Lignin as a Bio-Sourced Secondary Template for ZSM-5 Zeolite Synthesis
Abstract
:1. Introduction
2. Results and Discussion
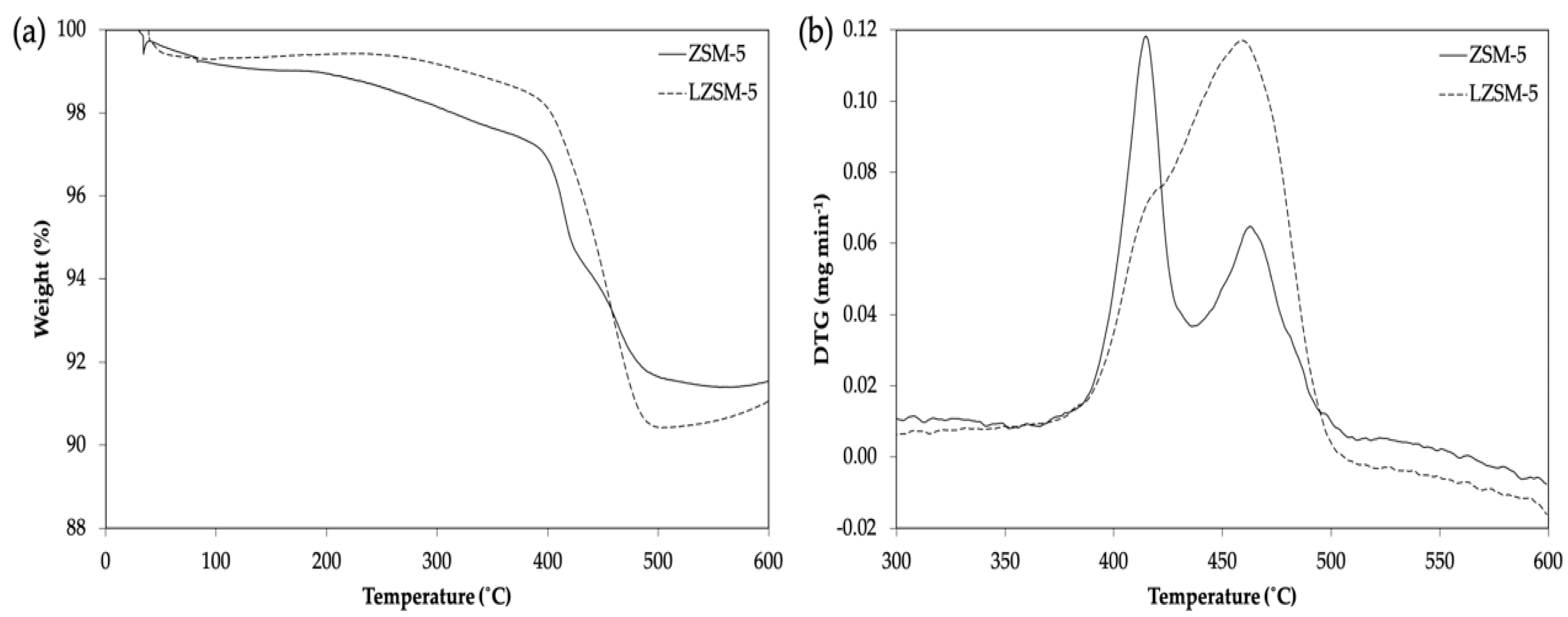
ZSM-5 Prepared Using Lignin as BSST
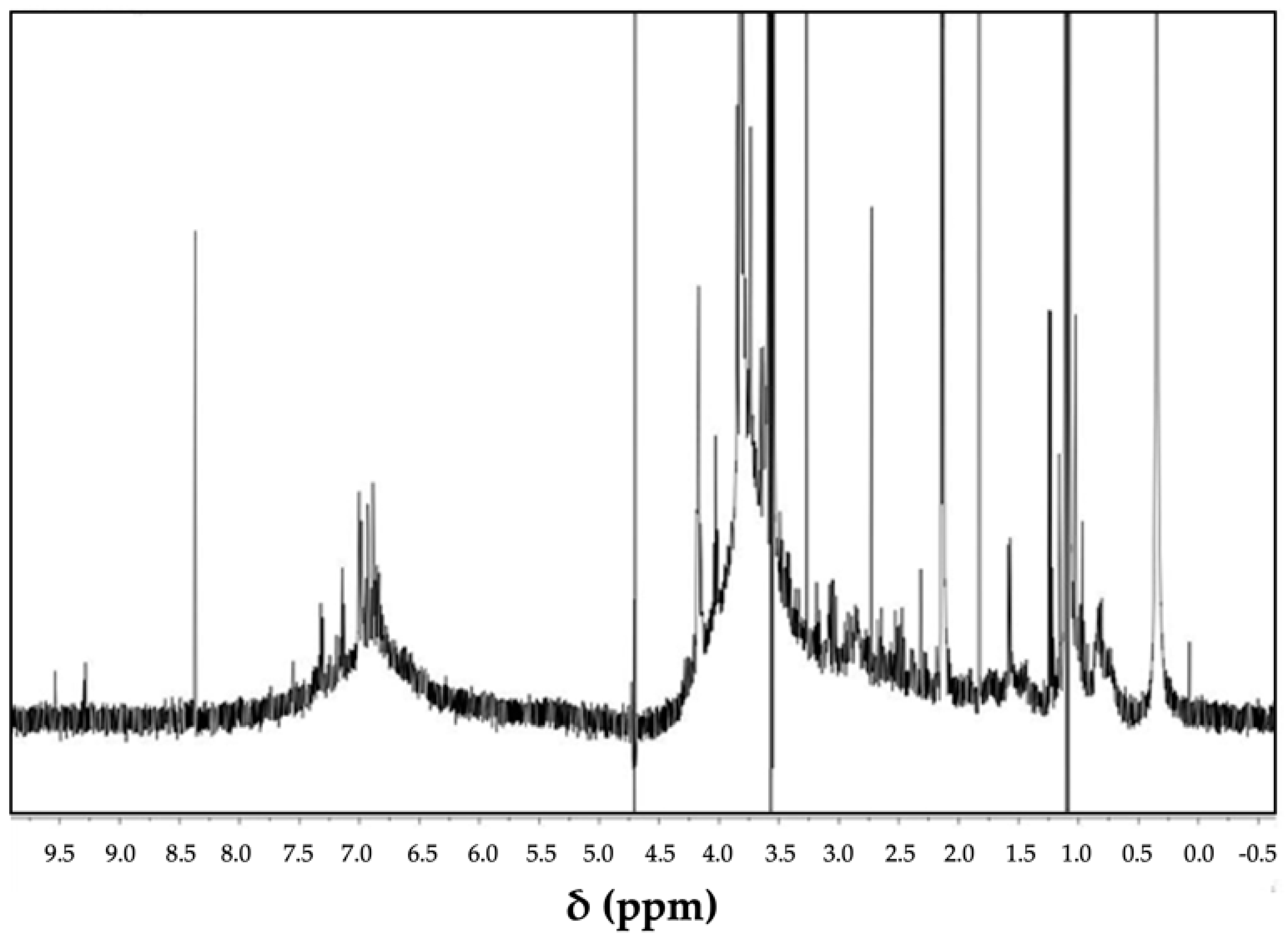
3. State and Role of Lignin
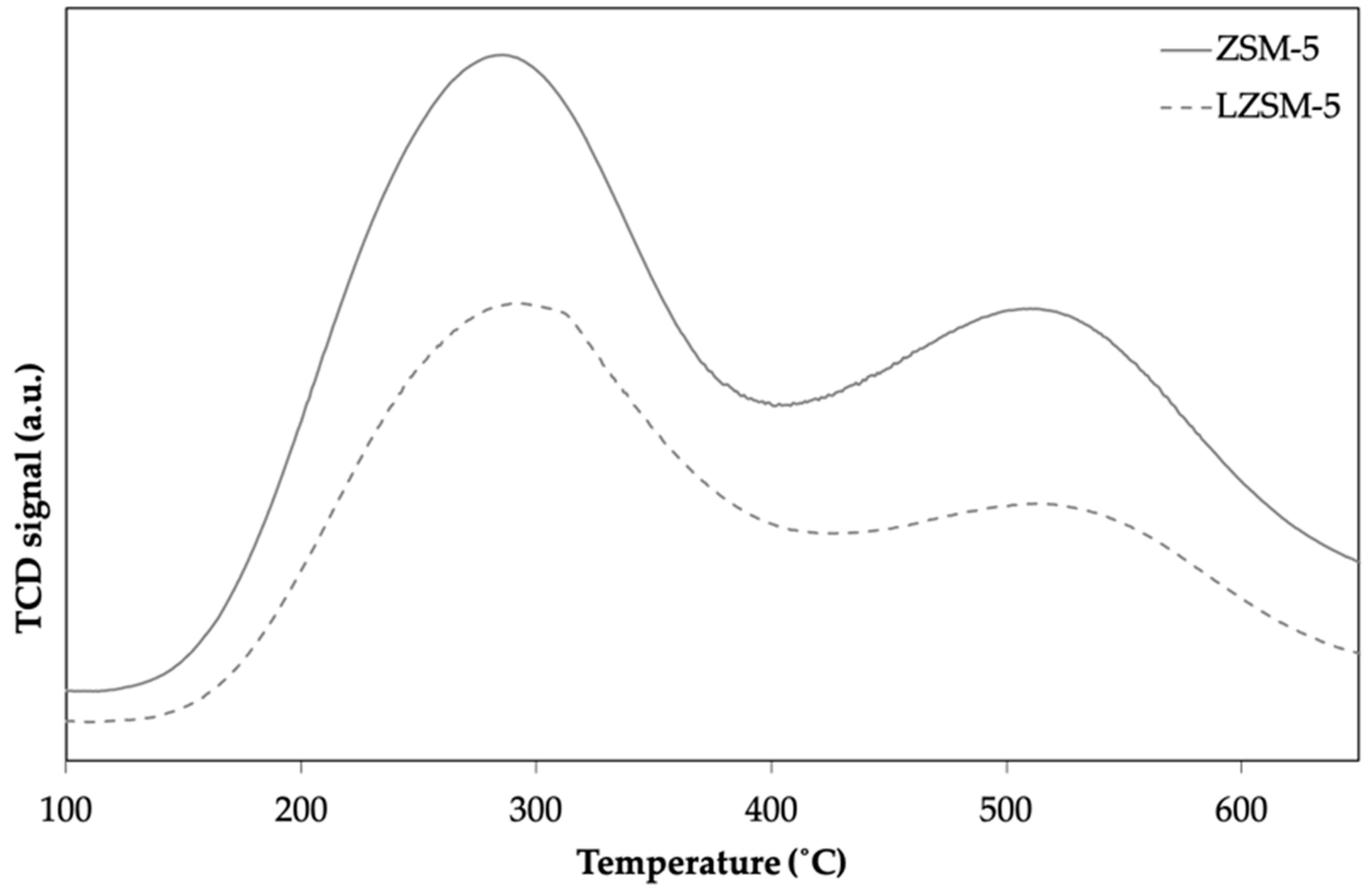
4. Catalyst Evaluation
5. Materials and Methods
ZSM-5 Zeolite Synthesis
6. Catalyst Characterization
7. Catalytic Tests
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luo, Y.; Funke, H.H.; Falconer, J.L.; Noble, R.D. Adsorption of CO2, CH4, C3H8, and H2O in SSZ-13, SAPO-34, and T-Type Zeolites. Ind. Eng. Chem. Res. 2016, 55, 9749–9757. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Bingre, R.; Huang, L.; Lutzweiler, G.; Wang, Q.; Louis, B. CO2 Adsorption Capacities in Zeolites and Layered Double Hydroxide Materials. Front. Chem. 2019, 7, 551. [Google Scholar] [CrossRef] [Green Version]
- Demirci, S.; Ustaoǧlu, Z.; Yilmazer, G.A.; Sahin, F.; Baç, N. Antimicrobial Properties of Zeolite-X and Zeolite-A Ion-Exchanged with Silver, Copper, and Zinc against a Broad Range of Microorganisms. Appl. Biochem. Biotechnol. 2014, 172, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.G.; Schneider, H.; Ferret, L.; Marcilio, N.R.; Oliveira, J.C.P. Potassic Zeolites from Brazilian Coal Ash for Use as a Fertilizer in Agriculture. Waste Manag. 2017, 70, 263–271. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, X.; Font, O.; Moreno, N.; Vallejo, V.R.; Querol, X.; Tobias, A. Synthesis of Merlinoite from Chinese Coal Fly Ashes and Its Potential Utilization as Slow Release K-Fertilizer. J. Hazard. Mater. 2014, 265, 242–252. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.C.; Souza, M.; Esteves, L.M.; Batalha, N.; Lam, Y.L.; Pereira, M.M. Hydroconversion of Xylose Derived Ketals: A Key Strategy for Producing A Broad Range of Green-Hydrocarbons Suitable as Fuels and Petrochemicals. Appl. Catal. A Gen. 2021, 609, 117911. [Google Scholar] [CrossRef]
- Flores, C.; Batalha, N.; Ordomsky, V.V.; Zholobenko, V.L.; Baaziz, W.; Marcilio, N.R.; Khodakov, A.Y. Direct Production of Iso-Paraffins from Syngas over Hierarchical Cobalt-ZSM-5 Nanocomposites Synthetized by Using Carbon Nanotubes as Sacrificial Templates. ChemCatChem 2018, 10, 2291–2299. [Google Scholar] [CrossRef]
- Flores, C.; Batalha, N.; Marcilio, N.R.; Ordomsky, V.V.; Khodakov, A.Y. Influence of Impregnation and Ion Exchange Sequence on Metal Localization, Acidity and Catalytic Performance of Cobalt BEA Zeolite Catalysts in Fischer-Tropsch Synthesis. ChemCatChem 2018, 11, 568–574. [Google Scholar] [CrossRef]
- Van Daele, S.; Minoux, D.; Nesterenko, N.; Maury, S.; Coupard, V.; Valtchev, V.; Travert, A.; Gilson, J.P. A Highly Selective FER-Based Catalyst to Produce n-Butenes from Isobutanol. Appl. Catal. B Environ. 2021, 284, 119699. [Google Scholar] [CrossRef]
- Martínez, C.; Corma, A. Inorganic Molecular Sieves: Preparation, Modification and Industrial Application in Catalytic Processes. Coord. Chem. Rev. 2011, 255, 1558–1580. [Google Scholar] [CrossRef] [Green Version]
- Primo, A.; Garcia, H. Zeolites as Catalysts in Oil Refining. Chem. Soc. Rev. 2014, 43, 7548–7561. [Google Scholar] [CrossRef]
- Weitkamp, J. Zeolites and Catalysis. Solide State Ionics 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Jae, J.; Tompsett, G.A.; Foster, A.J.; Hammond, K.D.; Auerbach, S.M.; Lobo, R.F.; Huber, G.W. Investigation into the Shape Selectivity of Zeolite Catalysts for Biomass Conversion. J. Catal. 2011, 279, 257–268. [Google Scholar] [CrossRef]
- Mores, D.; Kornatowski, J.; Olsbye, U.; Weckhuysen, B.M. Coke Formation during the Methanol-to-Olefin Conversion: In Situ Microspectroscopy on Individual H-ZSM-5 Crystals with Different Brønsted Acidity. Chem. A Eur. J. 2011, 17, 2874–2884. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Wu, W.; Li, G.; Wang, C.; Yang, H.; Zhang, D. Effect of SiO2/Al2O3 Ratio on the Performance of Nanocrystal ZSM-5 Zeolite Catalysts in Methanol to Gasoline Conversion. Appl. Catal. A Gen. 2016, 523, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Shirazi, L.; Jamshidi, E.; Ghasemi, M.R. The Effect of Si/Al Ratio of ZSM-5 Zeolite on Its Morphology, Acidity and Crystal Size. Cryst. Res. Technol. 2008, 43, 1300–1306. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.M.; Pizarro, P. Synthesis Strategies in the Search for Hierarchical Zeolites. Chem. Soc. Rev. 2013, 42, 4004–4035. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Lobo, R.F. Zeolite and Molecular Sieve Synthesis. Chem. Mater. 1992, 4, 756–768. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Bennett, J.M.; Grose, R.W.; Cohen, J.P.; Patton, R.L.; Kirchner, R.M.; Smith, J.V. Silicalite, a New Hydrophobic Crystalline Silica Molecular Sieve. Nature 1978, 271, 512–516. [Google Scholar] [CrossRef]
- Burkett, S.L.; Davis, M.E. Mechanism of Structure Direction in the Synthesis of Si-ZSM-5: An Investigation by Intermolecular 1H-29Si CP MAS NMR. J. Phys. Chem. 1994, 98, 4647–4653. [Google Scholar] [CrossRef]
- Sang, S.; Chang, F.; Liu, Z.; He, C.; He, Y.; Xu, L. Difference of ZSM-5 Zeolites Synthesized with Various Templates. Catal. Today 2004, 93–95, 729–734. [Google Scholar] [CrossRef]
- Meng, X.; Xiao, F.S. Green Routes for Synthesis of Zeolites. Chem. Rev. 2014, 114, 1521–1543. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zones, S.I.; Davis, M.E. A Combustion-Free Methodology for Synthesizing Zeolites and Zeolite-like Materials. Nature 2003, 425, 385–388. [Google Scholar] [CrossRef]
- Grand, J.; Awala, H.; Mintova, S. Mechanism of Zeolites Crystal Growth: New Findings and Open Questions. CrystEngComm 2016, 18, 650–664. [Google Scholar] [CrossRef]
- Maldonado, M.; Oleksiak, M.D.; Chinta, S.; Rimer, J.D. Controlling Crystal Polymorphism in Organic-Free Synthesis of Na-Zeolites. J. Am. Chem. Soc. 2013, 135, 2641–2652. [Google Scholar] [CrossRef]
- Louis, B.; Gomes, E.S.; Losch, P.; Lutzweiler, G.; Coelho, T.; Faro, A.; Pinto, J.F.; Cardoso, C.S.; Silva, A.V.; Pereira, M.M. Biomass-Assisted Zeolite Syntheses as a Tool for Designing New Acid Catalysts. ChemCatChem 2017, 9, 2065–2079. [Google Scholar] [CrossRef]
- Bingre, R.; Sayago, C.M.; Losch, P.; Huang, L.; Wang, Q.; Pereira, M.M.; Louis, B. Recent Progress in the Biomass-Mediated Synthesis of Porous Materials. Inorg. Chim. Acta 2019, 487, 379–386. [Google Scholar] [CrossRef]
- Gomes, E.S.; Lutzweiler, G.; Losch, P.; Silva, A.V.; Bernardon, C.; Parkhomenko, K.; Pereira, M.M.; Louis, B. Strategy to Design Zeolite Catalysts in the Presence of Biomass. Microporous Mesoporous Mater. 2017, 254, 28–36. [Google Scholar] [CrossRef]
- Ocampo, F.; Cunha, J.A.; De Lima Santos, M.R.; Tessonnier, J.P.; Pereira, M.M.; Louis, B. Synthesis of Zeolite Crystals with Unusual Morphology: Application in Acid Catalysis. Appl. Catal. A Gen. 2010, 390, 102–109. [Google Scholar] [CrossRef]
- Gomes, E.S.; Aranda, D.A.G.; Pereira, M.M.; Louis, B. ZSM-5 Synthesis by the Assistance of Biomass and Biomass-Derivate Compounds. Microporous Mesoporous Mater. 2018, 263, 251–256. [Google Scholar] [CrossRef]
- Silva, A.V.; Miranda, L.S.M.; Nele, M.; Louis, B.; Pereira, M.M. Insights to Achieve a Better Control of Silicon-Aluminum Ratio and ZSM-5 Zeolite Crystal Morphology through the Assistance of Biomass. Catalysts 2016, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.O.; Thabet, M.S.; El-Nasser, K.S.; Hassan, A.M.; Salama, T.M. Synthesis of Nanosized ZSM-5 Zeolite from Rice Straw Using Lignin as a Template: Surface-Modified Zeolite with Quaternary Ammonium Cation for Removal of Chromium from Aqueous Solution. Microporous Mesoporous Mater. 2012, 160, 97–105. [Google Scholar] [CrossRef]
- Mintova, S.; Barrier, N. Verified Syntheses of Zeolitic Materials, 3rd ed.; Synthesis Commission of the International Zeolite Association: Amsterdam, The Netherlands, 2016; ISBN 9780692685396. [Google Scholar]
- Burton, A.W.; Zones, S.I. Chapter 5: Organic Molecules in Zeolite Synthesis: Their Preparation and Structure-Directing Effects. Stud. Surf. Sci. Catal. 2007, 68, 137–179. [Google Scholar]
- Peter, A.; Jacobs, J.A.M. Synthesis of ZSM-5 Zeolites in the Presence of Tetrapropylammonium Ions. Stud. Surf. Sci. Catal. 1987, 33, 47–111. [Google Scholar]
- Qian, M.; Lei, H.; Zhao, Y.; Villota, E.; Huo, E.; Wang, C.; Zhang, X. Lignin-Mediated Preparation of Hierarchical ZSM-5 Catalysts and Their Effects in the Catalytic Co-Pyrolysis of Softwood Biomass and Low-Density Polyethylene Mixtures. ACS Sustain. Chem. Eng. 2021, 9, 12602–12613. [Google Scholar] [CrossRef]
- Qian, M.; Lei, H.; Villota, E.; Zhao, Y.; Huo, E.; Wang, C.; Mateo, W.; Zou, R. Enhanced Production of Renewable Aromatic Hydrocarbons for Jet-Fuel from Softwood Biomass and Plastic Waste Using Hierarchical ZSM-5 Modified with Lignin-Assisted Re-Assembly. Energy Convers. Manag. 2021, 236, 114020. [Google Scholar] [CrossRef]
- Van Grieken, R.; Sotelo, J.L.; Menendez, J.M.; Melero, J.A. Anomalous Crystallization Mechanism in the Synthesis of Nanocrystalline ZSM-5. Microporous Mesoporous Mater. 2000, 39, 135–147. [Google Scholar] [CrossRef]
- Pereira, M.M.; Gomes, E.S.; Silva, A.V.; Pinar, A.B.; Willinger, M.G.; Shanmugam, S.; Chizallet, C.; Laugel, G.; Losch, P.; Louis, B. Biomass-Mediated ZSM-5 Zeolite Synthesis: When Self-Assembly Allows to Cross the Si/Al Lower Limit. Chem. Sci. 2018, 9, 6532–6539. [Google Scholar] [CrossRef] [Green Version]
- Alipour, S.M.; Halladj, R.; Askari, S. Effects of the Different Synthetic Parameters on the Crystallinity and Crystal Size of Nanosized ZSM-5 Zeolite. Rev. Chem. Eng. 2014, 30, 289–322. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Fouad, O.A.; Ismail, A.A.; Ibrahim, I.A. Influence of Crystallization Times on the Synthesis of Nanosized ZSM-5. Mater. Lett. 2005, 59, 3441–3444. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Humphreys, J.M.; Chapple, C. Rewriting the Lignin Roadmap. Curr. Opin. Plant Biol. 2002, 5, 224–229. [Google Scholar] [CrossRef]
- Serrano, D.P.; Pinnavaia, T.J.; Aguado, J.; Escola, J.M.; Peral, A.; Villalba, L. Hierarchical ZSM-5 Zeolites Synthesized by Silanization of Protozeolitic Units: Mediating the Mesoporosity Contribution by Chanching the Organosilane Type. Catal. Today 2003, 227, 15–25. [Google Scholar] [CrossRef]
- Serrano, D.P.; Aguado, J.; Escola, J.M.; Peral, A.; Morales, G.; Abella, E. Synthesis of Hierarchical ZSM-5 by Silanization and Alkoxylation of Protozeolitic Units. Catal. Today 2011, 168, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ma, J.; Ren, F.; Du, J.; Li, R. Hierarchical Architectures of ZSM-5 Nanocrystalline Aggregates with Particular Catalysis for Lager Molecule Reaction. Microporous Mesoporous Mater. 2017, 240, 22–30. [Google Scholar] [CrossRef]
- Parker, L.M.; Bibby, D.M.; Patterson, J.E. Thermal Decomposition of ZSM-5 and Silicalite Precursors. Zeolites 1984, 4, 168–174. [Google Scholar] [CrossRef]
- Ház, A.; Jablonský, M.; Šurina, I.; Kačík, F.; Bubeníková, T.; Ďurkovič, J. Chemical Composition and Thermal Behavior of Kraft Lignins. Forests 2019, 10, 483. [Google Scholar] [CrossRef] [Green Version]
- El Moustaqim, M.; El Kaihal, A.; El Marouani, M.; Men-La-Yakhaf, S.; Taibi, M.; Sebbahi, S.; El Hajjaji, S.; Kifani-Sahban, F. Thermal and Thermomechanical Analyses of Lignin. Sustain. Chem. Pharm. 2018, 9, 63–68. [Google Scholar] [CrossRef]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal Degradation of Softwood Lignin and Hardwood Lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chiang, A.S.T.; Selvin, R.; Thompson, R.W. Rapid Synthesis of MFI Zeolite Nanocrystals. J. Phys. Chem. B 2005, 109, 18804–18814. [Google Scholar] [CrossRef]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and Characterization of Lignin from Different Biomass Resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Katada, N.; Igi, H.; Kim, J.H.; Niwa, M. Determination of the Acidic Properties of Zeolite by Theoretical Analysis of Temperature-Programmed Desorption of Ammonia Based on Adsorption Equilibrium. J. Phys. Chem. B 1997, 101, 5969–5977. [Google Scholar] [CrossRef]
- Sato, H. Acidity Control and Catalysis of Pentasil Zeolites. Catal. Rev. 1997, 39, 395–424. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, H.; Yan, W. Effect of Ethanol on the Crystallinity and Acid Sites of MFI Zeolite Nanosheets. RSC Adv. 2014, 4, 56938–56944. [Google Scholar] [CrossRef]
- Lónyi, F.; Valyon, J. On the interpretation of the NH3-TPD patterns of H-ZSM-5 and H-mordenite. Microporous Mesoporous Mater. 2001, 47, 293–301. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.C.; Hu, H.Q.; Xie, F.J.; Wei, X.Y.; Fan, X. Structural Characterization of Lignin and Its Degradation Products with Spectroscopic Methods. J. Spectrosc. 2017, 2017, 8951658. [Google Scholar] [CrossRef] [Green Version]
- García, A.; Toledano, A.; Serrano, L.; Egüés, I.; González, M.; Marín, F.; Labidi, J. Characterization of Lignins Obtained by Selective Precipitation. Sep. Purif. Technol. 2009, 68, 193–198. [Google Scholar] [CrossRef]
- Diop, A.; Jradi, K.; Daneault, C.; Montplaisir, D. Kraft Lignin Depolymerization in an Ionic Liquid without a Catalyst. BioResources 2015, 10, 4933–4946. [Google Scholar] [CrossRef]
- Modenbach, A.A. Effects of Sodium Hydroxide Pretreatment on Structural Components of Biomass. Biosyst. Agricutural Eng. 2014, 57, 1187–1198. [Google Scholar] [CrossRef]
- Dahl, I.M.; Kolboe, S. On the Reaction Mechanism for Hydrocarbon Formation from Methanol over SAPO-34. I. Isotopic Labeling Studies of the Co-Reaction of Ethene and Methanol. J. Catal. 1994, 149, 458–464. [Google Scholar] [CrossRef]
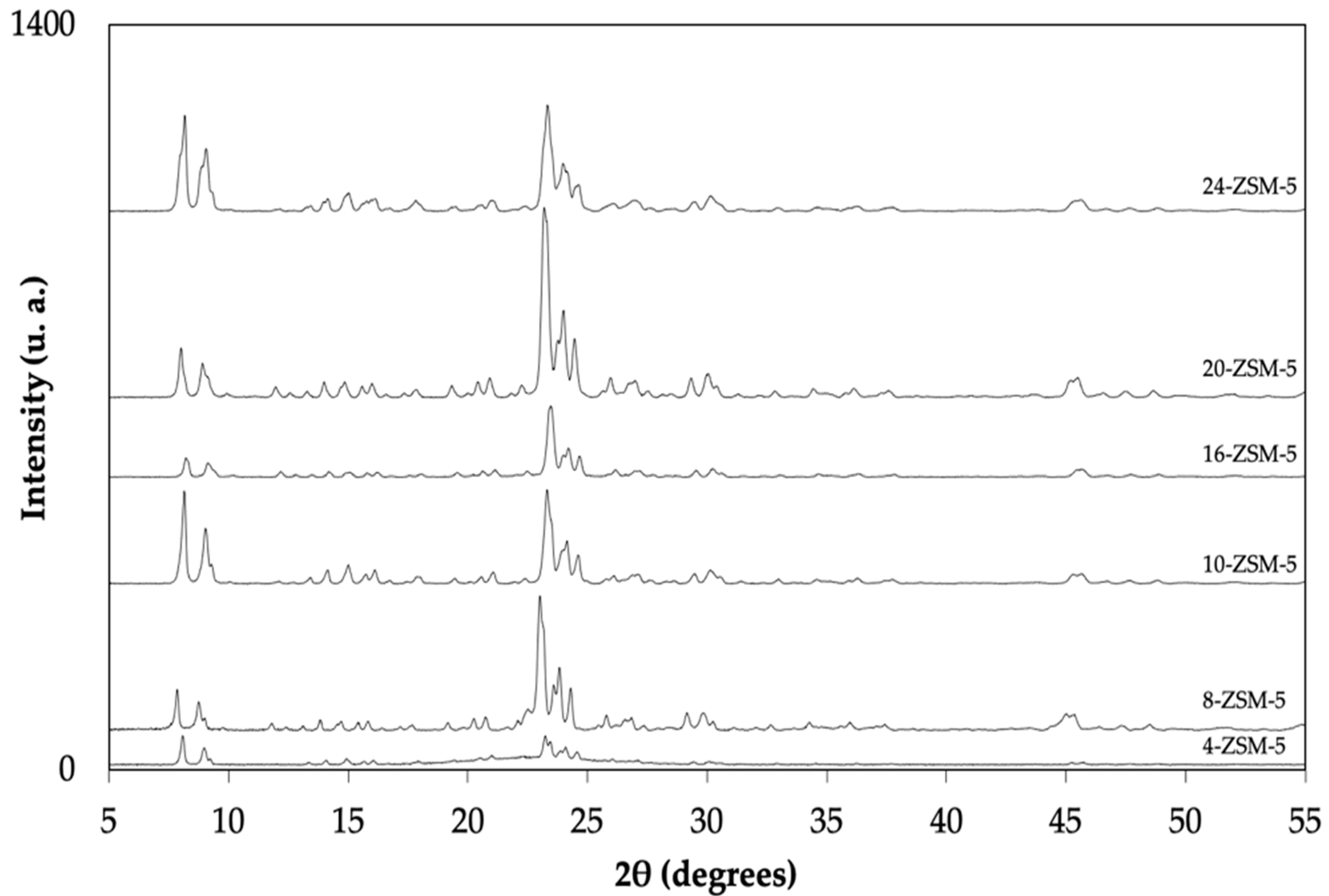
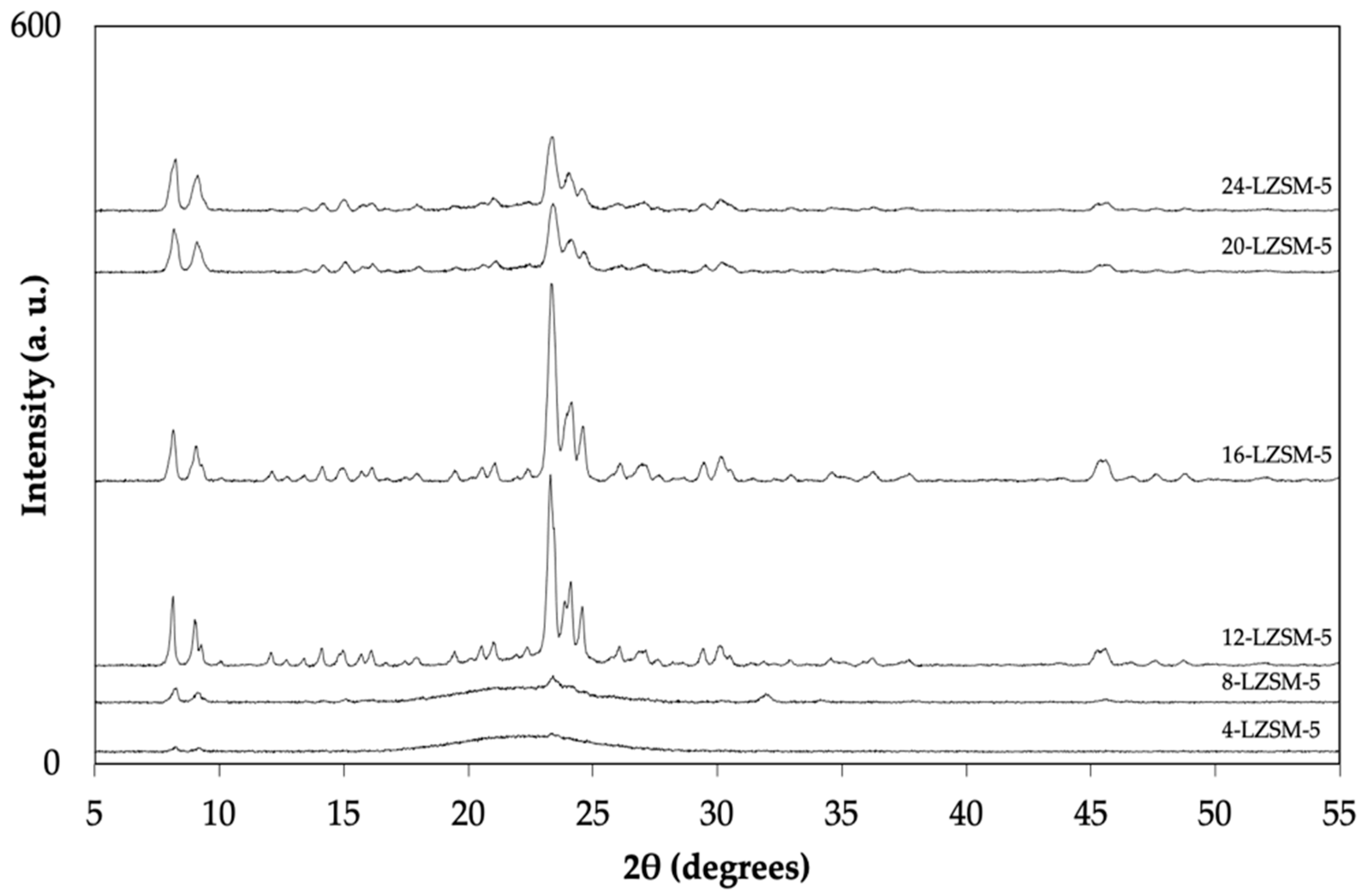
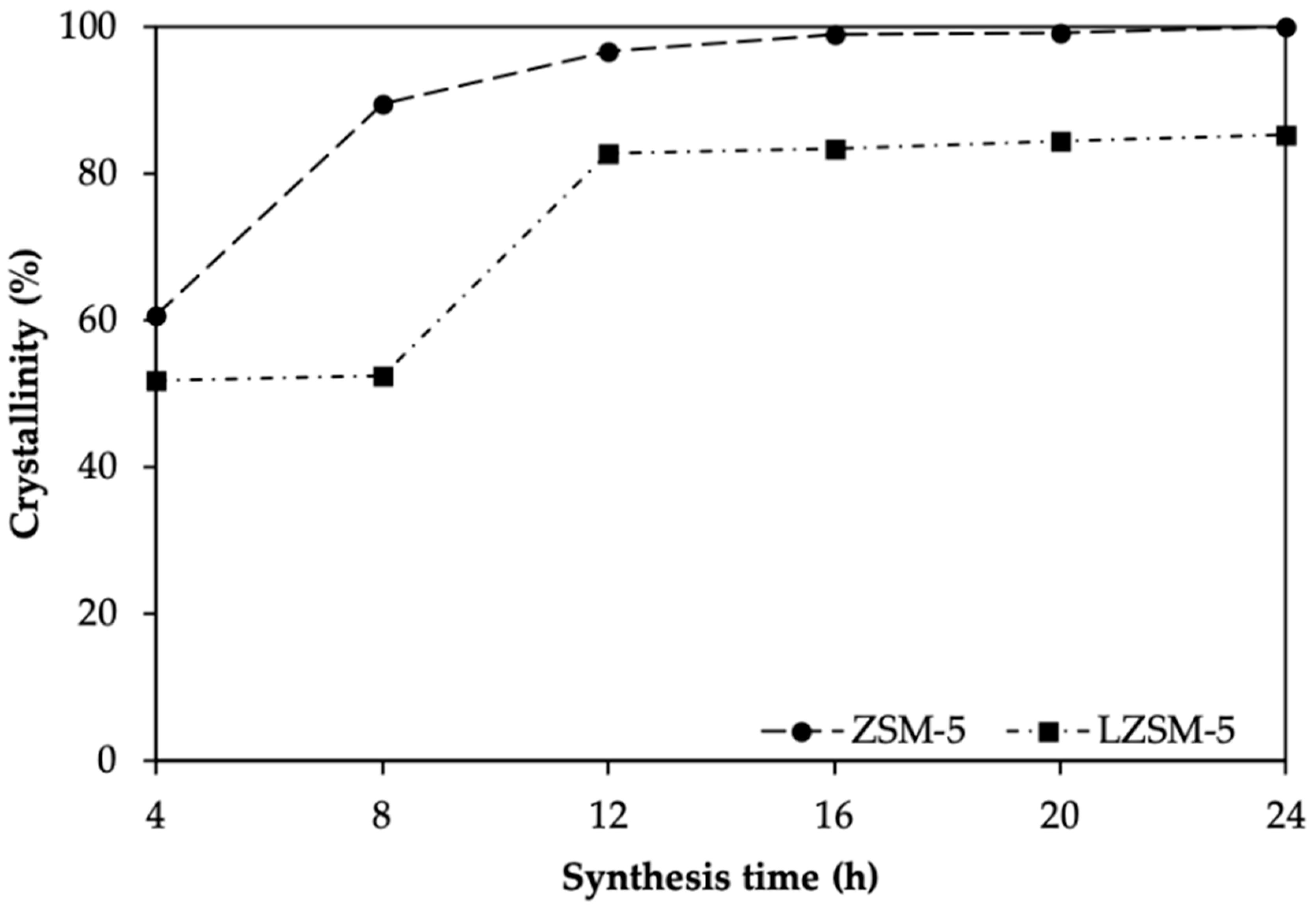
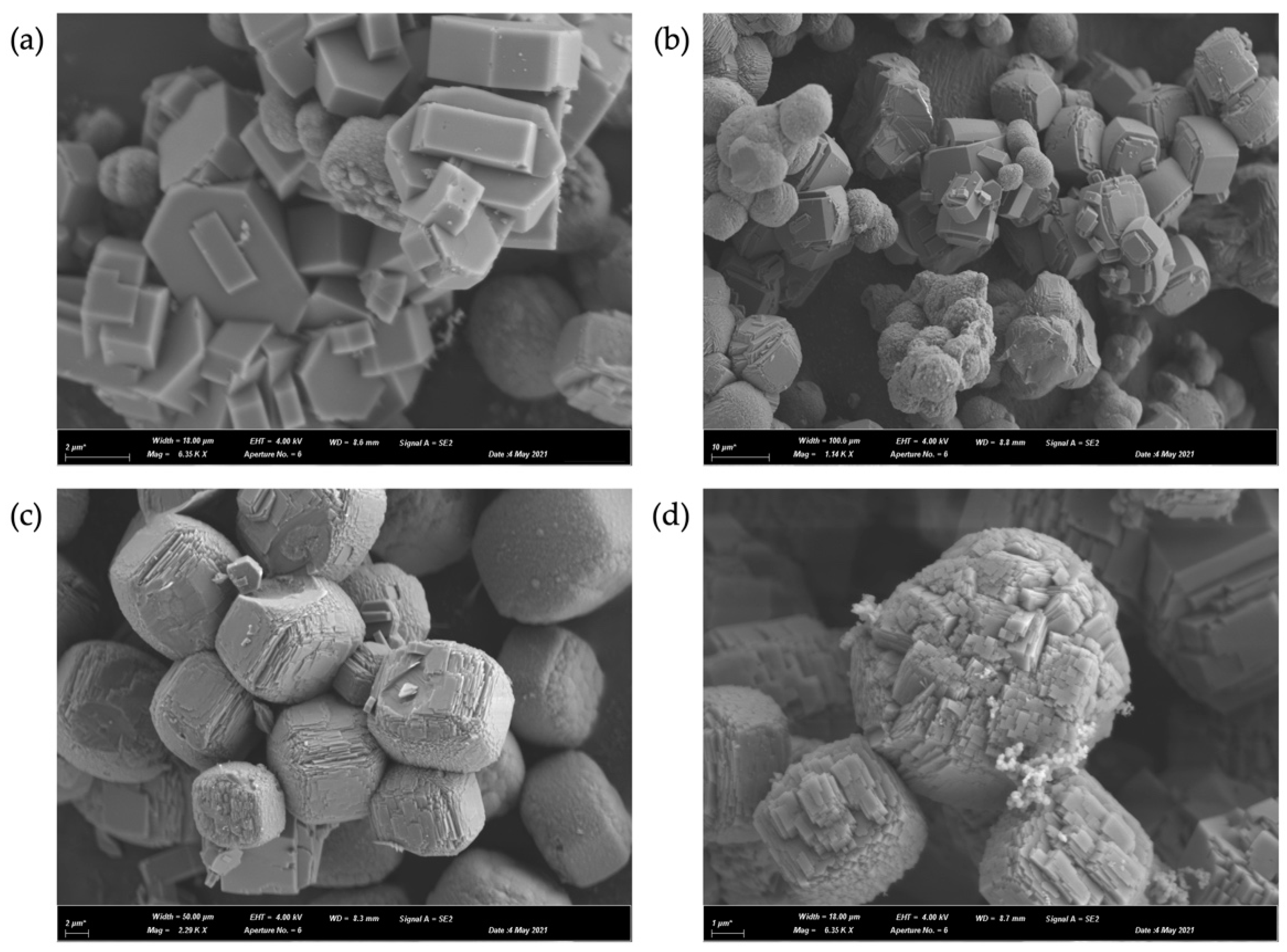
| Sample | SBET a (m2 g−1) | Vtotal b (cm3 g−1) | Vmicro c (cm3 g−1) | Relative Crystallinity d (%) |
|---|---|---|---|---|
| 4-ZSM-5 | 192 | 0.917 | 0.012 | 61 |
| 8-ZSM-5 | 143 | 0.098 | 0.044 | 90 |
| 12-ZSM-5 | 357 | 0.207 | 0.115 | 97 |
| 16-ZSM-5 | 387 | 0.211 | 0.123 | 99 |
| 20-ZSM-5 | 368 | 0.192 | 0.114 | 99 |
| 24-ZSM-5 | 348 | 0.184 | 0.110 | 100 |
| 4-LZSM-5 | 46 | 0.050 | 0.009 | 52 |
| 8-LZSM-5 | 86 | 0.450 | 0.004 | 53 |
| 12-LZSM-5 | 197 | 0.110 | 0.060 | 83 |
| 16-LZSM-5 | 318 | 0.180 | 0.102 | 77 |
| 20-LZSM-5 | 352 | 0.120 | 0.052 | 85 |
| 24-LZSM-5 | 334 | 0.110 | 0.052 | 85 |
| Sample | Si/Al | Acidity (mmol g−1) | ||
|---|---|---|---|---|
| Total | Weak | Strong | ||
| 20-ZSM-5 | 24 | 3.14 | 2.44 | 0.70 |
| 20-LZSM-5 | 28 | 1.07 | 0.96 | 0.11 |
| Catalyst | Conversion (%) | Selectivity (%) | kd (h−1) | |||||
|---|---|---|---|---|---|---|---|---|
| C1 | C2 = C4 | C3 | i-C4 | DME | C5+ | |||
| 12-ZSM-5 | 65 | - | 4 | 3 | - | 91 | 2 | 0.161 |
| 12-LZSM-5 | 57 | - | 7 | 7 | 85 | 1 | 0.205 | |
| 16-ZSM-5 | 50 | - | 29 | 26 | - | 20 | 25 | 0.020 |
| 16-LZSM-5 | 61 | - | 3 | 4 | 92 | 1 | 0.013 | |
| 20-ZSM-5 | 100 | - | 31 | 25 | 13 | 1 | 29 | 0.026 |
| 20-LZSM-5 | 81 | - | 18 | 10 | 68 | 4 | 0.287 | |
| 24-ZSM-5 | 100 | 2 | 44 | 46 | 3 | - | 5 | * |
| 24-LZSM-5 | 100 | - | 31 | 25 | 12 | 16 | 17 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, C.G.; Schneider, H.; Louis, B. Lignin as a Bio-Sourced Secondary Template for ZSM-5 Zeolite Synthesis. Catalysts 2022, 12, 368. https://doi.org/10.3390/catal12040368
Flores CG, Schneider H, Louis B. Lignin as a Bio-Sourced Secondary Template for ZSM-5 Zeolite Synthesis. Catalysts. 2022; 12(4):368. https://doi.org/10.3390/catal12040368
Chicago/Turabian StyleFlores, Camila Gomes, Helena Schneider, and Benoit Louis. 2022. "Lignin as a Bio-Sourced Secondary Template for ZSM-5 Zeolite Synthesis" Catalysts 12, no. 4: 368. https://doi.org/10.3390/catal12040368
APA StyleFlores, C. G., Schneider, H., & Louis, B. (2022). Lignin as a Bio-Sourced Secondary Template for ZSM-5 Zeolite Synthesis. Catalysts, 12(4), 368. https://doi.org/10.3390/catal12040368







