Two-Dimensional Nanostructures in the World of Advanced Oxidation Processes
Abstract
:1. Introduction
2. Two-Dimensional Nanostructures
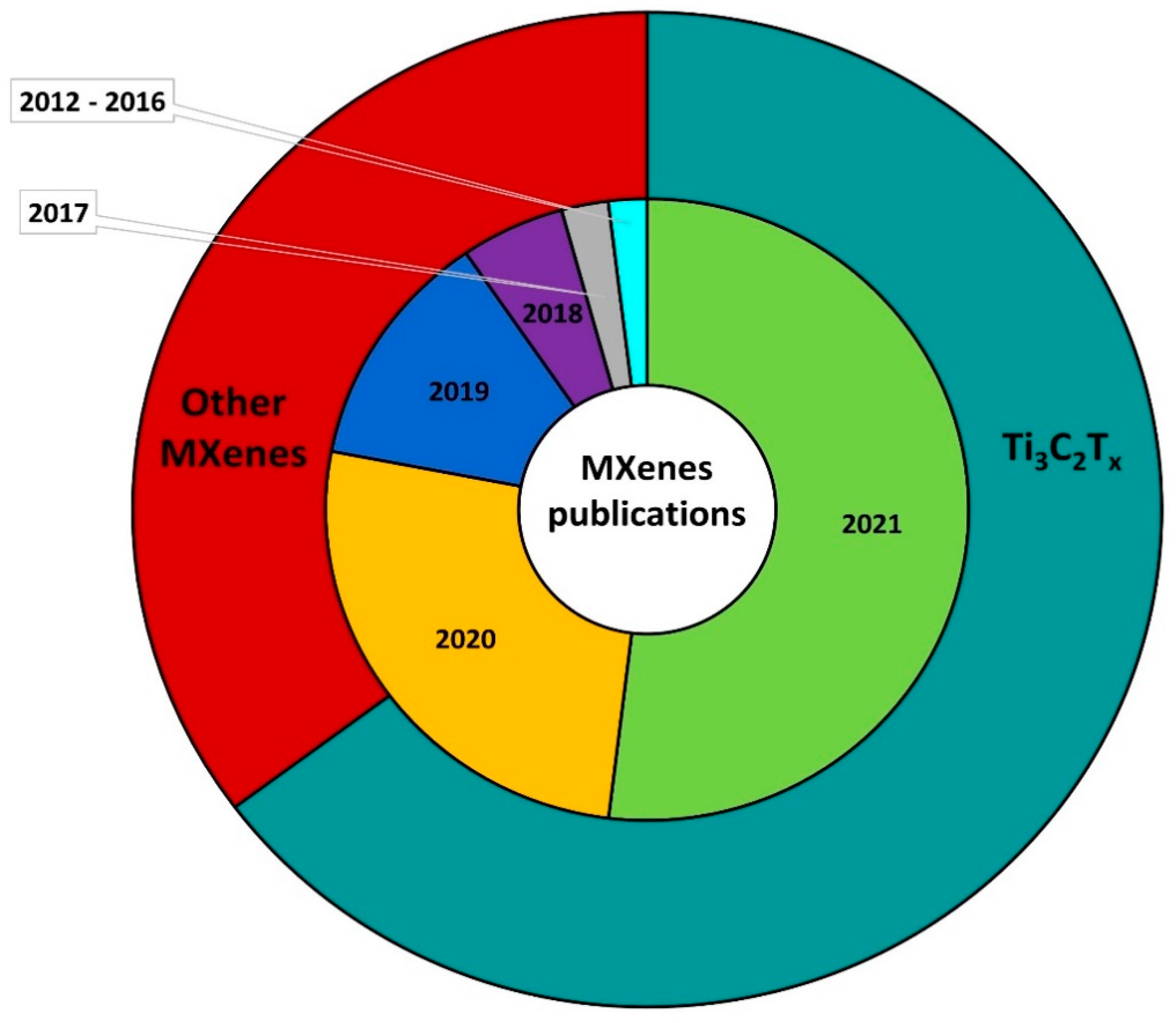
3. MXenes
4. Bi2WO6 (BWO)
5. MOF
6. Mechanism of Modified Fenton Process with 2D Structures
7. Potential Environment Applications and Efficiency of Degradation Process
8. Stability and Recyclability
9. Toxicity of Process
10. Summary and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- de Oliveira Neto, G.C.; Correia, J.M.F.; Silva, P.C.; de Oliveira Sanches, A.G.; Lucato, W.C. Cleaner Production in the textile industry and its relationship to sustainable development goals. J. Clean. Prod. 2019, 228, 1514–1525. [Google Scholar] [CrossRef]
- Tekin, H.; Bilkay, O.; Ataberk, S.S.; Balta, T.H.; Ceribasi, I.H.; Sanin, F.D.; Dilek, F.B.; Yetis, U. Use of Fenton oxidation to improve the biodegradability of a pharmaceutical wastewater. J. Hazard. Mater. 2006, 136, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, D.; Gujer, W. Evolution of a wastewater treatment plant challenges traditional design concepts. Water Res. 2006, 40, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Simultaneous removal of organics and heavy metals from industrial wastewater: A review. Chemosphere 2021, 262, 128379. [Google Scholar] [CrossRef] [PubMed]
- Eggen, R.I.L.; Hollender, J.; Joss, A.; Schärer, M.; Stamm, C. Reducing the Discharge of Micropollutants in the Aquatic Environment: The Benefits of Upgrading Wastewater Treatment Plants. Environ. Sci. Technol. 2014, 48, 7683–7689. [Google Scholar] [CrossRef]
- de Andrade, P.M.; Dufrayer, C.R.; Ionashiro, E.Y.; de Brito, N.N. The use of metallurgical waste for heterogeneous photo Fenton-Like treatment of cosmetic effluent. J. Environ. Chem. Eng. 2020, 8, 104148. [Google Scholar] [CrossRef]
- Cai, Q.Q.; Lee, B.C.Y.; Ong, S.L.; Hu, J.Y. Fluidized-bed Fenton technologies for recalcitrant industrial wastewater treatment–Recent advances, challenges and perspective. Water Res. 2021, 190, 116692. [Google Scholar] [CrossRef]
- Zajda, M.; Aleksander-Kwaterczak, U. Wastewater Treatment Methods for Effluents from the Confectionery Industry—An Overview. J. Ecol. Eng. 2019, 20, 293–304. [Google Scholar] [CrossRef]
- Ghime, D.; Ghosh, P. Advanced Oxidation Processes: A Powerful Treatment Option for the Removal of Recalcitrant Organic Compounds. In Advanced Oxidation Processes: Applications, Trends, and Prospects; IntechOpen: London, UK, 2020; pp. 1–12. [Google Scholar]
- Bogacki, J.; Naumczyk, J.; Marcinowski, P.; Kucharska, M. Oczyszczanie ścieków kosmetycznych metodami fizykochemicznymi i chemicznymi. Chemik 2011, 65, 94–97. [Google Scholar]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A review on industrial wastewater treatment via electrocoagulation processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Nunes, M.I. Recent trends and developments in Fenton processes for industrial wastewater treatment—A critical review. Environ. Res. 2021, 197, 110957. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.C.; Oliveira, L.C.A.; Murad, E. Iron oxide catalysts: Fenton and Fentonlike reactions—A review. Clay Miner. 2012, 47, 285–302. [Google Scholar] [CrossRef]
- Bogacki, J.; Marcinowski, P.; Bury, D.; Krupa, M.; Ścieżyńska, D.; Prabhu, P. Magnetite, Hematite and Zero-Valent Iron as Co-Catalysts in Advanced Oxidation Processes Application for Cosmetic Wastewater Treatment. Catalysts 2020, 11, 9. [Google Scholar] [CrossRef]
- Bogacki, J.; Marcinowski, P.; Zapałowska, E.; Maksymiec, J.; Naumczyk, J. Cosmetic wastewater treatment by the ZVI/H2O2 process. Environ. Technol. 2017, 38, 2589–2600. [Google Scholar] [CrossRef]
- Marcinowski, P.; Bury, D.; Krupa, M.; Ścieżyńska, D.; Prabhu, P.; Bogacki, J. Magnetite and Hematite in Advanced Oxidation Processes Application for Cosmetic Wastewater Treatment. Processes 2020, 8, 1343. [Google Scholar] [CrossRef]
- Muszyński, A.; Marcinowski, P.; Maksymiec, J.; Beskowska, K.; Kalwarczyk, E.; Bogacki, J. Cosmetic wastewater treatment with combined light/Fe0/H2O2 process coupled with activated sludge. J. Hazard. Mater. 2019, 378, 120732. [Google Scholar] [CrossRef]
- Poza-Nogueiras, V.; Rosales, E.; Pazos, M.; Sanromán, M.Á. Current advances and trends in electro-Fenton process using heterogeneous catalysts—A review. Chemosphere 2018, 201, 399–416. [Google Scholar] [CrossRef]
- Carey, J.H. An Introduction to Advanced Oxidation Processes (AOP) for Destruction of Organics in Wastewater. Water Qual. Res. J. 1992, 27, 1–22. [Google Scholar] [CrossRef]
- Iuga, C.; Campero, A.; Vivier-Bunge, A. Antioxidant vs. prooxidant action of phenothiazine in a biological environment in the presence of hydroxyl and hydroperoxyl radicals: A quantum chemistry study. RSC Adv. 2015, 5, 14678–14689. [Google Scholar] [CrossRef]
- Valange, S.; Védrine, J.C. General and Prospective Views on Oxidation Reactions in Heterogeneous Catalysis. Catalysts 2018, 8, 483. [Google Scholar] [CrossRef] [Green Version]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Sriprom, P.; Krobthong, W.; Assawasaengrat, P. Investigation of important parameters for Photo-Fenton degradation of methyl orange over Fe/TiO2 catalyst. Energy Rep. 2020, 6, 731–736. [Google Scholar] [CrossRef]
- Sabour, M.R.; Lak, M.G.; Rabbani, O. Evaluation of the main parameters affecting the Fenton oxidation process in municipal landfill leachate treatment. Waste Manag. Res. 2011, 29, 397–405. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Stavila, V. Crystal engineering, structure–function relationships, and the future of metal-organic frameworks. CrystEngComm 2015, 17, 229–246. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, Y.; Zhao, R.; Zhou, R. Effects of pH and particle size on kinetics of nitrobenzene reduction by zero-valent iron. J. Environ. Sci. 2010, 22, 1741–1747. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: Advances and prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef]
- Xiao, K.; Pei, K.; Wang, H.; Yu, W.; Liang, S.; Hu, J.; Hou, H.; Liu, B.; Yang, J. Citric acid assisted Fenton-like process for enhanced dewaterability of waste activated sludge with in-situ generation of hydrogen peroxide. Water Res. 2018, 140, 232–242. [Google Scholar] [CrossRef]
- Sheng, H.; Janes, A.N.; Ross, R.D.; Kaiman, D.; Huang, J.; Song, B.; Schmidt, J.R.; Jin, S. Stable and selective electrosynthesis of hydrogen peroxide and the electro-Fenton process on CoSe2 polymorph catalysts. Energy Environ. Sci. 2020, 13, 4189–4203. [Google Scholar] [CrossRef]
- Yang, H.; Shi, B.; Wang, S. Fe Oxides Loaded on Carbon Cloth by Hydrothermal Process as an Effective and Reusable Heterogenous Fenton Catalyst. Catalysts 2018, 8, 207. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Breslin, C.B. Graphene-Modified Composites and Electrodes and Their Potential Applications in the Electro-Fenton Process. Materials 2020, 13, 2254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-H.; Dong, H.; Zhao, L.; Wang, D.-X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Ebrahiem, E.E.; Al-Maghrabi, M.N.; Mobarki, A.R. Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab. J. Chem. 2017, 10, S1674–S1679. [Google Scholar] [CrossRef]
- Lan, H.; Wang, A.; Liu, R.; Liu, H.; Qu, J. Heterogeneous photo-Fenton degradation of acid red B over Fe2O3 supported on activated carbon fiber. J. Hazard. Mater. 2014, 285, 167–172. [Google Scholar] [CrossRef]
- Velichkova, F.; Delmas, H.; Julcour, C.; Koumanova, B. Heterogeneous fenton and photo-fenton oxidation for paracetamol removal using iron containing ZSM-5 zeolite as catalyst. AIChE J. 2017, 63, 669–679. [Google Scholar] [CrossRef] [Green Version]
- Giannakis, S. A review of the concepts, recent advances and niche applications of the (photo) Fenton process, beyond water/wastewater treatment: Surface functionalization, biomass treatment, combatting cancer and other medical uses. Appl. Catal. B Environ. 2019, 248, 309–319. [Google Scholar] [CrossRef]
- He, H.; Zhou, Z. Electro-Fenton process for water and wastewater treatment. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2100–2131. [Google Scholar] [CrossRef]
- Kang, M.-H.; Lee, D.; Sung, J.; Kim, J.; Kim, B.H.; Park, J. 2.04-Structure and Chemistry of 2D Materials. In Comprehensive Nanoscience and Nanotechnology, 2nd ed.; Andrews, D.L., Lipson, R.H., Nann, T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 55–90. [Google Scholar]
- Wang, Z.; Zhu, W.; Qiu, Y.; Yi, X.; Bussche, A.V.D.; Kane, A.; Gao, H.; Koski, K.; Hurt, R. Biological and environmental interactions of emerging two-dimensional nanomaterials. Chem. Soc. Rev. 2016, 45, 1750–1780. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhang, S.; Shi, R.; Waterhouse, G.I.N.; Tang, J.; Zhang, T. Two-dimensional photocatalyst design: A critical review of recent experimental and computational advances. Mater. Today 2020, 34, 78–91. [Google Scholar] [CrossRef]
- Xu, J.; Li, W.; Hou, Y. Two-Dimensional Magnetic Nanostructures. Trends Chem. 2020, 2, 163–173. [Google Scholar] [CrossRef]
- Anichini, C.; Czepa, W.; Pakulski, D.; Aliprandi, A.; Ciesielski, A.; Samorì, P. Chemical sensing with 2D materials. Chem. Soc. Rev. 2018, 47, 4860–4908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Bai, Y.; Shi, X.; Su, N.; Nie, G.; Zhang, R.; Nie, H.; Ye, L. Applications of MXene (Ti3C2Tx) in photocatalysis: A review. Mater. Adv. 2021, 2, 1570–1594. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Katsnelson, M.I. Graphene: Carbon in two dimensions. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Mas-Ballesté, R.; Navarro, C.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Szuplewska, A.; Kulpińska, D.; Jakubczak, M.; Dybko, A.; Chudy, M.; Olszyna, A.; Brzózka, Z.; Jastrzębska, A.M. The 10th anniversary of MXenes: Challenges and prospects for their surface modification toward future biotechnological applications. Adv. Drug Deliv. Rev. 2022, 182, 114099. [Google Scholar] [CrossRef]
- Jakubczak, M.; Szuplewska, A.; Rozmysłowska-Wojciechowska, A.; Rosenkranz, A.; Jastrzębska, A.M. Novel 2D MBenes—Synthesis, Structure, and Biotechnological Potential. Adv. Funct. Mater. 2021, 31, 2103048. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Andreescu, S. MXenes-Based Bioanalytical Sensors: Design, Characterization, and Applications. Sensors 2020, 20, 5434. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, X.; Zhao, S.; Huang, Q.; Xue, J. High adsorption capacity of heavy metals on two-dimensional MXenes: An ab initio study with molecular dynamics simulation. Phys. Chem. Chem. Phys. 2016, 18, 228–233. [Google Scholar] [CrossRef]
- Lim, G.P.; Soon, C.F.; Jastrzębska, A.M.; Ma, N.L.; Wojciechowska, A.R.; Szuplewska, A.; Omar, W.I.W.; Morsin, M.; Nayan, N.; Tee, K.S. Synthesis, characterization and biophysical evaluation of the 2D Ti2CTx MXene using 3D spheroid-type cultures. Ceram. Int. 2021, 47, 22567–22577. [Google Scholar] [CrossRef]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of MXenes at every step, from their precursors to single flakes and assembled films. Prog. Mater. Sci. 2021, 120, 100757. [Google Scholar] [CrossRef]
- Ihsanullah, I. Potential of MXenes in Water Desalination: Current Status and Perspectives. Nano-Micro Lett. 2020, 12, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubczak, M.; Karwowska, E.; Rozmysłowska-Wojciechowska, A.; Petrus, M.; Woźniak, J.; Mitrzak, J.; Jastrzębska, A.M. Filtration Materials Modified with 2D Nanocomposites—A New Perspective for Point-of-Use Water Treatment. Materials 2021, 14, 182. [Google Scholar] [CrossRef]
- Szuplewska, A.; Rozmysłowska-Wojciechowska, A.; Poźniak, S.; Wojciechowski, T.; Birowska, M.; Popielski, M.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Moszczyńska, D.; et al. Multilayered stable 2D nano-sheets of Ti2NTx MXene: Synthesis, characterization, and anticancer activity. J. Nanobiotechnol. 2019, 17, 114. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef]
- Morales-García, Á.; Calle-Vallejo, F.; Illas, F. MXenes: New Horizons in Catalysis. ACS Catal. 2020, 10, 13487–13503. [Google Scholar] [CrossRef]
- Xin, M.; Li, J.; Ma, Z.; Pan, L.; Shi, Y. MXenes and Their Applications in Wearable Sensors. Front. Chem. 2020, 8, 297. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Peng, J.; Ong, W.-J.; Ma, T.; Arramel; Zhang, P.; Jiang, J.; Yuan, X.; Zhang, C. MXenes: An Emerging Platform for Wearable Electronics and Looking Beyond. Matter 2021, 4, 377–407. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Mao, Y.; Li, Z. Progress and biomedical applications of MXenes. Nano Select 2021, 2, 1480–1508. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Huang, Q. MXenes: Two-Dimensional Building Blocks for Future Materials and Devices. ACS Nano 2021, 15, 5775–5780. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Li, Y.; Huo, M.; Lin, H.; Chen, Y. Triggering Sequential Catalytic Fenton Reaction on 2D MXenes for Hyperthermia-Augmented Synergistic Nanocatalytic Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 42917–42931. [Google Scholar] [CrossRef]
- Szuplewska, A.; Kulpińska, D.; Dybko, A.; Chudy, M.; Jastrzębska, A.M.; Olszyna, A.; Brzózka, Z. Future Applications of MXenes in Biotechnology, Nanomedicine, and Sensors. Trends Biotechnol. 2020, 38, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, D.; Shen, K.; Nie, S.; Liu, M.; Huang, H.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. Biomimetic anchoring of Fe3O4 onto Ti3C2 MXene for highly efficient removal of organic dyes by Fenton reaction. J. Environ. Chem. Eng. 2020, 8, 104369. [Google Scholar] [CrossRef]
- Ma, Y.; Lv, X.; Xiong, D.; Zhao, X.; Zhang, Z. Catalytic degradation of ranitidine using novel magnetic Ti3C2-based MXene nanosheets modified with nanoscale zero-valent iron particles. Appl. Catal. B Environ. 2020, 284, 119720. [Google Scholar] [CrossRef]
- Wojciechowska, A. Colloidal Properties and Stability of 2D Ti3C2 and Ti2C MXenes in Water. Int. J. Electrochem. Sci. 2018, 13, 10837–10847. [Google Scholar]
- Orimolade, B.O.; Idris, A.O.; Feleni, U.; Mamba, B. Recent advances in degradation of pharmaceuticals using Bi2WO6 mediated photocatalysis—A comprehensive review. Environ. Pollut. 2021, 289, 117891. [Google Scholar] [CrossRef]
- Jiang, X.; Kuklin, A.V.; Baev, A.; Ge, Y.; Ågren, H.; Zhang, H.; Prasad, P.N. Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications. Phys. Rep. 2020, 848, 1–58. [Google Scholar] [CrossRef]
- Cheng, X.; Zu, L.; Jiang, Y.; Shi, D.; Cai, X.; Ni, Y.; Lin, S.; Qin, Y. A titanium-based photo-Fenton bifunctional catalyst of mp-MXene/TiO2−x nanodots for dramatic enhancement of catalytic efficiency in advanced oxidation processes. Chem. Comm. 2018, 54, 11622–11625. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, H.; Chen, Z.; Wong, P.K.; Liu, J. Bi2WO6 micro/nanostructures: Synthesis, modifications and visible-light-driven photocatalytic applications. Appl. Catal. B Environ. 2011, 106, 1–13. [Google Scholar]
- Kong, X.Y.; Lee, W.Q.; Mohamed, A.R.; Chai, S.-P. Effective steering of charge flow through synergistic inducing oxygen vacancy defects and p-n heterojunctions in 2D/2D surface-engineered Bi2WO6/BiOI cascade: Towards superior photocatalytic CO2 reduction activity. Chem. Eng. J. 2019, 372, 1183–1193. [Google Scholar] [CrossRef]
- Huang, T.; Li, Y.; Wu, X.; Lv, K.; Li, Q.; Li, M.; Du, D.; Ye, H. In-situ transformation of Bi2WO6 to highly photoreactive Bi2WO6@Bi2S3 nanoplate via ion exchange. Chin. J. Catal. 2018, 39, 718–727. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Lu, J.; Ding, Y.; Ma, C.; Liu, X.; Meng, M.; Yan, Y. Fabrication of Bi2WO6/In2O3 photocatalysts with efficient photocatalytic performance for the degradation of organic pollutants: Insight into the role of oxygen vacancy and heterojunction. Adv. Powder Technol. 2020, 31, 2890–2900. [Google Scholar] [CrossRef]
- Yang, R.; Zhong, S.; Zhang, L.; Liu, B. PW12/CN@Bi2WO6 composite photocatalyst prepared based on organic-inorganic hybrid system for removing pollutants in water. Sep. Purif. Technol. 2020, 235, 116270. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, G.; Lv, C.; Yao, Y.; Xu, Y.; Jin, X.; Meng, Q. Enabling Nitrogen Fixation on Bi2WO6 Photocatalyst by c-PAN Surface Decoration. ACS Sustain. Chem. Eng. 2018, 6, 11190–11195. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, K.; Han, X.; Zhao, Q.; Zhang, Y.; Qi, M.; Wang, D.; Fu, F. 2D/2D type-II Cu2ZnSnS4/Bi2WO6 heterojunctions to promote visible-light-driven photo-Fenton catalytic activity. Chin. J. Catal. 2020, 41, 503–513. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, J.; Niu, S.; Wang, X.; Li, T.; Liu, S.; Lin, Y.; Xie, T.; Dong, S. Comparing dark- and photo-Fenton-like degradation of emerging pollutant over photo-switchable Bi(2)WO(6)/CuFe(2)O(4): Investigation on dominant reactive oxidation species. J. Environ. Sci. 2021, 106, 147–160. [Google Scholar] [CrossRef]
- Zhong, X.; Wu, W.-T.; Jie, H.-N.; Tang, W.-Y.; Chen, D.-Y.; Ruan, T.; Bai, H.-P. Degradation of norfloxacin by copper-doped Bi2WO6-induced sulfate radical-based visible light-Fenton reaction. RSC Adv. 2020, 10, 38024–38032. [Google Scholar] [CrossRef]
- Pamei, M.; Puzari, A. Luminescent transition metal-organic frameworks: An emerging sensor for detecting biologically essential metal ions. Nano-Struct. Nano-Objects 2019, 19, 100364. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Yaghi, O.M. Deconstructing the crystal structures of metal-organic frameworks and related materials into their underlying nets. Chem. Rev. 2012, 112, 675–702. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal-organic frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, Y.; Peng, Y.; Huang, Z.; Ma, Q.; Zhang, H. Two-dimensional metal-organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal-organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Huang, C.-W.; Nguyen, V.-H.; Zhou, S.-R.; Hsu, S.-Y.; Tan, J.-X.; Wu, K.C.W. Metal-organic frameworks: Preparation and applications in highly efficient heterogeneous photocatalysis. Sustain. Energy Fuels 2020, 4, 504–521. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, H.; Kang, L.; Gao, Z.; Ren, F. Fe-based metal-organic frameworks as Fenton-like catalysts for highly efficient degradation of tetracycline hydrochloride over a wide pH range: Acceleration of Fe(II)/Fe(III) cycle under visible light irradiation. Appl. Catal. B Environ. 2020, 263, 118282. [Google Scholar] [CrossRef]
- Bai, W.; Li, S.; Ma, J.; Cao, W.; Zheng, J. Ultrathin 2D metal-organic framework (nanosheets and nanofilms)-based xD–2D hybrid nanostructures as biomimetic enzymes and supercapacitors. J. Mater. Chem. A 2019, 7, 9086–9098. [Google Scholar] [CrossRef]
- Mu, J.; Liu, J.; Ran, Z.; Arif, M.; Gao, M.; Wang, C.; Ji, S. Critical Role of CUS in the Au/MOF-808(Zr) Catalyst for Reaction of CO2 with Amine/H2 via N-Methylation and N-Formylation. Ind. Eng. Chem. Res. 2020, 59, 6543–6555. [Google Scholar] [CrossRef]
- Ma, D.; Li, B.; Liu, K.; Zhang, X.; Zou, W.; Yang, Y.; Li, G.; Shi, Z.; Feng, S. Bifunctional MOF heterogeneous catalysts based on the synergy of dual functional sites for efficient conversion of CO2 under mild and co-catalyst free conditions. J. Mater. Chem. A 2015, 3, 23136–23142. [Google Scholar] [CrossRef]
- Yoo, D.K.; Bhadra, B.N.; Jhung, S.H. Adsorptive removal of hazardous organics from water and fuel with functionalized metal-organic frameworks: Contribution of functional groups. J. Hazard. Mater. 2021, 403, 123655. [Google Scholar] [CrossRef]
- Liu, H.; Yin, H.; Zhu, M.; Dang, Z. Degradation of organophosphorus flame retardants in heterogeneous photo-Fenton system driven by Fe(III)-based metal organic framework: Intermediates and their potential interference on bacterial metabolism. Chemosphere 2021, 291, 133072. [Google Scholar] [CrossRef] [PubMed]
- Ghasempour, H.; Wang, K.-Y.; Powell, J.A.; ZareKarizi, F.; Lv, X.-L.; Morsali, A.; Zhou, H.-C. Metal-organic frameworks based on multicarboxylate linkers. Coord. Chem. Rev. 2021, 426, 213542. [Google Scholar] [CrossRef]
- Mu, W.; Liu, D.; Yang, Q.; Zhong, C. Computational study of the effect of organic linkers on natural gas upgrading in metal-organic frameworks. Microporous Mesoporous Mater. 2010, 130, 76–82. [Google Scholar] [CrossRef]
- Virender, K.S. Water depollution using metal-organic frameworks-catalyzed advanced oxidation processes: A review. J. Hazard. Mater. 2019, 372, 3–16. [Google Scholar]
- Laurier, K.G.M.; Fron, E.; Atienzar, P.; Kennes, K.; Garcia, H.; Van der Auweraer, M.; De Vos, D.E.; Hofkens, J.; Roeffaers, M.B.J. Delayed electron-hole pair recombination in iron(iii)-oxo metal-organic frameworks. Phys. Chem. Chem. Phys. 2014, 16, 5044–5047. [Google Scholar] [CrossRef]
- Zheng, F.; Xiang, D.; Li, P.; Zhang, Z.; Du, C.; Zhuang, Z.; Li, X.; Chen, W. Highly Conductive Bimetallic Ni-Fe Metal Organic Framework as a Novel Electrocatalyst for Water Oxidation. ACS Sustain. Chem. Eng. 2019, 7, 9743–9749. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, W.; Wu, Y.-P.; Tian, J.-W.; Wang, X.-K.; Huang, D.-D.; Zhao, J.; Li, D.-S. Two facile routes to an AB & Cu-MOF composite with improved hydrogen evolution reaction. J. Alloys Compd. 2018, 753, 228–233. [Google Scholar]
- Soetaredjo, F.E.; Santoso, S.P.; Lunardi, V.B.; Kurniawan, A.; Shuwanto, H.; Lie, J.; Foe, K.; Irawaty, W.; Yuliana, M.; Putro, J.N.; et al. Highly efficient degradation of organic pollutant mixtures by a Fe(III)-based MOF-catalyzed Fenton-like process in subcritical water. J. Mol. Liq. 2021, 347, 117989. [Google Scholar] [CrossRef]
- Ye, Z.; Schukraft, G.E.M.; L’Hermitte, A.; Xiong, Y.; Brillas, E.; Petit, C.; Sirés, I. Mechanism and stability of an Fe-based 2D MOF during the photoelectro-Fenton treatment of organic micropollutants under UVA and visible light irradiation. Water Res. 2020, 184, 115986. [Google Scholar] [CrossRef]
- Wang, F.-X.; Wang, C.-C.; Du, X.; Li, Y.; Wang, F.; Wang, P. Efficient removal of emerging organic contaminants via photo-Fenton process over micron-sized Fe-MOF sheet. Chem. Eng. J. 2022, 429, 132495. [Google Scholar] [CrossRef]
- Ameta, R.; Chohadia, A.K.; Jain, A.; Punjabi, P.B. Chapter 3—Fenton and Photo-Fenton Processes. In Advanced Oxidation Processes for Waste Water Treatment; Ameta, S.C., Ameta, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 49–87. [Google Scholar]
- Liao, C.-H.; Gurol, M.D. Chemical Oxidation by Photolytic Decomposition of Hydrogen Peroxide. Environ. Sci. Technol. 1995, 29, 3007–3014. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-H.; Kang, S.-F.; Wu, F.-A. Hydroxyl radical scavenging role of chloride and bicarbonate ions in the H2O2/UV process. Chemosphere 2001, 44, 1193–1200. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zhao, H.; Yao, F.; Cao, J.; Chen, Z.; Ma, F.; Wang, D.; Yang, Q. 2D/2D FeNi-layered double hydroxide/bimetal-MOFs nanosheets for enhanced photo-Fenton degradation of antibiotics: Performance and synergetic degradation mechanism. Chemosphere 2021, 287 Pt 1, 132061. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Yuan, X.; Huang, L.; Shang, S.; Xu, D. Fe-based metal-organic frameworks as heterogeneous catalysts for highly efficient degradation of wastewater in plasma/Fenton-like systems. RSC Adv. 2020, 10, 36363–36370. [Google Scholar] [CrossRef]
- Barreiro, J.C.; Capelato, M.D.; Martin-Neto, L.; Bruun Hansen, H.C. Oxidative decomposition of atrazine by a Fenton-like reaction in a H2O2/ferrihydrite system. Water Res. 2007, 41, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Pliego, G.; Zazo, J.A.; Garcia-Muñoz, P.; Munoz, M.; Casas, J.A.; Rodriguez, J.J. Trends in the Intensification of the Fenton Process for Wastewater Treatment: An Overview. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2611–2692. [Google Scholar] [CrossRef]
- Jiang, C.-C.; Zhang, J.-F. Progress and prospect in electro-Fenton process for wastewater treatment. J. Zhejiang Univ. Sci. A 2007, 8, 1118–1125. [Google Scholar] [CrossRef]
- Pala, A.; Erden, G. Decolorization of a baker’s yeast industry effluent by Fenton oxidation. J. Hazard. Mater. 2005, 127, 141–148. [Google Scholar] [CrossRef]
- Seo, D.C.; Lee, H.J.; Hwang, H.N.; Park, M.R.; Kwak, N.W.; Cho, I.J.; Cho, J.S.; Seo, J.Y.; Joo, W.H.; Park, K.H.; et al. Treatment of non-biodegradable cutting oil wastewater by ultrasonication-Fenton oxidation process. Water Sci. Technol. 2007, 55, 251–259. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, A.; Sharma, G.; Naushad, M.; Dhiman, P.; Kumari, A.; Stadler, F.J. Recent advances in nano-Fenton catalytic degradation of emerging pharmaceutical contaminants. J. Mol. Liq. 2019, 290, 111177. [Google Scholar] [CrossRef]
- Ihsanullah, I. MXenes (two-dimensional metal carbides) as emerging nanomaterials for water purification: Progress, challenges and prospects. Chem. Eng. J. 2020, 388, 124340. [Google Scholar] [CrossRef]
- Du, X.; Fu, W.; Su, P.; Cai, J.; Zhou, M. Internal-micro-electrolysis-enhanced heterogeneous electro-Fenton process catalyzed by Fe/Fe3C@PC core-shell hybrid for sulfamethazine degradation. Chem. Eng. J. 2020, 398, 125681. [Google Scholar] [CrossRef]
- Casado, J. Towards industrial implementation of Electro-Fenton and derived technologies for wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 102823. [Google Scholar] [CrossRef]
- Luo, S.; Wang, R.; Yin, J.; Jiao, T.; Chen, K.; Zou, G.; Peng, Q. Preparation and Dye Degradation Performances of Self-Assembled MXene-Co3O4 Nanocomposites Synthesized via Solvothermal Approach. ACS Omega 2019, 4, 3946–3953. [Google Scholar] [CrossRef] [Green Version]
- Rosales, E.; Pazos, M.; Sanromán, M.A. Advances in the Electro-Fenton Process for Remediation of Recalcitrant Organic Compounds. Chem. Eng. Technol. 2012, 35, 609–617. [Google Scholar] [CrossRef]
- Muñoz-Écija, T.; Vargas-Quesada, B.; Chinchilla-Rodríguez, Z. Identification and visualization of the intellectual structure and the main research lines in nanoscience and nanotechnology at the worldwide level. J. Nanopart. Res. 2017, 19, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J. Environ. Sci. Health C 2009, 27, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Kumar, S.; Kumar, V. Challenges for Assessing Toxicity of Nanomaterials. In Biochemical Toxicology—Heavy Metals and Nanomaterials; IntechOpen: London, UK, 2019. [Google Scholar]
- Arvidsson, R.; Molander, S.; Sandén, B.A.; Hassellöv, M. Challenges in Exposure Modeling of Nanoparticles in Aquatic Environments. Hum. Ecol. Risk Assess. 2011, 17, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Jahnel, J. Chapter 3.2—Addressing the Challenges to the Risk Assessment of Nanomaterials. In Nanoengineering; Dolez, P.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 485–521. [Google Scholar]
- Sun, W.; Wu, F.-G. Two-Dimensional Materials for Antimicrobial Applications: Graphene Materials and Beyond. Chem. Asian J. 2018, 13, 3378–3410. [Google Scholar] [CrossRef]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Ivanova, E.P. Mechano-bactericidal mechanism of graphene nanomaterials. Interface Focus 2018, 8, 20170060. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Feng, X.; Werber, J.R.; Chu, C.; Zucker, I.; Kim, J.H.; Osuji, C.O.; Elimelech, M. Enhanced antibacterial activity through the controlled alignment of graphene oxide nanosheets. Proc. Natl. Acad. Sci. USA 2017, 114, E9793–E9801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, D.; Wang, J.; Wang, J. Bioaccumulation of Fe2O3(magnetic) nanoparticles in Ceriodaphnia dubia. Environ. Pollut. 2012, 162, 216–222. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Umasuthan, N.; Mohan, R.; Lee, J.; Kim, S.-J. Antibacterial Activity of Graphene Oxide Nanosheets. Sci. Adv. Mater. 2012, 4, 1–7. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Park, M.R.; Kwon, D.N.; Kim, J.H. Antibacterial activity of dithiothreitol reduced graphene oxide. J. Ind. Eng. Chem. 2013, 19, 1280–1288. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Han, H. A new function of graphene oxide emerges: Inactivating phytopathogenic bacterium Xanthomonas oryzae pv. Oryzae. J. Nanopart. Res. 2013, 15, 1658. [Google Scholar] [CrossRef]
- Karwowska, E. Antibacterial potential of nanocomposite-based materials—A short review. Nanotechnol. Rev. 2017, 6, 243–254. [Google Scholar] [CrossRef]
- Jakubczak, M.; Jastrzębska, A.M. A Review on Development of Ceramic-Graphene Based Nanohybrid Composite Systems in Biological Applications. Front. Chem. 2021, 9, 685014. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojnacka, K.; Mikulewicz, M. Bioaccumulation. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 456–460. [Google Scholar]
- Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Sharma, V.K. Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ. Chem. Lett. 2018, 16, 947–967. [Google Scholar] [CrossRef] [Green Version]
- Saratale, R.G.; Sivapathan, S.; Saratale, G.D.; Banu, J.R.; Kim, D.S. Hydroxamic acid mediated heterogeneous Fenton-like catalysts for the efficient removal of Acid Red 88, textile wastewater and their phytotoxicity studies. Ecotoxicol. Environ. Saf. 2019, 167, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Kwarciak-Kozłowska, A. Chapter 7—Removal of pharmaceuticals and personal care products by ozonation, advance oxidation processes, and membrane separation. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Prasad, M.N.V., Vithanage, M., Kapley, A., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 151–171. [Google Scholar]
- Wu, Y.; Song, X.; Xu, W.; Sun, K.-Y.; Wang, Z.; Lv, Z.; Wang, Y.; Wang, Y.; Zhong, W.; Wei, J.; et al. NIR-Activated Multimodal Photothermal/Chemodynamic/Magnetic Resonance Imaging Nanoplatform for Anticancer Therapy by Fe(II) Ions Doped MXenes (Fe-Ti3C2). Small 2021, 17, 2101705. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Biosynthesis and functions of glutathione, an essential biofactor. J. Nutr. Sci. Vitaminol. 1992, 38, 1–6. [Google Scholar] [CrossRef]
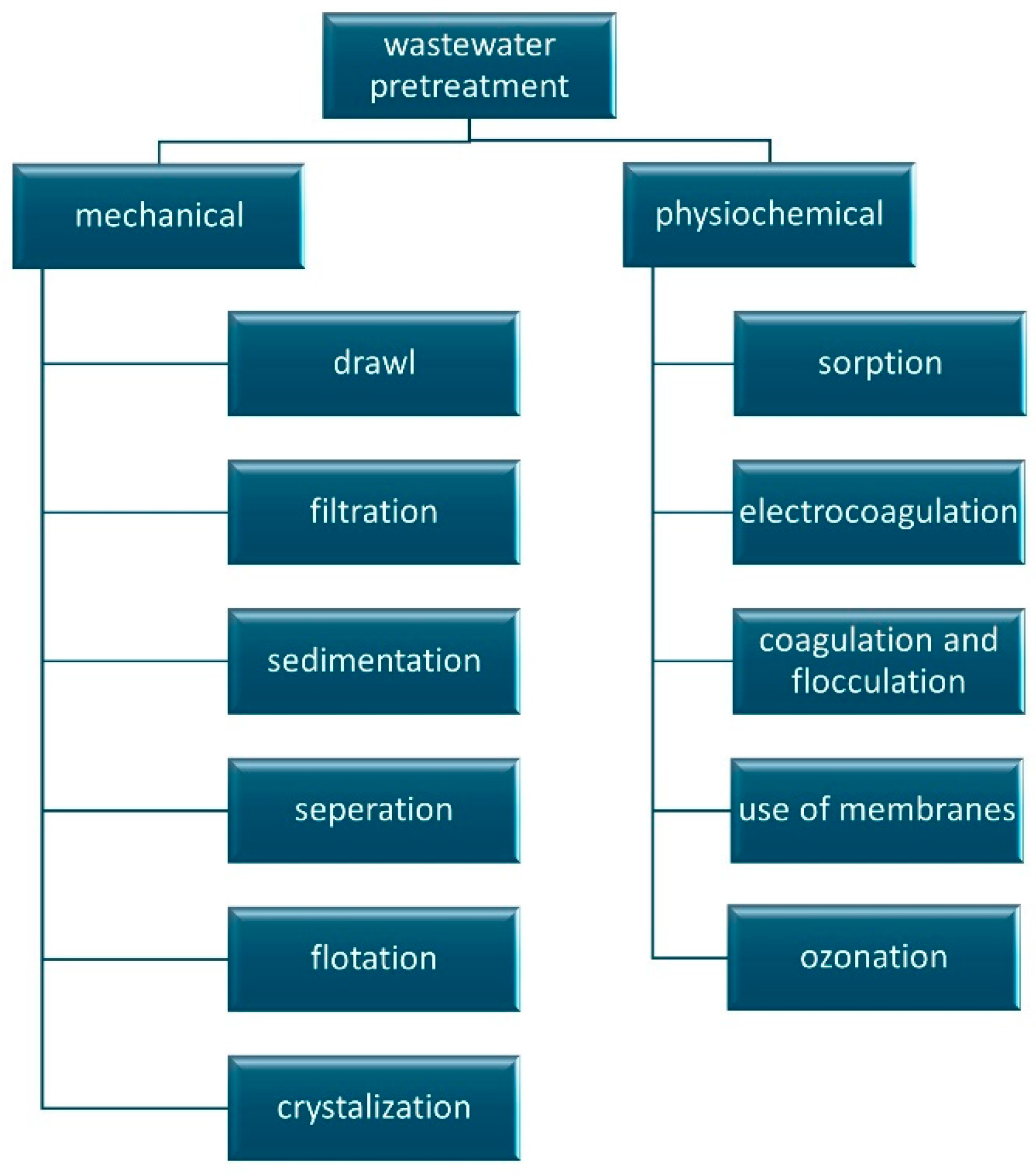

| Oxidizing Agent | Potential (V) |
|---|---|
| Fluorine (F2) | 3.03 |
| Hydroxyl radical (HO•) | 2.80 |
| Atomic oxygen (O) | 2.42 |
| Ozone (O3) | 2.07 |
| Hydrogen peroxide (H2O2) | 1.78 |
| Perhydroxyl radical (•OOH) | 1.70 |
| Permanganate (KMnO4) | 1.68 |
| Chlorine (Cl2) | 1.36 |
| Molecular oxygen (O2) | 1.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ścieżyńska, D.; Bury, D.; Marcinowski, P.; Bogacki, J.; Jakubczak, M.; Jastrzębska, A. Two-Dimensional Nanostructures in the World of Advanced Oxidation Processes. Catalysts 2022, 12, 358. https://doi.org/10.3390/catal12040358
Ścieżyńska D, Bury D, Marcinowski P, Bogacki J, Jakubczak M, Jastrzębska A. Two-Dimensional Nanostructures in the World of Advanced Oxidation Processes. Catalysts. 2022; 12(4):358. https://doi.org/10.3390/catal12040358
Chicago/Turabian StyleŚcieżyńska, Dominika, Dominika Bury, Piotr Marcinowski, Jan Bogacki, Michał Jakubczak, and Agnieszka Jastrzębska. 2022. "Two-Dimensional Nanostructures in the World of Advanced Oxidation Processes" Catalysts 12, no. 4: 358. https://doi.org/10.3390/catal12040358
APA StyleŚcieżyńska, D., Bury, D., Marcinowski, P., Bogacki, J., Jakubczak, M., & Jastrzębska, A. (2022). Two-Dimensional Nanostructures in the World of Advanced Oxidation Processes. Catalysts, 12(4), 358. https://doi.org/10.3390/catal12040358








