Abstract
With emission control regulations getting stricter, multi-functional catalyst systems are increasingly important for low-temperature operation. We investigate a wide range of multi-component catalyst systems, as physical mixtures and in multi-bed configurations, while varying the ratios of hydrocarbon traps (HCT), passive NOx adsorbers (PNAs), and diesel oxidation catalysts (DOC). Using industrially guided protocols, we measured the ability of these complex catalyst systems to reduce emissions during a 40 °C/min temperature ramp to simulate cold-start conditions. Using a temperature boundary condition of 250 °C, the average conversion was calculated for each regulated pollutant: CO, NOx, and total hydrocarbons (THC). An emissions merit function was developed to evaluate the effectiveness of each system relative to the relevant emission standards and expected engine exhaust concentrations. This merit function identified that a 1:1:4 ratio of PNA:HCT:DOC was the most effective emissions reduction configuration and had similar reactivity as a physical mixture or as a PNA→HCT→DOC multi-bed reactor.
1. Introduction
Stricter emission control regulations, coupled with the need for improved vehicle efficiency, have created significant challenges for the combustion engine-powered sectors [1,2,3,4,5]. This has led to the introduction of several new technologies over the past 15 years, especially for the diesel-powered vehicles with lean exhaust (excess O2). Diesel oxidation catalysts (DOC), diesel particulate filters (DPF), and urea-based selective catalytic reduction (SCR) of NOx catalysts are now widely deployed across most new on-road diesel-powered vehicles [6,7]. These technologies continue to be viable and are continually being improved, but with new regulations on the horizon [8,9,10] more advanced solutions will be needed. The most promising technologies that have been reported recently are hydrocarbon traps (HCT) [10,11,12,13,14,15,16,17,18,19,20] and passive NOx adsorbers (PNA) [16,17,18,19,20,21,22,23,24,25,26,27,28].
HCT is a technology that has been deployed and studied at several points over the past 25 years [29,30]. The general HCT concept is that a small pore material, such as a zeolite, is used to trap and store hydrocarbons at low temperatures where the DOC is not active for hydrocarbon oxidation. Upon further heating, the hydrocarbons are released and then converted over a platinum group metal (PGM) oxidation catalyst. This oxidation catalyst can be a part of the HCT, be mixed in close proximity to the HCT, or be a downstream DOC. Some reports highlight the need for a metal to be exchanged in the zeolite for optimal adsorption [12,20], especially for light hydrocarbons (C3 or less). Other reports have shown that a significant quantity of heavy hydrocarbons can be adsorbed on un-exchanged zeolites [13,20]. BEA and ZSM-5 zeolites are widely reported to be effective as HCTs, with the slightly larger pore size of BEA being more favorable to the large hydrocarbons that are common in diesel exhaust and its durability to 800 °C in the un-exchanged state providing a longer potential lifetime [13].
PNAs are a relatively new technology that were first reported in 2013 [27] and have been the focus of immense research in the years that followed [16,17,18,19,20,21,22,23,24,25,26,27,28,31]. Similar to HCTs, PNAs trap NOx at low temperatures, where either the SCR catalyst is not active or urea, the NH3 precursor, cannot be injected yet. Generally, 200 °C is the target SCR temperature for urea injection [32]. A key difference with PNAs compared to HCTs is that a PGM component is required for good NOx adsorption [28]. Pd-exchanged zeolites and Pd- or Pt-ceria were the materials that showed the most promise [16,17,18,19,20,21,22,23,24,25,26,27,28]. Pd-CHA is hydrothermally durable up to 800 °C [13,23,26,27,28,33,34], is tolerant to sulfur [27,28], and is still the most promising technology currently reported, although it has shown other deactivation mechanisms [26,35,36,37,38]. NO generally interacts with ion-exchanged Pd on a 1:1 basis and has little to no interaction with Pd that is present as nanoparticles [28]. Under typical exhaust conditions, NO is released from Pd/CHA between 200 and 400 °C [26,36,37,38,39,40].
Both HCTs and PNAs can be evaluated using the emissions control protocols established by U.S. DRIVE [41]. These materials would typically be evaluated using the low-temperature storage protocol, which calls for a 30-min saturation phase at 100 °C, followed by a 20 °C/min ramp up to 600 °C. In contrast, an evaluation of DOCs would employ a low-temperature oxidation catalyst approach, and the gases would be established at 100 °C over the catalyst, followed by a ramp up to 600 °C at 2 °C/min. Having established materials that are of interest, and protocols on how to evaluate them individually, it is important to evaluate how these technologies work together and then determine how they should be used in an emissions control system. Should they all be mixed together? Or should they be deployed one after the other? Additionally, the evaluation methods for these multicomponent systems are not well established. DOC activity is relatively straightforward to assess by itself since the lowest light-off temperature results in the best catalyst. Light-off temperature is typically defined as the temperature of 50% or 90% conversion (T50 or T90). However, in a combined system with DOC, HCT, and PNA, there is storage occurring at low temperatures, followed by a release. Thus, a simple light-off temperature evaluation does not give a fair representation of the activity, and an alternative analysis approach is necessary, but questions about that analysis remain. What ramp rate should be deployed? Should there be a storage period at the beginning?
Evaluating how effective these materials are in a combined system becomes very challenging to do in a way that is representative and is the focus of the research reported here. We outline the development of an emissions merit function that accounts for the emission regulation standards [2,3,4,5] and can be tailored to the expected engine-out emissions for a variety of combustion approaches [41]. If desired, it can also account for the mass of precious metal being used in the emissions control system. We present this approach in two steps. First, we evaluate physical mixtures of catalyst beds with HCT, PNA, and DOC components with varying ratios. After establishing the best ratio of HCT:PNA:DOC in physical mixtures, we then apply this to multi-bed evaluations, where the beds are physically separated. The rigorous method outlined here is designed to establish a standard technique that can be broadly used across emissions control evaluation studies.
2. Results and Discussion
The initial series of experiments was designed to identify the best ratio of PNA:HCT:DOC to use in the catalytic system. We evaluated eight unique combinations, including one with only the DOC. Flowing all of the gases that are required in the protocol and ramping at 40 °C/min results in complex reactor effluents that can be difficult to use in identifying which system yields the best results. Figure 1 shows two characteristic concentration profiles for CO, THC, and NOx concentrations during the evaluation procedure. The first concentration profile is for the DOC-only evaluation and is denoted by a lighter color in the figures. Additionally, the results from a mixture of PNA:HCT:DOC in a 1:1:4 ratio are shown in a darker color. It should be noted that the plots shown in Figure 1 are the average of the three trials that were performed. Because the temperature of the reactor does not immediately start increasing when the reactants are introduced, it is necessary to show the plots versus time and the temperature in a secondary y-axis. This delay is simply due to the thermal mass of the system and the time component associated with heat transfer.
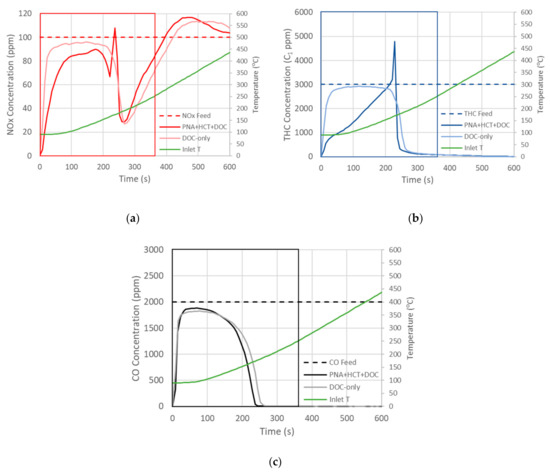
Figure 1.
Time-based reactor effluents for (a) NO, (b) THC, and (c) CO during temperature ramps for DOC-only and PNA + HCT + DOC reactor beds; temperature plotted on right y-axis. Boxes indicate area that is below 250 °C and is the primary focus for reactivity analysis.
Not surprisingly, the impact of adding the PNA and HCT is most notable in the NOx and THC plots (Figure 1a,b); however, an improvement in CO reactivity is also observed as the physical mixture of PNA + HCT + DOC reaches full conversion at a lower temperature (Figure 1c). The NOx concentration profiles in the PNA + HCT + DOC show a modest adsorption early and approaches saturation just before 200 s. After 200 s, we see a decrease in NOx concentration, again at an inlet temperature around 150 °C. This has been reported as a release of H2O from the Pd/CHA zeolite pores, which allows more NOx adsorption [35,39,42,43,44]. A sharp release of NOx is observed at 220 s and is associated with an exotherm from CO oxidation as the CO conversion approaches 90%; in fact, the bed temperature jumps to 36 °C higher than the inlet temperature (not shown). This peak is followed by a sharp decrease that also occurs on the DOC and, thus, is not associated with typical NOx adsorption on the PNA. As the furnace is heated further, we observe the NOx concentration rises and, in fact, it rises above the feed concentration for both the DOC and the PNA + HCT + DOC. This is likely due to the stored NOx that is being released unconverted as expected. The observation that both the DOC and PNA are releasing NOx above 300 °C (temperature at 425 s) suggests that the NOx is being stored by another mechanism that is independent of ion-exchanged Pd. In several of our evaluations we observed this NOx conversion, followed by a release at higher temperatures. This behavior is consistent with NOx storage on Al2O3 analogous to lean NOx traps (LNT) or NOx storage release (NSR) catalysts [45]. LNTs also typically have Ba or K to stabilize the stored NOx at higher temperatures, but NOx adsorption on Al2O3 has also been shown in this temperature range [46,47,48,49,50,51]. One other possibility is the HC-SCR of NOx where the partially oxidized HCs react with the NOx to reduce it to N2 and N2O. Although we do not have an FTIR in our system to measure N2O and, thus, confirm/refute HC-SCR, this has been reported by others [52,53] and is a typical signature product of HC-SCR.
The THC concentration plots have similar trends as the NOx plots up to 200 s. It is clear that a significant fraction of the THC is being adsorbed on the HCT, as the PNA + HCT + DOC evaluation has a notably lower concentration than the DOC-only up to this point. Above 200 s, the hydrocarbons start to release as the inlet temperature reaches 150 °C. Shortly afterwards, there is a sharp increase in THC concentration that coincides with the NOx peak and the exothermic oxidation of CO. This is followed by a steep decrease to 270 ppm and then a gradual decline to the baseline. Although not shown, this gradual decrease is primarily due to the presence of propane, as it is the least reactive of the HCs that we are introducing.
As noted above, the CO profiles have subtle differences, with the PNA + HCT + DOC having a slightly earlier light-off temperature than the DOC-only. Of the three criteria pollutants, the CO behavior is most like a typical DOC evaluation and, thus, T50 s and T90 s accurately reflect the activity of a given catalyst system. Figure 2a shows these for all of the physical mixtures evaluated, and the lowest T90 for CO is the HCT + DOC (1:2). The light-off temperatures can also be calculated for THC, as shown in Figure 2b, and if we only use this metric, then the PNA + HCT + DOC (1:1:4) has a slightly lower T90 than the other samples. From these results, a couple of interesting observations can be made. The first item is the notably higher CO and THC T90 for the HCT + DOC (1:1) sample. This is likely simply due to this sample having the lowest PGM content—50% of the DOC-only sample. The other interesting result is that the CO T90 for the HCT + DOC (1:2) sample decreases by 7 °C compared to the DOC-only, even though it has 2/3 of the PGM of the DOC-only sample; not surprisingly, the T90 THC increases for this sample. This is likely due to the hydrocarbons being adsorbed in the HCT and, thus, not absorbing on or near the DOC’s PGM, which results in more adsorption/reaction sites for CO.

Figure 2.
Inlet temperatures associated with 50% and 90% conversion (T50 and T90, respectively) for (a) CO and (b) THC over several multi-component physical mixture reactor beds. Ratios in parentheses represent the mass ratio of the different components in the sample, respectively.
As mentioned earlier, simply relying on T90 s does not account for the storage that is occurring on these samples. Additionally, NOx is not expected to be converted over these samples and will rely on a downstream SCR. Thus, it is difficult to fully assess how these mixtures are helping with NOx emissions control. One option is to evaluate how much NOx, THC, and CO are converted or stored before the SCR is capable of converting NOx. As discussed earlier, ~200 °C is the desired temperature before urea should be injected and, since the DOC is upstream of the SCR, it is reasonable to choose a DOC inlet temperature of 250 °C as a threshold temperature before it is possible to rely on the SCR to convert the NOx. Using this approach, we calculated the overall fraction of NOx/THC/CO converted or stored before the inlet temperature reaches 250 °C, which is the time window highlighted in Figure 1 with a box. It should be noted that this time window includes the initial release of NOx and HCs, but intentionally does not include the later release of NOx that would be possible to convert over a downstream SCR. The conversion/storage values are shown in Figure 3 for all samples. Interpreting the data from this graph is relatively complicated, as the best catalyst combination for NOx is different from the best conversion for THC, and the best combination for CO conversion is different still. Due to these complicating factors, we developed a merit function approach that accounts for both the feed concentration and emission standards for NOx, THC, and CO.
The emission standards that are most relevant to the LTC-D combustion mode chosen here are for heavy-duty (HD) diesel trucks. The emission regulations that are proposed to be implemented by 2027 are listed in Table 1 in g/bhp-hr [2,3,4,5]. The NOx requirement is the lowest of the three—775 times lower than CO; thus, from a weighting factor (WF) standpoint, it is logical to normalize to it, as shown in Table 1. NOx is also expected to be the lowest criteria pollutant from a low-temperature combustion mode, as illustrated by the feed concentrations used in this study (displayed in the Experimental section), and since the regulations are listed in a mass basis and the gas feed concentrations are listed in volumetric or molar basis, it is necessary to make some assumptions on the chemical makeup of both the NOx and THC. Diesel fuel is composed of a wide range of saturated and unsaturated hydrocarbons that include aromatic benzyl groups and, thus, a common C:H ratio reported is 1.8 [54]. This can be used to calculate a molecular weight of the THC on a C1-basis of 13.8 g/mol. The NOx emissions, which result from nitrogen and oxygen combining at high temperatures during combustion, are composed of NO and NO2. NO is thermodynamically favored at combustion temperatures, but 5–16% of the NOx emissions are measured to be NO2 [55]; thus, 10% NO2 is reasonable to use here. This results in a molecular weight of 31.6 g/mol for NOx, where x = 1.1. Having these molecular weights, it is then possible to calculate the representative mass flowrate for each criteria pollutant at 333 s-cm3/min; these values are listed at the bottom of Table 1.



Figure 3.
Overall conversions for CO, THC, and NOx in the temperature window below 250 °C over several multi-component physical mixture reactor beds. Ratios in parentheses represent the mass ratio of the different components in the sample, respectively.

Table 1.
Key parameters and variables used for emissions merit function approach.
Table 1.
Key parameters and variables used for emissions merit function approach.
| CO | THC | NOx | ||
|---|---|---|---|---|
| (CO) | (C1H1.8) | (NO1.1) | ||
| Future heavy-duty regulation | 15.5 | 0.14 | 0.02 | g/bhp/hr |
| Tailpipe ratios | 775.0 | 7.0 | 1.0 | |
| Weighting Factor (WF) | 0.0013 | 0.14 | 1 | 1/tailpipe ratios |
| Molecular weight | 28.0 | 13.8 | 31.6 | g/mol |
| Feed Concentration (LTC-D) | 2000 | 3000 | 100 | ppm (vol) |
| Mass flowrate (at 333 s-cm3/min) | 50.0 | 37.0 | 2.8 | g/hr |
Having both the weighting factors and the mass flowrates for NOx, THC, and CO, a weighted emissions mass flow rate (ṁWE) can be calculated according to the following:
ṁWE = ṁNOxWFNOx + ṁTHCWFTHC + ṁCOWFCO
This flowrate is designed to apply relative merit to each of the criteria pollutants as a function of both the emissions standard and the expected engine-out emissions. This weighted emissions flowrate will change depending on the regulations and the engine-out emissions, and this flexibility is a key feature of the merit function.
Having this merit function approach allows us to compare the effectiveness of the different catalyst loadings. Continuing to focus on the results before the catalyst inlet temperature reaches 250 °C (Figure 3), we can calculate an average weighted emissions flow rate leaving the catalyst bed, (ṁWE)out. Subtracting this value from the inlet flowrate, (ṁWE)in, the average weighted emissions conversion rate, (ṁWE)converted, can be calculated for this period:
(ṁWE)converted = (ṁWE)in − (ṁWE)out
Another factor that is important in deciding the best emissions control approach is how much PGM is in the catalyst. We account for this by simply dividing the weighted emission flowrates by the mass of the PGM, which, for the catalysts studied here, are listed in the last column in Table 2 and Table 3. Both of these weighted emission approaches are applied to the physical mixtures evaluated in this study, as shown Figure 4a,b. Additionally, the individual contributions of (■) CO, (■) THC, and (■) NOx are shown. From this analysis, it is clear that the ability of a sample to reduce THC and NOx emissions is the most important factor; in fact, the contribution from CO is very small, less than 0.1 g/h or g/h/mgPGM, and nearly impossible to see on the graphs. This is the case even though nearly 50% of the CO was converted below 250 °C (Figure 3). It is now possible to see that the PNA + HCT + DOC with a ratio of 1:1:4 converts the most weighted emissions, while normalizing to PGM mass shows that the HCT + DOC (1:1) is the most productive. In each instance, the addition of either PNA or HCT leads to an improvement over the DOC-only case.

Table 2.
Physical mixture experimental matrix with varying PNA, HCT, and DOC ratios.

Table 3.
Multi-bed experimental matrix with a fixed PNA:HCT:DOC ratio of 1:1:4.

Figure 4.
(a) Average weighted emissions conversion rate up to 250 °C, as calculated from the parameters in Table 1 and Equations (1) and (2), for a series of physical mixture reactor beds; (b) also shows the emissions when normalized to PGM content. Contributions from (■) CO, (■) THC, and (■) NOx are shown as a portion of the total for each graph. Ratios in parentheses represent the mass ratio of the different components in the sample, respectively.
A simpler way to represent this reactivity is to convert these weighted emission flowrates to conversions, XWE. This can be calculated in a straightforward manner:
XWE = 1 − (ṁWE)out/(ṁWE)in
These results are shown in Figure 5 for the physical mixtures up to 250 °C and continue to show that the most effective catalyst mixture is PNA + HCT + DOC with a ratio of 1:1:4. A drawback of only comparing conversions is that the importance of PGM is lost. However, LTC-D, a commercial emissions control solution, has not been developed for the combustion mode we are studying, so the importance of PGM is not as critical as identifying an approach that will enable emissions compliance. This suggests that the 1:1:4 ratio is the best catalyst system, although it is only marginally better than the HCT + DOC (1:1) mixture. This approach continues to highlight the importance of THC and NOx conversion in these samples compared to CO conversion.
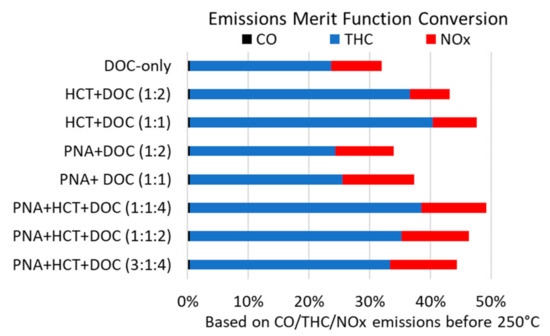
Figure 5.
Emissions merit function conversion for a series of physical mixture reactor beds calculated from Equation (3) that represents the portion of the emissions that are converted up to 250 °C. Contributions from (■) CO, (■) THC, and (■) NOx are shown as a portion of the total for each graph. Ratios in parentheses represent the mass ratio of the different components in the sample, respectively.
Physical mixtures are a straightforward way to measure the reactivity of multi-component systems and represent a possible commercial solution. However, catalysts are often prepared in zones where functionalities are placed one in front of the other. To replicate this, we used the best PNA:HCT:DOC ratio from the physical mixtures (1:1:4) and then created a series of beds in the reactor separated by a thin layer of ground quartz wool, as described in the Experimental Section. Operating the same experimental procedure and applying the same temperature window as before (up to 250 °C), we evaluated four multi-bed configurations and compared the NOx/THC/CO results to the 1:1:4 physical mixture and DOC-only results, as shown in Figure 6 and Figure 7. Once again, it is difficult to differentiate between the different evaluations with just this comparison, so it makes sense to apply the merit function approach to this series of data. The average conversion rates and overall conversions are shown in Figure 8 and Figure 9, and, once again, all of the multi-component evaluations yield significant improvements compared to the DOC-only reactor bed, while using less PGM content. Of the multi-bed evaluations, it is apparent that the PNA→HCT→DOC configuration yields the best results; in fact, the multi-bed approach is even more effective than the physical mixture approach: 53% weighted emissions conversion versus 49%. This is particularly interesting since this arrangement had the highest T90 for both CO and THC (Figure 7). However, the separation of the different phases allowed each of the components to avoid the exotherm that occurs over the downstream DOC. With this gap, the large spike of HCs and NOx does not occur, and they are released at higher inlet temperatures. This is illustrated in Figure 10 for both THC and NOx, as it is clear the release occurs over much longer periods of time. This allows more of the released HCs to be converted over the DOC and, critically, allows the release of NOx to occur at temperatures where SCRs are active for NOx reduction.

Figure 6.
Overall conversions for CO, THC, and NOx in the temperature window below 250 °C over several multibed reactor configurations.

Figure 7.
Inlet temperatures associated with 50% and 90% conversion (T50 and T90, respectively) for (a) CO and (b) THC over several multibed reactor configurations.

Figure 8.
(a) Average weighted emissions conversion rate up to 250 °C, as calculated from the parameters in Table 1 and Equations (1) and (2), for a series of multibed reactor systems; (b) also shows the emissions when normalized to PGM content. Contributions from (■) CO, (■) THC, and (■) NOx are shown as a portion of the total for each graph.

Figure 9.
Emissions merit function conversion for a series of multibed reactor systems calculated from Equation (3) that represents the portion of the emissions that are converted up to 250 °C. Contributions from (■) CO, (■) THC, and (■) NOx are shown as a portion of the total for each graph.

Figure 10.
Time-based reactor effluents for (a) NO and (b) THC during temperature ramps for physical mixture (PNA + HCT + DOC) and multi-bed (PNA→HCT→DOC) reactor beds; temperature plotted on right y-axis. Boxes indicate area that is below 250 °C and is the primary focus for reactivity analysis.
3. Experimental
3.1. Catalyst Preparation
Both the HCT and the DOC for this study were used directly from the vendor. The HCT was NH4+-exchanged BEA zeolite from Zeolyst International with a SiO2:Al2O3 molar ratio (SAR) of 25. The model DOC was a mixture of 1% Pt/alumina and 1% Pd/alumina obtained from Sigma-Aldrich; the two catalysts were always combined in a 1:1 mixture by weight. The PNA was Pd/CHA, and the CHA zeolite was purchased from ACS Materials LLC with a SAR of 25. The Pd precursor, Pd(NO3)2·2H2O, was purchased from Sigma-Aldrich (St. Louis, MO, USA). CHA was functionalized with Pd using an ion-exchange method. Briefly, an aqueous solution of Pd(NO3)2·2H2O was added dropwise to a suspension of NH4-SSZ-13 (3.960 g in 50 mL water; 250 RPM stirring), and the reaction was performed at 80 °C for 20 h. The catalyst was air calcined at 500 °C for 5 h, and the Pd loading was confirmed to be ~1 wt% prior to evaluation. All catalytic materials were pressed into pellets, ground, and sieved to 250–500 μm particles before being introduced to the reactor.
3.2. Reactor
A quartz powder reactor with an inner diameter of 8 mm was employed in this study. The U-tube shaped reactor is placed in a cylindrical ceramic heater that is 200 mm long, with a 150 mm long heated zone, and a 150 mm inner diameter. Quartz wool is place inside the furnace between the reactor and the furnace walls to distribute the heat evenly throughout the reactor and avoid radiant heat transfer. A type-K thermocouple is placed on the outside of the reactor near the catalyst bed and is connected to a Cole-Parmer temperature control unit. An additional type-K thermocouple is placed outside the reactor in a similar location and is connected to the data acquisition software. Two more type-K thermocouples are placed inside the reactor. One is approximately 4 mm from the front of the catalyst bed and provides the inlet temperature. This inlet thermocouple is used in reporting the data shown in the results. The second internal thermocouple is placed 1–2 mm into the catalyst bed and can be used to measure exotherms. If more than one bed is used, this thermocouple will always be in the first bed.
The gas concentrations in the catalyst effluent were measured using four analyzers. The total hydrocarbons (THC) were measured using a flame ionization detector (FID), and the CO concentration was measured using a non-dispersive infrared (NDIR) analyzer, both from California Analytical Instruments (CAI, Orange, CA, USA). The NO/NO2/NOx was measured using a chemiluminescent system from EcoPhysics. Additionally, a mass spectrometer from Stanford Research Systems (SRS) was employed to ensure all of the gases were flowing as intended and stable concentrations were maintained.
The experimental matrix was evaluated in two parts. The first set of experiments was performed while varying the PNA, HCT, and DOC components, as shown in Table 2. In all cases a target sample loading of 100 mg was maintained, and the flow rate was kept constant. The catalysts were physically mixed together after loading into the reactor using an agitator for at least 1 min (Fisher Scientific Touch Mixer Model 231). This approach is designed to mimic a single washcoat that includes all of the components on a monolith and is the same volume of existing DOCs, i.e., the two layers would be in one DOC and the emissions control device volume would be the same. The second set of evaluations focused on a multi-bed approach with a single ratio of components—PNA:HCT:DOC of 1:1:4. The compositions are listed in Table 3, and the arrows (→) in the table indicate that more than one bed was used, while a plus sign (+) indicates a physical mixture. Thus, in the case of HCT + PNA→DOC, a physical mixture of HCT and PNA are in the first powder reactor bed, and a second powder bed of DOC follows. When two beds were used, a thin layer of 25 mg of ground quartz wool was used to separate the beds. This second approach is designed to mimic a zoned washcoat or a multi-brick catalytic system. As in the first set of evaluations, the total target catalyst loading and flowrate were held constant.
3.3. Catalyst Evaluation
Although there is an extensive set of protocols provided by the U.S. DRIVE Advanced Combustion and Emission Control Tech Team [41], adaptation is necessary in the case of a multicomponent system. Since our primary goal was to measure the impact on the oxidation reactivity in the presence of trap materials and illustrate improvements on DOC functionality, we focused on the oxidation protocol and modified the ramp rate. The gas concentrations from that protocol are listed in Table 4 for the low-temperature combustion-diesel (LTC-D) combustion mode. The primary attributes that distinguish this combustion mode from the other ones listed are the relatively high concentrations of both CO (2000 ppm) and total hydrocarbons or THC (3000 ppm C1), and the low concentration of NOx (100 ppm NO). This is generally consistent with combustion studies investigating LTC [41]. Since our project was aimed at investigating emission control systems that would enable a LTC approach, it was natural to select these gas feeds. To enable the use of a mass spectrometer for monitoring multiple species of interest, argon was used as the balance gas in these experiments.

Table 4.
Low-Temperature Combustion-Diesel (LTC-D) exhaust constituents and concentrations.
As mentioned earlier, the catalyst loading and flowrates were kept constant at 100 mg and 333 mL (STP)/min, respectively. This weight-to-flow ratio was recommended in the protocols, such that a weight-hourly space velocity (WHSV) of 200 L/g/h was achieved. The ramp rate was increased to 40 °C/min, which was the fastest repeatable ramp rate that we could achieve in our reactor system. The reactor was equipped with a four-way switching valve that allowed a fast, seamless transition from the THC/CO/NOx flows to an argon flow, while maintaining a flow of H2O, CO2, and O2 at all times. With this setup we were able to implement the following procedure in an automated fashion using our custom LabVIEW program:
- Heat reactor to 700 °C in H2O, CO2, and O2 at 20 °C/min, and hold for 4 h;
- Cool to 100 °C in H2O, CO2, and O2 as fast as possible, and stabilize for 2 h;
- Simultaneously introduce THC/CO/NOx to reactor and immediately begin ramp up to 600 °C at 40 °C/min, then hold for 1 h;
- Switch off THC/CO/NOx flows and cool to 100 °C for repeated measurements;
- Record a total of three 40 °C/min ramps to 600 °C.
This approach is designed to represent the cold-start immediately after a vehicle engine is initially started.
4. Conclusions
In this multi-pronged study, we evaluated a series of multifunctional catalyst beds that contained PNA, HCT, and DOC functionalities in a wide array of ratios and configurations. To fairly evaluate which configuration yielded the best results, we developed a merit function approach that is flexible to different combustion strategies and quantifiably accounts for:
- Emission standards for NOx, THC, and CO;
- Expected emission concentrations and mass flow rates;
- Platinum group metal (PGM) content (if desired).
This merit function is used to determine weighting factors that can be adjusted based on the relevant emission standards and combustion mode being employed. We then applied this to illustrate that a ratio of 1:1:4 in a series of physical mixtures of PNA + HCT + DOC yielded the most reactive catalyst bed without considering PGM content. This approach was extended to multi-bed evaluations, and when using this same PNA:HCT:DOC ratio of 1:1:4, the PNA→HCT→DOC configuration yields the best results. The improvement observed in the multi-bed approach points to the importance of balancing the quantity of stored emission constituents with the reactivity of the catalysts while maintaining optimal release kinetics. This flexible merit function can be adapted across a wide range of emission control strategies, including three-way catalysts and stoichiometric combustion.
Author Contributions
Conceptualization, T.J.T. and P.K.; methodology, T.J.T. and P.K.; validation, T.J.T.; formal analysis, T.J.T.; investigation, T.J.T. and P.K.; resources, T.J.T.; data curation, T.J.T.; writing—original draft preparation, T.J.T.; writing—review and editing, P.K.; visualization, T.J.T.; supervision, T.J.T.; project administration, T.J.T.; funding acquisition, T.J.T. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by the U.S. Department of Energy’s Vehicle Technologies Office (VTO). The authors greatly appreciate support from Ken Howden, Siddiq Khan, and Gurpreet Singh at VTO.
Acknowledgments
This manuscript has been authored by UT-Battelle, LLC., under contract DE-AC05-00OR22725 with the US Department of Energy (DOE). I would also like to acknowledge many fruitful conversations in the development of this evaluation approach with the entire Applied Catalysis and Emissions Research group at ORNL, particularly Austin Ladshaw, Sreshtha Majumdar, and Josh Pihl.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zammit, M.; Di Maggio, C.; Kim, C.; Lambert, C.; Muntean, G.; Peden, C.; Parks, J.; Howden, K. Future Automotive Aftertreatment Solutions: The 150 °C Challenge Workshop Report. Available online: https://cleers.org/wp-content/uploads/2012_The_150C_Challenge_Workshop_Report.pdf (accessed on 4 April 2022).
- U.S. Environmental Protection Agency. EPA Proposes Tier 3 Motor Vehicle Emission and Fuel Standards; EPA-420-F-13-016a; U.S. Environmental Protection Agency: Washington, DC, USA, 2013; pp. 1–4. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/P100G84Y.PDF?Dockey=P100G84Y.PDF (accessed on 4 April 2022).
- U.S. Environmental Protection Agency; U.S. Department of Transportation; National Highway Traffic Safety Administration. 77 FR 62623—2017 and Later Model Year Light-Duty Vehicle Greenhouse Gas Emissions and Corporate Average Fuel Economy Standards; Office of the Federal Register, National Archives and Records Administration: College Park, MD, USA, 2012; Volume 77, pp. 62623–63200.
- U.S. Environmental Protection Agency. Certification and Compliance. Available online: https://www.epa.gov/vehicles-and-engines (accessed on 10 November 2016).
- California Environmental Protection Agency Air Resources Board. Amendments to the Low-Emission Vehicle Program—LEV III. Available online: www.arb.ca.gov/msprog/levprog/leviii/leviii.htm (accessed on 28 July 2016).
- Chilumukuru, K.; Gupta, A.; Ruth, M.; Cunningham, M.; Kothandaraman, G.; Cumaranatunge, L.; Hess, H. Aftertreatment Architecture and Control Methodologies for Future Light Duty Diesel Emission Regulations. SAE Int. J. Engines 2017, 10, 1580–1587. [Google Scholar] [CrossRef]
- Lou, D.; Zhao, Y.; Zhang, Y.; Sun, Y. Modeling and Analysis on Emission Characteristics of Light-Duty Diesel Engine After-Treatment System Based on Neural Network; SAE Technical Paper, 2021-01-0595; SAE International: Warrendale, PA, USA, 2021. [Google Scholar] [CrossRef]
- Dahodwala, M.; Joshi, S.; Koehler, E.; Franke, M.; Tomazic, D. Strategies for Meeting Phase 2 GHG and Ultra-Low NOx Emission Standards for Heavy-Duty Diesel Engines. SAE Int. J. Engines 2018, 11, 1109–1122. [Google Scholar] [CrossRef]
- DieselNet. United States: Heavy-Duty Onroad Engines. Available online: https://dieselnet.com/standards/us/hd.php (accessed on 28 October 2021).
- Joshi, A. Review of Vehicle Engine Efficiency and Emissions; SAE Technical Paper, 2021-01-0575; SAE International: Warrendale, PA, USA, 2021; Volume 1. [Google Scholar] [CrossRef]
- Park, J.; Park, S.J.; Nam, I.; Yeo, G.K.; Kil, J.K.; Youn, Y.K. A fast and quantitative assay for developing zeolite-type hydrocarbon trap catalyst. Microporous Mesoporous Mater. 2007, 101, 264–270. [Google Scholar] [CrossRef]
- Park, J.; Park, S.J.; Ahn, H.A.; Nam, I.; Yeo, G.K.; Kil, J.K.; Youn, Y.K. Promising zeolite-type hydrocarbon trap catalyst by a knowledge-based combinatorial approach. Microporous Mesoporous Mater. 2009, 117, 178–184. [Google Scholar] [CrossRef]
- Toops, T.; Binder, A.; Kunal, P.; Kyriakidou, E.; Choi, J.-S. Analysis of Ion-Exchanged ZSM-5, BEA, and SSZ-13 Zeolite Trapping Materials under Realistic Exhaust Conditions. Catalysts 2021, 11, 449. [Google Scholar] [CrossRef]
- Endo, Y.; Nishikawa, J.; Iwakura, H.; Inamura, M.; Wakabayashi, T.; Nakahara, Y.; Ogasawara, M.; Sumio, K. Development of Highly Durable Zeolites as Hydrocarbon Trap Materials for Automotive Catalysts; SAE Technical Paper, 2018-01-0947; SAE International: Warrendale, PA, USA, 2018. [Google Scholar] [CrossRef]
- Luo, J.; McCabe, R.W.; Dearth, M.A.; Gorte, R.J. Transient adsorption studies of automotive hydrocarbon traps. AIChE J. 2014, 60, 2875–2881. [Google Scholar] [CrossRef]
- Moser, D.H.; Nipunage, S.; Nunan, J.; Day, R.; Alltizer, C. An Unconventional Application of a HC Trap to Meet SULEV20; SAE Technical Paper 2021-01-0574; SAE International: Warrendale, PA, USA, 2021. [Google Scholar] [CrossRef]
- Malamis, S.A.; Harold, M.P. Optimizing the lean hydrocarbon NOx trap: Sequential and dual-layer configurations. Catal. Today 2021, 360, 388–400. [Google Scholar] [CrossRef]
- Malamis, S.A.; Harold, M.P.; Epling, W.S. Coupled NO and C3H6 Trapping, Release and Conversion on Pd/BEA: Evaluation of the Lean Hydrocarbon NOx Trap. Ind. Eng. Chem. Res. 2019, 58, 22912–22923. [Google Scholar] [CrossRef]
- Lee, J. Zeolite-Based Hydrocarbon Traps and Passive NOx Adsorbers for Vehicle Cold Start Applications (Order No. 28417752). Ph.D. Thesis, State University of New York at Buffalo, New York, NY, USA, 2021; p. 2555619491. [Google Scholar]
- Lee, J.; Theis, J.R.; Kyriakidou, E.A. Vehicle emissions trapping materials: Successes, challenges, and the path forward. Appl. Catal. B 2019, 243, 397–414. [Google Scholar] [CrossRef]
- Moliner, M.; Corma, A. From metal-supported oxides to well-defined metal site zeolites: The next generation of passive NOx adsorbers for low-temperature control of emissions from diesel engines. React. Chem. Eng. 2019, 4, 223–234. [Google Scholar] [CrossRef]
- Khivantsev, K.; Jaegers, N.R.; Kovarik, L.; Hu, J.Z.; Gao, F.; Wang, Y.; Szanyi, J. Palladium/Zeolite Low Temperature Passive NOx Adsorbers (PNA): Structure-Adsorption Property Relationships for Hydrothermally Aged PNA Materials. Emiss. Control Sci. Technol. 2020, 6, 126–138. [Google Scholar] [CrossRef]
- Gu, Y.; Epling, W.S. Passive NOx adsorber: An overview of catalyst performance and reaction chemistry. Appl. Catal. A Gen. 2019, 570, 1–14. [Google Scholar] [CrossRef]
- Ji, Y.; Bai, S.; Crocker, M. Al2O3-based passive NOx adsorbers for low temperature applications. Appl. Catal. B Environ. 2015, 170, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Berndt, C.T. An Experimental Study of a Passive NOx Adsorber (PNA) for the Reduction of Cold Start Diesel Emissions (Order No. 27668392). Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2019; p. 2377950409. [Google Scholar]
- Kunal, P.; Toops, T.J.; Kidder, M.K.; Lance, M.J. Deactivation trends of Pd/SSZ-13 under the simultaneous presence of NO, CO, hydrocarbons and water for passive NOx adsorption. Appl. Catal. B Environ. 2021, 299, 120591. [Google Scholar] [CrossRef]
- HChen, Y.; Mulla, S.; Weigert, E.; Camm, K.; Ballinger, T.; Cox, J.; Blakeman, P. Cold Start Concept (CSCTM): A Novel Catalyst for Cold Start Emission Control; SAE Technal Paper; SAE International: Warrendale, PA, USA, 2013; Volume 2, pp. 372–381. [Google Scholar]
- Chen, H.Y.; Collier, J.E.; Liu, D.; Mantarosie, L.; Duran-Martín, D.; Novak, V.; Rajaram, R.R.; Thompsett, D. Low temperature NO storage of zeolite supported Pd for low temperature diesel engine emission control. Catal. Lett. 2016, 146, 1706–1711. [Google Scholar] [CrossRef]
- Ballinger, T.; Manning, W.; Lafyatis, D. Hydrocarbon Trap Technology for the Reduction of Cold-Start Hydrocarbon Emissions; SAE Technical Paper 970741; SAE International: Warrendale, PA, USA, 1997. [Google Scholar] [CrossRef]
- Murakami, K.; Tominaga, S.; Hamada, I.; Nagayama, T.; Kijima, Y.; Katougi, K.; Nakagawa, S. Development of a High Performance Catalyzed Hydrocarbon Trap Using Ag-Zeolite; SAE Technical Paper 2004-01-1275; SAE International: Warrendale, PA, USA, 2004. [Google Scholar] [CrossRef]
- Murata, Y.; Morita, T.; Wada, K.; Ohno, H. NOx Trap Three-Way Catalyst (N-TWC) Concept: TWC with NOx Adsorption Properties at Low Temperatures for Cold-Start Emission Control. SAE Int. J. Fuels Lubr. 2015, 8, 454–459. [Google Scholar] [CrossRef]
- Addy Majewski, W. Urea Dosing and Injection Systems, DieselNet. Available online: https://dieselnet.com/tech/cat_scr_diesel_urea_dosing.php#:~:text=The%20highest%20risk%20of%20deposit,about%20125%2D175%C2%B0C (accessed on 28 October 2021).
- Lee, J.; Ryou, Y.S.; Hwang, S.; Kim, Y.; Cho, S.J.; Lee, H.; Kim, C.H.; Kim, D.H. Comparative study of the mobility of Pd species in SSZ-13 and ZSM-5, and its implication for their activity as passive NOx adsorbers (PNAs) after hydro-thermal aging. Catal. Sci. Technol. 2019, 9, 163–173. [Google Scholar] [CrossRef]
- Wang, A.; Lindgren, K.; Di, M.; Bernin, D.; Carlsson, P.-A.; Thuvander, M.; Olsson, L. Insight into hydrothermal aging effect on Pd sites over Pd/LTA and Pd/SSZ-13 as PNA and CO oxidation monolith catalysts. Appl. Catal. B Environ. 2020, 278, 119315. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Hwang, S.; Lee, E.; Lee, H.; Kim, C.H.; Kim, D.H. Deactivation of Pd/Zeolites passive NOx adsorber induced by NO and H2O: Comparative study of Pd/ZSM-5 and Pd/SSZ-13. Catal. Today 2021, 360, 350–355. [Google Scholar] [CrossRef]
- Theis, J.R.; Ura, J.A. Assessment of zeolite-based Low temperature NOx adsorbers: Effect of reductants during multiple sequential cold starts. Catal. Today 2021, 360, 340–349. [Google Scholar] [CrossRef]
- Zhao, H.; Hill, A.J.; Ma, L.; Bhat, A.; Jing, G.; Schwank, J.W. Progress and future challenges in passive NO adsorption over Pd/zeolite catalysts. Catal. Sci. Technol. 2021, 11, 5986–6000. [Google Scholar] [CrossRef]
- Gu, Y.; Marino, S.; Cortés-Reyes, M.; Pieta, I.S.; Pihl, J.A.; Epling, W.S. Integration of an Oxidation Catalyst with Pd/Zeolite-Based Passive NOx Adsorbers: Impacts on Degradation Resistance and Desorption Characteristics. Ind. Eng. Chem. Res. 2021, 60, 6455–6464. [Google Scholar] [CrossRef]
- Gu, Y.; Zelinsky, R.P.; Chen, Y.; Epling, W.S. Investigation of an irreversible NOx storage degradation Mode on a Pd/BEA passive NOx adsorber. Appl. Catal. B Environ. 2019, 258, 118032. [Google Scholar] [CrossRef]
- Gu, Y.; Majumdar, S.S.; Pihl, J.A.; Epling, W.S. Investigation of NO adsorption and desorption phenomena on a Pd/ZSM-5 passive NOx adsorber. Appl. Catal. B Environ. 2021, 298, 120561. [Google Scholar] [CrossRef]
- Rappé, K.; DiMaggio, C.; Pihl, J.; Theis, J.R.; Oh, S.H.; Fisher, G.B.; Parks, J.; Easterling, V.G.; Yang, M.; Stewart, M.L.; et al. Aftertreatment Protocols for Catalyst Characterization and Performance Evaluation: Low-Temperature Oxidation, Storage, Three-Way, and NH3-SCR Catalyst Test Protocols. Emiss. Control. Sci. Technol. 2019, 5, 183. [Google Scholar] [CrossRef]
- Villamaina, R.; Iacobone, U.; Nova, I.; Tronconi, E.; Ruggeri, M.P.; Mantarosie, L.; Collier, J.; Thompsett, D. Mechanistic insight in NO trapping on Pd/Chabazite systems for the low-temperature NOx removal from Diesel exhausts. Appl. Catal. B Environ. 2021, 284, 119724. [Google Scholar] [CrossRef]
- Khivantsev, K.; Gao, F.; Kovarik, L.; Wang, Y.; Szanyi, J. Molecular Level Understanding of How Oxygen and Carbon Monoxide Improve NOx Storage in Palladium/SSZ-13 Passive NOx Adsorbers: The Role of NO+ and Pd(II)(CO)(NO) Species. J. Phys. Chem. C 2018, 122, 10820–10827. [Google Scholar] [CrossRef]
- Zelinsky, R.; Epling, W.S. Effects of CO and H2O Co-Feed on the Adsorption and Oxidation Properties of a Pd/BEA Hydrocarbon Trap. Catalysts 2021, 11, 348. [Google Scholar] [CrossRef]
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks, J.E., II. Overview of the Fundamental Reactions and Degradation Mechanisms of NOx Storage/Reduction Catalysts. Catal. Rev. 2004, 46, 163–245. [Google Scholar] [CrossRef]
- Medhekar, V.; Balakotaiah, V.; Harold, M.P. TAP study of NOx storage and reduction on Pt/Al2O3 and Pt/Ba/Al2O3. Catal. Today 2007, 121, 226–236. [Google Scholar] [CrossRef]
- Fridell, E.; Persson, H.; Westerberg, B.; Olsson, L.; Skoglundh, M. The mechanism for NOx storage. Catal. Lett. 2000, 66, 71–74. [Google Scholar] [CrossRef]
- Szailer, T.; Kwak, J.H.; Kim, D.H.; Hanson, J.C.; Peden, C.H.F.; Szanyi, J. Reduction of stored NOx on Pt/Al2O3 and Pt/BaO/Al2O3 catalysts with H2 and CO. J. Catal. 2006, 239, 51–64. [Google Scholar] [CrossRef]
- Olsson, L.; Fridell, E. The Influence of Pt Oxide Formation and Pt Dispersion on the Reactions NO2⇔NO+1/2 O2 over Pt/Al2O3 and Pt/BaO/Al2O3. J. Catal. 2002, 210, 340–353. [Google Scholar] [CrossRef]
- Olsson, L.; Jozsa, P.; Nilsson, M.; Jobson, E. Fundamental studies of NOx storage at low temperatures. Top. Catal. 2007, 42, 95–98. [Google Scholar] [CrossRef]
- Toops, T.J.; Smith, D.B.; Epling, W.S.; Parks, J.E.; Partridge, W.P. Quantified NOx adsorption on Pt/K/gamma-Al2O3 and the effects of CO2 and H2O. Appl. Catal. B Environ. 2007, 58, 255–264. [Google Scholar] [CrossRef]
- Burch, R.; Millington, P.J. Selective Reduction of NOx by Hydrocarbons in Excess Oxygen by Alumina and Silica-supported Catalysts. Catal. Today 1996, 29, 37–42. [Google Scholar] [CrossRef]
- Burch, R.; Watling, T.C. The Effect of Promoters on Pt/A12O3 Catalysts for The Reduction of NO by C3H6 under Lean-burn Conditions. Appl. Catal. B 1997, 11, 207–216. [Google Scholar] [CrossRef]
- IEA-AMF. Composition of Gasoline and Diesel. Available online: https://www.iea-amf.org/content/fuel_information/diesel_gasoline (accessed on 29 October 2021).
- DieselNet. Gaseous Emissions. Available online: https://dieselnet.com/tech/emi_gas.php (accessed on 29 October 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).