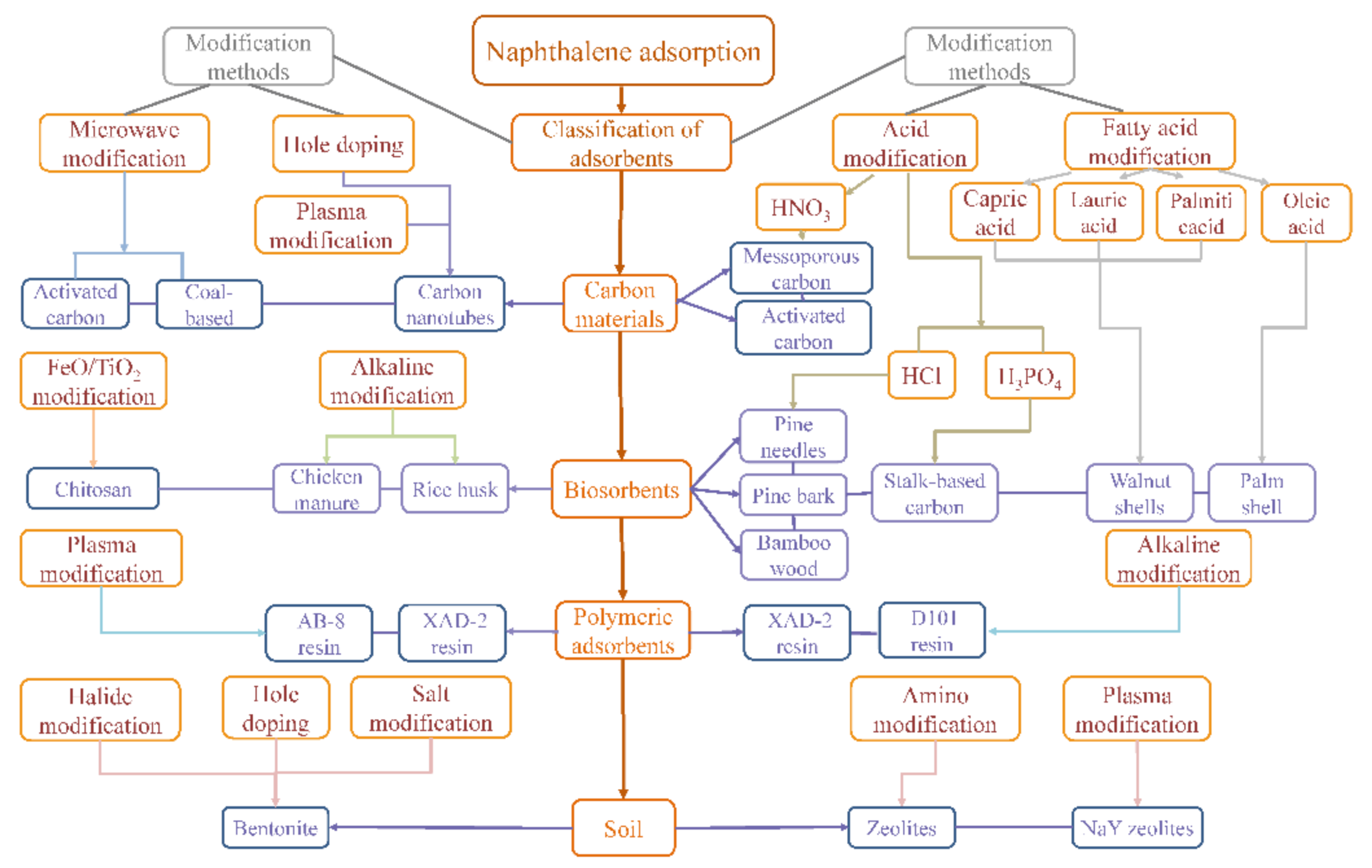
A Review on Modification Methods of Adsorbents for Naphthalene in Environment
Abstract
:1. Introduction
2. Acid and Alkaline Modification
2.1. Acid Modification
| Raw Adsorbent | Modification Reagents | Modified Adsorbents’ Name | Nap Adsorption Efficiency | Adsorption Capacity (μg g−1) | Ref. |
|---|---|---|---|---|---|
| MC | HNO3 | COMC | / | 1.6 × 103 | [49] |
| MC | HNO3 | Oxidized MC | 100% | / | [53] |
| ACC/ ACNU | HNO3/O2 | NO-ACC/NO-ACNU/O-ACC/O-ACNU | 100% | / | [54] |
| MCs | H2SO4/(NH4)2S2O8 | MCS-S/MCS-N/MCS-NS | 10% | / | [55] |
| B | HNO3/(NH4)2S2O8 | BN20/BN60/BS | / | 4.7 × 105 | |
| BW/PW/PN/PB | HCl | BW-DS/PW-DS/PN-DS/PB-DS | / | 2.8 × 103 | [57] |
| Soybean stalk-based carbon | H3PO4 | / | 100% | 3.02 × 104 | [58] |
| Bentonite | DPC | Organoclays | The Kd value of Nap increased from 0.151 to 1.675 L g−1 when the amount of DPC increased to 2.00 times the CEC. | 1.75 × 103 | [59] |
| Kaolinite and Halloysite | HDTMA | - | When the HDTMA was 42% of CEC, the Kd of Nap increased from 0.9 to 109. | About 9.0 × 102 | [60] |
2.2. Fatty Acid Modification
2.3. Alkaline Modification
3. Salt Modification
3.1. Bromide Modification
| Raw Adsorbent | Modification Reagents | Name of Modified Adsorbents | Nap Adsorption Efficiency | Adsorption Capacity | Ref. |
|---|---|---|---|---|---|
| WNS | fatty acid | OWNS/CWNS/LWNS/PWNS | 85.3% | 7210 mg g−1 | [62] |
| PSAC | oleic acid | / | / | 44.87 mg g−1 | [63] |
| bentonites | DTAB/SDBs | modified bentonite | 99.1% | 0.604 mg g−1 | [72] |
| bentonite | ammonium bromide | modified bentonite | 99.4% | / | [73] |
| bentonite | DPC | CEC | 55–60% | 1.675 L g−1 | [59] |
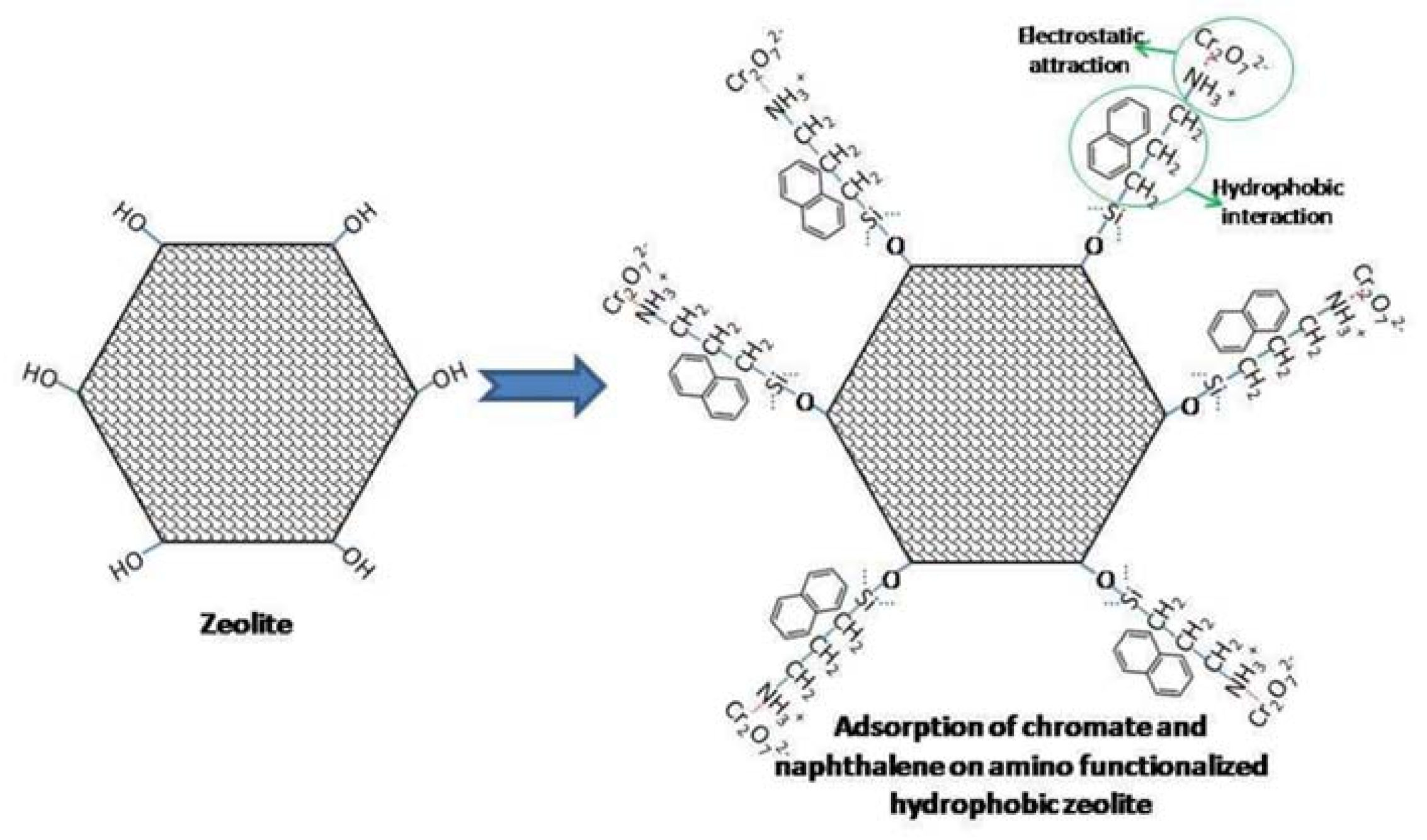
| zeolite | APTMS | NH2-zeolite | 93.95% | 1.88 mg g−1 | [74] |
| SWNTs | hole doping | (10, 0) SWNTs | / | / | [75] |
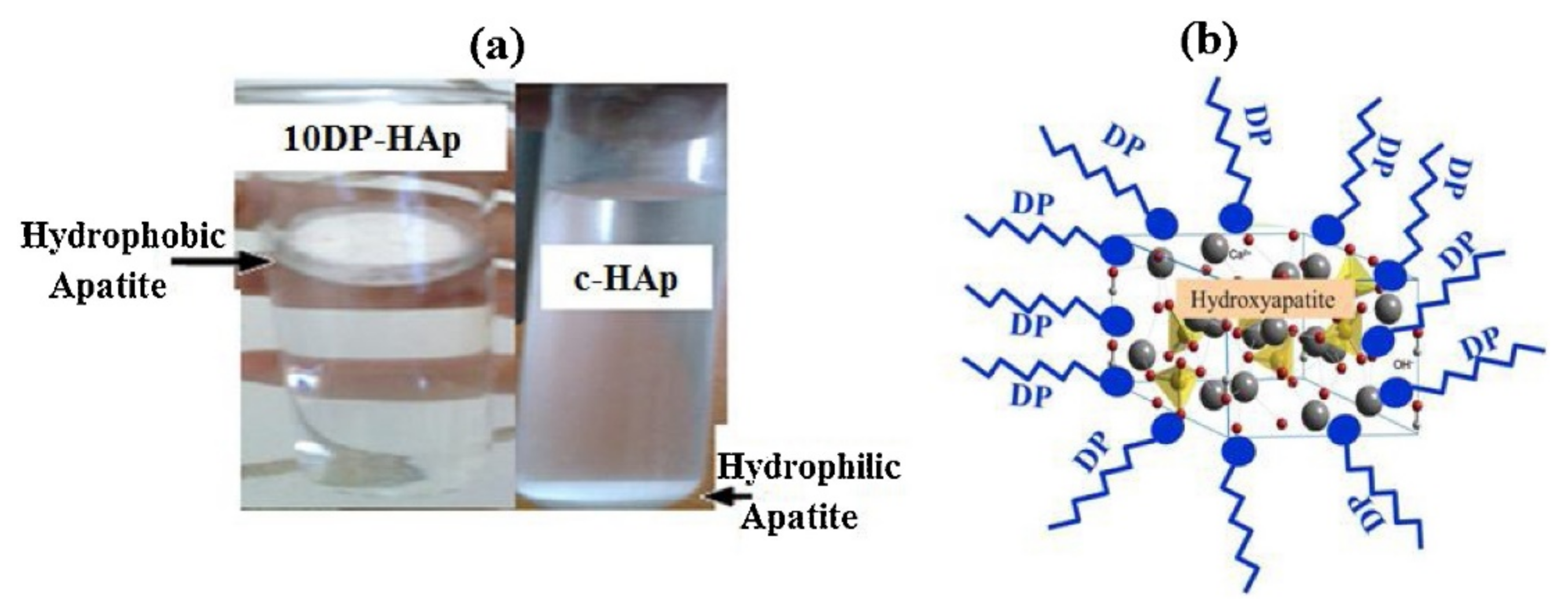
| c-HAp | DP | 10 DP-HAp | / | 7.22 mg g−1 | [76] |
| SBA-15 | NH2 | NH2-SBA-15 | 79.3% | 1.92 mg g−1 | [11] |
| un-Ch | FeO/TiO2 | Ch- FeO/TiO2 | 90% | 149.3 mg g−1 | [77] |
3.2. Chloride Modification
4. Amino Modification
5. Doping Modification
6. Microwave and Plasma Modification
6.1. Microwave Modification
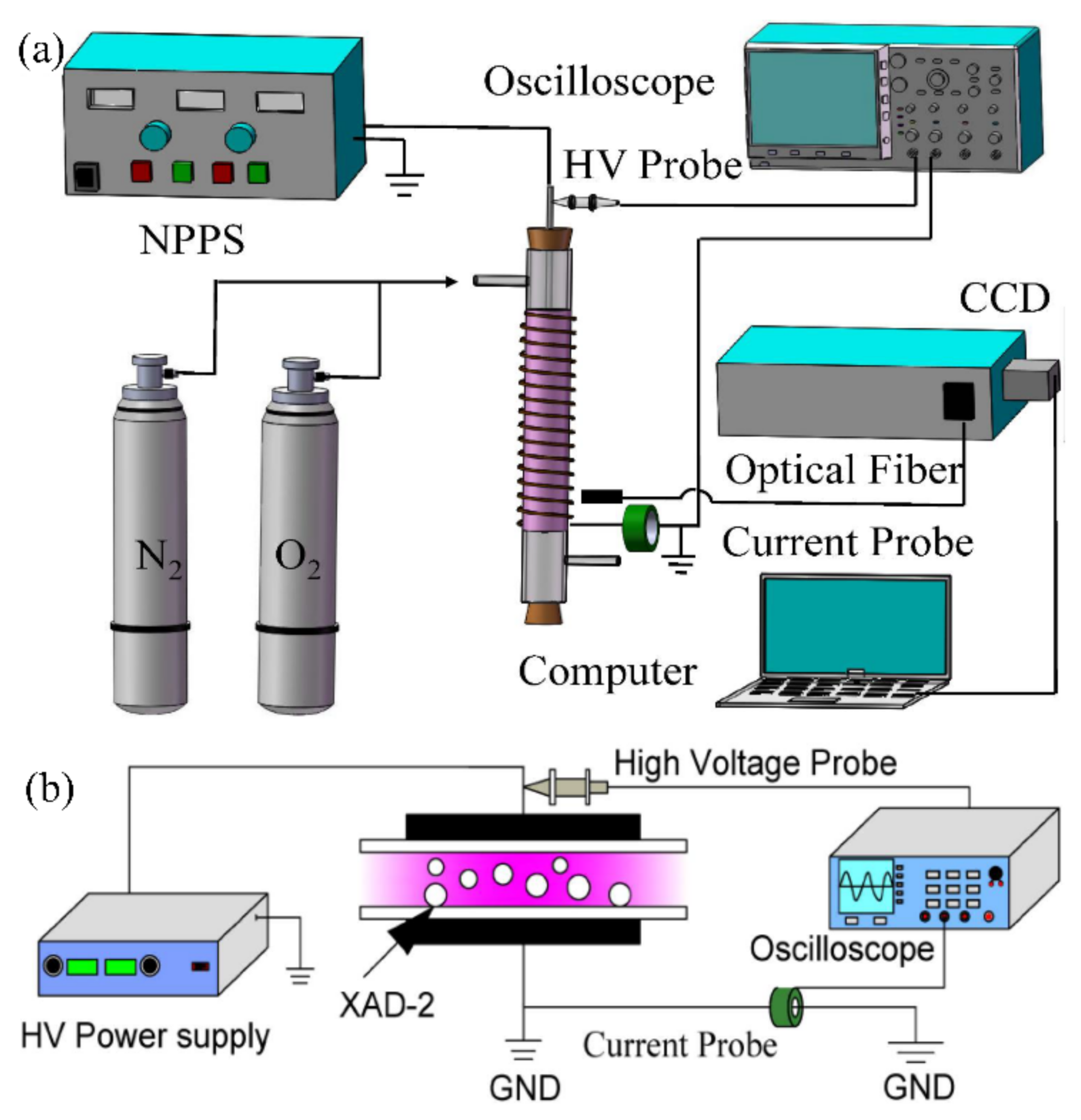
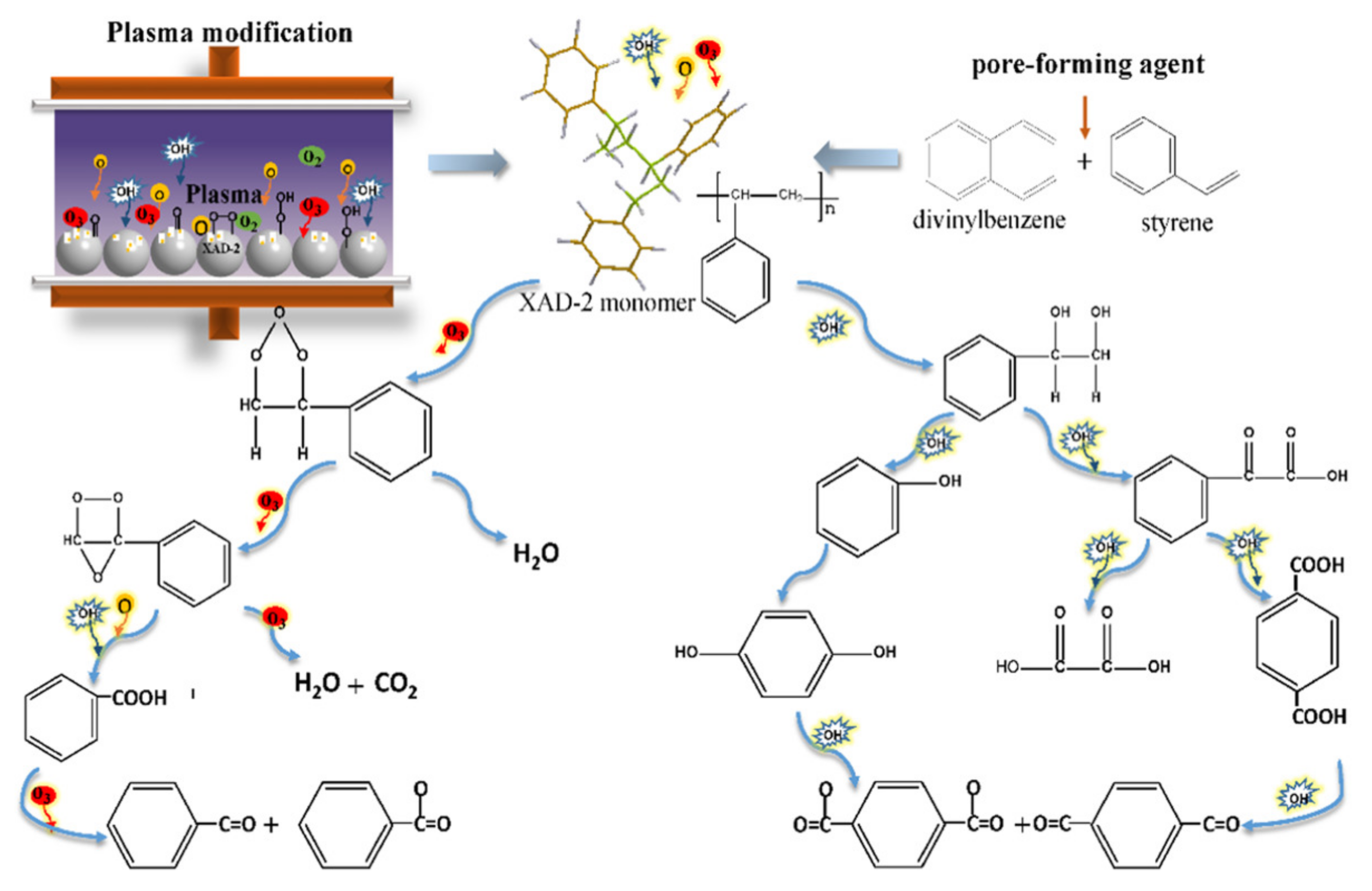
6.2. Plasma Modification
7. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Lamichhane, S.; Krishna, K.C.B.; Sarukkalige, R. Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: A review. Chemosphere 2016, 148, 336–353. [Google Scholar] [CrossRef] [PubMed]
- WHO. Polynuclear Aromatic Hydrocarbons in Drinking-Water. Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2003; p. 2. [Google Scholar]
- Magnusson, R.; Arnoldsson, K.; Lejon, C.; Hagglund, L.; Wingfors, H. Field evaluation and calibration of a small axial passive air sampler for gaseous and particle bound polycyclic aromatic hydrocarbons (PAHs) and oxygenated PAHs. Environ. Pollut. 2016, 216, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wu, Z.; Liu, D.; He, X. Microwave-assisted modification of activated carbon with cationic surfactants for enhancement of naphthalene adsorption. Korean J. Chem. Eng. 2018, 35, 557–566. [Google Scholar] [CrossRef]
- Chang, C.F.; Chang, C.Y.; Chen, K.H.; Tsai, W.T.; Shie, J.L.; Chen, Y.H. Adsorption of naphthalene on zeolite from aqueous solution. J. Colloid Interface Sci. 2004, 277, 29–34. [Google Scholar] [CrossRef]
- Luo, X.J.; Mai, B.X.; Yang, Q.S.; Fu, J.M.; Sheng, G.Y.; Wang, Z.S. Polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides in water columns from the Pearl River and the Macao harbor in the Pearl River Delta in South China. Mar. Pollut. Bull. 2004, 48, 1102–1115. [Google Scholar] [CrossRef]
- Cargnin, R.S.; do Nascimento, P.C.; Ferraz, L.M.; Barichello, M.M.; Brudi, L.C.; da Rosa, M.B.; de Carvalho, L.M.; do Nascimento, D.B.; Cravo, M.C.; Cravo, L.A.H. Investigation of Extraction and Collection of Polycyclic Aromatic Hydrocarbons from Aqueous Solutions at Different Temperatures. Polycycl. Aromat. Compd. 2020, 40, 40–49. [Google Scholar] [CrossRef]
- Vane, C.H.; Kim, A.W.; Beriro, D.J.; Cave, M.R.; Knights, K.; Moss-Hayes, V.; Nathanail, P.C. Polycyclic aromatic hydrocarbons (PAH) and polychlorinated biphenyls (PCB) in urban soils of Greater London, UK. Appl. Geochem. 2014, 51, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gao, Y.; Yu, N.; Zhang, C.K.; Wang, S.Y.; Ma, L.M.; Zhao, J.F.; Lohmann, R. Particulate matter, gaseous and particulate polycyclic aromatic hydrocarbons (PAHs) in an urban traffic tunnel of China: Emission from on-road vehicles and gas-particle partitioning. Chemosphere 2015, 134, 52–59. [Google Scholar] [CrossRef]
- Balati, A.; Shahbazi, A.; Amini, M.M.; Hashemi, S.H. Adsorption of polycyclic aromatic hydrocarbons from wastewater by using silica-based organic-inorganic nanohybrid material. J. Water Reuse Desalination 2015, 5, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.J.; Wang, X.C.; Fan, B. Characteristics of PAHs adsorption on inorganic particles and activated sludge in domestic wastewater treatment. Bioresour. Technol. 2011, 102, 5305–5311. [Google Scholar] [CrossRef]
- Wolowiec, M.; Muir, B.; Zieba, K.; Bajda, T.; Kowalik, M.; Franus, W. Experimental Study on the Removal of VOCs and PAHs by Zeolites and Surfactant-Modified Zeolites. Energy Fuels 2017, 31, 8803–8812. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Zave, Z.G.; Bahrami, A.; Shahna, F.G.; Farhadian, M. Application of a needle trap device packed with XAD-2 polyaniline composite for sampling naphthalene and phenanthrene in air. J. Chromatogr. A 2019, 1602, 74–82. [Google Scholar] [CrossRef]
- IARC. Polynuclear Aromatic Compounds: International Agency for Research on Cancer, France; International Agency for Research on Cancer: Lyons, France, 2010. [Google Scholar]
- Kristensen, P.; Eilertsen, E.; Einarsdottir, E.; Haugen, A.; Skaug, V.; Ovrebo, S. Fertility in Mice after Prenatal Exposure to Benzo a Pyrene and Inorganic lead. Environ. Health Perspect. 1995, 103, 588–590. [Google Scholar] [CrossRef]
- Xu, X.H.; Hu, H.; Kearney, G.D.; Kan, H.D.; Sheps, D.S. Studying the effects of polycyclic aromatic hydrocarbons on peripheral arterial disease in the United States. Sci. Total. Environ. 2013, 461, 341–347. [Google Scholar] [CrossRef]
- Siddens, L.K.; Larkin, A.; Krueger, S.K.; Bradfield, C.A.; Waters, K.M.; Tilton, S.C.; Pereira, C.B.; Lohr, C.V.; Arlt, V.M.; Phillips, D.H.; et al. Polycyclic aromatic hydrocarbons as skin carcinogens: Comparison of benzo a pyrene, dibenzo def,p chrysene and three environmental mixtures in the FVB/N mouse. Toxicol. Appl. Pharmacol. 2012, 264, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Song, T.; Tian, W.; Qiao, K.; Zhao, J.; Chu, M.; Du, Z.; Wang, L.; Xie, W. Adsorption Behaviors of Polycyclic Aromatic Hydrocarbons and Oxygen Derivatives in Wastewater on N-Doped Reduced Graphene Oxide. Sep. Purif. Technol. 2021, 254, 117565. [Google Scholar] [CrossRef]
- Tang, T.; Li, J.; Yang, Z.; Xiang, F. Research Progress on Biodegradation and Transformation Pathways of Polycyclic Aromatic Hydrocarbons. Acta Pet. Sinica. Pet. Process. Sect. 2019, 35, 403–413. [Google Scholar]
- Peng, R.H.; Fu, X.Y.; Zhao, W.; Tian, Y.S.; Zhu, B.; Han, H.J.; Xu, J.; Yao, Q.H. Phytoremediation of Phenanthrene by Transgenic Plants Transformed with a Naphthalene Dioxygenase System from Pseudomonas. Environ. Sci. Technol. 2014, 48, 12824–12832. [Google Scholar] [CrossRef]
- Li, C.H.; Wong, Y.S.; Wang, H.Y.; Tam, N.F.Y. Anaerobic biodegradation of PAHs in mangrove sediment with amendment of NaHCO3. J. Environ. Sci. 2015, 30, 148–156. [Google Scholar] [CrossRef]
- Yu, K.; Huang, L.Y.; Lou, L.L.; Chang, Y.; Dong, Y.L.; Wang, H.; Liu, S.X. Degradation of polycyclic aromatic hydrocarbons in crumb tyre rubber catalysed by rutile TiO2 under UV irradiation. Environ. Technol. 2015, 36, 1008–1015. [Google Scholar] [CrossRef]
- Dong, D.B.; Li, P.J.; Li, X.J.; Xu, C.B.; Gong, D.W.; Zhang, Y.Q.; Zhao, Q.; Li, P. Photocatalytic degradation of phenanthrene and pyrene on soil surfaces in the presence of nanometer rutile TiO2 under UV-irradiation. Chem. Eng. J. 2010, 158, 378–383. [Google Scholar] [CrossRef]
- Zhou, W.J.; Wang, X.H.; Chen, C.P.; Zhu, L.Z. Removal of polycyclic aromatic hydrocarbons from surfactant solutions by selective sorption with organo-bentonite. Chem. Eng. J. 2013, 233, 251–257. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.Y.; Wang, X.W.; Wei, C.H. Efficient adsorption of phenanthrene by simply synthesized hydrophobic MCM-41 molecular sieves. Appl. Surf. Sci. 2014, 311, 825–830. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B.; Zhu, L. Enhanced sorption of polycyclic aromatic hydrocarbons from aqueous solution by modified pine bark. Bioresour. Technol. 2010, 101, 7307–7313. [Google Scholar] [CrossRef]
- Duan, S.; Liu, X.; Wang, Y.; Meng, Y.; Alsaedi, A.; Hayat, T.; Li, J. Plasma surface modification of materials and their entrapment of water contaminant: A review. Plasma Process. Polym. 2017, 14, 1600218. [Google Scholar] [CrossRef]
- Chunyun, C.; Ke, Y.U.E.; Zhenming, C.; Xiaoyun, Z.; Hongqi, Z. Advances in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs). J. Mirobiology 2007, 27, 100–106. [Google Scholar]
- Dzombak, D.A.; Luthy, R.G. Estimating Adsorption of Polycyclic Aromatic-Hydrocarbons on soils. Soil Sci. 1984, 137, 292–308. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sanchez-Perez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination-A review. Sci. Total. Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef]
- Sakulthaew, C.; Comfort, S.; Chokejaroenrat, C.; Harris, C.; Li, X. A combined chemical and biological approach to transforming and mineralizing PAHs in runoff water. Chemosphere 2014, 117, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, X.S.; Yu, S.L.; Shi, W.X.; Sun, N.; Jin, L.M.; Wang, S.; Zhang, B.; Ma, C.; Sun, L.P. The influence of important factors on ultrafiltration of oil/water emulsion using PVDF membrane modified by nano-sized TiO2/Al2O3. Desalination 2011, 281, 179–184. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Chong, M.F.; Bhatia, S.; Ismail, S. Drinking water reclamation from palm oil mill effluent (POME) using membrane technology. Desalination 2006, 191, 35–44. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Ates, H.; Argun, M.E. Removal of PAHs from leachate using a combination of chemical precipitation and Fenton and ozone oxidation. Water Sci. Technol. 2018, 78, 1064–1070. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Clemente, A.; Torres-Palma, R.A.; Penuela, G.A. Removal of polycyclic aromatic hydrocarbons in aqueous environment by chemical treatments: A review. Sci. Total. Environ. 2014, 478, 201–225. [Google Scholar] [CrossRef]
- Liu, J.F.; Chen, J.J.; Jiang, L.; Yin, X. Adsorption of mixed polycyclic aromatic hydrocarbons in surfactant solutions by activated carbon. J. Ind. Eng. Chem. 2014, 20, 616–623. [Google Scholar] [CrossRef]
- Liu, J.F.; Zhang, Y.S.; Sun, X.J.; Hu, W.L. Separation of polycyclic aromatic hydrocarbons from rhamnolipid solution by activated carbon adsorption. Environ. Earth Sci. 2016, 75, 1453. [Google Scholar] [CrossRef]
- Grassi, M.; Kaykioglu, G.; Belgiorno, V.; Lofrano, G. Removal of Emerging Contaminants from Water and Wastewater by Adsorption Process; Springer: Berlin/Heidelberg, Germany, 2012; pp. 15–37. [Google Scholar] [CrossRef]
- Ania, C.O.; Cabal, B.; Pevida, C.; Arenillas, A.; Parra, J.B.; Rubiera, F.; Pis, J.J. Effects of activated carbon properties on the adsorption of naphthalene from aqueous solutions. Appl. Surf. Sci. 2007, 253, 5741–5746. [Google Scholar] [CrossRef] [Green Version]
- Tseng, H.H.; Wey, M.Y.; Chen, J.C.; Lu, C.Y. The adsorption of PAHs, BTEX, and heavy metals on surfactant-modified desulfurization sorbents in a dry scrubber. Fuel 2002, 81, 2407–2416. [Google Scholar] [CrossRef]
- Liu, Z.S.; Wey, M.Y.; Lin, C.L. Simultaneous control of acid gases and PAHs using a spray dryer combined with a fabric filter using different additives. J. Hazard. Mater. 2002, 91, 129–141. [Google Scholar] [CrossRef]
- Mei, L.; He, L.; Fan, C.; Hao, L.; Li, S.; Song, W. Adsorption of Naphthalene at Low Concentration on Activated Carbon. Chin. J. Process. Eng. 2014, 14, 253–257. [Google Scholar]
- Saleh, T.A.; Sari, A.; Tuzen, M. Simultaneous removal of polyaromatic hydrocarbons from water using polymer modified carbon. Biomass Convers. Biorefinery 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Yu, H.D.; Yang, B.; Waigi, M.G.; Peng, F.; Li, Z.K.; Hu, X.J. The effects of functional groups on the sorption of naphthalene on microplastics. Chemosphere 2020, 261, 127592. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Z.; Sun, Z.; Ye, J. Comparison study of naphthalene adsorption on activated carbons prepared from different raws. Korean J. Chem. Eng. 2018, 35, 2086–2096. [Google Scholar] [CrossRef]
- Anbia, M.; Moradi, S.E. Adsorption of naphthalene-derived compounds from water by chemically oxidized nanoporous carbon. Chem. Eng. J. 2009, 148, 452–458. [Google Scholar] [CrossRef]
- Bazula, P.A.; Lu, A.H.; Nitz, J.J.; Schuth, F. Surface and pore structure modification of ordered mesoporous carbons via a chemical oxidation approach. Microporous Mesoporous Mater. 2008, 108, 266–275. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Y.; Zhou, Q.; Zhu, C.; She, M.; Ding, W. Adsorptive removal of gas-phase mercury by oxygen non-thermal plasma modified activated carbon. Chem. Eng. J. 2016, 294, 281–289. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, P.; Qiu, Y.; Yu, Q.; Ma, J.; Wu, H.; Luo, G.; Xu, M.; Yao, H. Increasing oxygen functional groups of activated carbon with non-thermal plasma to enhance mercury removal efficiency for flue gases. Chem. Eng. J. 2015, 263, 1–8. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, G.; Bao, Y.; Zeng, D.; Chen, Y. Effect of oxidative modification of coal tar pitch-based mesoporous activated carbon on the adsorption of benzothiophene and dibenzothiophene. Fuel Process. Technol. 2015, 129, 85–90. [Google Scholar] [CrossRef]
- Zhou, A.; Ma, X.; Song, C. Effects of oxidative modification of carbon surface on the adsorption of sulfur compounds in diesel fuel. Appl. Catal. B Environ. 2009, 87, 190–199. [Google Scholar] [CrossRef]
- Wen, J.; Sun, W.; Yang, W. Effects of oxidative modification of carbon surface on selective removal of nitrogen compounds from model fuel. J. Funct. Mater. 2013, 44, 2954–2958. [Google Scholar]
- Ania, C.O.; Cabal, B.; Parra, J.B.; Arenillas, A.; Arias, B.; Pis, J.J. Naphthalene adsorption on activated carbons using solvents of different polarity. Adsorpt. J. Int. Adsorpt. Soc. 2008, 14, 343–355. [Google Scholar] [CrossRef]
- Xi, Z.M.; Chen, B.L. Removal of polycyclic aromatic hydrocarbons from aqueous solution by raw and modified plant residue materials as biosorbents. J. Environ. Sci. 2014, 26, 737–748. [Google Scholar] [CrossRef]
- Kong, H.L.; He, J.; Gao, Y.Z.; Han, J.; Zhu, X.Z. Removal of Polycyclic Aromatic Hydrocarbons from Aqueous Solution on Soybean Stalk-based Carbon. J. Environ. Qual. 2011, 40, 1737–1744. [Google Scholar] [CrossRef]
- Changchaivong, S.; Khaodhiar, S. Adsorption of naphthalene and phenanthrene on dodecylpyridinium-modified bentonite. Appl. Clay Sci. 2009, 43, 317–321. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, S.J. Adsorption of naphthalene by HDTMA modified kaolinite and halloysite. Appl. Clay Sci. 2002, 22, 55–63. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Joshi, M.V.; Jayaram, R.V. Treatment of oil spill by sorption technique using fatty acid grafted sawdust. Chemosphere 2006, 64, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.J.; Yao, J.; Dong, L.F.; Sun, J.J. Adsorption of naphthalene from aqueous solution onto fatty acid modified walnut shells. Chemosphere 2016, 144, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.A.; Amarnath, D.J.; Kumar, P.S.; Kaushik, C.S.; Varghese, M.E.; Saravanan, A. Mass transfer and thermodynamic analysis on the removal of naphthalene from aqueous solution using oleic acid modified palm shell activated carbon. Desalination Water Treat. 2018, 106, 238–250. [Google Scholar] [CrossRef]
- Xu, W.; Hussain, A.; Liu, Y. A review on modification methods of adsorbents for elemental mercury from flue gas. Chem. Eng. J. 2018, 346, 692–711. [Google Scholar] [CrossRef]
- Aryee, A.A.; Mpatani, F.M.; Kani, A.N.; Dovi, E.; Han, R.; Li, Z.; Qu, L. A review on functionalized adsorbents based on peanut husk for the sequestration of pollutants in wastewater: Modification methods and adsorption study. J. Clean. Prod. 2021, 310, 127502. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Vargas, A.M.M.; Nogami, E.M.; Kunita, M.H.; Guilherme, M.R.; Martins, A.C.; Silva, T.L.; Moraes, J.C.G.; Almeida, V.C. NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption. Chem. Eng. J. 2011, 174, 117–125. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef]
- Wahi, R.; Chuah, L.A.; Choong, T.S.Y.; Ngaini, Z.; Nourouzi, M.M. Oil removal from aqueous state by natural fibrous sorbent: An overview. Sep. Purif. Technol. 2013, 113, 51–63. [Google Scholar] [CrossRef]
- Peng, Z.; Gong, X.; Xiong, W.; Wang, Z.; Ren, D. Effect and the Mechanism of Modified Biochar on Adsorption of Naphthalene. J. Ecol. Rural. Environ. 2021, 37, 1080–1088. [Google Scholar]
- Liu, C.C.; Yin, Z.H.; Hu, D.; Mo, F.; Chu, R.Y.; Zhu, L.D.; Hu, C.Z. Biochar derived from chicken manure as a green adsorbent for naphthalene removal. Environ. Sci. Pollut. Res. 2021, 28, 36585–36597. [Google Scholar] [CrossRef]
- Long, C.; Li, A.M.; Wu, H.S.; Zhang, Q.X. Adsorption of naphthalene onto macroporous and hypercrosslinked polymeric adsorbent: Effect of pore structure of adsorbents on thermodynamic and kinetic properties. Colloids Surf. A Physicochem. Eng. Asp. 2009, 333, 150–155. [Google Scholar] [CrossRef]
- Shi, B.; Zuo, W.; Tong, H. Adsorption of polycyclic aromatic hydrocarbons from aqueous solutions using modified bentonite. Chin. J. Environ. Eng. 2015, 9, 1680–1686. [Google Scholar]
- Gan, L.X.; Mao, Y.; Liu, X.; Lin, W. Study on adsorption of naphthalin with modified bentonite. J. Hubei Univ. 2010, 32, 458–462. [Google Scholar]
- Wang, C.; Leng, S.Z.; Xu, Y.; Tian, Q.Y.; Zhang, X.M.; Cao, L.Y.; Huang, J.F. Preparation of Amino Functionalized Hydrophobic Zeolite and Its Adsorption Properties for Chromate and Naphthalene. Minerals 2018, 8, 145. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Lu, J.; Lai, L.; Ni, M.; Mei, W.N.; Li, G.; Nagase, S.; Maeda, Y.; Akasaka, T.; Gao, Z.; et al. Effects of hole doping on selectivity of naphthalene towards single-wall carbon nanotubes. Comput. Mater. Sci. 2007, 40, 354–358. [Google Scholar] [CrossRef]
- Bouiahya, K.; Oulguidoum, A.; Laghzizil, A.; Shalabi, M.; Nunzi, J.M. Hydrophobic chemical surface functionalization of hydroxyapatite nanoparticles for naphthalene removal. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124706. [Google Scholar] [CrossRef]
- Patino-Ruiz, D.A.; De Avila, G.; Alarcon-Suesca, C.; Gonzalez-Delgado, A.D.; Herrera, A. Ionic Cross-Linking Fabrication of Chitosan-Based Beads Modified with FeO and TiO2 Nanoparticles: Adsorption Mechanism toward Naphthalene Removal in Seawater from Cartagena Bay Area. Acs. Omega 2020, 5, 26463–26475. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Tian, F.; Wu, Z.; Yan, Y.; Cravotto, G.; Wu, Z. Adsorption of naphthalene from aqueous solution on coal-based activated carbon modified by microwave induction: Microwave power effects. Chem. Eng. Process. Process. Intensif. 2015, 91, 67–77. [Google Scholar] [CrossRef]
- Shen, B.X.; Li, G.L.; Wang, F.M.; Wang, Y.Y.; He, C.; Zhang, M.; Singh, S. Elemental mercury removal by the modified bio-char from medicinal residues. Chem. Eng. J. 2015, 272, 28–37. [Google Scholar] [CrossRef]
- Alijani, H.; Shariatinia, Z. Synthesis of high growth rate SWCNTs and their magnetite cobalt sulfide nanohybrid as super-adsorbent for mercury removal. Chem. Eng. Res. Des. 2018, 129, 132–149. [Google Scholar] [CrossRef]
- Venkatesh, M.S.; Raghavan, G.S.V. An overview of microwave processing and dielectric properties of agri-food materials. Biosyst. Eng. 2004, 88, 1–18. [Google Scholar] [CrossRef]
- Ge, X.Y.; Ma, X.F.; Wu, Z.S.; Xiao, X.M.; Yan, Y.J. Modification of coal-based activated carbon with nitric acid using microwave radiation for adsorption of phenanthrene and naphthalene. Res. Chem. Intermed. 2015, 41, 7327–7347. [Google Scholar] [CrossRef]
- Ge, X.Y.; Wu, Z.S.; Wu, Z.L.; Yan, Y.J.; Cravotto, G.; Ye, B.C. Enhanced PAHs adsorption using iron-modified coal-based activated carbon via microwave radiation. J. Taiwan Inst. Chem. Eng. 2016, 64, 235–243. [Google Scholar] [CrossRef]
- Wu, Z.S.; Liu, P.Y.; Wu, Z.L.; Cravotto, G. In Situ Modification of Activated Carbons by Oleic Acid under Microwave Heating to Improve Adsorptive Removal of Naphthalene in Aqueous Solutions. Processes 2021, 9, 391. [Google Scholar] [CrossRef]
- Wang, H.; Yang, D.; Xu, Q.; Yuan, H.; Zhou, X.; Wang, W. XAD-2 resin modified by nanosecond pulsed discharge to improve the adsorption capacity of polycyclic aromatic hydrocarbons. J. Phys. D Appl. Phys. 2021, 54, 025202. [Google Scholar] [CrossRef]
- Xu, Q.N.; Yang, D.Z.; Wang, H.L.; Yuan, H.; Jia, Z.X.; Yang, H.; Zhou, X.F.; Wang, W.C.; Xu, Y.; Yan, E.Y. Enhancing the adsorption property of macroporous XAD-2 resin by nanosecond-pulsed discharge plasma modification. Plasma Process. Polym. 2021, 18, 2000117. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, D.Z.; Jia, Z.X.; Zhou, X.F.; Wang, H.L.; Xu, Q.N.; Wang, W.C.; Xu, Y. Activated Carbon Modified by Nanosecond Pulsed Discharge for Polycyclic Aromatic Hydrocarbons Detection. Plasma Chem. Plasma Process. 2020, 40, 1539–1553. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Q.; Zhou, X.; Liang, J.; Yuan, H.; Yang, D.; Wang, W. Highly efficient adsorptive removal of persistent organic pollutants using NPD-acid combined modified NaY zeolites. Chem. Eng. J. 2021, 431, 133858. [Google Scholar] [CrossRef]
- Dou, S.; Tao, L.; Wang, R.L.; El Hankari, S.; Chen, R.; Wang, S.Y. Plasma-Assisted Synthesis and Surface Modification of Electrode Materials for Renewable Energy. Adv. Mater. 2018, 30, 1705850. [Google Scholar] [CrossRef]
| Formula | Structure | λ | Molecular Weight (g/mol) | Melting Point (K) | Boiling Point (K) | Log Kow | Possibly Carcinogenic to Humans |
|---|---|---|---|---|---|---|---|
| C10H8 | 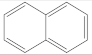 | 254 | 128.16 | 354.15 | 491.05 | 3.36 | 2B |
| Methods | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Biodegradation | Good efficiency Less expensive | Refractory high molecular weight PAHs Long incubation time PH sensitive | [30,31] |
| Photocatalysis | Good efficiency | Toxic products Requires skilled operator | [32] |
| Physical and chemical processes | Good efficiency | High operation cost Corrosion Secondary pollution | [33] |
| Membrane filtration | High removal efficiency | High initial and operating cost Membrane fouling | [34,35] |
| Advanced oxidation processes | High removal efficiency Strong oxidation Easy operation control | High cost Instability High requirements for reaction equipment | [36,37,38] |
| Adsorption | High efficiency Simple operation Low processing cost | Labor intensive Poor removal of fine emulsions | [39,40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Yuan, H.; Wang, H.; Xu, Y.; Yang, D. A Review on Modification Methods of Adsorbents for Naphthalene in Environment. Catalysts 2022, 12, 398. https://doi.org/10.3390/catal12040398
Xu Q, Yuan H, Wang H, Xu Y, Yang D. A Review on Modification Methods of Adsorbents for Naphthalene in Environment. Catalysts. 2022; 12(4):398. https://doi.org/10.3390/catal12040398
Chicago/Turabian StyleXu, Qingnan, Hao Yuan, Hongli Wang, Yong Xu, and Dezheng Yang. 2022. "A Review on Modification Methods of Adsorbents for Naphthalene in Environment" Catalysts 12, no. 4: 398. https://doi.org/10.3390/catal12040398
APA StyleXu, Q., Yuan, H., Wang, H., Xu, Y., & Yang, D. (2022). A Review on Modification Methods of Adsorbents for Naphthalene in Environment. Catalysts, 12(4), 398. https://doi.org/10.3390/catal12040398






