Abstract
Direct carboxylation of thiophene with CO2 has been achieved under a relatively mild solvent-free carbonate and carboxylate medium. This base-mediated medium can cleave the very weakly acidic C–H bond without using other limiting reagents, which is one indispensable step in the carboxylation reaction. Product yield varies with different carboxylate salts, and cesium pivalate is the most suitable base additive among targeted simple carboxylate salts. Furthermore, the detailed mechanism of this carboxylation reaction is studied, which involves initial proton abstraction, rendered by carbonate and C–C bond formation, by inserting CO2. The activation energy barrier of the C–H activation step is higher than the following CO2 insertion step, whether for the formation of the mono- and/or di-carboxylate, which indicates that the C–H deprotonation induced by the base is slow and the resulting carbon-centered nucleophile reacts rapidly with CO2.
1. Introduction
Direct conversion with CO2 into high-valued carboxylic acids is an economic and environmental strategy because CO2 is regarded as the abundant, non-toxic, and renewable C1 source [1,2]. Among various carboxylation routes, C–H carboxylation with unactivated arenes has attracted enormous attention because of the high atom economy and potential commercial value, although certain limitations for this carboxylation pathway have not been addressed effectively [3]. Thermodynamical stability of CO2 and the exceptional inertness of unactivated arenes result in the difficulty of C–H carboxylation under moderate conditions [3,4]. Specific limiting reagents should be employed in the carboxylation system to enhance the inactive reactivity, such as Lewis acid [5,6,7,8,9,10,11,12], carbene metal complexes [13,14] or alkali metal tert-butoxides [15,16,17,18,19], and transition metal catalysis [20,21], but the majority of these metal additives are expensive, non-renewable, or unstable in the air environment. Although relatively high product yield can be obtained through consuming these reagents, a more environment-friendly and effective synthetic route should be developed to prevent the above issues.
In addition to the aforementioned imperfect limiting reagents, carbonate salt can achieve the carboxylation of aromatic compounds through cleaving C–H bond(s) in the solvent or solvent-free environment [22,23,24,25]. However, this carboxylation strategy is ineffective once the pKa value of aromatic substrate exceeds 27 [22]. In another solvent-free condition, aromatic substrate with weaker acidic C–H bonds, such as cesium furan-2-carboxylate (pKa ≈ 35), can achieve carboxylation at 195–260 °C, through abstracting proton and reacting with CO2 in the molten salt CO2 interface [23]. Apart from these solid reaction mixtures, more challenging liquid-phase benzene (pKa > 40) [26] can also be converted into carboxylic acids in the presence of cesium carbonate, but the additional co-salt of cesium isobutyrate is indispensable to provide the necessary molten phase [23]. However, an extremely high reaction temperature (340–380 °C) and pressure (70 bar) are required in this base-mediated system, and the combined yield of carboxylic acids is relatively poor due to the chemical inertness of benzene and its lower solubility in this molten salt.
It is also very urgent work to realize the direct carboxylation of thiophene (pKa ≈ 32.5) [26] with CO2 to produce the thiophene carboxylic acid products, due to its difficulty in C–H deprotonation [22,27], because most of the thiophene are broken as organic sulfur impurities in the coking process and are not reasonably utilized. The properties of thiophene and benzene are very similar, so it is a substitute for benzene in many fields. Inspired by benzene carboxylation in the solvent-free cesium salt medium [23], direct carboxylation of thiophene can also be explored in a similar medium with a combination of cesium carbonate and carboxylate. The carboxylate species can affect the reaction efficiency of the hydrogenation of CO2 into multicarbon carboxylates in the carbonate system [28]. However, the serial of carboxylate additives have not been selected in the carboxylation of substrate benzene [23]. Therefore, the effective carboxylate salt should be determined in this carboxylation pathway, which might affect the rate of proton abstraction. Simple cesium or potassium carboxylates, such as acetate, can be prioritized in this base-catalyzed experiment because of their simple chemical structure and inexpensive price. In addition, cesium or potassium pivalate co-salts can also be used in this reaction because the pivalate anion is capable of activating the inert C–H bond in the proton abstraction route [29,30,31].
In this paper, we develop the direct carboxylation of thiophene with CO2 under a base-mediated system with carbonate and carboxylate salts. The combination of carbonate and simple carboxylate exhibits the synergetic effect in this carboxylation reaction, and cesium pivalate is the most effective base additive among these carboxylate salts. A feasible carboxylation mechanism, involving initial proton abstraction and C–C bond formation by inserting CO2, is proposed.
2. Results and Discussions
2.1. Carboxylation Reaction in the Carbonate/Carboxylate Mixed Salts
A direct thiophene carboxylation reaction is performed in the solvent-free salt containing the carbonate M2CO3 (M = Cs or K) and the correspondingly concomitant carboxylate (Cs or K). Table 1 elucidates the feasibility of this small-scale C–H carboxylation reaction. Initially taking the cesium acetate as an illustrating example, only the thiophene-2-carboxylate product is obtained at a reaction temperature of 200 °C and the thiophene-2, 5-dicarboxylate product is obtained at a reaction temperature of over 220 °C. The corresponding carboxylate yield gradually improves as the temperature increases, and the maximum carboxylate yield (4.98%) is reached at a temperature of 300 °C, in which 152.96 μmol/g of carboxylates are generated per gram of the carbonate as the ratio of monocarboxylate to dicarboxylate is 1:3.5. A decreased carboxylate yield is observed when the temperature exceeds 300 °C, probably due to the thermal decomposition of the carboxylate products and mixed salts. Among these alternatively assisted carboxylate bases, the improvement effect of the carboxylation reaction changes with the variation of the carboxylate salts. As observed in Table 1, the stronger the alkalinity of carboxylate, the better the reaction effect. There is no doubt that the cesium pivalate system, owing to the fact it has the strongest alkalinity, has the best reaction effect among these alternative carboxylates. Besides, note that there are no products generations as CO2 is replaced by nitrogen, which means that the carboxylic acid anion in the carboxylate product comes from the CO2. Furthermore, when the auxiliary base remains unchanged, the reaction effect of potassium carbonate is also less than that of cesium carbonate since the former is less basic than the latter. Therefore, based on the above results, we can draw the following conclusions: that the more electron-donating groups in the auxiliary base, the stronger its basicity, and the better the reaction result when the carbonate remains unchanged; that the stronger the alkalinity of the carbonate, when the assisted carboxylate salt remains constant, the better the reaction effect.

Table 1.
Summary reaction results for thiophene carboxylation as the function of temperature and salts.
As seen in Table 2, the product yield increases at the CO2 pressure variation of 2 bar to 8 bar, and then decreases (Entry 1~5). The substrate thiophene amount is also influenced by the product yield, and the optimal is 10 mmol (Entry 6~9). When the carbonate proportion in the mixed salts changes, the corresponding yield also varies, and the appropriate carbonate proportion is 40% (Entry 10~12).

Table 2.
The effects of various factors on the product yield and selectivity in the Cs2CO3/CsOPiv mixtures.
2.2. Reaction Mechanism of Cs2CO3-Promoted C–H Carboxylation with CO2
All the density functional theory (DFT) calculations were performed via the quantum chemistry package of Gaussian 09 (Rev.D01) suites [32]. Geometric optimizations and frequency calculations for all referred structures of reactants or reactant complexes, intermediates and transition states, and products or product complexes were carried out at the B3LYP-D3(BJ) theory level with the mixed basis set, including the Stuttgart effective core potential SDD [33] for Cs atom and triple-ζ split-valence 6-311G** for all other atoms. The electronic single-point energies were computed by the M06-2X theory level with the def2-TZVPP basis set for the above obtained structures.
This reaction mainly consists of two consecutive steps for the formation of presumed C4 carbanion through the cleavage of the C–H bond(s) via the strong base CO32− and the C4 nucleophile attacking the weak electrophile CO2 to form the C–C bond(s). The counter-cation Cs+ is considered in this present study, owing to the inherent instability of the formed carbanion in the gas phase [34]. Therefore, we used cesium carbonate Cs2CO3, as a whole, as a catalyst to demonstrate the detailed reaction mechanism.
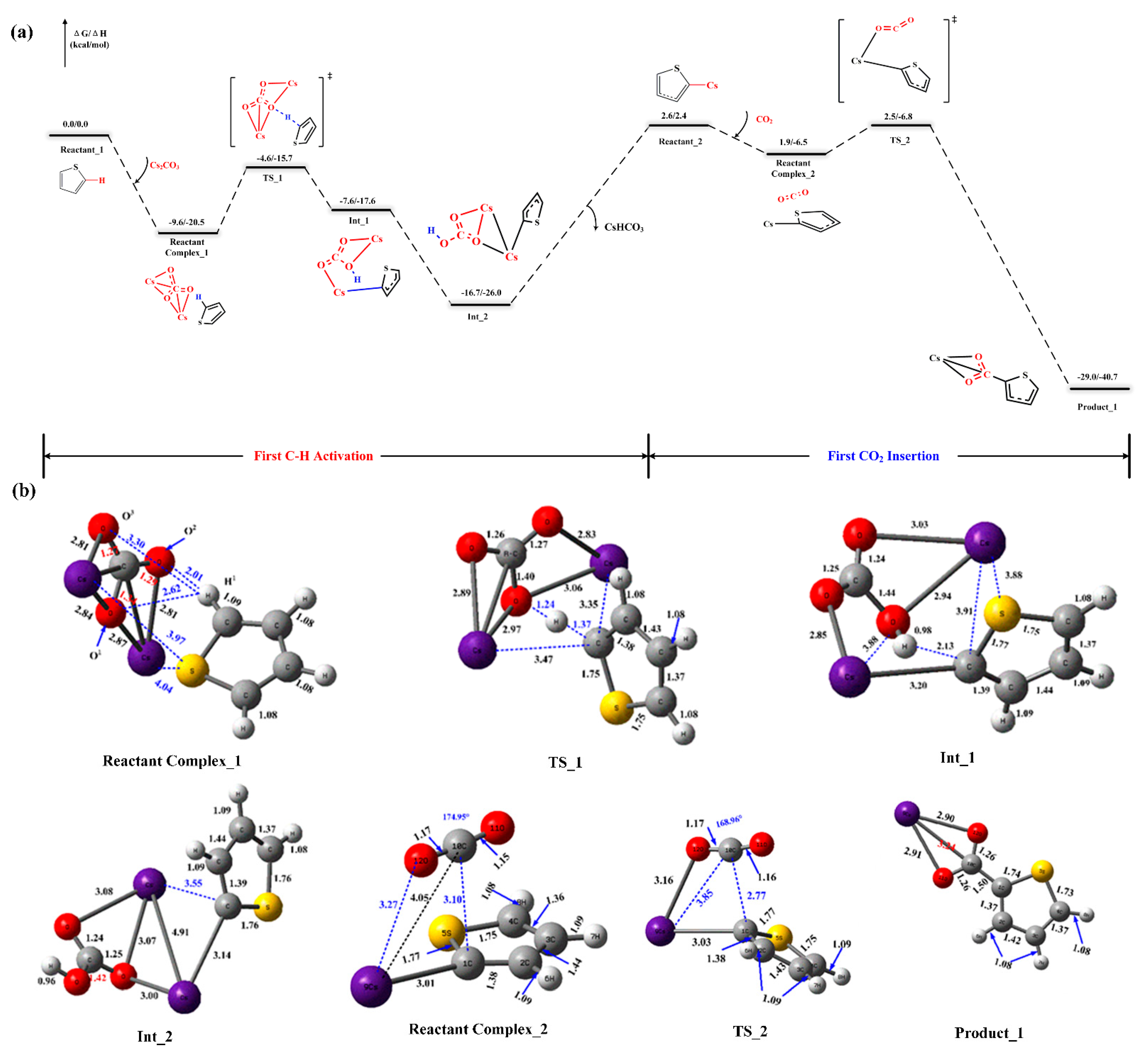
The addition of the cesium carbonate Cs2CO3 into the thiophene system leads to the formation of the stable reactant intermediate Reactant Complex_1 with 9.6 kcal/mol exergonic (Figure 1a). Illustrated in the geometric parameters of this reactant complex, Figure 1b shows that the distances between H1 of thiophene and O1 and O2 of Cs2CO3 are within the hydrogen bond range [35] which is, respectively, 2.62 Å and 2.01 Å. The NPA charge for these two oxygen atoms is, respectively, −1.014 (a.u.) and −0.912 (a.u.). Therefore, as concluded from these two insights, the atom O1 in Cs2CO3 possibly acts as a proton acceptor, which is demonstrated by the structure of the transition state TS_1. In TS_1, the activated C–H bond is elongated from 1.09 Å to 1.37 Å, while it is shortened from 2.62 Å to 1.24 Å for the O1-H1 bond. Meanwhile, the distance between the counter-cation Cs+ and the carbanion is also gradually decreased. Considering the energy of the reactant compound as the reference, the activation barrier is 11.9 kcal/mol in the thiophene deprotonation step. The gradual increasing of the distance in the C–H bond results in producing the less thermodynamically stable intermediate Int_1. The more stable intermediate Int_2 is then obtained after the isomerization. Meanwhile, the bicarbonate is thermally unstable, and it will undergo the thermal decomposition (175 °C) into the carbonate. Therefore, in this reaction mechanism, the detailed decomposition steps are not considered, although the onset temperature of this carboxylation reaction is higher than the thermal decomposition temperature of the bicarbonate. In order to more clearly judge the difference in the activation energy barrier between the key steps in this reaction process, we simply took the energy difference between the generated nucleophile and the bicarbonate as the starting point for the next step. The elimination of the cesium bicarbonate from the intermediate Int_2 leads to the formation of the complex Reactant_2 with the metal–nucleophile bond, which requires the Gibbs free energy of 19.3 kcal/mol.

Figure 1.
(a) Reaction energy profiles of the first carboxylation reaction. Energies are relative to the reactants of Reactant_1 and Cs2CO3. (b) All reported structures and their important geometric parameters (distances are in Angstroms (Å)).
Figure 1a also provides detailed mechanistic insights into the step where the nucleophile attacks the CO2. The stable compound, Reactant Complex_2, is generated with 0.7 kcal/mol exergonic under the comprehensive interaction of the nucleophile with the CO2 in the presence of counter-cation Cs+. These interactions lead to a change in the bond angle of CO2 (180° to 174.95°) and the bond length of the C–C bond (Figure 1b). The corresponding transition state structure TS_2 is formed when the carbanion gradually attacks the CO2, wherein the distance between the C-center of the CO2 and nucleophile C of the thiophene ring is shortened from 3.10 Å to 2.77 Å, and the corresponding bond angle is bent to 168.96°. Considering the energy of the compound Reactant Complex_2 as the reference, the activation barrier is 0.8 kcal/mol in this CO2 insertion step. Gradually reducing the distance in the activated C–C bond results in producing a more thermodynamically stable product (Product_1).
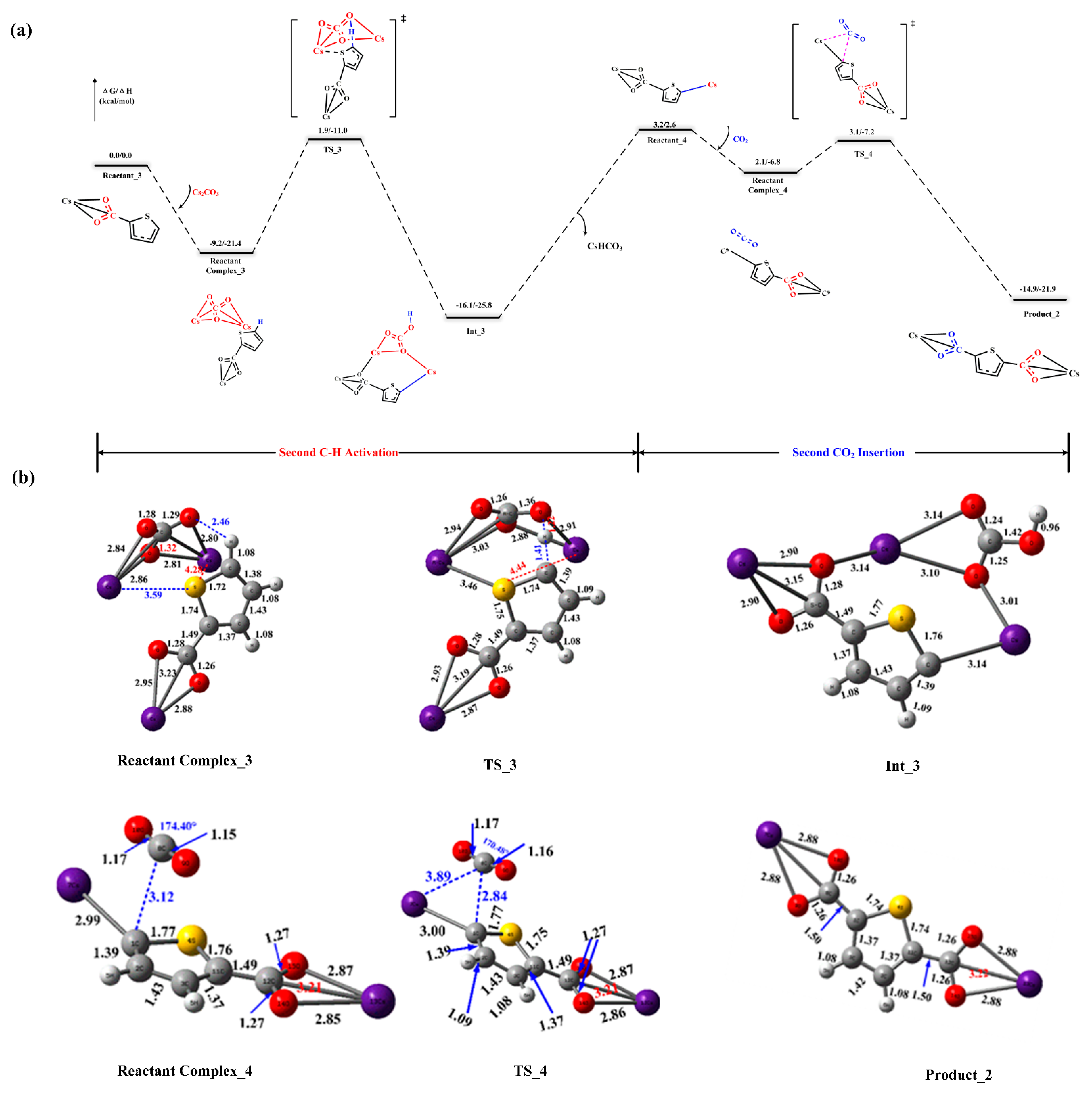
Figure 2a also provides mechanistic insights into how monocarboxylate carboxylation produces dicarboxylate, which are the similar to the aforementioned steps. Note that the difference between the second and the first C–H abstraction step is that there are interactions between the counter-cation Cs+ and heteroatom S in the transition state structure, which will strengthen the acidity of C–H bond and reduce the height of energy barrier. And the corresponding interaction analysis results are shown in Figure S3 of Supplementary Materials. Figure 2b presents these, paired with the geometric structure parameters of the reactant complexes, transition state, and intermediates. Considering the reactant compound Reactant Complex_3 and Reactant_Complex_4 as the reference, the activation barrier is 18.3 kcal/mol and 1.0 kcal/mol in this deprotonation and CO2 insertion step, respectively. We found that the energy barrier of the C–H activation step is higher than that of the following CO2 insertion step, whether for the formation of the mono- and/or di-carboxylate. In other words, C–H deprotonation induced by the base is slow and the resulting carbon-centered nucleophile reacts rapidly with CO2.

Figure 2.
(a) Reaction energy profiles of the second carboxylation reaction. Energies are relative to the reactants of Reactant_3 and Cs2CO3. (b) All reported structures and their important geometric parameters (distances are in Angstroms (Å)).
2.3. Carboxylate-Assisted Cs2CO3-Mediated C–H Carboxylation with CO2
The chemical reactivity is different with the aid of alternative carboxylates, according to the aforementioned experimental results. The substitution of cesium acetate for cesium formate increases the carboxylate yield by 5.8 times, while, for cesium pivalate (CsOPiv), the yield increases by a factor of 9.7 in the small-scale reaction processes. There are also some differences in the selectivity of reaction products for acetate and pivalate. As a result, in this section, we will investigate the roles of assisted carboxylate(s) in the stoichiometric reaction of carbonate and thiophene.
As indicated from the mechanistic insights, the cleavage of the C–H bond is the rate-limiting step in this reaction process, as well as the (regio)selectivity-determined step. Therefore, as long as we can find the difference in the C–H activation step(s) of different carboxylates, the reason for the difference in the effects of alternative salts can be identified. The NPA charge difference in the intermediate Reactant Complex_1 between the proton acceptor and proton donor is 1.204 (a.u.), 1.229 (a.u.) and 1.231 (a.u.), respectively, for the formate, acetate, and pivalate-assisted Cs2CO3 systems, which indicates that the cesium formate-assisted system is the least capable of deprotonation, that is, the activation energy barrier in the proton abstraction step could be the highest. This can also explain why its reaction effect is poorer than the others. The deprotonation pathways of the cesium carbonate system, aided by several carboxylate salts, are explored in mechanism to clearly demonstrate this point of view.
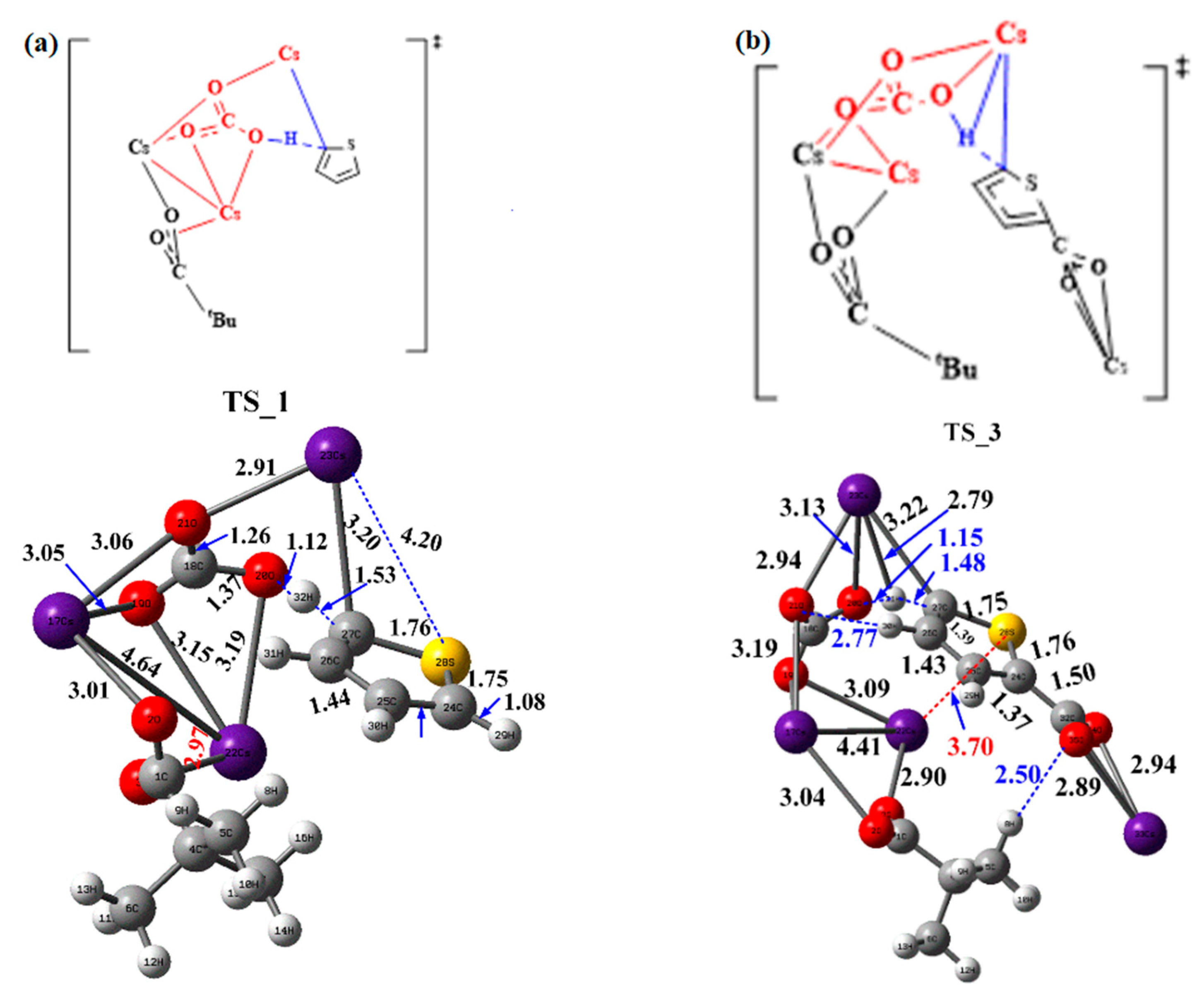
The summary of the results of the alternative carboxylate-assisted proton abstraction paths is enumerated in Table 3. The free energy barriers in the breaking of the C–H bond pathway of the carboxylate-assisted Cs2CO3 systems are all greater than those of the bare cesium carbonate process, and the difference in the activation energy barrier of these two breaking C–H bond steps is smaller than that of the bare Cs2CO3 process. The largest activation energy barrier occurs in the rate-determining step for formate among these three alternative salts, leading to the transition state shifting to the later stage, and the corresponding results are in line with that of the NPA charge difference. The most different point is that the free energy barrier in the cleavage of the C–H bond of thiophene is higher than that of monocarboxylate, indicating that the rate-determining step is the C–H activation step in thiophene. This is the reason why most of the resultant compounds are the dicarboxylate. In addition, both the activation energy barriers of the proton abstraction steps are the minimum for the cesium pivalate among these three alternative additives. The corresponding transition state structure for the CsOPiv–Cs2CO3 system is shown in Figure 3.

Table 3.
Important parameters for C–H activation step for alternative carboxylates.

Figure 3.
The transition structure for CsOPiv–Cs2CO3 system: (a) TS_1 and (b) TS_3.
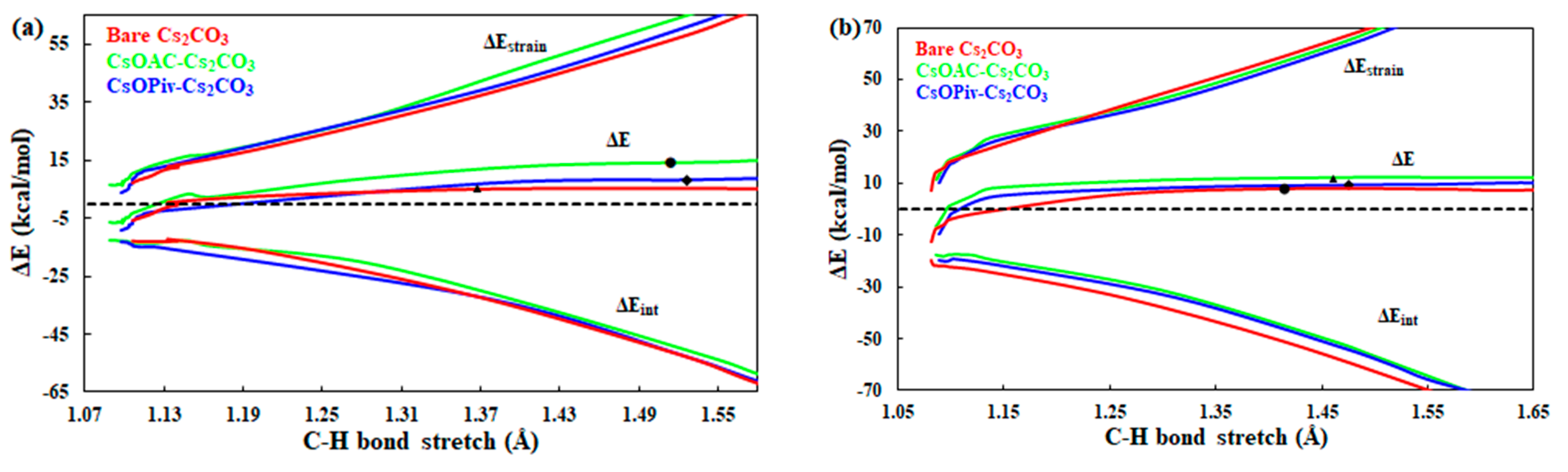
Aiming to precisely disclose the origin of the activation energy barrier in this carboxylate-assisted, carbonate-promoted C–H carboxylation reaction process, the distortion/interaction–activation strain analysis [36] along the entire reaction coordinate is employed. Figure 4 shows the results of activation strain analyses in the proton abstraction steps for the bare and carboxylate-assisted Cs2CO3 systems. As discovered from Figure 4a, these two alternative additive possibilities have the similar strain energy term along the reaction coordinate, and both are greater than that of the bare Cs2CO3 process. After the transition state position of the bare Cs2CO3 process, the distortion energy of the cesium acetate alternative exceeds that of the cesium pivalate process. The interaction energy between this carboxylate-carbonate system and thiophene in the pivalate-assisted process is higher than that of the other two possibilities, while the interaction energy term in the cesium acetate process is the lowest. Therefore, the higher activation energy barrier in the acetate-assisted thiophene proton abstraction step derives from the combination effect of the more destabilizing strain energy term and the least stabilizing interaction energy term, which will make the reaction more endothermic and results in the shifting of the transition state to a later stage and the product side. However, the origin of the larger free energy barrier in the pivalate pathway results from the stronger strain energy term with the help of the stabilizing factor. Figure 4b indicates that the interaction energy terms in these two carboxylate-assisted systems are weaker than that of the bare Cs2CO3 pathway. There is little difference in the interaction energy curve between the pivalate and acetate processes, but it is still larger for the pivalate system. As the reaction proceeds, the distortion energy in the bare carbonate process is increases and gradually exceeds that of the carboxylate-assisted systems. Hence, the formation of the later stage transition structure for these two carboxylate pathways is caused by the less stabilizing factor with the softer strain term. The higher activation energy barrier in the acetate-assisted monocarboxylate proton abstraction step derives from the combination effect of the more destabilizing strain energy term and the least stabilizing interaction energy term when compared with that of the pivalate-assisted case.

Figure 4.
Distortion/Interaction–activation strain analyses for the proton abstraction steps along the reaction coordinate: (a) first, the C–H cleavage step; (b) second, the C–H cleavage step. A dot designates a TS.
3. Conclusions
The direct C–H carboxylation of thiophene with CO2 was carried out in the presence of mixed cesium carbonate and carboxylate salts from the perspective of the experiment and DFT calculations. The carboxylate salt created the synergistic effect on the carbonate-promoted carboxylation, and the reaction effect varied with the carboxylates owing to their different deprotonation abilities. The pivalate-assisted carbonate-promoted C–H carboxylation reaction had the best reaction effect since both the activation energy barriers of the proton abstraction steps were the minimal. The activation energy barrier of C–H activation step was higher than the following CO2 insertion step, whether for the formation of the mono- and/or di-carboxylate. This indicates that the C–H deprotonation induced by the base was slow and the resulting carbon-centered nucleophile reacted rapidly with CO2. Furthermore, the origin and difference of the activation energy barrier was also investigated through the distortion/interaction model analysis, taking the acetate and pivalate co-salts as the illustrating example. The softer strain terms in the pivalate system in comparison with those in the acetate system, led to the lower activation energy barrier in the thiophene deprotonation step, while the stronger interaction term was the main source of this phenomenon for the secondary carboxylation process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12040369/s1, Figure S1: 1H-NMR (500 MHz) spectra for the C-H carboxylation of thiophene (2 mmol) in the presence of 40 mol% Cs2CO3/60 mol% CsOPiv. a, reaction temperature of 280 °C, 8 bar CO2 initial pressure and 3 mmol total cesium salts; b, reaction temperature of 300 °C, 8 bar CO2 initial pressure and 3 mmol total cesium salts.; Figure S2: The standard IRI color-bar and the corresponding chemical explanations of sign(λ2)ρ on IRI isosurface. Figure S3: The IRI analysis results for TS_1 and TS_3 structures [37,38].
Author Contributions
Data curation, Q.Z.; Formal analysis, Q.Z. and P.S.; Investigation, Q.Z. and P.S.; Project administration, A.Z.; Supervision, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of carbon dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Larrosa, I. C–H carboxylation of aromatic compounds through CO2 fixation. ChemSusChem 2017, 10, 3317–3332. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Li, M.; Zhang, J.; Sun, B.; Mo, F. C-H bond carboxylation with carbon dioxide. ChemSusChem 2019, 12, 6–39. [Google Scholar] [CrossRef]
- Liu, A.H.; Yu, B.; He, L.N. Catalytic conversion of carbon dioxide to carboxylic acid derivatives. Greenh. Gases 2015, 5, 17–33. [Google Scholar] [CrossRef]
- Olah, G.A.; Torok, B.; Joschek, J.P.; Bucsi, I.; Esteves, P.M.; Rasul, G.; Prakash, G.K.S. Efficient chemoselective carboxylation of aromatics to arylcarboxylic acids with a superelectrophilically activated carbon dioxide-Al2Cl6/Al system. J. Am. Chem. Soc. 2002, 124, 11379–11391. [Google Scholar] [CrossRef] [PubMed]
- Munshi, P.; Beckman, E.J. Effect of incubation of CO2 and Lewis acid on the generation of toluic acid from toluene and CO2. Ind. Eng. Chem. Res. 2009, 48, 1059–1062. [Google Scholar] [CrossRef]
- Munshi, P.; Beckman, E.J.; Padmanabhan, S. Combined influence of fluorinated solvent and base in Friedel-Crafts reaction of toluene and CO2. Ind. Eng. Chem. Res. 2010, 49, 6678–6682. [Google Scholar] [CrossRef]
- Nemoto, K.; Yoshida, H.; Egusa, N.; Morohashi, N.; Hattori, T. Direct carboxylation of arenes and halobenzenes with CO2 by the combined use of AlBr3 and R3SiCl. J. Org. Chem. 2010, 75, 7855–7862. [Google Scholar] [CrossRef]
- Nemoto, K.; Onozawa, S.; Egusa, N.; Morohashi, N.; Hattori, T. Carboxylation of indoles and pyrroles with CO2 in the presence of dialkylaluminum halides. Tetrahedron Lett. 2009, 50, 4512–4514. [Google Scholar] [CrossRef]
- Nemoto, K.; Onozawa, S.; Konno, M.; Morohashi, N.; Hattori, T. Direct carboxylation of thiophenes and benzothiophenes with the aid of EtAlCl2. Bull. Chem. Soc. Jpn. 2012, 85, 369–371. [Google Scholar] [CrossRef]
- Tanaka, S.; Watanabe, K.; Tanaka, Y.; Hattori, T. EtAlCl2/2,6-disubstituted pyridine-mediated carboxylation of alkenes with carbon dioxide. Org. Lett. 2016, 18, 2576–2579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, Z. Performance of combined use of chlorosilanes and AlCl3 in the carboxylation of toluene with CO2. AIChE J. 2017, 63, 185–191. [Google Scholar] [CrossRef]
- Boogaerts, I.I.F.; Nolan, S.P. Carboxylation of C-H Bonds using N-heterocyclic carbene gold(I) complexes. J. Am. Chem. Soc. 2010, 132, 8858–8859. [Google Scholar] [CrossRef] [PubMed]
- Boogaerts, I.I.F.; Fortman, G.C.; Furst, M.R.L.; Cazin, C.S.J.; Nolan, S.P. Carboxylation of N-H/C-H bonds using N-heterocyclic carbene copper(I) complexes. Angew. Chem. Int. Edit. 2010, 49, 8674–8677. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.J.; Capdevila, M.G.; Du, X.; Kobayashi, S. Base-mediated carboxylation of unprotected indole derivatives with carbon dioxide. Org. Lett. 2012, 14, 5326–5329. [Google Scholar] [CrossRef] [PubMed]
- Fenner, S.; Ackermann, L. C-H carboxylation of heteroarenes with ambient CO2. Green Chem. 2016, 18, 3804–3807. [Google Scholar] [CrossRef] [Green Version]
- Shigeno, M.; Hanasaka, K.; Sasaki, K.; Kumada, N.K.; Kondo, Y. Direct carboxylation of electron-rich heteroarenes promoted by LiO-tBu with CsF and [18]crown-6. Chem. Eur. J. 2019, 25, 3235–3239. [Google Scholar]
- Shigeno, M.; Sasaki, K.; Kumada, N.K.; Kondo, Y. Double-carboxylation of two C-H bonds in 2-alkylheteroarenes using LiO-t-Bu/CsF. Org. Lett. 2019, 21, 4515–4519. [Google Scholar] [CrossRef]
- Shigeno, M.; Tohara, I.; Kumada, N.K.; Kondo, Y. Direct C-2 carboxylation of 3-substituted indoles using a combined Brønsted base consisting of LiO-tBu/CsF/18-crown-6. Eur. J. Org. Chem. 2020, 2020, 1987–1991. [Google Scholar] [CrossRef]
- Lee, M.; Hwang, Y.K.; Kwak, J. Ag(I)-catalyzed C-H carboxylation of thiophene derivatives. Organometallics 2021, 40, 3136–3144. [Google Scholar] [CrossRef]
- Sugimoto, H.; Kawata, I.; Taniguchi, H.; Fujiwara, Y. Palladium-catalyzed carboxylation of aromatic compounds with carbon dioxide. J. Organomet. Chem. 1984, 266, C44–C46. [Google Scholar] [CrossRef]
- Vechorkin, O.; Hirt, N.; Hu, X. Carbon dioxide as the C1 source for direct C-H functionalization of aromatic heterocycles. Org. Lett. 2010, 12, 3567–3569. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Dick, G.R.; Yoshino, T.; Kanan, M.W. Carbon dioxide utilization via carbonate-promoted C-H carboxylation. Nature 2016, 531, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.R.; Frankhouser, A.D.; Banerjee, A.; Kanan, M.W. A scalable carboxylation route to furan-2,5-dicarboxylic acid. Green Chem. 2017, 19, 2966–2972. [Google Scholar] [CrossRef]
- Lankenau, A.W.; Kanan, M.W. Polyamide monomers via carbonate-promoted C-H carboxylation of furfurylamine. Chem. Sci. 2020, 11, 248–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, K.; Fu, Y.; Li, J.N.; Liu, L.; Guo, Q.X. What are the pKa values of C-H bonds in aromatic heterocyclic compounds in DMSO? Tetrahedron 2007, 63, 1568–1576. [Google Scholar] [CrossRef]
- Porter, T.M.; Kanan, M.W. Carbonate-promoted C-H carboxylation of electron-rich heteroarenes. Chem. Sci. 2020, 11, 11936–11944. [Google Scholar] [CrossRef]
- Banerjee, A.; Kanan, M.W. Carbonate-promoted hydrogenation of carbon dioxide to multicarbon carboxylates. ACS Cent. Sci. 2018, 4, 606–613. [Google Scholar] [CrossRef]
- Lafrance, M.; Fagnou, K. Palladium-Catalyzed Benzene Arylation: Incorporation of catalytic pivalic acid as a proton shuttle and a key element in catalyst design. J. Am. Chem. Soc. 2006, 128, 16496–16497. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, W.; Lian, S.; Yang, F.; Lan, J.; You, J. Phosphine-free, palladium-catalyzed arylation of heterocycles through C-H bond activation with pivalic acid as a cocatalyst. Chem. Eur. J. 2009, 15, 1337–1340. [Google Scholar] [CrossRef]
- Xu, H.; Muto, K.; Yamaguchi, J.; Zhao, C.; Itami, K.; Musaev, D.G. Key mechanistic features of Ni-catalyzed C−H/C−O biaryl coupling of azoles and naphthalen-2-yl pivalates. J. Am. Chem. Soc. 2014, 136, 14834–14844. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013; Available online: https://www.scienceopen.com/document?vid=839f33cc-9114-4a55-8f1a-3f1520324ef5 (accessed on 5 February 2022).
- Wang, Y.; Guo, C.; Shen, J.; Sun, Y.; Niu, Y.; Li, P.; Liu, G.; Wei, X. A sustainable and green route to futan-2,5-dicarboxylaic acid by direct carboxylation of 2-furoic acid and CO2. J. CO2 Util. 2021, 48, 101524. [Google Scholar] [CrossRef]
- Anslyn, E.V.; Dougherty, D.A. Modern Physical Organic Chemistry; University Science Book: Sausalito, CA, USA, 2006; pp. 87–95. [Google Scholar]
- Xu, L.; Haines, B.; Ajitha, M.; Murakami, K.; Itami, K.; Musaev, D. Roles of base in the Pd-catalyzed annulative chlorophenylene dimerization. ACS Catal. 2020, 10, 3059–3073. [Google Scholar] [CrossRef]
- Wolters, L.P.; Bickelhaupt, F.M. The activation strain model and molecular orbital theory. WIREs Comput. Mol. Sci. 2015, 5, 324–343. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Interaction region indicator: A simple real space function clearly revealing both chemical bonds and weak interactions. Chem.-Methods 2021, 1, 231–239. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).