Hydrogen Storage in Complex Metal Hydrides NaBH4: Hydrolysis Reaction and Experimental Strategies
Abstract
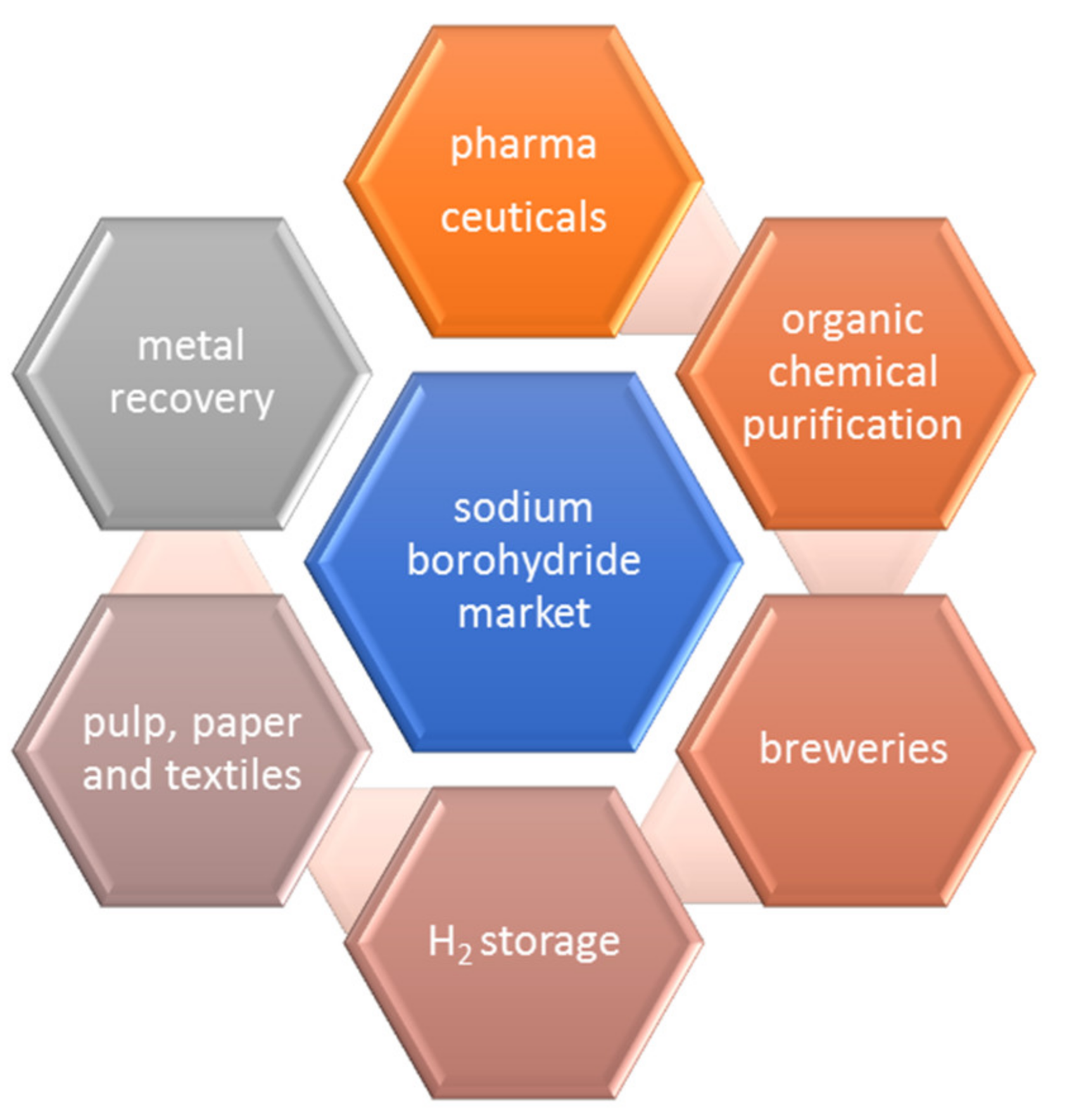
:1. Introduction
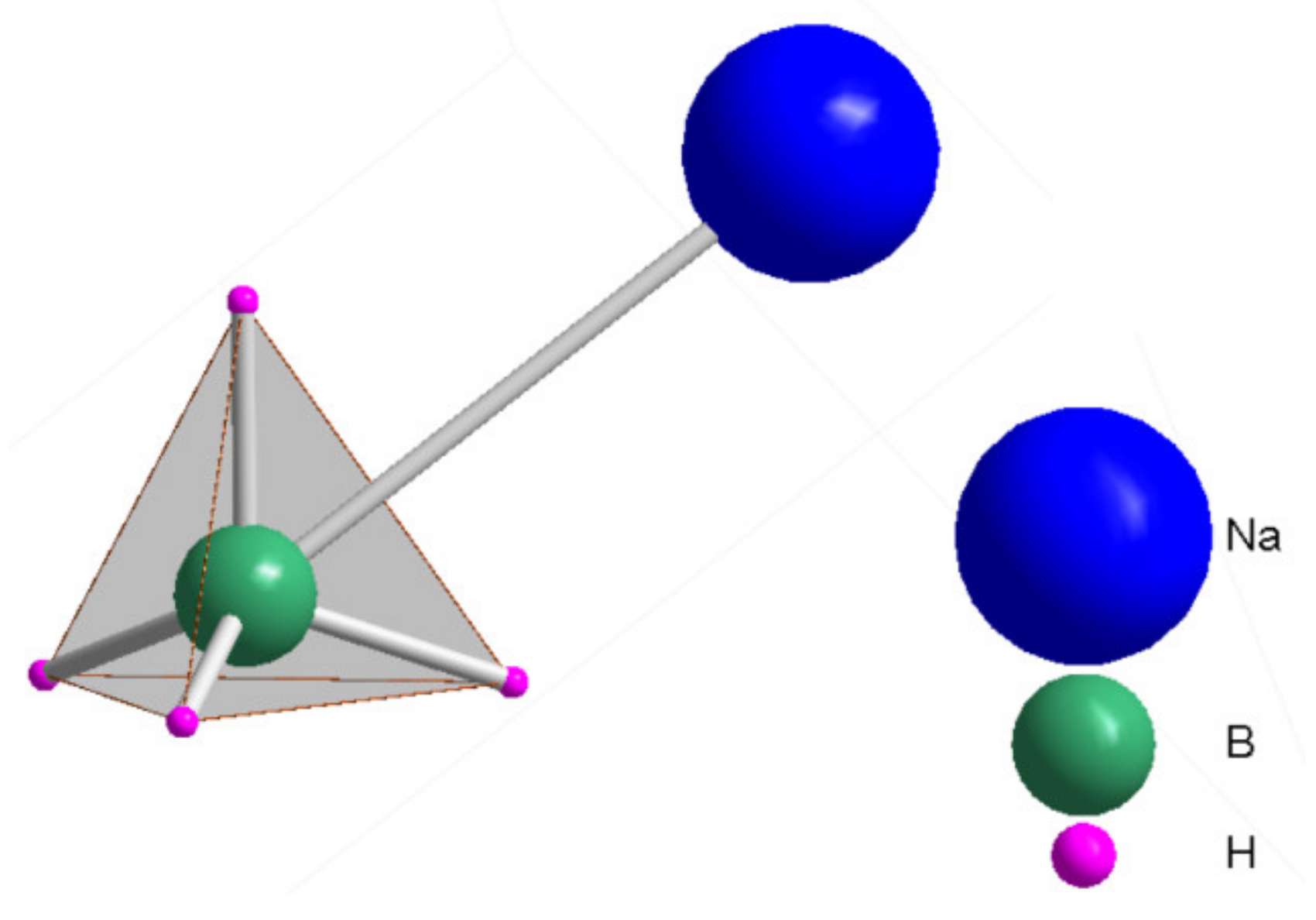
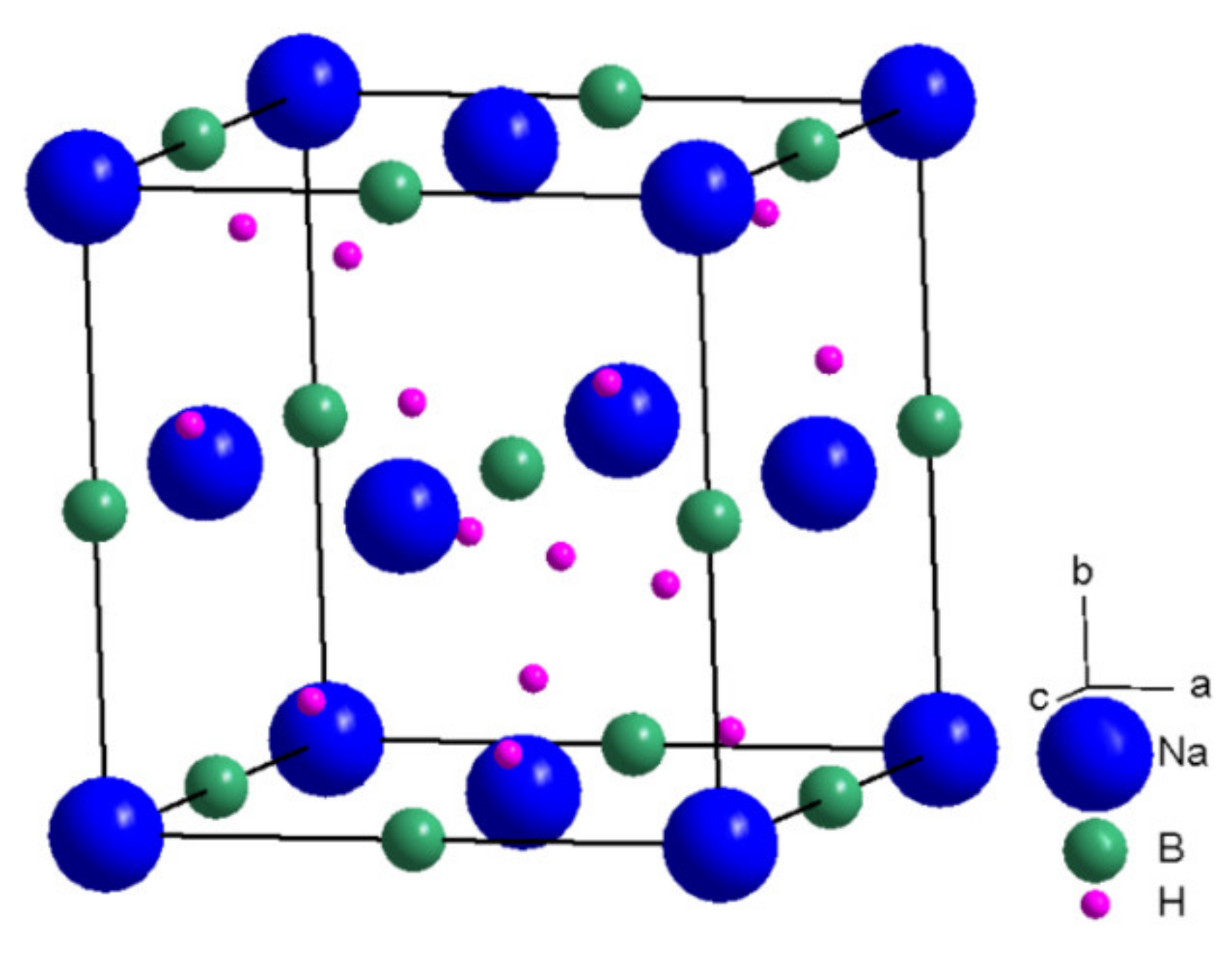
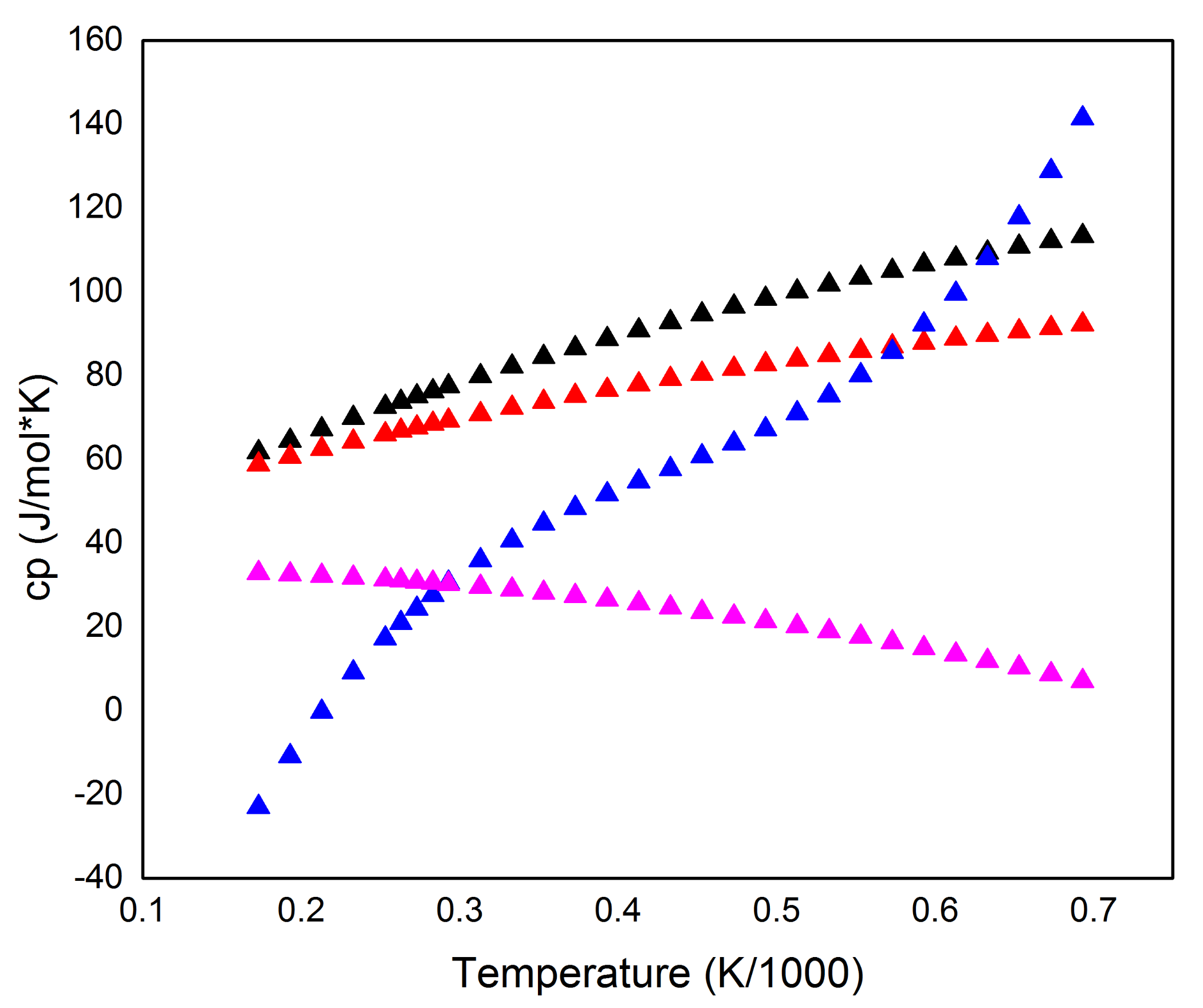
2. Structure of NaBH4
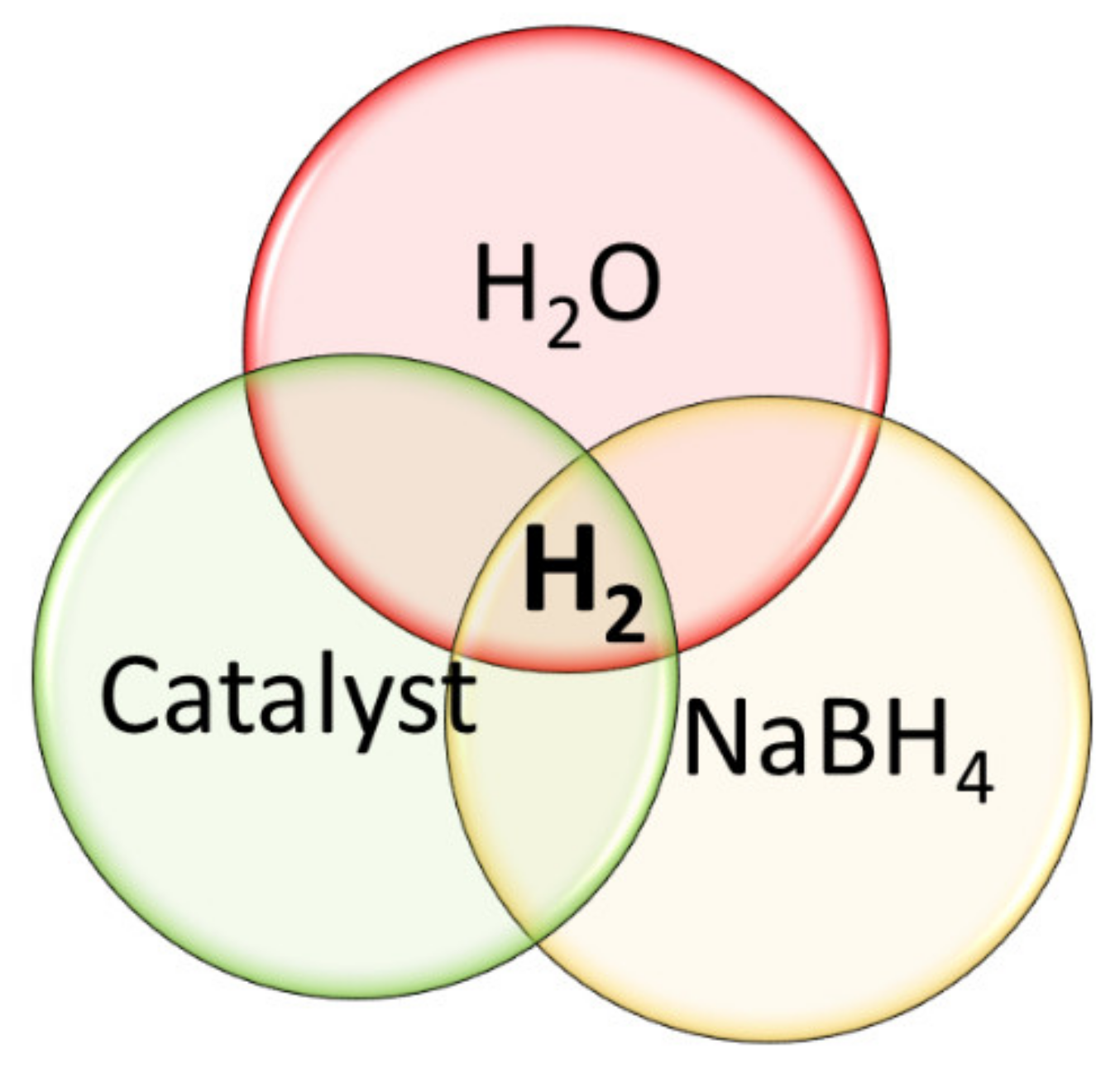
3. Reaction Mechanism
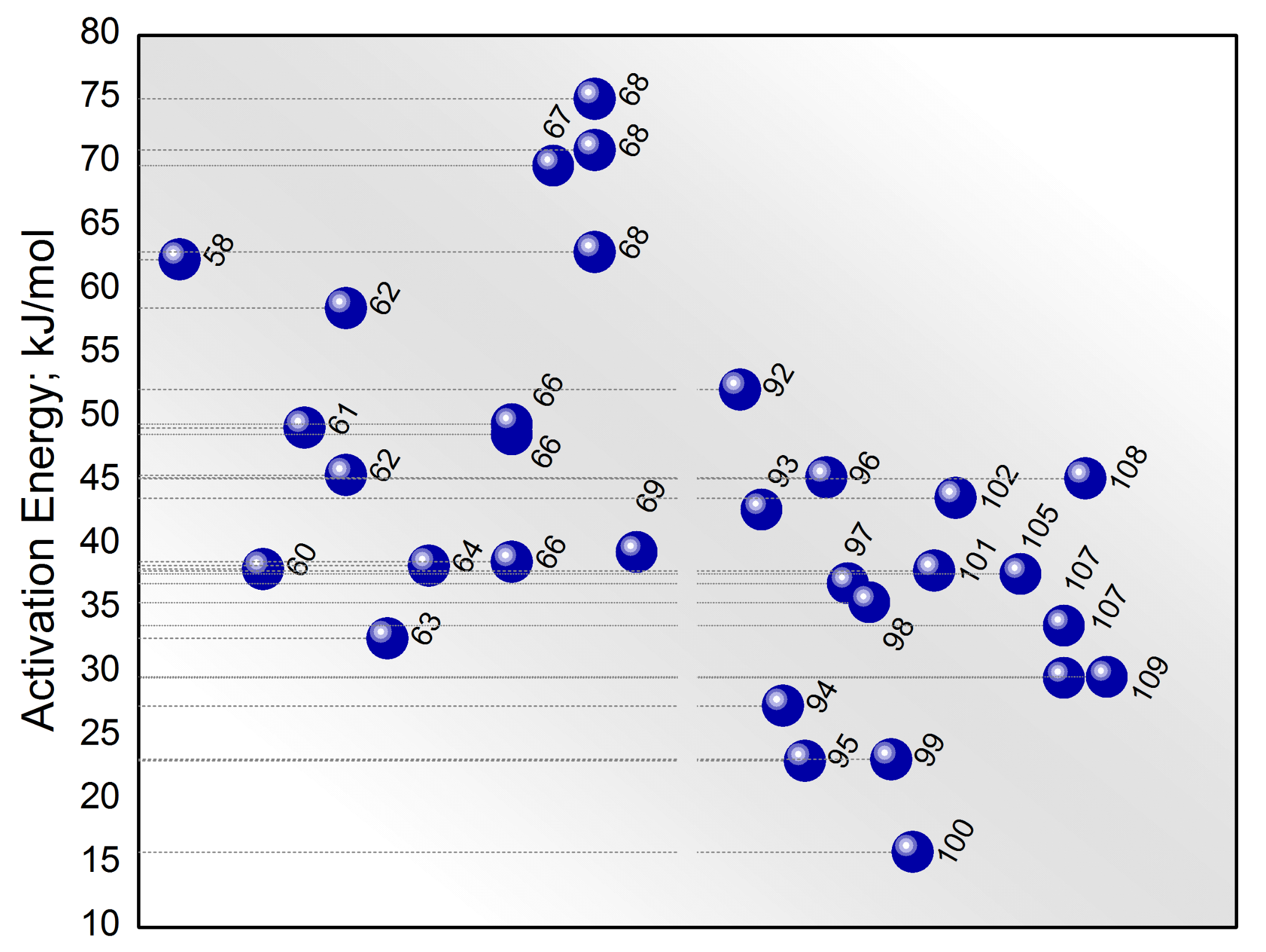
4. Catalysts
5. The Role of Method and Reactor on Carrying Out the NaBH4 Hydrolysis Reaction
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 24 January 2022).
- U.S. Department of Energy, Office of Basic Energy Sciences. Basic Research Needs for the Hydrogen Economy, Report. 2003. Available online: http://www.sc.doe.gov/bes/hydrogen.pdf (accessed on 24 January 2022).
- Ross, D.K. Hydrogen storage: The major technological barrier to the development of hydrogen fuel cell cars. Vacuum 2006, 80, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Maestre, V.M.; Ortiz, A.; Ortiz, I. Challenges and prospects of renewable hydrogen-based strategies for full decarbonization of stationary power applications. Renew. Sustain. Energy Rev. 2021, 152, 111628. [Google Scholar] [CrossRef]
- Egeland-Eriksen, T.; Hajizadeh, A.; Sartori, S. Hydrogen-based systems for integration of renewable energy in power systems: Achievements and perspectives. Int. J. Hydrogen Energy 2021, 46, 31963–31983. [Google Scholar] [CrossRef]
- Reedijk, J.; Poeppelmeier, K. (Eds.) Comprehensive Inorganic Chemistry-II; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780080977744. [Google Scholar]
- Mao, J.; Gregory, D.H. Recent Advances in the Use of Sodium Borohydride as a Solid State Hydrogen Store. Energies 2015, 8, 430–453. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. A review on hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 726–765. [Google Scholar] [CrossRef]
- Schlesinger, H.J.; Brown, H.C.; Sheft, I.; Ritter, D.M. Addition compounds of alkali metal hydrides sodium trimethoxyborohydride and related compounds. J. Am. Chem. Soc. 1953, 75, 192–195. [Google Scholar]
- U.S. Department of Energy Hydrogen Program. Go/no-go recommendation for sodium borohydride for on-board vehicular hydrogen storage. Indep. Rev. 2007, 150, 1–21. [Google Scholar]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E. The Preparation of Sodium Borohydride by the High Temperature Reaction of Sodium Hydride with Borate Esters. J. Am. Chem. Soc. 1953, 75, 205–209. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Abraham, B.; Bond, A.C.; Davidson, N.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.; Horvitz, L.; Hyde, E.K.; et al. New Developments in the Chemistry of Diborane and the Borohydrides. I. General Summary. J. Am. Chem. Soc. 1953, 75, 186–190. [Google Scholar] [CrossRef]
- Broja, G.; Schlabacher, W. Process for the Production of Alkali Metal Borohydrides. U.S. Patent US3259474A, 5 July 1966. [Google Scholar]
- Schlesinger, H.I.; Brown, H.C.; Hoekstra, H.R.; Rapp, L.R. Reactions of diborane with alkali metal hydrides and their addition compounds. New syntheses of borohydrides. Sodium and potassium borohydrides1. J. Am. Chem. Soc. 1953, 75, 199–204. [Google Scholar] [CrossRef]
- Umeda, H.; Sasaki, A.; Takahashi, K.; Haga, K.; Yasushi Takasaki, Y.; Shibayama, A. Recovery and Concentration of Precious Metals from Strong Acidic Wastewater. Mater. Trans. 2011, 52, 1462–1470. [Google Scholar] [CrossRef] [Green Version]
- Vielstich, W.; Lamm, A.; Hubert, A. (Eds.) Handbook of Fuel Cells: Fundamentals, Technology, Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; ISBN 978-0-471-49926-8. [Google Scholar]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.R.; Hyde, E.K. Sodium Borohydride, Its Hydrolysis and its Use as a Reducing Agent and in the Generation of Hydrogen1. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Richardson, B.S.; Birdwell, J.F.; Pin, F.G.; Jansen, J.F.; Lind, R.F. Sodium borohydride based hybrid power system. J. Power Sources 2005, 145, 21–29. [Google Scholar] [CrossRef]
- Duggan, P.J.; Johnson, A.A.; Rogers, R.L. Chemical reaction hazards associated with the use of sodium borohydride. In Institution of Chemical Engineers Symposium Series; IChemE Symposium Series No. 134—Hazards; Hemsphere Publishing Corporation: London, UK, 1994; Volume 12, pp. 553–561. [Google Scholar]
- Cook, M.M.; Lander, J.A. Use of Sodium Borohydride to Control Heavy Metal Discharge in the Photographic Industry. J. Appl. Photogr. Eng. 1979, 5, 144–147. [Google Scholar]
- Stevens, M.R.; Pirnazari, M.; Saavedra, F. Removal of Silver from Photographic Solutions; Pawlowski, L., Verdier, A.J., Lacy, W.J., Eds.; Studies in Environmental Science; Elsevier: Amsterdam, The Netherlands, 1984; Volume 23, pp. 525–534. [Google Scholar] [CrossRef]
- Tang, L.C. Stabilization of Paper Through Sodium Borohydride Treatment. Chapter 24—Historic Textile and Paper Materials. Adv. Chem. 1986, 212, 427–441. [Google Scholar] [CrossRef]
- Westbroek, P. Chapter 7—Advantages of electrocatalytic reactions in textile applications: Example—Electrocatalytic oxidation of sodium dithionite at a phthalocyanine and porphyrin cobalt(II)-modified gold electrode. In Analytical Electrochemistry in Textiles; Westbroek, P., Priniotakis, G., Kiekens, P., Eds.; Woodhead Publishing Series in Textile; Elsevier: Amsterdam, The Netherlands, 2007; pp. 198–211. [Google Scholar]
- Source of hydrogen & other borohydrides; bleacher of wood pulp; blowing agent for plastics. In Patty’s Industrial Hygiene and Toxicology; Clayton, G.D.; Clayton, F.E. (Eds.) John Wiley & Sons: Hoboken, NJ, USA, 1991; (2A, 2B, 2C), 2995. [Google Scholar]
- Soyeon, C. Foxing on Paper: A Literature Review. J. Am. Inst. Conserv. 2020, 46, 137–152. [Google Scholar] [CrossRef]
- Yilmazer, D.; Kanik, M. Bleaching of Wool with Sodium Borohydride. J. Eng. Fibers Fabr. 2009, 4, 45–50. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Li, C.; Xu, P. Stereoselective Synthesis of (−)-Chloramphenicol, (+)-Thiamphenicol and (+)-Sphinganine via Chiral Tricyclic Iminolactone. Chin. J. Chem. 2013, 31, 149–153. [Google Scholar] [CrossRef]
- Odinokov, V.N.; Spivak, A.Y.; Emelyanova, G.A.; Mallyabaeva, M.I.; Nazarova, O.V.; Dzhemilev, U.M. Synthesis of α-tocopherol (vitamin E), vitamin K1-chromanol, and their analogs in the presence of aluminosilicate catalysts Tseokar-10 and Pentasil. Arkivoc Arch. Org. Chem. 2003, 13, 101–118. [Google Scholar] [CrossRef]
- De Keukeleire, D. Fundamentals of beer and hop chemistry. Quím. Nova 2000, 23, 108–112. [Google Scholar] [CrossRef]
- Chaikin, S.W.; Brown, W.G. Reduction of Aldehydes, Ketones and Acid Chlorides by Sodium Borohydride. J. Am. Chem. Soc. 1949, 71, 122–125. [Google Scholar] [CrossRef]
- O’Neil, M.J. The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed.; The Royal Society of Chemistry: London, UK, 2001; p. 1537. [Google Scholar]
- Viswanathan, B. Chapter 10—Hydrogen Storage. Energy Sources; Viswanathan, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 185–212. [Google Scholar] [CrossRef]
- Abanades, S.; Flamant, G. Thermochemical hydrogen production from a two-step solar-driven water-splitting cycle based on cerium oxides. Sol. Energy 2006, 80, 1611–1623. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M.A. Chapter 17—Exergy Analysis of Hydrogen Production Systems; Elsevier: Amsterdam, The Netherlands, 2013; pp. 347–362. [Google Scholar]
- Ma, J.; Choudhury, N.A.; Sahai, Y. A comprehensive review of direct borohydride fuel cells. Renew. Sustain. Energy Rev. 2001, 14, 183–199. [Google Scholar] [CrossRef]
- Sundén, B. Chapter 3—Hydrogen, Hydrogen, Batteries and Fuel Cells; Academic Press: Cambridge, MA, USA, 2001; pp. 37–55. [Google Scholar]
- Filinchuk, Y.; Hagemann, H. Structure and Properties of NaBH4·2H2O and NaBH4. Eur. J. Inorg. Chem. 2008, 20, 3127–3133. [Google Scholar] [CrossRef] [Green Version]
- Diamond—Crystal and Molecular Structure Visualization, Crystal Impact—Dr. H. Putz & Dr. K. Brandenburg GbR, Kreuzherrenstr. 102, 53227 Bonn, Germany. Available online: https://www.crystalimpact.de/diamond (accessed on 24 January 2022).
- Johnston, H.L.; Hallett, N.C. Low Temperature Heat Capacities of Inorganic Solids. XIV. Heat Capacity of Sodium Borohydride from 15–300 °K. J. Am. Chem. Soc. 1953, 75, 1467–1468. [Google Scholar] [CrossRef]
- Stockmayer, W.H.; Stephenson, C.C. The Nature of the Gradual Transition in Sodium Borohydride. J. Chem. Phys. 1953, 21, 1311–1312. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Kalnajs, J. The Lattice Constants of the Alkali Borohydrides and the Low-Temperature Phase of Sodium Borohydride. J. Chem. Phys. 1954, 22, 434. [Google Scholar] [CrossRef]
- Stockmayer, W.H.; Rice, D.W.; Stephenson, C.C. Thermodynamic Properties of Sodium Borohydride and Aqueous Borohydride Ion. J. Am. Chem. Soc. 1955, 77, 1980–1983. [Google Scholar] [CrossRef]
- Fischer, P.; Züttel, A. Order-Disorder Phase Transition in NaBD4. Mater. Sci. Forum 2004, 443–444, 287–290. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Talyzin, A.V.; Chernyshov, D.; Dmitriev, V. High-pressure phase of NaBH4: Crystal structure from synchrotron powder diffraction data. Phys. Rev. B 2007, 76, 092104. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Kumar, R.; Weck, P.F.; Cornelius, A.L.; Nicol, M.; Vogel, S.C.; Zhang, J.; Hartl, M.; Stowe, A.C.; Daemen, L.; et al. Pressure-driven phase transitions in NaBH4: Theory and experiments. Phys. Rev. B 2007, 111, 13873–13876. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Haga, T. Recycling process of sodium metaborate to sodium borohydride. Int. J. Hydrog. Energy 2003, 28, 989–993. [Google Scholar] [CrossRef]
- Zhang, J.; Delgass, W.; Fisher, T.; Gore, J. Kinetics of Ru catalyzed sodium borohydride hydrolysis. J. Power Sources 2007, 164, 772–781. [Google Scholar] [CrossRef]
- Wee, J.H.; Lee, K.Y.; Kim, S.H. Sodium borohydride as the hydrogen supplier for proton exchange membrane fuel cell systems. Fuel Process. Technol. 2006, 87, 811–819. [Google Scholar] [CrossRef]
- Maier Chas, G.; Kelley, K.K. An equation for the representation of high-temperature of high heat content data. J. Am. Chem. Soc. 1932, 54, 3243–3246. [Google Scholar] [CrossRef]
- Grimvall, G. (Ed.) Thermophysical Properties of Materials; Elsevier Science B.V.: Amsterdam, The Netherlands, 1999; ISBN 9780444827944. [Google Scholar] [CrossRef]
- Lide, D.R.; Kehiaian, H.V. CRC Handbook of Thermophysical and Thermochemical Data; CRC: Boca Raton, FL, USA, 1994. [Google Scholar]
- Mochalov, K.N.; Khain, V.S.; Gil’manshin, G.G. A generalized scheme for the hydrolysis of the borohydride ion and diborane. Dokl. Akad. Nauk SSSR 1965, 162, 613–616. [Google Scholar]
- Demirci, U.B.; Mielea, P. Cobalt-based catalysts for the hydrolysis of NaBH4 and NH3BH3. Phys. Chem. Chem. Phys. 2014, 16, 6872–6885. [Google Scholar] [CrossRef]
- Seven, F.; Sahiner, N. Enhanced catalytic performance in hydrogen generation from NaBH4 hydrolysis by super porous cryogel supported Co and Ni catalysts. J. Power Sources 2014, 272, 128–136. [Google Scholar] [CrossRef]
- Netskina, O.V.; Tayban, E.S.; Rogov, V.A.; Ozerova, A.M.; Mukha, S.A.; Simagina, V.I.; Komova, O.V. Solid-state NaBH4 composites for hydrogen generation: Catalytic activity of nickel and cobalt catalysts. Int. J. Hydrogen Energy 2021, 46, 5459–5471. [Google Scholar] [CrossRef]
- Brown, H.C.; Subba, R.B.C. A New Powerful Reducing Agent—Sodium Borohydride in the Presence of Aluminum Chloride and Other Polyvalent Metal Halides. J. Am. Chem. Soc. 1956, 78, 2582–2588. [Google Scholar] [CrossRef]
- Jung, S.C.; Nahm, S.W.; Jung, H.Y.; Park, Y.K.; Seo, S.G.; Kim, S.C. Preparations of Platinum Nanoparticles and Their Catalytic Performances. J. Nanosci. Nanotechnol. 2015, 15, 5461–5465. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Zabihi, M. Hydrogen generation by catalytic hydrolysis of sodium borohydride using the nano-bimetallic catalysts supported on the core-shell magnetic nanocomposite of activated carbon. Int. J. Hydrogen Energy 2020, 45, 12331–12346. [Google Scholar] [CrossRef]
- Brown, C.A. Catalytic hydrogenation. V. Reaction of sodium borohydride with aqueous nickel salts. P-1 nickel boride, a convenient, highly active nickel hydrogenation catalyst. J. Org. Chem. 1970, 35, 1900–1904. [Google Scholar] [CrossRef]
- Amendola, S.C.; Binder, M.; Kelly, M.T.; Petillo, P.J.; Sharp-Goldman, S.L. A Novel Catalytic Process for Generating Hydrogen Gas from Aqueous Borohydride Solutions. In Advances in Hydrogen Energy; Padró, C.E.G., Lau, F., Eds.; Springer: Boston, MA, USA, 2002. [Google Scholar] [CrossRef]
- Paksoy, A.; Kurtoğlu, S.F.; Dizaji, A.K.; Altıntaş, Z.; Khoshsima, S.; Uzun, A.; Balcı, Ö. Nanocrystalline cobalt–nickel–boron (metal boride) catalysts for efficient hydrogen production from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 7974–7988. [Google Scholar] [CrossRef]
- Damjanović, L.; Majchrzak, M.; Bennici, S.; Auroux, A. Determination of the heat evolved during sodium borohydride hydrolysis catalyzed by Co3O4. Int. J. Hydrogen Energy 2011, 36, 1991–1997. [Google Scholar] [CrossRef]
- Fernandes, R.; Patel, N.; Miotello, A.; Jaiswal, R.; Kothari, D.C. Dehydrogenation of Ammonia Borane with transition metal-doped Co–B alloy catalysts. Int. J. Hydrogen Energy 2012, 37, 2397–2406. [Google Scholar] [CrossRef]
- Yang, C.C.; Chen, M.S.; Chen, Y.W. Hydrogen generation by hydrolysis of sodium borohydride on CoB/SiO2 catalyst. Int. J. Hydrogen Energy 2011, 36, 1418–1423. [Google Scholar] [CrossRef]
- Sahiner, N.; Ozay, O.; Inger, E.; Aktas, N. Superabsorbent hydrogels for cobalt nanoparticle synthesis and hydrogen production from hydrolysis of sodium boron hydride. Appl. Catal. B Environ. 2011, 102, 201–206. [Google Scholar] [CrossRef]
- Chen, B.; Chen, S.; Bandal, H.A.; Appiah-Ntiamoah, R.; Jadhav, A.R.; Kim, H. Cobalt nanoparticles supported on magnetic core-shell structured carbon as a highly efficient catalyst for hydrogen generation from NaBH4 hydrolysis. Int. J. Hydrogen Energy 2018, 43, 9296–9306. [Google Scholar] [CrossRef]
- Ma, H.; Ji, W.; Zhao, J.; Liang, J.; Chen, J. Preparation, characterization and catalytic NaBH4 hydrolysis of Co-B hollow spheres. J. Alloys Compd. 2009, 474, 584–589. [Google Scholar] [CrossRef]
- Sun, L.; Meng, Y.; Kong, X.; Ge, H.; Chen, X.; Ding, C.; Yang, H.; Li, D.; Gao, X.; Dou, J. Novel high dispersion and high stability cobalt-inlaid carbon sphere catalyst for hydrogen generation from the hydrolysis of sodium borohydride. Fuel 2022, 31, 122276. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Reaction mechanisms of the hydrolysis of sodium borohydride: A discussion focusing on cobalt-based catalysts. Comptes Rendus Chim. 2014, 17, 707–716. [Google Scholar] [CrossRef]
- Hua, D.; Hanxi, Y.; Xinping, A.; Chuansin, C. Hydrogen production from catalytic hydrolysis of sodium borohydride solution using nickel boride catalyst. Int. J. Hydrogen Energy 2003, 28, 1095–1100. [Google Scholar] [CrossRef]
- Tignol, P.; Demirci, U.B. Nickel-based catalysts for hydrogen evolution by hydrolysis of sodium borohydride: From structured nickel hydrazine nitrate complexes to reduced counterparts. Int. J. Hydrogen Energy 2019, 44, 14207–14216. [Google Scholar] [CrossRef]
- Ghodke, N.P.; Rayaprol, S.; Bhoraskar, S.V.; Mathe, V.L. Catalytic hydrolysis of sodium borohydride solution for hydrogen production using thermal plasma synthesized nickel nanoparticles. Int. J. Hydrogen Energy 2020, 45, 16591–16605. [Google Scholar] [CrossRef]
- Kaufman, C.M.; Sen, B. Hydrogen generation by hydrolysis of sodium tetrahydroborate: Effects of acids and transition metals and their salts. J. Chem. Soc. Dalton Trans. 1985, 307–313. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Wei, L.; Zhao, X.; Zhou, X.; Liu, H. Highly efficient and reactivated electrocatalyst of ruthenium electrodeposited on nickel foam for hydrogen evolution from NaBH4 alkaline solution. Int. J. Hydrogen Energy 2018, 43, 592–600. [Google Scholar] [CrossRef]
- Liang, Z.; Li, Q.; Li, F.; Zhao, S.; Xia, X. Hydrogen generation from hydrolysis of NaBH4 based on high stable NiB/NiFe2O4 catalyst. Int. J. Hydrogen Energy 2017, 42, 3971–3980. [Google Scholar] [CrossRef]
- Amendola, S.C.; Sharp-Goldman, S.L.; Janjua, M.S.; Spencer, N.C.; Kelly, M.T.; Petillo, P.J.; Binder, M. A safe, portable, hydrogen gas generator using aqueous borohydride solution and Ru catalyst. Int. J. Hydrogen Energy 2000, 25, 969–975. [Google Scholar] [CrossRef]
- Brown, H.C.; Brown, C.A. Reaction of Sodium Borohydride with Platinum Metal Salts in the Presence of Decolorizing Carbon-A Supported Platinum Catalyst of Markedly Enhanced Activity for Hydrogenations. J. Am. Chem. Soc. 1962, 84, 2827. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, B.; Chan, S.H.; Tang, E.H.; Hong, L. Pt and Ru dispersed on LiCoO2 for hydrogen generation from sodium borohydride solutions. J. Power Sources 2008, 176, 306–311. [Google Scholar] [CrossRef]
- Chen, Y.H.; Pan, C.Y. Effect of various Co–B catalyst synthesis conditions on catalyst surface morphology and NaBH4 hydrolysis reaction kinetic parameters. Int. J. Hydrogen Energy 2014, 39, 1648–1663. [Google Scholar] [CrossRef]
- Liang, Y.; Dai, H.B.; Ma, L.P.; Wang, P.; Cheng, H.M. Hydrogen generation from sodium borohydride solution using a ruthenium supported on graphite catalyst. Int. J. Hydrogen Energy 2010, 35, 3023–3028. [Google Scholar] [CrossRef]
- Senliang, X.; Xiaojun, W.; Dan, W.; Xingtao, H.; Shixue, Z.; Hao, Y. Efficient Hydrogen Generation from Hydrolysis of Sodium Borohydride in Seawater Catalyzed by Polyoxometalate Supported on Activated Carbon. Front. Chem. 2020, 8, 676. [Google Scholar] [CrossRef]
- Jadhav, A.R.; Bandal, H.A.; Kim, H. NiCo2O4 hollow sphere as an efficient catalyst for hydrogen generation by NaBH4 hydrolysis. Mater. Lett. 2017, 198, 50–53. [Google Scholar] [CrossRef]
- Bandal, H.A.; Jadhav, A.R.; Kim, H. Cobalt impregnated magnetite-multiwalled carbon nanotube nanocomposite as magnetically separable efficient catalyst for hydrogen generation by NaBH4 hydrolysis. J. Alloys Compd. 2017, 699, 1057–1067. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.S.; Li, W.; Fortner, J.D. Towards optimizing cobalt based metal oxide nanocrystals for hydrogen generation via NaBH4 hydrolysis. Appl. Catal. A Gen. 2020, 589, 117303. [Google Scholar] [CrossRef]
- Tiri, R.N.E.; Gulbagca, F.; Aygun, A.; Cherif, A.; Sen, F. Biosynthesis of Ag–Pt bimetallic nanoparticles using propolis extract: Antibacterial effects and catalytic activity on NaBH4 hydrolysis. Environ. Res. 2022, 206, 112622. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, Y.; Zhang, W.; Rao, X.; Yan, P.; Isimjan, T.T.; Yang, X. Structure-regulated Ru particles decorated P-vacancy-rich CoP as a highly active and durable catalyst for NaBH4 hydrolysis. J. Colloid Interface Sci. 2021, 591, 221–228. [Google Scholar] [CrossRef]
- Wang, J.; Ke, D.; Yuan Li, Y.; Zhang, H.; Wang, C.; Zhao, X.; Yuan, Y.; Han, S. Efficient hydrolysis of alkaline sodium borohydride catalyzed by cobalt nanoparticles supported on three–dimensional graphene oxide. Mater. Res. Bull. 2017, 95, 204–210. [Google Scholar] [CrossRef]
- İzgi, M.S.; Baytar, O.; Şahin, Ö.; Kazıcı, H.Ç. CeO2 supported multimetallic nano materials as an efficient catalyst for hydrogen generation from the hydrolysis of NaBH4. Int. J. Hydrogen Energy 2020, 45, 34857–34866. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Magnetic sensitive Hericium erinaceus residue chitin/Cu hydrogel nanocomposites for H2 generation by catalyzing NaBH4 hydrolysis. Carbohydr. Polym. 2020, 229, 115426. [Google Scholar] [CrossRef] [PubMed]
- Prasad, D.; Patil, K.N.; Sandhya, N.; Chaitra, C.R.; Bhanushali, J.T.; Samal, A.K.; Keri, R.S.; Jadhav, A.H.; Nagaraja, B.M. Highly efficient hydrogen production by hydrolysis of NaBH4 using eminently competent recyclable Fe2O3 decorated oxidized MWCNTs robust catalyst. Appl. Surf. Sci. 2019, 489, 538–551. [Google Scholar] [CrossRef]
- Zahmakiran, M.; Özkar, S. Zeolite-Confined Ruthenium(0) Nanoclusters Catalyst: Record Catalytic Activity, Reusability, and Lifetime in Hydrogen Generation from the Hydrolysis of Sodium Borohydride. Langmuir 2009, 25, 2667–2678. [Google Scholar] [CrossRef]
- Hung, A.J.; Tsai, S.F.; Hsu, Y.Y.; Ku, J.R.; Chen, Y.H.; Yu, C.C. Kinetics of sodium borohydride hydrolysis reaction for hydrogen generation. Int. J. Hydrogen Energy 2008, 33, 6205–6215. [Google Scholar] [CrossRef]
- Sousa, T.; Fernandes, V.R.; Pinto, P.J.R.; Slavkov, Y.; Bosukov, L.; Rangel, C.M. A sodium borohydride hydrogen generation reactor for stationary applications: Experimental and reactor simulation studies. Chem. Eng. Sci. 2012, 84, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Brewer, J.H.; Heer, A.A.; McLaughlin, C.B. The use of sodium borohydride for producing hydrogen in an anaerobe jar. Appl. Microbiol. 1955, 3, 136. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Gore, J.P.; Fisher, T.S. 1 kWe sodium borohydride hydrogen generation system: Part I: Experimental study. J. Power Sources 2007, 165, 844–853. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Gore, J.P.; Mudawar, I.; Fisher, T.S. 1 kWe sodium borohydride hydrogen generation system: Part II: Reactor modeling. J. Power Sources 2007, 170, 150–159. [Google Scholar] [CrossRef]
- Oronzio, R.; Monteleone, G.; Pozio, A.; De Francesco, M.; Galli, S. New reactor design for catalytic sodium borohydride hydrolysis. Int. J. Hydrogen Energy 2009, 34, 4555–4560. [Google Scholar] [CrossRef]
- Gislon, P.; Monteleone, G.; Prosini, P.P. Hydrogen production from solid sodium borohydride. Int. J. Hydrogen Energy 2009, 34, 929–937. [Google Scholar] [CrossRef]
- Ferreira, M.J.F.; Rangel, C.M.; Pinto, A.M.F.R. Water handling challenge on hydrolysis of sodium borohydride in batch reactors. Int. J. Hydrogen Energy 2012, 37, 6985–6994. [Google Scholar] [CrossRef]
- Marchionni, A.; Bevilacqua, M.; Filippi, J.; Folliero, M.G.; Innocenti, M.; Lavacchi, A.; Miller, H.A.; Pagliaro, M.V.; Vizza, F. High volume hydrogen production from the hydrolysis of sodium borohydride using a cobalt catalyst supported on a honeycomb matrix. J. Power Sources 2015, 299, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.J.; Kim, T. Hydrogen supply system employing direct decomposition of solid-state NaBH4. Int. J. Hydrogen Energy 2015, 40, 2274–2282. [Google Scholar] [CrossRef]
- Minkina, V.M.; Shabunya, S.I.; Kalinin, V.I.; Smirnova, A. Hydrogen generation from sodium borohydride solutions for stationary applications. Int. J. Hydrogen Energy 2016, 41, 9227–9233. [Google Scholar] [CrossRef]
- Cento, C.; Gislon, P.; Prosini, P.P. Hydrogen generation by hydrolysis of NaBH4. Int. J. Hydrogen Energy 2009, 34, 4551–4554. [Google Scholar] [CrossRef]
- Özkan, G.; Akkuş, M.S.; Özkan, G. The effects of operating conditions on hydrogen production from sodium borohydride using Box-Wilson optimization technique. Int. J. Hydrogen Energy 2019, 44, 9811–9816. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Rodríguez-Pérez, I.A.; Miao, W.; Chen, K.; Wang, W.; Li, W.; Han, S. Carbon nanospheres supported bimetallic Pt-Co as an efficient catalyst for NaBH4 hydrolysis. Appl. Surf. Sci. 2021, 540, 148296. [Google Scholar] [CrossRef]
- Ke, D.; Tao, Y.; Li, Y.; Zhao, X.; Zhang, L.; Wang, J.; Han, S. Kinetics study on hydrolytic dehydrogenation of alkaline sodium borohydride catalyzed by Mo-modified Co–B nanoparticles. Int. J. Hydrogen Energy 2015, 40, 7308–7317. [Google Scholar] [CrossRef]
- Nunes, H.X.; Ferreira, M.J.F.; Rangel, C.M.; Pinto, A.M.F.R. Hydrogen generation and storage by aqueous sodium borohydride (NaBH4) hydrolysis for small portable fuel cells (H2—PEMFC). Int. J. Hydrog. Energy 2016, 41, 15426–15432. [Google Scholar] [CrossRef]
- Akkuş, M.S.; Murathan, H.B.; Özgür, D.Ö.; Özkan, G.; Göksel Özkan, G. New insights on the mechanism of vapour phase hydrolysis of sodium borohydride in a fed-batch reactor. Int. J. Hydrogen Energy 2018, 43, 10734–10740. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Chen, L.; Dong, Y.; Xie, P.; Li, Q. Preparation of CoB nanoparticles decorated PANI nanotubes as catalysts for hydrogen generation from NaBH4 hydrolysis. J. Taiwan Inst. Chem. Eng. 2021, 122, 148–156. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lin, J.-C. Reactant Feeding Strategy Analysis of Sodium Borohydride Hydrolysis Reaction Systems for Instantaneous Hydrogen Generation. Energies 2020, 13, 4674. [Google Scholar] [CrossRef]
- Li, Q.; Wang, F.; Zhou, X.; Chen, J.; Tang, C.; Zhang, L. Synergistical photo-thermal-catalysis of Zn2GeO4:xFe3+ for H2 evolution in NaBH4 hydrolysis reaction. Catal. Commun. 2021, 156, 106321. [Google Scholar] [CrossRef]
- Lim, D.; Özkan, G.; Özkan, G. Ni–B and Zr–Ni–B in-situ catalytic performance for hydrogen generation from sodium borohydride, ammonia borane and their mixtures. Int. J. Hydrogen Energy 2022, 47, 3396–3408. [Google Scholar] [CrossRef]
- Pinto, A.M.F.R.; Ferreira, M.J.F.; Fernandes, V.R.; Rangel, C.M. Durability and reutilization capabilities of a Ni–Ru catalyst for the hydrolysis of sodium borohydride in batch reactors. Catal. Today 2011, 170, 40–49. [Google Scholar] [CrossRef]
- Li, R.; Zhang, F.; Zhang, J.; Dong, H. Catalytic hydrolysis of NaBH4 over titanate nanotube supported Co for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 5260–5268. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragan, M. Hydrogen Storage in Complex Metal Hydrides NaBH4: Hydrolysis Reaction and Experimental Strategies. Catalysts 2022, 12, 356. https://doi.org/10.3390/catal12040356
Dragan M. Hydrogen Storage in Complex Metal Hydrides NaBH4: Hydrolysis Reaction and Experimental Strategies. Catalysts. 2022; 12(4):356. https://doi.org/10.3390/catal12040356
Chicago/Turabian StyleDragan, Mirela. 2022. "Hydrogen Storage in Complex Metal Hydrides NaBH4: Hydrolysis Reaction and Experimental Strategies" Catalysts 12, no. 4: 356. https://doi.org/10.3390/catal12040356
APA StyleDragan, M. (2022). Hydrogen Storage in Complex Metal Hydrides NaBH4: Hydrolysis Reaction and Experimental Strategies. Catalysts, 12(4), 356. https://doi.org/10.3390/catal12040356




