Advanced Nickel-Based Catalysts for Urea Oxidation Reaction: Challenges and Developments
Abstract
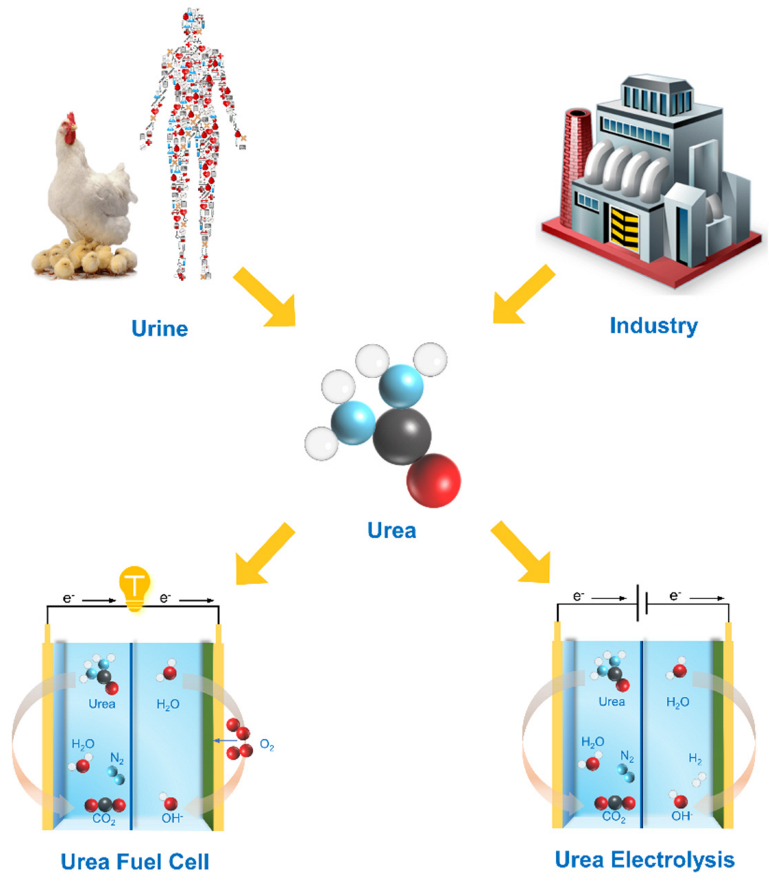
:1. Introduction
2. Mechanism of UOR in Alkaline Media on Ni-Based Catalysts
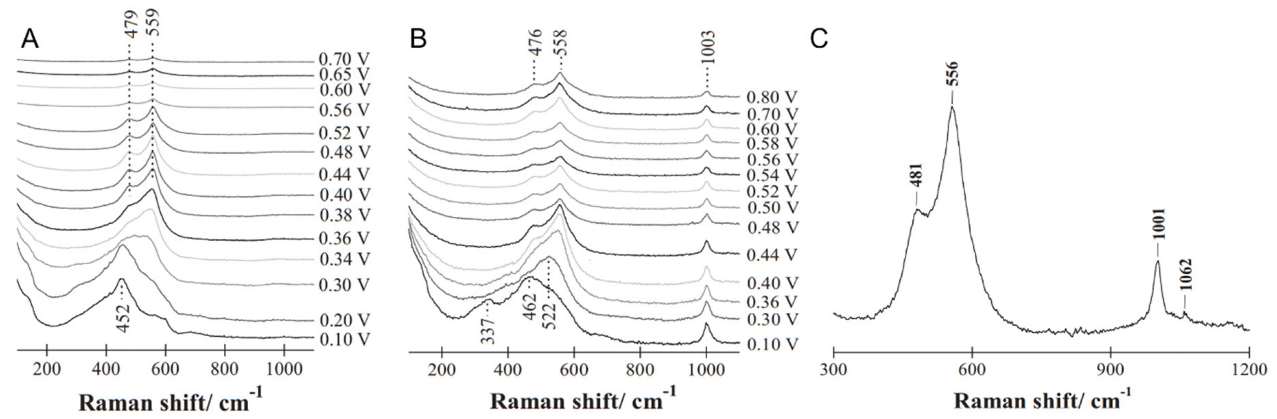
2.1. The Oxidation and Electrochemical Oxidation of Metallic Nickel
2.2. Inactivation or Poisoning of Nickel-Based Catalysts
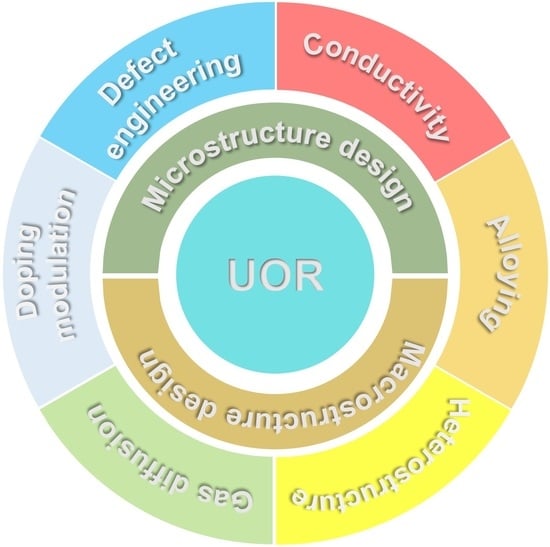
3. Tailoring Ni-Based Catalysts for UOR
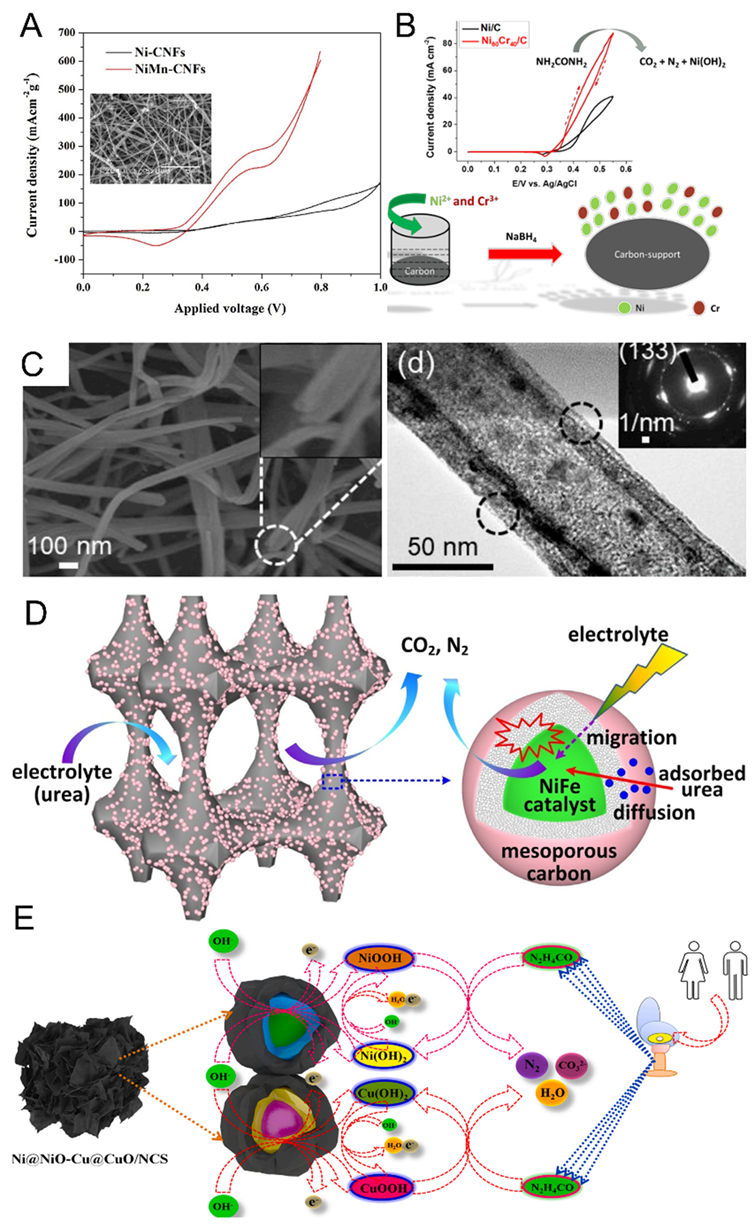
3.1. Other Metals Compounded with Nickel Hydroxides
3.2. Alloying Nickel-Based Catalysts
3.2.1. Alloying with Noble-Metal Nickel-Based Catalysts
3.2.2. Alloying with Non-Noble-Metal Nickel-Based Catalysts
3.3. Heteroatom Modified Nickel-Based Catalysts
3.3.1. Nickel-Based Chalcogenides
3.3.2. Nickel-Based Nitrogen-Element-Doped Compounds
3.3.3. Nickel-Based Sulfides and Nitrogen-Dual-Doped Compounds
3.3.4. Heteroatom-Doped Nickel-Based Metal Compounds
3.4. Nickel-Based Materials Compound with Carbon-Based Materials
3.5. Others Structural Modifications of Nickel-Based Materials
4. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, Z.-Y.; Lang, C.-C.; Gao, M.-R.; Chen, Y.; Fu, Q.-Q.; Duan, Y.; Yu, S.-H. Ni–Mo–O nanorod-derived composite catalysts for efficient alkaline water-to-hydrogen conversion via urea electrolysis. Energy Environ. Sci. 2018, 11, 1890–1897. [Google Scholar] [CrossRef]
- Keshet, R.; Szlosarek, P.; Carracedo, A.; Erez, A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat. Rev. Cancer 2018, 18, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Seredych, M.; Chen, C.; Gura, V.; Mikhalovsky, S.; Sandeman, S.; Ingavle, G.; Ozulumba, T.; Miao, L.; Anasori, B.; et al. MXene sorbents for removal of urea from dialysate: A step toward the wearable artificial kidney. ACS Nano 2018, 12, 10518–10528. [Google Scholar] [CrossRef]
- Batshaw, M.L.; Brusilow, S.; Waber, L.; Blom, W.; Brubakk, A.M.; Burton, B.K.; Cann, H.M.; Kerr, D.; Mamunes, P.; Matalon, R.; et al. Treatment of inborn errors of urea synthesis—activation of alternative pathways of waste nitrogen synthesis and excretion. N. Engl. J. Med. 1982, 306, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhu, X.; Wen, X.; Zhou, Y.; Zhou, L.; Li, H.; Tao, L.; Li, Q.; Du, S.; Liu, T.; et al. Coupling N2 and CO2 in H2O to synthesize urea under ambient conditions. Nat. Chem. 2020, 12, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Adler, L.; Karathia, H.; Carmel, N.; Rabinovich, S.; Auslander, N.; Keshet, R.; Stettner, N.; Silberman, A.; Agemy, L.; et al. Urea Cycle Dysregulation Generates Clinically Relevant Genomic and Biochemical Signatures. Cell 2018, 174, 1559–1570.e22. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Ling, Y.; Lu, X.; Wang, H.; Qian, F.; Tong, Y.; Li, Y. Solar driven hydrogen releasing from urea and human urine. Energy Environ. Sci. 2012, 5, 8215. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Zhang, H.; Liu, W.; Zhu, W.; Zhu, Y. A highly crystalline perylene imide polymer with the robust built-in electric field for efficient photocatalytic water oxidation. Adv. Mater. 2020, 32, e1907746. [Google Scholar] [CrossRef]
- Shukla, K.; Srivastava, V.C. Synthesis of organic carbonates from alcoholysis of urea: A review. Catal. Rev. 2017, 59, 1–43. [Google Scholar] [CrossRef]
- Forslund, R.P.; Mefford, J.T.; Hardin, W.G.; Alexander, C.T.; Johnston, K.P.; Stevenson, K.J. Nanostructured LaNiO3 perovskite electrocatalyst for enhanced urea oxidation. ACS Catal. 2016, 6, 5044–5051. [Google Scholar] [CrossRef]
- Van Gelder, M.K.; Jong, J.A.W.; Folkertsma, L.; Guo, Y.; Bluchel, C.; Verhaar, M.C.; Odijk, M.; Van Nostrum, C.F.; Hennink, W.E.; Gerritsen, K.G.F. Urea removal strategies for dialysate regeneration in a wearable artificial kidney. Biomaterials 2020, 234, 119735. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Stelter, D.; Keyes, T.; Reinhard, B.M. Plasmonic Photocatalysis of urea oxidation and visible-light fuel cells. Chem 2019, 5, 2228–2242. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Zhuo, Z.; Ju, H.; Li, D.; Guo, Y.; Wu, X.; Li, H.; Zhai, T. Local Charge Distribution Engineered by Schottky Heterojunctions toward Urea Electrolysis. Adv. Energy Mater. 2018, 8, 1801775. [Google Scholar] [CrossRef]
- Krajewska, B. Ureases I. Functional, catalytic and kinetic properties: A review. J. Mol. Catal. B Enzym. 2009, 59, 9–21. [Google Scholar] [CrossRef]
- Xue, C.; Wilson, L.D. Kinetic study on urea uptake with chitosan based sorbent materials. Carbohydr. Polym. 2016, 135, 180–186. [Google Scholar] [CrossRef]
- Liu, X.; Sun, S.; Tang, Y.; Li, S.; Chang, J.; Guo, L.A.; Zhao, Y. Preparation and kinetic modeling of cross-linked chitosan microspheres immobilized Zn(II) for urea adsorption. Anal. Lett. 2012, 45, 1632–1644. [Google Scholar] [CrossRef]
- Zhu, L.-J.; Liu, F.; Yu, X.-M.; Gao, A.-L.; Xue, L.-X. Surface zwitterionization of hemocompatible poly(lactic acid) membranes for hemodiafiltration. J. Membr. Sci. 2015, 475, 469–479. [Google Scholar] [CrossRef]
- Simka, W.; Piotrowski, J.; Robak, A.; Nawrat, G. Electrochemical treatment of aqueous solutions containing urea. J. Appl. Electrochem. 2009, 39, 1137–1143. [Google Scholar] [CrossRef]
- Mahalik, K.; Sahu, J.N.; Patwardhan, A.V.; Meikap, B.C. Kinetic studies on hydrolysis of urea in a semi-batch reactor at atmospheric pressure for safe use of ammonia in a power plant for flue gas conditioning. J. Hazard. Mater. 2010, 175, 629–637. [Google Scholar] [CrossRef]
- Von Ahnen, M.; Pedersen, L.-F.; Pedersen, P.B.; Dalsgaard, J. Degradation of urea, ammonia and nitrite in moving bed biofilters operated at different feed loadings. Aquac. Eng. 2015, 69, 50–59. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Greenman, J.; Melhuish, C. Urine utilisation by microbial fuel cells; energy fuel for the future. Phys. Chem. Chem. Phys. 2012, 14, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Ananiev, A. The urea decomposition in the process of the heterogeneous catalytic denitration of nitric acid solutions Part I. Kinetics of the reaction. Appl. Catal. B Environ. 2003, 45, 189–196. [Google Scholar] [CrossRef]
- Shen, S.; Li, M.; Li, B.; Zhao, Z. Catalytic hydrolysis of urea from wastewater using different aluminas by a fixed bed reactor. Environ. Sci. Pollut. Res. Int. 2014, 21, 12563–12568. [Google Scholar] [CrossRef]
- Lundström, A.; Snelling, T.; Morsing, P.; Gabrielsson, P.; Senar, E.; Olsson, L. Urea decomposition and HNCO hydrolysis studied over titanium dioxide, Fe-Beta and γ-Alumina. Appl. Catal. B Environ. 2011, 106, 273–279. [Google Scholar] [CrossRef]
- Xu, H.; Ye, K.; Zhu, K.; Yin, J.; Yan, J.; Wang, G.; Cao, D. Template-directed assembly of urchin-like CoSx/Co-MOF as an efficient bifunctional electrocatalyst for overall water and urea electrolysis. Inorg. Chem. Front. 2020, 7, 2602–2610. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, S.; Liu, J.; Xu, G.; Jia, Q.; Chen, F.; Song, X. Ti-Mesh supported porous CoS2 nanosheet self-interconnected networks with high oxidation states for efficient hydrogen production via urea electrolysis. Nanoscale 2020, 12, 11573–11581. [Google Scholar] [CrossRef]
- Singh, T.I.; Rajeshkhanna, G.; Singh, S.B.; Kshetri, T.; Kim, N.H.; Lee, J.H. Metal-Organic Framework-derived Fe/Co-based Bifunctional electrode for h2 production through water and urea electrolysis. ChemSusChem 2019, 12, 4810–4823. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M. Energy, environment and sustainable development. Renew. Sustain. Energy Rev. 2008, 12, 2265–2300. [Google Scholar] [CrossRef]
- Satyapal, S.; Petrovic, J.; Read, C.; Thomas, G.; Ordaz, G. The U.S. Department of Energy’s National Hydrogen Storage Project: Progress towards meeting hydrogen-powered vehicle requirements. Catal. Today 2007, 120, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Aroonratsameruang, P.; Pattanasattayavong, P.; Dorcet, V.; Mériadec, C.; Ababou-Girard, S.; Fryars, S.; Loget, G. Structure–property relationships in redox-derivatized metal–insulator–semiconductor (MIS) Photoanodes. J. Phys. Chem. C 2020, 124, 25907–25916. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Shao, M.; Wei, M. Directed synthesis of SnO2@BiVO4/Co-Pi photoanode for highly efficient photoelectrochemical water splitting and urea oxidation. J. Mater. Chem. A 2019, 7, 6327–6336. [Google Scholar] [CrossRef]
- Loget, G.; Meriadec, C.; Dorcet, V.; Fabre, B.; Vacher, A.; Fryars, S.; Ababou-Girard, S. Tailoring the photoelectrochemistry of catalytic metal-insulator-semiconductor (MIS) photoanodes by a dissolution method. Nat. Commun. 2019, 10, 3522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Bao, W.W.; Li, M.Y.; Yang, C.M.; Zhang, N.N. Ultrafast formation of an FeOOH electrocatalyst on Ni for efficient alkaline water and urea oxidation. Chem. Commun. 2020, 56, 14713–14716. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhang, Z.; Li, Q.; Jin, W.; Wu, Z.; Fu, G.; Liu, X. Ni-foam supported Co(OH)F and Co–P nanoarrays for energy-efficient hydrogen production via urea electrolysis. J. Mater. Chem. A 2019, 7, 3697–3703. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, S.; Ning, J.; Zhang, Z.; Zhong, Y.; Hu, Y. A one-pot “shielding-to-etching” strategy to synthesize amorphous MoS2 modified CoS/Co0.85Se heterostructured nanotube arrays for boosted energy-saving H2 generation. Nanoscale 2020, 12, 991–1001. [Google Scholar] [CrossRef]
- Ghouri, Z.K.; Elsaid, K.; Al-Meer, S.; Barakat, N.A.M. Applicable anode based on Co3O4–SrCO3 heterostructure nanorods-incorporated CNFs with low-onset potential for DUFCs. Appl. Nanosci. 2017, 7, 625–631. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Ren, T.; Ren, K.; Yu, S.; Liu, M.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Metal-organic frameworks-derived Ru-doped Co2P/N-doped carbon composite nanosheet arrays as bifunctional electrocatalysts for hydrogen evolution and urea oxidation. Chem. Eng. J. 2021, 408, 127308. [Google Scholar] [CrossRef]
- Alotaibi, N.; Hammud, H.H.; Al Otaibi, N.; Prakasam, T. Electrocatalytic properties of 3D hierarchical graphitic carbon-cobalt nanoparticles for urea oxidation. ACS Omega 2020, 5, 26038–26048. [Google Scholar] [CrossRef]
- Xiao, C.; Li, S.; Zhang, X.; MacFarlane, D.R. MnO2/MnCo2O4/Ni heterostructure with quadruple hierarchy: A bifunctional electrode architecture for overall urea oxidation. J. Mater. Chem. A 2017, 5, 7825–7832. [Google Scholar] [CrossRef]
- Elbohy, H.; Bahrami, B.; Mabrouk, S.; Reza, K.M.; Gurung, A.; Pathak, R.; Liang, M.; Qiao, Q.; Zhu, K. Tuning Hole transport layer using urea for high-performance perovskite solar cells. Adv. Funct. Mater. 2018, 29, 1806740. [Google Scholar] [CrossRef]
- Li, P.; Zhuang, Z.; Du, C.; Xiang, D.; Zheng, F.; Zhang, Z.; Fang, Z.; Guo, J.; Zhu, S.; Chen, W. Insights into the Mo-doping effect on the electrocatalytic performance of hierarchical CoxMoyS nanosheet arrays for hydrogen generation and urea oxidation. ACS Appl. Mater. Interfaces 2020, 12, 40194–40203. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Si, J.; Lyu, S.; Zhang, T.; Li, Z.; Lei, C.; Lei, L.; Yuan, C.; Yang, B.; Gao, L.; et al. Highly effective electrochemical exfoliation of ultrathin tantalum disulfide nanosheets for energy-efficient hydrogen evolution electrocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 24675–24682. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Duan, J.; Vasileff, A.; Qiao, S.Z. Size fractionation of two-dimensional sub-nanometer thin manganese dioxide crystals towards superior urea electrocatalytic conversion. Angew. Chem. Int. Ed. 2016, 55, 3804–3808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, Y.; Liu, W.; Xu, L.; Guan, M.; Zhao, Y.; Bao, J.; Li, H. Manganese-modulated cobalt-based layered double hydroxide grown on nickel foam with 1D-2D-3D heterostructure for highly efficient oxygen evolution reaction and urea oxidation reaction. Chemistry 2020, 26, 9382–9388. [Google Scholar] [CrossRef]
- Liu, T.; Liu, D.; Qu, F.; Wang, D.; Zhang, L.; Ge, R.; Hao, S.; Ma, Y.; Du, G.; Asiri, A.M.; et al. Enhanced electrocatalysis for energy-efficient hydrogen production over CoP catalyst with nonelectroactive Zn as a promoter. Adv. Energy Mater. 2017, 7, 1700020. [Google Scholar] [CrossRef]
- Sun, Y.; Duan, J.; Zhu, J.; Chen, S.; Antonietti, M. Metal-cluster-directed surface charge manipulation of two-dimensional nanomaterials for efficient urea electrocatalytic conversion. ACS Appl. Nano Mater. 2018, 1, 6649–6655. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Direct evidence of the mechanism for the electro-oxidation of urea on Ni(OH)2 catalyst in alkaline medium. Electrochim. Acta 2013, 108, 660–665. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, H.; Li, G.; Wu, Z. Nickel-cobalt bimetallic anode catalysts for direct urea fuel cell. Sci. Rep. 2014, 4, 5863. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Huang, J.; Dong, P.; Ji, J.; Li, J.; Cao, L.; Feng, L.; Jin, P.; Wang, C. Decorating CoNi layered double hydroxides nanosheet arrays with fullerene quantum dot anchored on Ni foam for efficient electrocatalytic water splitting and urea electrolysis. Chem. Eng. J. 2020, 390, 124525. [Google Scholar] [CrossRef]
- Xie, J.; Liu, W.; Zhang, X.; Guo, Y.; Gao, L.; Lei, F.; Tang, B.; Xie, Y. Constructing hierarchical wire-on-sheet nanoarrays in phase-regulated cerium-doped nickel hydroxide for promoted urea electro-oxidation. ACS Mater. Lett. 2019, 1, 103–110. [Google Scholar] [CrossRef]
- Babar, P.; Lokhande, A.; Karade, V.; Pawar, B.; Gang, M.G.; Pawar, S.; Kim, J.H. Bifunctional 2D Electrocatalysts of transition metal hydroxide nanosheet arrays for water splitting and urea electrolysis. ACS Sustain. Chem. Eng. 2019, 7, 10035–10043. [Google Scholar] [CrossRef]
- Sha, L.; Ye, K.; Yin, J.; Zhu, K.; Cheng, K.; Yan, J.; Wang, G.; Cao, D. In situ grown 3D hierarchical MnCo2O4.5@Ni(OH)2 nanosheet arrays on Ni foam for efficient electrocatalytic urea oxidation. Chem. Eng. J. 2020, 381, 122603. [Google Scholar] [CrossRef]
- Yue, Z.; Yao, S.; Li, Y.; Zhu, W.; Zhang, W.; Wang, R.; Wang, J.; Huang, L.; Zhao, D.; Wang, J. Surface engineering of hierarchical Ni(OH)2 nanosheet@nanowire configuration toward superior urea electrolysis. Electrochim. Acta 2018, 268, 211–217. [Google Scholar] [CrossRef]
- Zeng, M.; Wu, J.; Li, Z.; Wu, H.; Wang, J.; Wang, H.; He, L.; Yang, X. Interlayer effect in NiCo layered double hydroxide for promoted electrocatalytic urea oxidation. ACS Sustain. Chem. Eng. 2019, 7, 4777–4783. [Google Scholar] [CrossRef]
- Wang, T.; Wu, H.; Feng, C.; Zhang, L.; Zhang, J. MoP@NiCo-LDH on nickel foam as bifunctional electrocatalyst for high efficiency water and urea–water electrolysis. J. Mater. Chem. A 2020, 8, 18106–18116. [Google Scholar] [CrossRef]
- Meng, J.; Chernev, P.; Mohammadi, M.R.; Klingan, K.; Loos, S.; Pasquini, C.; Kubella, P.; Jiang, S.; Yang, X.; Cui, Z.; et al. Self-supported Ni(OH)2/MnO2 on CFP as a flexible anode towards electrocatalytic urea conversion: The role of composition on activity, redox states and reaction dynamics. Electrochim. Acta 2019, 318, 32–41. [Google Scholar] [CrossRef]
- Yan, X.; Hu, Q.-T.; Liu, J.; Zhang, W.-D.; Gu, Z.-G. Ultrafine-grained NiCo layered double hydroxide nanosheets with abundant active edge sites for highly enhanced electro-oxidation of urea. Electrochim. Acta 2021, 368, 137648. [Google Scholar] [CrossRef]
- Sun, C.B.; Guo, M.W.; Siwal, S.S.; Zhang, Q.B. Efficient hydrogen production via urea electrolysis with cobalt doped nickel hydroxide-riched hybrid films: Cobalt doping effect and mechanism aspect. J. Catal. 2020, 381, 454–461. [Google Scholar] [CrossRef]
- Xia, L.; Liao, Y.; Qing, Y.; Xu, H.; Gao, Z.; Li, W.; Wu, Y. In Situ growth of porous ultrathin ni(oh)2 nanostructures on nickel foam: An efficient and durable catalysts for urea electrolysis. ACS Appl. Energy Mater. 2020, 3, 2996–3004. [Google Scholar] [CrossRef]
- Xie, J.; Gao, L.; Cao, S.; Liu, W.; Lei, F.; Hao, P.; Xia, X.; Tang, B. Copper-incorporated hierarchical wire-on-sheet α-Ni(OH)2 nanoarrays as robust trifunctional catalysts for synergistic hydrogen generation and urea oxidation. J. Mater. Chem. A 2019, 7, 13577–13584. [Google Scholar] [CrossRef]
- Lan, R.; Tao, S.; Irvine, J.T.S. A direct urea fuel cell—Power from fertiliser and waste. Energy Environ. Sci. 2010, 3, 438. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Lee, D.; Lee, Y.N.; Yoon, Y.S.; Kim, D.-J. Solid solution palladium-nickel bimetallic anode catalysts by co-sputtering for direct urea fuel cells (DUFC). J. Power Sources 2019, 431, 259–264. [Google Scholar] [CrossRef]
- Ding, R.; Qi, L.; Jia, M.; Wang, H. Correction: Facile synthesis of mesoporous spinel NiCo2O4 nanostructures as highly efficient electrocatalysts for urea electro-oxidation. Nanoscale 2015, 7, 9946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, R.L.; Botte, G.G. Hydrogen production via urea electrolysis using a gel electrolyte. J. Power Sources 2011, 196, 2773–2778. [Google Scholar] [CrossRef]
- Guo, F.; Cao, D.; Du, M.; Ye, K.; Wang, G.; Zhang, W.; Gao, Y.; Cheng, K. Enhancement of direct urea-hydrogen peroxide fuel cell performance by three-dimensional porous nickel-cobalt anode. J. Power Sources 2016, 307, 697–704. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Botte, G.G. Nickel and cobalt bimetallic hydroxide catalysts for urea electro-oxidation. Electrochim. Acta 2012, 61, 25–30. [Google Scholar] [CrossRef]
- Vidotti, M.; Silva, M.R.; Salvador, R.P.; de Torresi, S.I.C.; Dall’Antonia, L.H. Electrocatalytic oxidation of urea by nanostructured nickel/cobalt hydroxide electrodes. Electrochim. Acta 2008, 53, 4030–4034. [Google Scholar] [CrossRef]
- Periyasamy, S.; Subramanian, P.; Levi, E.; Aurbach, D.; Gedanken, A.; Schechter, A. Exceptionally active and stable spinel nickel manganese oxide electrocatalysts for urea oxidation reaction. ACS Appl. Mater. Interfaces 2016, 8, 12176–12185. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; El-Newehy, M.H.; Yasin, A.S.; Ghouri, Z.K.; Al-Deyab, S.S. Ni&Mn nanoparticles-decorated carbon nanofibers as effective electrocatalyst for urea oxidation. Appl. Catal. A Gen. 2016, 510, 180–188. [Google Scholar]
- Barakat, N.A.M.; Alajami, M.; Al Haj, Y.; Obaid, M.; Al-Meer, S. Enhanced onset potential NiMn-decorated activated carbon as effective and applicable anode in urea fuel cells. Catal. Commun. 2017, 97, 32–36. [Google Scholar] [CrossRef]
- Vilana, J.; Gómez, E.; Vallés, E. Influence of the composition and crystalline phase of electrodeposited CoNi films in the preparation of CoNi oxidized surfaces as electrodes for urea electro-oxidation. Appl. Surf. Sci. 2016, 360, 816–825. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Wang, D.; Botte, G.G. Electrochemical decomposition of urea with Ni-based catalysts. Appl. Catal. B Environ. 2012, 127, 221–226. [Google Scholar] [CrossRef]
- Kakati, N.; Maiti, J.; Lee, K.S.; Viswanathan, B.; Yoon, Y.S. Hollow sodium nickel fluoride nanocubes deposited MWCNT as an efficient electrocatalyst for urea oxidation. Electrochim. Acta 2017, 240, 175–185. [Google Scholar] [CrossRef]
- Wang, L.; Du, T.; Cheng, J.; Xie, X.; Yang, B.; Li, M. Enhanced activity of urea electrooxidation on nickel catalysts supported on tungsten carbides/carbon nanotubes. J. Power Sources 2015, 280, 550–554. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Understanding the electro-catalytic oxidation mechanism of urea on nickel electrodes in alkaline medium. Electrochim. Acta 2012, 81, 292–300. [Google Scholar] [CrossRef]
- Xu, W.; Du, D.; Lan, R.; Humphreys, J.; Wu, Z.; Tao, S. Highly active Ni–Fe double hydroxides as anode catalysts for electrooxidation of urea. New J. Chem. 2017, 41, 4190–4196. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Al Haj, Y.; Alajami, M.; Alawadhi, H.; Barakat, N.A.M. Ni-Cd carbon nanofibers as an effective catalyst for urea fuel cell. J. Environ. Chem. Eng. 2018, 6, 332–337. [Google Scholar] [CrossRef]
- Kim, B.; Das, G.; Park, B.J.; Lee, D.H.; Yoon, H.H. A free-standing NiCr-CNT@C anode mat by electrospinning for a high-performance urea/H2O2 fuel cell. Electrochim. Acta 2020, 354, 136657. [Google Scholar] [CrossRef]
- Singh, R.K.; Schechter, A. Electroactivity of NiCr catalysts for urea oxidation in alkaline electrolyte. ChemCatChem 2017, 9, 3374–3379. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Yasin, A.S.; Barakat, N.A.M.; Song, S.A.; Lee, H.E.; Kim, S.S. Electrocatalytic behavior of a nanocomposite of Ni/Pd supported by carbonized PVA nanofibers towards formic acid, ethanol and urea oxidation: A physicochemical and electro-analysis study. Appl. Surf. Sci. 2018, 435, 122–129. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, W.; Li, J.-G.; Li, Z.; Ao, X.; Xue, K.-H.; Ostrikov, K.K.; Tang, J.; Wang, C. Rh-engineered ultrathin NiFe-LDH nanosheets enable highly-efficient overall water splitting and urea electrolysis. Appl. Catal. B Environ. 2021, 284, 119740. [Google Scholar] [CrossRef]
- Xu, Y.; Chai, X.; Ren, T.; Yu, S.; Yu, H.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Ir-Doped Ni-based metal-organic framework ultrathin nanosheets on Ni foam for enhanced urea electro-oxidation. Chem. Commun. 2020, 56, 2151–2154. [Google Scholar] [CrossRef] [PubMed]
- Khalafallah, D.; Xiaoyu, L.; Zhi, M.; Hong, Z. 3D Hierarchical NiCo layered double hydroxide nanosheet arrays decorated with noble metal nanoparticles for enhanced urea electrocatalysis. ChemElectroChem 2019, 7, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Yan, W.; Vijapur, S.H.; Botte, G.G. Enhanced electrocatalytic oxidation of urea based on nickel hydroxide nanoribbons. J. Power Sources 2012, 217, 498–502. [Google Scholar] [CrossRef]
- Ye, K.; Zhang, D.; Guo, F.; Cheng, K.; Wang, G.; Cao, D. Highly porous nickel@carbon sponge as a novel type of three-dimensional anode with low cost for high catalytic performance of urea electro-oxidation in alkaline medium. J. Power Sources 2015, 283, 408–415. [Google Scholar] [CrossRef]
- Guo, F.; Ye, K.; Cheng, K.; Wang, G.; Cao, D. Preparation of nickel nanowire arrays electrode for urea electro-oxidation in alkaline medium. J. Power Sources 2015, 278, 562–568. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Diaz, L.A.; Botte, G.G. Nickel nanowires as effective catalysts for urea electro-oxidation. Electrochim. Acta 2014, 134, 266–271. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; He, T.; Wang, M.; Qi, R.; Yan, Y.; Dong, Z.; Liu, H.; Wang, H.; Xia, B.Y. Energy-saving hydrogen production coupling urea oxidation over a bifunctional nickel-molybdenum nanotube array. Nano Energy 2019, 60, 894–902. [Google Scholar] [CrossRef]
- Cao, J.; Li, H.; Zhu, R.; Ma, L.; Zhou, K.; Wei, Q.; Luo, F. Improved hydrogen generation via a urea-assisted method over 3D hierarchical NiMo-based composite microrod arrays. J. Alloy. Compd. 2020, 844, 155382. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Liu, C. Chemical design of novel electrospun CoNi/Cr nanoparticles encapsulated in C-nanofibers as highly efficient material for urea oxidation in alkaline media. Appl. Surf. Sci. 2019, 475, 532–541. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Li, W.; Li, F.; Arandiyan, H.; Sun, H.; Chen, Y. Free-standing Ni-Co alloy nanowire arrays: Efficient and robust catalysts toward urea electro-oxidation. Electrochim. Acta 2018, 283, 1277–1283. [Google Scholar] [CrossRef]
- Wu, M.-S.; Jao, C.-Y.; Chuang, F.-Y.; Chen, F.-Y. Carbon-encapsulated nickel-iron nanoparticles supported on nickel foam as a catalyst electrode for urea electrolysis. Electrochim. Acta 2017, 227, 210–216. [Google Scholar] [CrossRef]
- Nangan, S.; Ding, Y.; Alhakemy, A.Z.; Liu, Y.; Wen, Z. Hybrid alkali-acid urea-nitrate fuel cell for degrading nitrogen-rich wastewater. Appl. Catal. B Environ. 2021, 286, 119892. [Google Scholar] [CrossRef]
- Singh, R.K.; Subramanian, P.; Schechter, A. enhanced urea activity of oxidation on nickel-deposited tin dendrites. ChemElectroChem 2017, 4, 1037–1043. [Google Scholar] [CrossRef]
- Wang, S.; Xu, P.; Tian, J.; Liu, Z.; Feng, L. Phase structure tuning of graphene supported Ni-NiO Nanoparticles for enhanced urea oxidation performance. Electrochim. Acta 2021, 370, 137755. [Google Scholar] [CrossRef]
- Liu, M.; Jiao, Y.; Zhan, S.; Wang, H. Ni3S2 nanowires supported on Ni foam as efficient bifunctional electrocatalyst for urea-assisted electrolytic hydrogen production. Catal. Today 2020, 355, 596–601. [Google Scholar] [CrossRef]
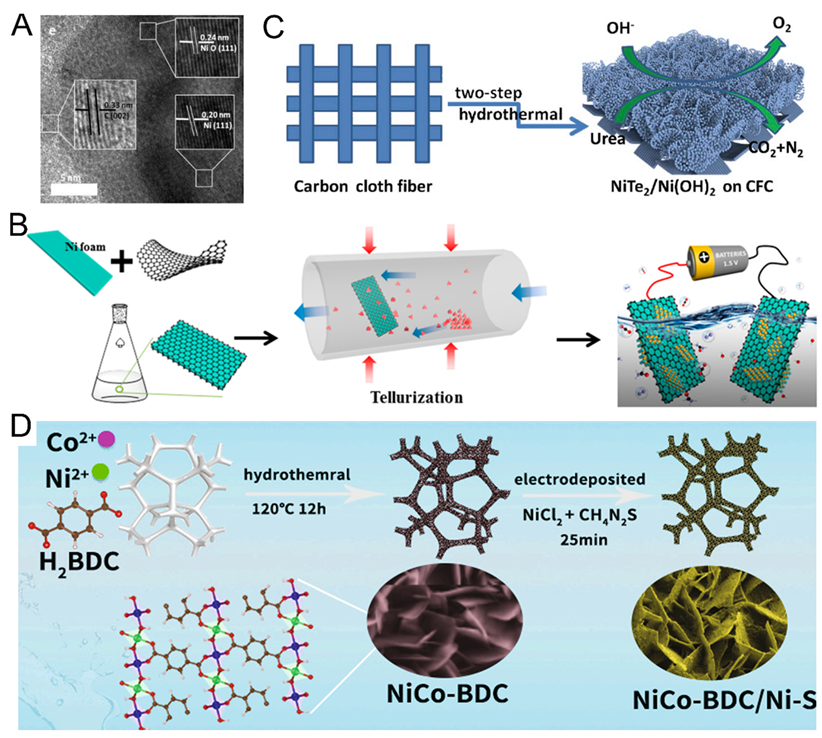
- Xu, B.; Yang, X.; Liu, X.; Song, W.; Sun, Y.; Liu, Q.; Yang, H.; Li, C. Lattice distortion in hybrid NiTe2/Ni(OH)2 nanosheets as efficient synergistic electrocatalyst for water and urea oxidation. J. Power Sources 2020, 449, 227585. [Google Scholar] [CrossRef]
- Li, M.; Ao, X.; Li, J.-G.; Sun, H.; Zheng, L.; Wang, C. NiCo-BDC nanosheets coated with amorphous Ni-S thin film for high-efficiency oxygen evolution reaction and urea oxidation reaction. FlatChem 2021, 25, 100222. [Google Scholar] [CrossRef]
- Song, W.; Xu, M.; Teng, X.; Niu, Y.; Gong, S.; Liu, X.; He, X.; Chen, Z. Construction of self-supporting, hierarchically structured caterpillar-like NiCo2S4 arrays as an efficient trifunctional electrocatalyst for water and urea electrolysis. Nanoscale 2021, 13, 1680–1688. [Google Scholar] [CrossRef]
- Lu, S.; Gu, Z.; Hummel, M.; Zhou, Y.; Wang, K.; Xu, B.B.; Wang, Y.; Li, Y.; Qi, X.; Liu, X. Nickel Oxide Immobilized on the Carbonized Eggshell Membrane for Electrochemical Detection of Urea. J. Electrochem. Soc. 2020, 167, 106509. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, P.; Liu, M.; Guo, C.; Liu, H.; Wei, S.; Zhang, J.; Lu, X. Rational design of metallic NiTex (x = 1 or 2) as bifunctional electrocatalysts for efficient urea conversion. ACS Appl. Energy Mater. 2019, 2, 3363–3372. [Google Scholar] [CrossRef]
- Hu, S.; Wang, S.; Feng, C.; Wu, H.; Zhang, J.; Mei, H. Novel MOF-derived nickel nitride as high-performance bifunctional electrocatalysts for hydrogen evolution and urea oxidation. ACS Sustain. Chem. Eng. 2020, 8, 7414–7422. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, L.; Qu, F.; Liu, Z.; Du, G.; Asiri, A.M.; Sun, X. A porous Ni3N nanosheet array as a high-performance non-noble-metal catalyst for urea-assisted electrochemical hydrogen production. Inorg. Chem. Front. 2017, 4, 1120–1124. [Google Scholar] [CrossRef]
- Khalafallah, D.; Zhi, M.; Hong, Z. Shape-dependent electrocatalytic activity of carbon reinforced Ni2P hybrids toward urea electrocatalysis. Energy Technol. 2019, 7, 1900548. [Google Scholar] [CrossRef]
- Wang, G.; Ye, K.; Shao, J.; Zhang, Y.; Zhu, K.; Cheng, K.; Yan, J.; Wang, G.; Cao, D. Porous Ni2P nanoflower supported on nickel foam as an efficient three-dimensional electrode for urea electro-oxidation in alkaline medium. Int. J. Hydrog. Energy 2018, 43, 9316–9325. [Google Scholar] [CrossRef]
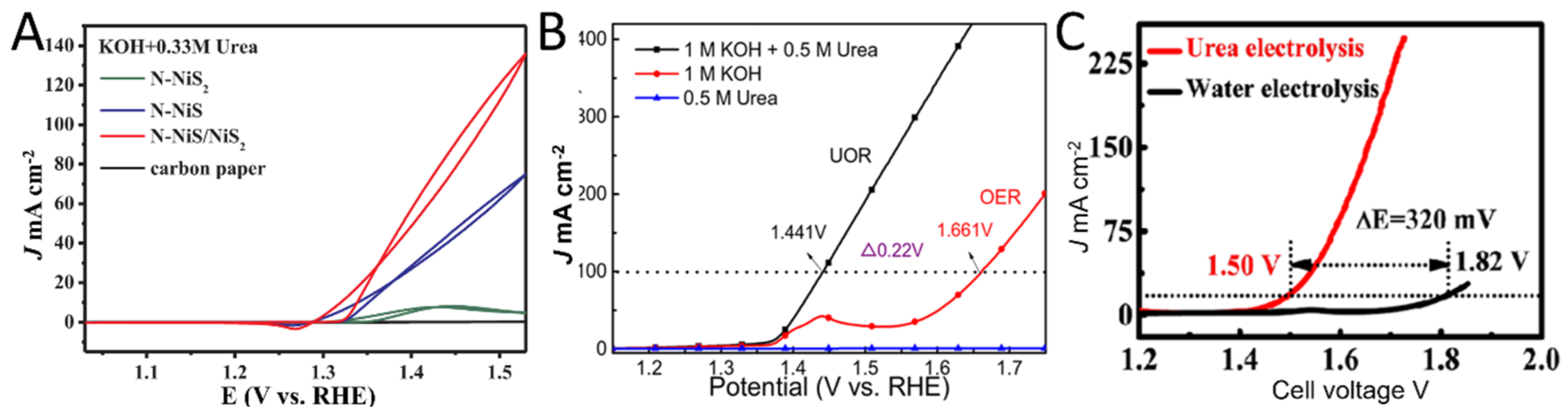
- Liu, H.; Liu, Z.; Wang, F.; Feng, L. Efficient catalysis of N doped NiS/NiS2 heterogeneous structure. Chem. Eng. J. 2020, 397, 125507. [Google Scholar] [CrossRef]
- He, M.; Feng, C.; Liao, T.; Hu, S.; Wu, H.; Sun, Z. Low-cost Ni2P/Ni0.96S heterostructured bifunctional electrocatalyst toward highly efficient overall urea-water electrolysis. ACS Appl. Mater. Interfaces 2020, 12, 2225–2233. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, Y.; Wang, Y.; Li, B.; Zhang, Y.; Ma, Z.; Liu, S. Coaxial Ni-S@N-doped carbon nanofibers derived hierarchical electrodes for efficient H2 production via urea electrolysis. ACS Appl. Mater. Interfaces 2021, 13, 3937–3948. [Google Scholar] [CrossRef]
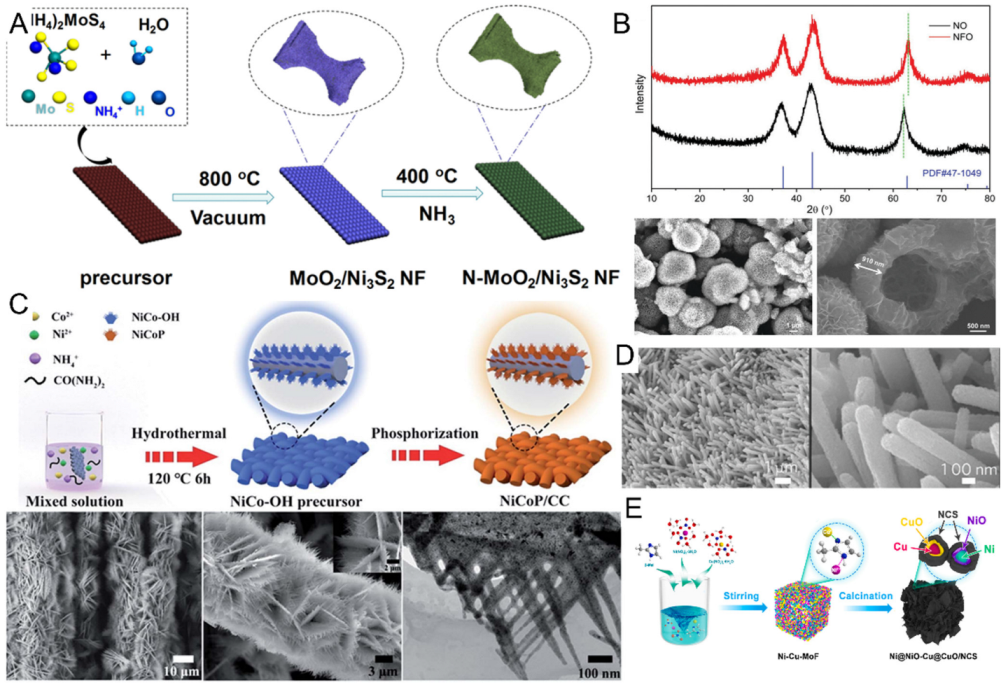
- Wang, L.; Cao, J.; Lei, C.; Dai, Q.; Yang, B.; Li, Z.; Zhang, X.; Yuan, C.; Lei, L.; Hou, Y. Strongly coupled 3D N-doped MoO2/Ni3S2 hybrid for high current density hydrogen evolution electrocatalysis and biomass upgrading. ACS Appl. Mater. Interfaces 2019, 11, 27743–27750. [Google Scholar] [CrossRef]
- Wu, F.; Ou, G.; Yang, J.; Li, H.; Gao, Y.; Chen, F.; Wang, Y.; Shi, Y. Bifunctional nickel oxide-based nanosheets for highly efficient overall urea splitting. Chem. Commun. 2019, 55, 6555–6558. [Google Scholar] [CrossRef]
- Sha, L.; Yin, J.; Ye, K.; Wang, G.; Zhu, K.; Cheng, K.; Yan, J.; Wang, G.; Cao, D. The construction of self-supported thorny leaf-like nickel-cobalt bimetal phosphides as efficient bifunctional electrocatalysts for urea electrolysis. J. Mater. Chem. A 2019, 7, 9078–9085. [Google Scholar] [CrossRef]
- Han, W.K.; Li, X.P.; Lu, L.N.; Ouyang, T.; Xiao, K.; Liu, Z.Q. Partial S substitution activates NiMoO4 for efficient and stable electrocatalytic urea oxidation. Chem. Commun. 2020, 56, 11038–11041. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, S.; Shahrokhian, S. 3D ternary NixCo2-xP/C nanoflower/nanourchin arrays grown on HCNs: A highly efficient bi-functional electrocatalyst for boosting hydrogen production via the urea electro-oxidation reaction. Nanoscale 2020, 12, 16123–16135. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yao, C.; Kong, X.; Li, Z.; Jiang, M.; Zhang, F.; Lei, X. Boosting hydrogen production by electrooxidation of urea over 3d hierarchical Ni4N/Cu3N nanotube arrays. ACS Sustain. Chem. Eng. 2019, 7, 13278–13285. [Google Scholar] [CrossRef]
- Zhu, W.; Ren, M.; Hu, N.; Zhang, W.; Luo, Z.; Wang, R.; Wang, J.; Huang, L.; Suo, Y.; Wang, J. Traditional NiCo2S4 phase with porous nanosheets array topology on carbon cloth: A flexible, versatile and fabulous electrocatalyst for overall water and urea electrolysis. ACS Sustain. Chem. Eng. 2018, 6, 5011–5020. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Zhu, S.; Shao, M.; Yang, B.; Chen, J.G. Tungsten carbide and cobalt modified nickel nanoparticles supported on multiwall carbon nanotubes as highly efficient electrocatalysts for urea oxidation in alkaline electrolyte. ACS Appl. Mater. Interfaces 2018, 10, 41338–41343. [Google Scholar] [CrossRef]
- Wang, L.; Ren, L.; Wang, X.; Feng, X.; Zhou, J.; Wang, B. Multivariate MOF-templated pomegranate-Like Ni/C as efficient bifunctional electrocatalyst for hydrogen evolution and urea oxidation. ACS Appl. Mater. Interfaces 2018, 10, 4750–4756. [Google Scholar] [CrossRef]
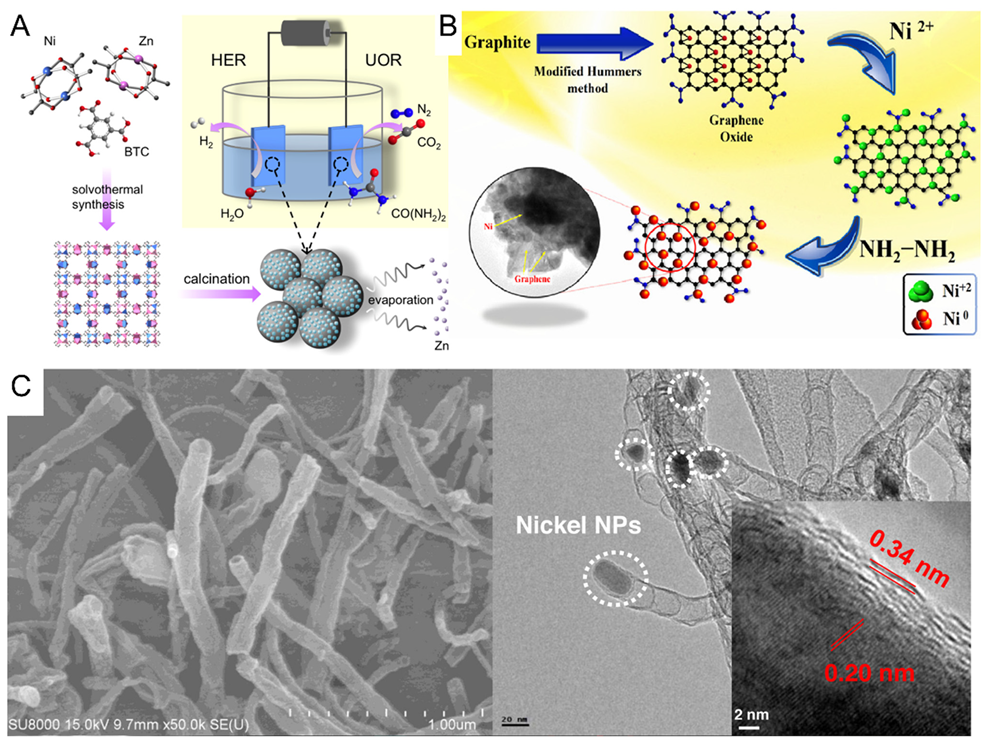
- Zhang, Q.; Kazim, F.M.D.; Ma, S.; Qu, K.; Li, M.; Wang, Y.; Hu, H.; Cai, W.; Yang, Z. Nitrogen dopants in nickel nanoparticles embedded carbon nanotubes promote overall urea oxidation. Appl. Catal. B Environ. 2021, 280, 119436. [Google Scholar] [CrossRef]
- Munde, A.V.; Mulik, B.B.; Chavan, P.P.; Sathe, B.R. Enhanced electrocatalytic activity towards urea oxidation on Ni nanoparticle decorated graphene oxide nanocomposite. Electrochim. Acta 2020, 349, 136386. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Lin, H.; Liu, Y.; Ye, J.; Wen, Y.; Chen, A.; Wang, L.; Ni, F.; Zhou, Z.; et al. A lattice-oxygen-involved reaction pathway to boost urea oxidation. Angew. Chem. Int. Ed. Engl. 2019, 58, 16820–16825. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Mao, Z.; Yan, C.; Shen, G.; Wang, X. Bimetal schottky heterojunction boosting energy-saving hydrogen production from alkaline water via urea electrocatalysis. Adv. Funct. Mater. 2020, 30, 2000556. [Google Scholar] [CrossRef]
- He, Q.; Wan, Y.; Jiang, H.; Pan, Z.; Wu, C.; Wang, M.; Wu, X.; Ye, B.; Ajayan, P.M.; Song, L. Nickel vacancies boost reconstruction in nickel hydroxide electrocatalyst. ACS Energy Lett. 2018, 3, 1373–1380. [Google Scholar] [CrossRef]
- Tong, Y.; Chen, P.; Zhang, M.; Zhou, T.; Zhang, L.; Chu, W.; Wu, C.; Xie, Y. oxygen vacancies confined in nickel molybdenum oxide porous nanosheets for promoted electrocatalytic urea oxidation. ACS Catal. 2017, 8, 1–7. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Ma, C.; Wang, Y.; Wang, K. Advanced Nickel-Based Catalysts for Urea Oxidation Reaction: Challenges and Developments. Catalysts 2022, 12, 337. https://doi.org/10.3390/catal12030337
Ma Y, Ma C, Wang Y, Wang K. Advanced Nickel-Based Catalysts for Urea Oxidation Reaction: Challenges and Developments. Catalysts. 2022; 12(3):337. https://doi.org/10.3390/catal12030337
Chicago/Turabian StyleMa, Yaming, Chenxiang Ma, Yingche Wang, and Ke Wang. 2022. "Advanced Nickel-Based Catalysts for Urea Oxidation Reaction: Challenges and Developments" Catalysts 12, no. 3: 337. https://doi.org/10.3390/catal12030337
APA StyleMa, Y., Ma, C., Wang, Y., & Wang, K. (2022). Advanced Nickel-Based Catalysts for Urea Oxidation Reaction: Challenges and Developments. Catalysts, 12(3), 337. https://doi.org/10.3390/catal12030337