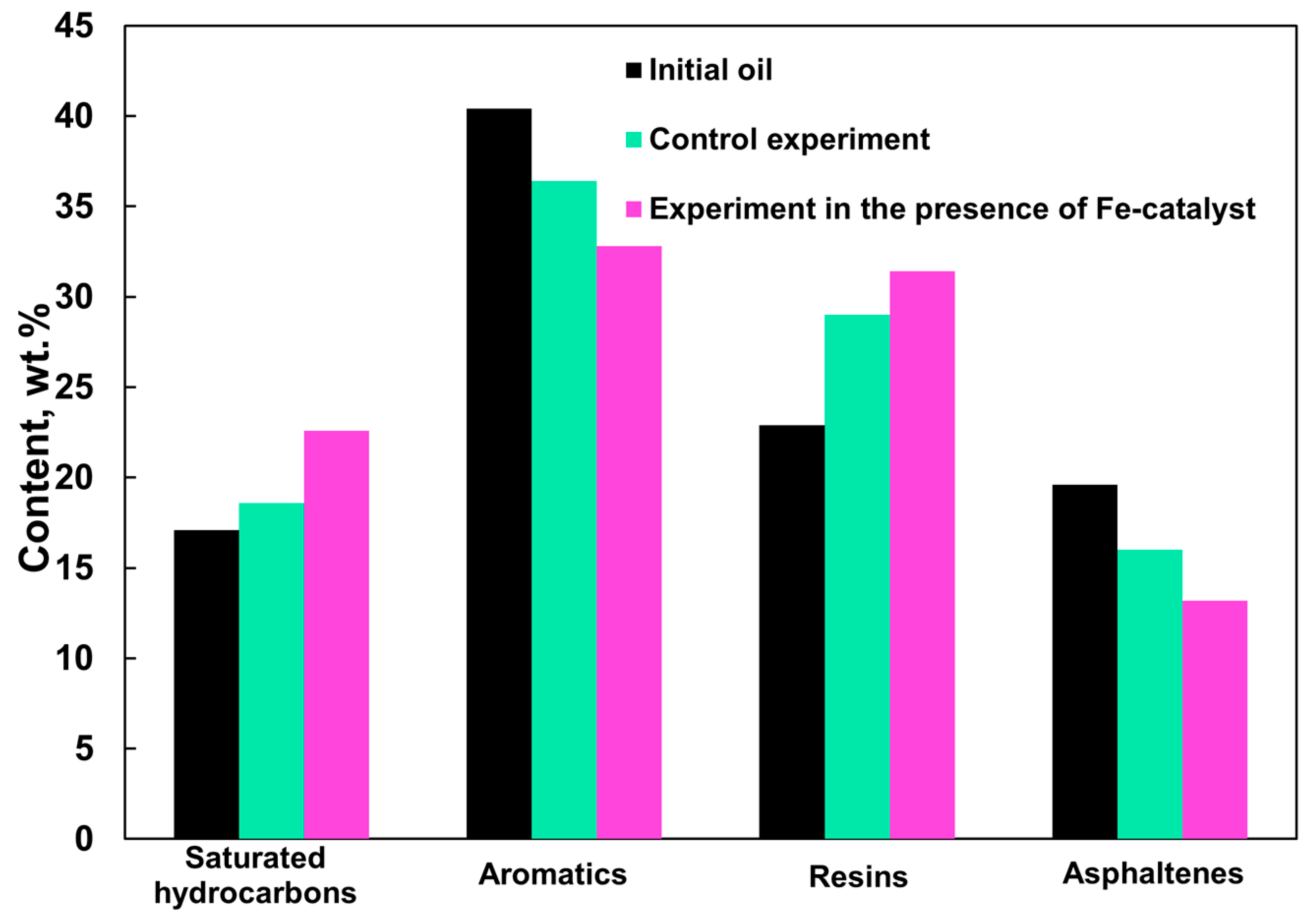
| Group Composition of Oil (SARA) | wt.% |
|---|---|
| Saturates | 17.1 |
| Aromatics | 40.4 |
| Resins | 22.9 |
| Asphaltenes | 19.6 |
| 9 | Elemental Analysis, wt.% | |||||
|---|---|---|---|---|---|---|
| C | H | N | S | O | ||
| Initial oil | oil | 75.47 | 10.12 | 0.41 | 5.60 | 8.40 |
| S | 76.66 | 13.42 | 0.06 | 4.42 | 5.44 | |
| A | 83.31 | 11.03 | 0.09 | 2.34 | 3.23 | |
| R | 76.28 | 10.33 | 0.49 | 5.54 | 7.36 | |
| A | 74.39 | 8.57 | 0.87 | 6.30 | 9.87 | |
| Model | Equation |
|---|---|
| Reaction of nth order (Fn) | f = (1 − α)n |
| Two-dimensional phase boundary (R2) | f = 2(1 − α)1/2 |
| Three-dimensional phase boundary (R3) | f = 3(1 − α)2/3 |
| N-dimensional nucleation according to Avrami–Erofeev (An) | f = n·(1 − α)·[−ln(1 − α)](n−1)/n |
| Expanded Prout–Tompkins equation (Bna) | f = (1 − α)n·αAutocatOrder |
| The reaction of nth order with m-power autocatalysis by-product (Cnm) | f = (1 − α)n·(1 + AutocatOrder · αm) |
| Kamal–Sourur equation (KS) |
| Sample | Elemental Analysis, wt.% | |||||
|---|---|---|---|---|---|---|
| C | H | N | S | O | ||
| Non-catalytic steam thermal processing | oil | 69.34 | 9.30 | 0.36 | 8.40 | 12.60 |
| S | 79.17 | 13.92 | 0.06 | 2.74 | 4.11 | |
| A | 83.04 | 11.21 | 0.11 | 2.26 | 3.38 | |
| R | 76.48 | 10.69 | 0.55 | 4.91 | 7.37 | |
| A | 73.07 | 8.23 | 0.91 | 7.11 | 10.68 | |
| Catalytic steam thermal processing | oil | 73.60 | 9.95 | 0.37 | 6.43 | 9.65 |
| S | 80.92 | 14.04 | 0.06 | 2.09 | 2.89 | |
| A | 83.76 | 10.79 | 0.11 | 2.22 | 3.12 | |
| R | 67.09 | 9.29 | 0.38 | 9.76 | 13.48 | |
| A | 63.11 | 6.98 | 0.64 | 12.29 | 16.98 | |
| No | Experimental Conditions | C1 | C2 | C3 | C4 | C5 | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Initial oil | 1.50 | 0.14 | 0.56 | 4.17 | 0.04 | ||
| Batch reactor experiments | ||||||||
| Process | Pressure | T. °C | C1 | C2 | C3 | C4 | C5 | |
| 1 | Control experiment | 90 | 250 | 0.93 | 0.23 | 0.56 | 3.65 | 0.03 |
| 2 | The experiment in the presence of iron tallates | 1.13 | 0.22 | 0.68 | 3.62 | 0.10 | ||
| Non-Catalytic Pyrolysis | |||||
| ASTM E2890 | ASTM E2890 | ASTM E2890 | |||
| Eα, kJ·mol−1 | Eα, kJ·mol−1 | Eα, kJ·mol−1 | Eα, kJ·mol−1 | Eα, kJ·mol−1 | Eα, kJ·mol−1 |
| 224 ± 12 | 224 ± 12 | 224 ± 12 | 224 ± 12 | 224 ± 12 | 224 ± 12 |
| Catalytic Pyrolysis | |||||
| ASTM E2890 | ASTM E2890 | ASTM E2890 | |||
| Eα, kJ·mol−1 | Eα, kJ·mol−1 | Eα, kJ·mol−1 | Eα, kJ·mol−1 | Eα, kJ·mol−1 | Eα, kJ·mol−1 |
| 167 ± 14 | 167 ± 14 | 167 ± 14 | 167 ± 14 | 167 ± 14 | 167 ± 14 |
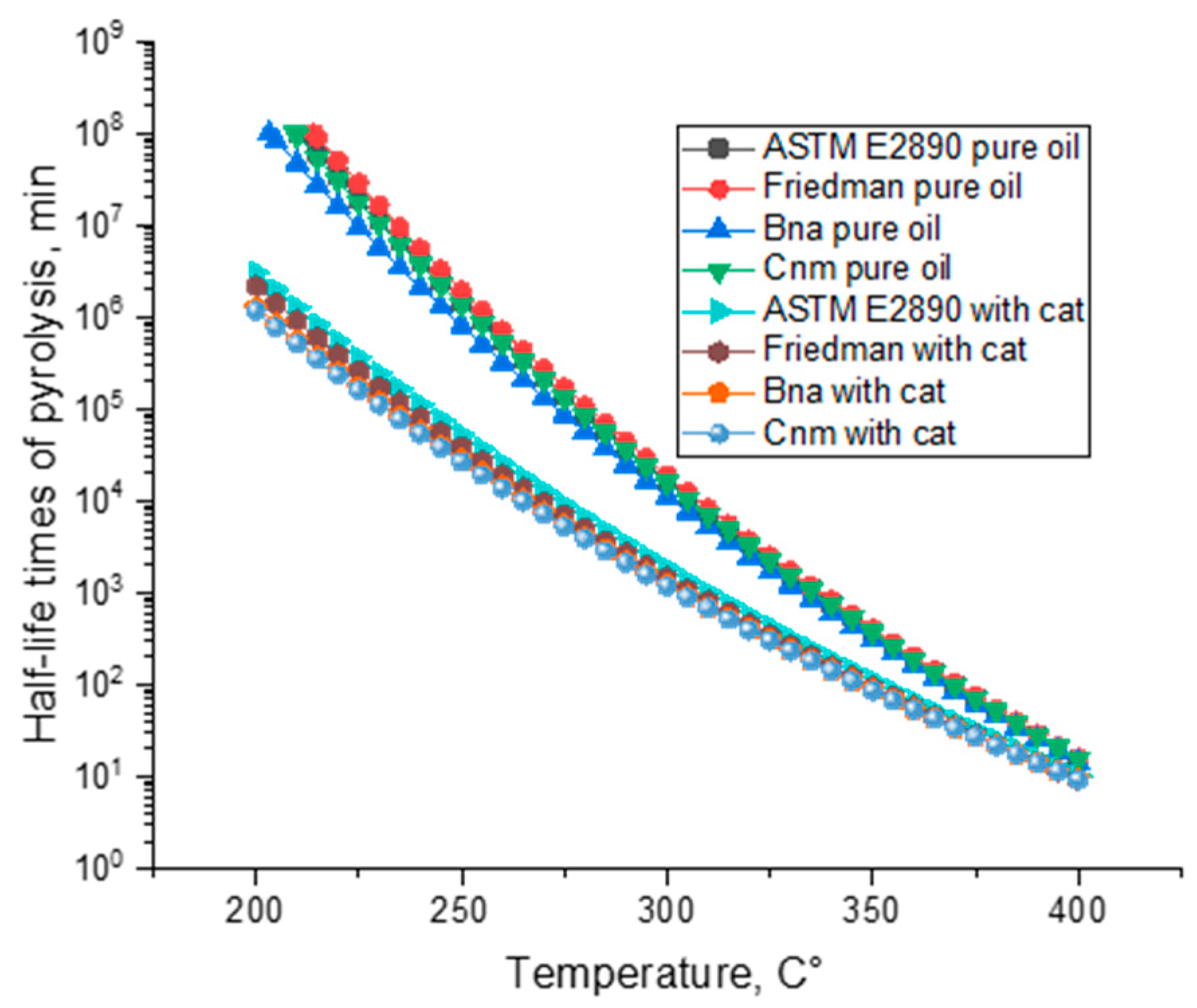
| Models | Pure Oil | Pure Oil with Catalyst | ||
|---|---|---|---|---|
| Kinetic Parameters | R2 | Kinetic Parameters | R2 | |
| Fn | E = 215.8 kJ·mol−1 lnA = 13.7 ReactOrder n = 1.1 | 0.99175 | E= 175.6 kJ·mol−1 lnA = 10.6 ReactOrder n = 0.8 | 0.99473 |
| R2 | E = 147.4 kJ·mol−1 lnA = 8.2 | 0.96275 | E = 141.5 kJ·mol−1 lnA = 7.8 | 0.98479 |
| R3 | E = 167.3 kJ·mol−1 lnA = 9.5 | 0.98117 | E = 161.1 kJ·mol−1 lnA = 9 | 0.99321 |
| An | E = 273.4 kJ·mol−1 lnA = 17.9 Dimension n = 0.8 | 0.99359 | E = 161 lnA = 9.6 Dimension n = 1.2 | 0.99517 |
| Bna | E = 213.5 kJ·mol−1 lnA = 13.5 ReactOrder n = 1.1 AutocatOrder 0.01 | 0.99160 | E = 156.4 lnA = 9.3 ReactOrder n = 0.8 AutocatOrder 0.14 | 0.99559 |
| Cnm | E = 222.3 kJ·mol−1 lnA = 13.8 ReactOrder n = 1.2 AutocatOrder 0.01 AutocatPower m = 0.01 | 0.99153 | E = 155.5 lnA = 5 ReactOrder n = 0.8 AutocatOrder 4.3 AutocatPower m = 0.15 | 0.99558 |
| KS | E = 604.3 kJ·mol−1 E2 = 227.3 kJ·mol−1 lnA = 11 ReactOrder n = 1.2 AutocatOrder 3.5 AutocatPower m = 0.01 | 0.99109 | E = 647 E2 = 163 lnA = 9.7 ReactOrder n = 0.9 AutocatOrder 0.02 AutocatPower m = 0.1 | 0.99536 |
| Pure Oil | Oil with Catalyst | |||||
|---|---|---|---|---|---|---|
| α (%) | ||||||
| Friedman method | ||||||
| 10 | 196 ± 25 | −19 ± 4 | 209 ± 25 | 147 ± 19 | −90 ± 3 | 211 ± 19 |
| 20 | 211 ± 21 | 3 ± 4 | 209 ± 21 | 153 ± 16 | −80.5 ± 2.8 | 210 ± 16 |
| 30 | 221 ± 18 | 17 ± 3 | 209 ± 18 | 155 ± 14 | −76.4 ± 2.5 | 210 ± 14 |
| 40 | 229 ± 18 | 27 ± 3 | 209 ± 18 | 157 ± 15 | −73.6 ± 2.5 | 210 ± 15 |
| 50 | 236 ± 19 | 37 ± 3 | 209 ± 19 | 158 ± 15 | −72.3 ± 2.6 | 209 ± 16 |
| 60 | 242 ± 21 | 44 ± 3 | 210 ± 21 | 159 ± 16 | −70.3 ± 2.7 | 209 ± 16 |
| 70 | 248 ± 22 | 52 ± 4 | 210 ± 22 | 160 ± 16 | −68.4 ± 2.7 | 209 ± 17 |
| 80 | 257 ± 23 | 65 ± 4 | 211 ± 24 | 161 ± 17 | −66.3 ± 2.8 | 208 ± 17 |
| 90 | 283 ± 31 | 99 ± 5 | 211 ± 31 | 167 ± 18 | −55.2 ± 2.9 | 207 ± 18 |
| Aver | 236 | 36 | 209 | 157 | −72.6 | 209 |
| KAS method | ||||||
| 10 | 196 ± 44 | −23 ± 8 | 212 ± 45 | 173 ± 14 | −54.2 ± 2.4 | 212 ± 14 |
| 20 | 202 ± 35 | −12 ± 6 | 211 ± 35 | 163 ± 13 | −67.5 ± 2.3 | 212 ± 14 |
| 30 | 209 ± 30 | −2 ± 5 | 210 ± 30 | 160 ± 14 | −71.1 ± 2.3 | 211 ± 14 |
| 40 | 215 ± 26 | 7 ± 4 | 210 ± 26 | 159 ± 14 | −72.2 ± 2.3 | 211 ± 14 |
| 50 | 220 ± 23 | 15 ± 4 | 210 ± 23 | 159 ± 14 | −72.8 ± 2.3 | 211 ± 14 |
| 60 | 226 ± 21 | 22 ± 4 | 210 ± 22 | 158 ± 14 | −72.9 ± 2.4 | 211 ± 14 |
| 70 | 231 ± 20 | 29 ± 3 | 210 ± 21 | 158 ± 15 | −72.4 ± 2.5 | 210 ± 15 |
| 80 | 237 ± 21 | 38 ± 3 | 210 ± 21 | 159 ± 15 | −71.7 ± 2.5 | 210 ± 15 |
| 90 | 249 ± 20 | 53 ± 3 | 211 ± 21 | 159 ± 16 | −70.1 ± 2.6 | 209 ± 16 |
| Aver | 221 | 14 | 210 | 161 | −69.4 | 211 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khelkhal, M.A.; Lapuk, S.E.; Buzyurov, A.V.; Ignashev, N.E.; Shmeleva, E.I.; Mukhamatdinov, I.I.; Vakhin, A.V. Thermal Behavior of Heavy Oil Catalytic Pyrolysis and Aquathermolysis. Catalysts 2022, 12, 449. https://doi.org/10.3390/catal12040449
Khelkhal MA, Lapuk SE, Buzyurov AV, Ignashev NE, Shmeleva EI, Mukhamatdinov II, Vakhin AV. Thermal Behavior of Heavy Oil Catalytic Pyrolysis and Aquathermolysis. Catalysts. 2022; 12(4):449. https://doi.org/10.3390/catal12040449
Chicago/Turabian StyleKhelkhal, Mohammed A., Semen E. Lapuk, Aleksey V. Buzyurov, Nikita E. Ignashev, Elvira I. Shmeleva, Irek I. Mukhamatdinov, and Alexey V. Vakhin. 2022. "Thermal Behavior of Heavy Oil Catalytic Pyrolysis and Aquathermolysis" Catalysts 12, no. 4: 449. https://doi.org/10.3390/catal12040449
APA StyleKhelkhal, M. A., Lapuk, S. E., Buzyurov, A. V., Ignashev, N. E., Shmeleva, E. I., Mukhamatdinov, I. I., & Vakhin, A. V. (2022). Thermal Behavior of Heavy Oil Catalytic Pyrolysis and Aquathermolysis. Catalysts, 12(4), 449. https://doi.org/10.3390/catal12040449