Abstract
The development of a new type of oxygen evolution reaction (OER) catalyst to reduce the energy loss in the process of water electrolysis is of great significance to the realization of the industrialization of hydrogen energy storage. Herein, we report the catalysts of NiFe double-layer hydroxide (NiFe-LDH) mixed with different equivalent terephthalic acid (TPA), synthesized by the hydrothermal method. The catalyst synthesized with the use of the precursor solution containing one equivalent of TPA shows the best performance with the current density of 2 mA cm−2 at an overpotential of 270 mV, the Tafel slope of 40 mV dec−1, and excellent stable electrocatalytic performance for OER. These catalysts were characterized in a variety of methods. X-ray diffraction (XRD), Fourier Transform Infrared Spectrometer (FTIR), and Raman spectrum proved the presence of TPA in the catalysts. The lamellar structure and the uniform distribution of Ni and Fe in the catalysts were observed by a scanning electron microscope (SEM) and a transmission electron microscope (TEM). In X-ray photoelectron spectroscopy (XPS) of NiFe-LDH with and without TPA, the changes in the peak positions of Ni and Fe spectra indicate strong electronic interactions between TPA and Ni and Fe atoms. These results suggest that a certain amount of TPA can boost catalytic activity.
1. Introduction
The electrolysis of water is regarded as one of the most promising energy conversion and storage technologies [1,2,3]. Generally, the reaction is composed of two half-reactions, oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), which occur on the surface of the positive and negative electrode, respectively. It is well known that the OER is the bottleneck of water electrolysis due to the complex transfer process of four electrons and protons, leading to a high overpotential (the difference between thermodynamic potential and the actual applied potential) and increased energy consumption to ensure the oxygen evolution [4,5]. Therefore, it is necessary to develop the catalysts with low overpotential.
The OER catalysts have been developing for many years and various excellent catalysts have been proposed. Among these catalysts, Iridium (Ir)-based and Ruthenium (Ru)-based catalysts have shown the best OER performance in acid [6,7]. For example, Paoli et al. synthesized mass-selected RuO2 nanoparticles that show 0.6 A mg−1 at 0.25 V overpotential [8]. Reier and co-workers compared the catalytic activity and durability of Ru-based and Ir-based nanoparticles and corresponding bulk materials [9]. Although Ir-based and Ru-based catalysts display extremely high catalytic efficiency in the OER process, their scarcity and expensiveness limit further industrial development. Evidently, finding a cheap, efficient, and stable OER catalyst is extremely important.
NiFe-based electrocatalysts have gradually attracted plenty of attention due to their earth-abundant compositions and excellent activity [10,11,12,13,14]. Corrigan et al. first explored the incorporation of iron into nickel oxide that could improve the OER kinetics in the 1980s [15]. In recent years, the research on NiFe-based catalysts as one of the most active transition metal-based electrocatalysts has been boosted due to the development of new materials and technologies [16,17,18,19]. In particular, NiFe-layered double hydroxide (LDH) shows great catalytic activity for OER under intense studies [20,21,22,23]. The reason for the improved catalytic performance may be attributed to the mutual adjustment of the d-band electronic structure between Ni and Fe atoms, which is more conducive to the transport of electrons during the OER [12,24].
Moreover, chemical tunability such as organic ligand modification can enhance catalytic activity [25,26,27,28]. For example, Chen et al. synthesized NiFe-bimetal-organic frameworks (NFF-MOF) with the use of nickel-iron foam as the support, exhibiting 250 mV at a current density of 10 mA cm−2 in the OER process [29]. Zhao’s group reported NiCo bimetal-organic framework nanosheets with an electrochemical overpotential of 250 mV at the current density of 10 mA cm−2 [27]. In fact, for metal-organic complexes, the binding energy of the d-band center of the catalytically active center element could be adjusted by negatively charged coordination groups or non-metallic atoms, which may significantly enhance the electrocatalytic rate [26,29,30,31]. In addition, free radicals may participate in the transmission of protons during the OER [32,33]. According to Li’s report, the uncoordinated carboxylates could serve as the proton transfer relays which would accelerate the transfer rate [32]. Thus, based on the above reports, introducing organic compounds with carboxylic acid groups into NiFe-LDH might improve OER catalytic ability.
Herein, we report the catalysts of NiFe-LDH mixed with different equivalent terephthalic acid (TPA), synthesized by the hydrothermal method. In these catalysts, nickel and iron atoms play the role of catalytic active center, and terephthalic acid (TPA) serves as adjusting the d-bond center of catalytic active atoms. In this article, the effect of the introduced TPA on catalyst structure, morphology, and electrochemical catalytic performance was investigated. In addition, Fourier transform infrared spectroscopy (FTIR), Raman spectra, X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscope (SEM), transmission electron microscopy (TEM), and inductively coupled plasma-optical emission spectrometry (ICP-OES) were measured to characterize the catalysts.
2. Results
In order to evaluate the effect of the amount of TPA on the catalytic performance of the catalyst, four precursor solutions with different molar equivalent (Eq) of TPA for catalyst synthesis were prepared by separately adding 0 Eq, 0.5 Eq, 1 Eq, and 2 Eq of TPA into the solution including 0.5 Eq Fe ion, 0.5 Eq Ni ion, and 1 Eq Urea. The corresponding synthesized catalysts are named NiFe-LDH-TPA0, NiFe-LDH-TPA0.5, NiFe-LDH-TPA1, and NiFe-LDH-TPA2, respectively. Among them, NiFe-LDH-TPA0 represents pure NiFe-LDH without TPA. The specific experimental details could be found in the section of Materials and Methods. According to reports in the published literature, the presence of carboxylic acid groups influences the deposition of oxide particles [34]. Therefore, we conducted a semi-quantitative analysis to verify. The IPC-OES was performed to investigate the molar ratio of nickel and iron and the result was shown in Table 1. The values of ratio (Fe:Ni) are 5.67, 4.09, 4.92, and 58.33 corresponding to NiFe-LDH-TPA0, NiFe-LDH-TPA0.5, NiFe-LDH-TPA1, and NiFe-LDH-TPA2, respectively, indicating the amount of TPA may affect the deposition ratio of Fe to Ni. Adding no more than 1 Eq of TPA in precursor has little effect on the ratio of Fe to Ni in the system, however, excessive TPA of 2 Eq (or even more) would cause the Ni deposition efficiency to decrease significantly.

Table 1.
ICP-OES results.
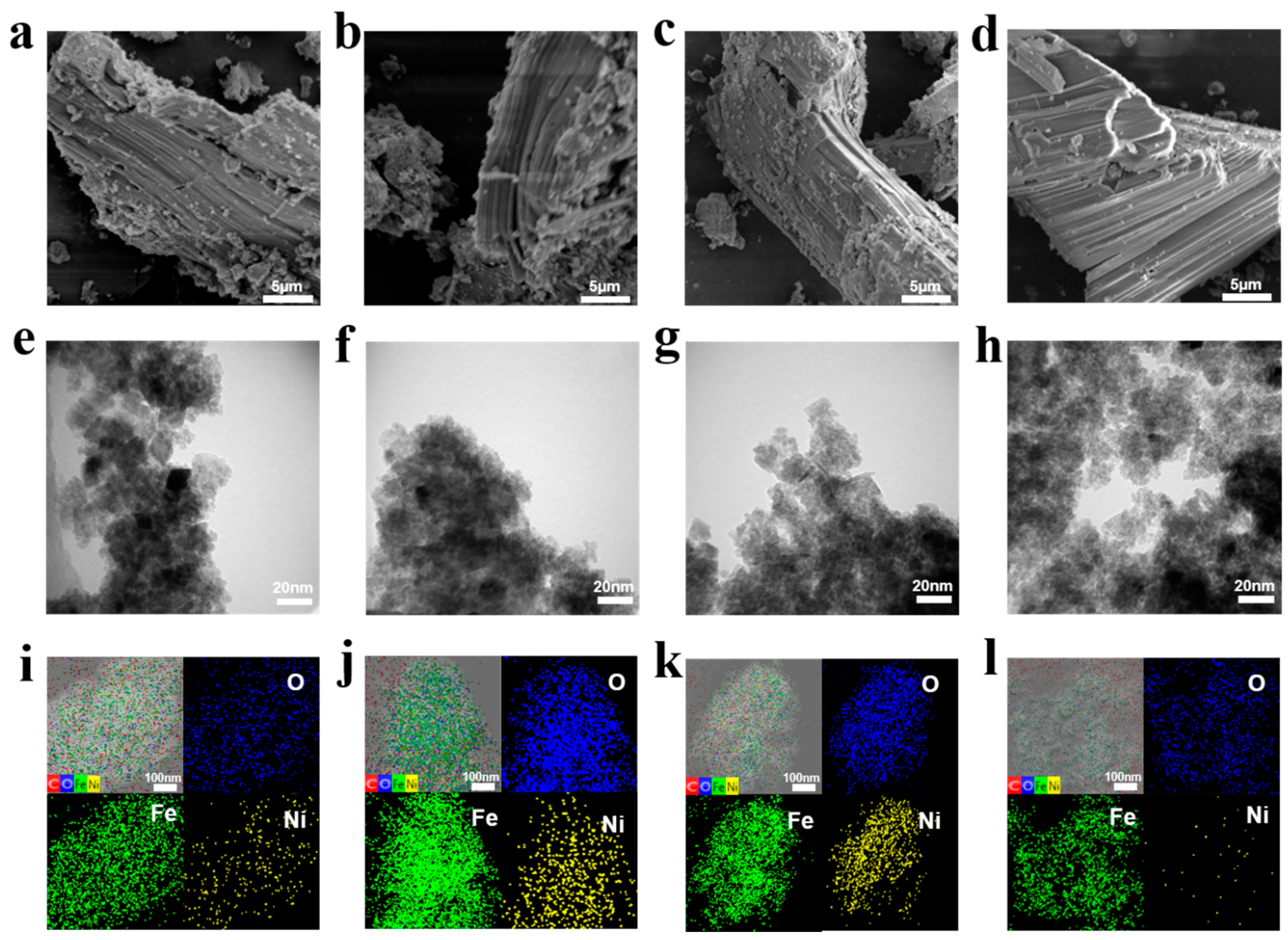
The morphology and structures of NiFe-LDH without and with TPA characterized by Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are shown in Figure 1. It can be seen from the SEM image that all NiFe-LDH samples present similar morphology and structure, including long block shape, layered structure on the surface and some small particles attached to the surface [35,36] (Figure 1a–d). TEM images of NiFe-LDH-TPA0 (without TPA) show the uneven distribution and large-area aggregation of particles. Meanwhile, NiFe-LDH samples with TPA show a similar appearance, indicating that TPA does not change the layered morphology of NiFe-LDH. (Figure 1e). HADDF-TEM and EDS show that the sample is mainly composed of C, O, Fe, and Ni. Moreover, the content of the N element detected by EDS can be ignored, which may be due to the small proportion of elements in the sample. The molar ratio of Fe to Ni derived from EDS is basically consistent with the results given by ICP. In the above mapping images, element C may appear in the non-sample area, which is mainly due to the influence of carbon film on the copper mesh. The mapping images demonstrate a uniform distribution of O, Fe, Ni atoms throughout entire surfaces of NiFe-LDH without and with TPA (Figure 1i–f). It can be concluded that the addition of TPA has no great effect on the morphology of NiFe-LDH and thus the four samples exhibit similar morphology.

Figure 1.
Characterizations on the morphologies and structures of NiFe-LDH without and with TPA. (a–d) SEM images, (e–h) TEM images, (i–l) High angular annular dark field (HADDF) TEM and EDS mapping images of NiFe-LDH-TPA0, NiFe-LDH-TPA0.5, NiFe-LDH-TPA1, and NiFe-LDH-TPA2, respectively. Scale bar: SEM, 5 µm; TEM, 20 nm.
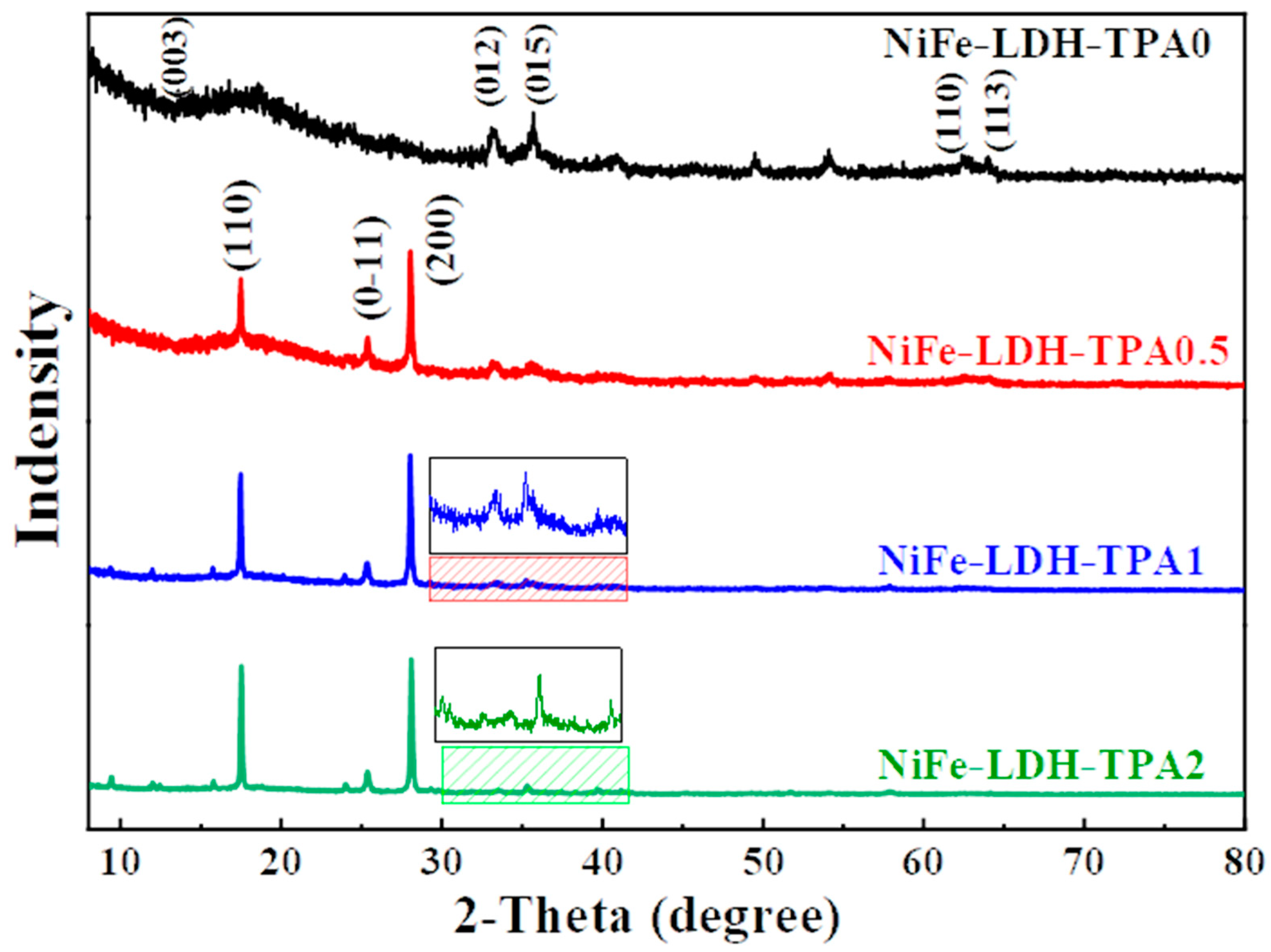
The X-ray diffraction (XRD) measurements were performed to investigate the crystal structures of catalysts of NiFe-LDH-TPA0, NiFe-LDH-TPA0.5, NiFe-LDH-TPA1, and NiFe-LDH-TPA2, as shown in Figure 2. According to the curve of NiFe-LDH-TPA0, the peaks at 33.2°, 35.8°, 62.4°, 63.9° might correspond to the (012), (015), (110), (113) planes of NiFe-LDH, respectively, which give agreement with the previous reports regarding the NiFe-LDH catalysts with weak crystal structure synthesized under acidic conditions [37]. Since the overall crystal signal of NiFe-LDH is relatively weak, it could be attributed to the weak acidity of the synthesis environment [38]. For the samples of NiFe-LDH with TPA, it could find that the strong peaks appeared at 17.4°, 25.2°, 27.9° which could be attributed to (110), (0-11), (200) planes of TPA, respectively (PDF card 31-1916). It should be noted that the XRD information of NiFe-LDH with TPA is not the same as the reported literature [27,28,29,32], which could be attributed to the different catalyst synthesis methods. It is also worth noting that after the addition of TPA, the signal-to-noise ratio of NiFe-LDH significantly deteriorated. This may be caused by a change in pH after the addition of TPA [38]. It can be seen from the published literature that pH could influence the crystallinity of NiFe-LDH. Moreover, the negatively charged carboxylate groups from TPA could attract metal ions which might also hinder the formation of NiFe-LDH. In short, it could be suggested from XRD results that NiFe-LDH based catalysts were successfully synthesized and TPA could be incorporated with NiFe-LDH by the hydrothermal method.
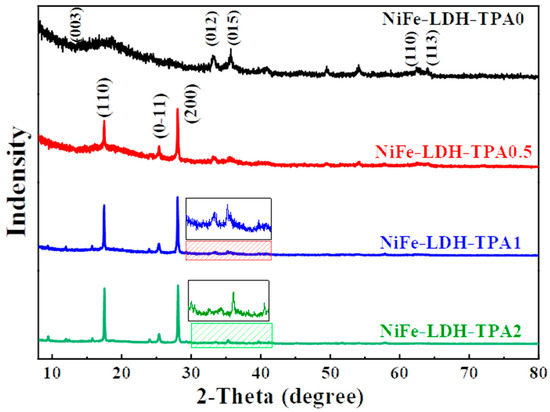
Figure 2.
XRD measurements. XRD for NiFe-LDH-TPA0 (black line), NiFe-LDH-TPA0.5 (red line), NiFe-LDH-TPA1 (blue line), and NiFe-LDH-TPA2 (green line).
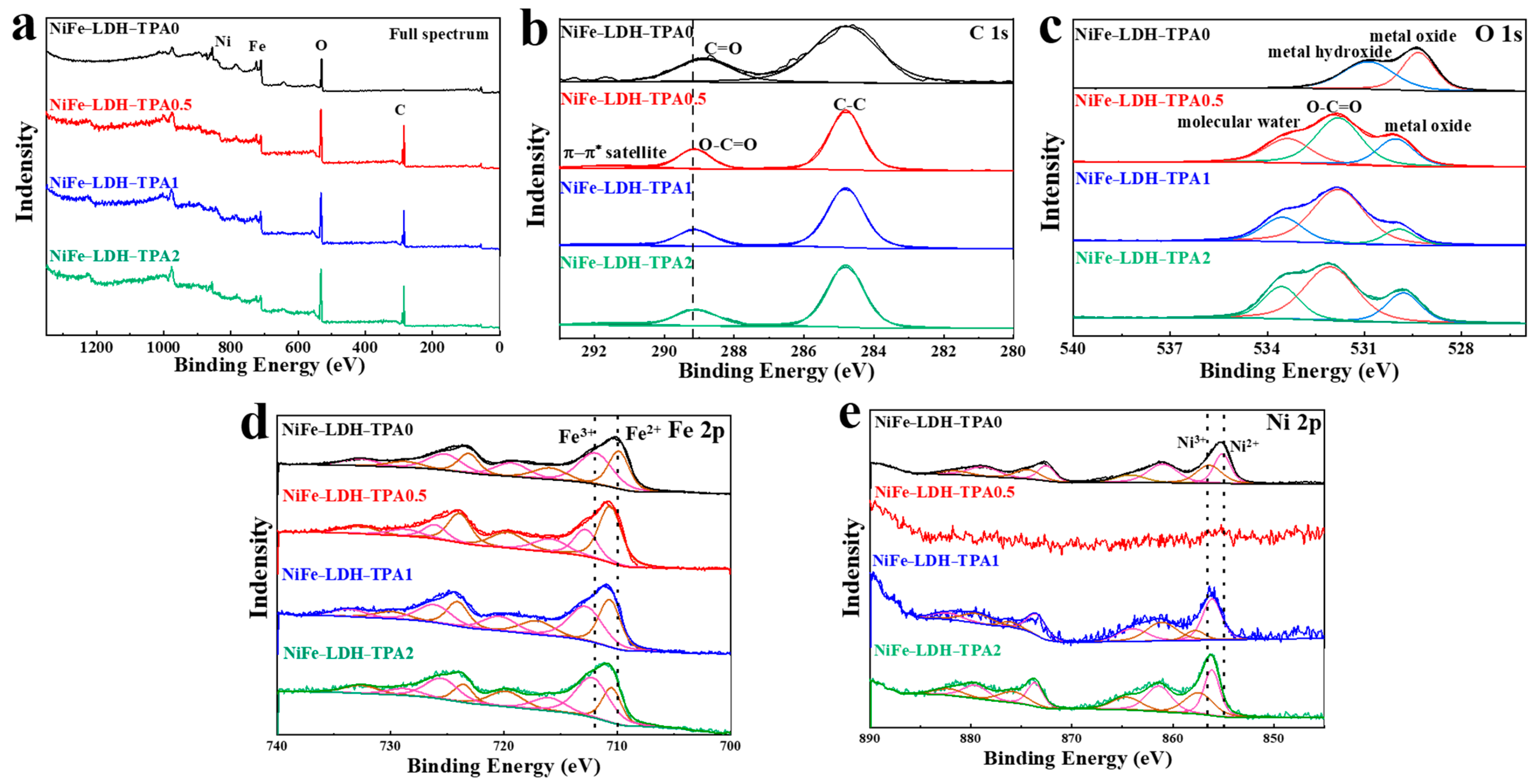
X-ray photoelectron spectroscopy (XPS) measurements were obtained to investigate the surface composition and the valance structure of the elements in catalysts. All the XPS peaks have been calibrated by 284.8 eV of C-C bond. As shown in Figure 3a, the peaks of C, O, Ni, Fe could be found in the full spectra, where the signal of C becomes very strong in the samples of NiFe-LDH with TPA most likely due to the contribution of TPA. The high-resolution spectrums of C 1s, O 1s, Fe 2p, and Ni 2p correspond to Figure 3b,e, respectively. The spectra of C 1s in Figure 3b could be deconvolved into two peaks, of which one peak at 284.8 eV shown in all samples is attributed to C-C. The other peak is located at 288.9 eV for NiFe-LDH-TPA0 and 289.2 eV for NiFe-LDH with TPA, attributed to C=O and O-C=O, respectively. The difference undoubtedly designates the existence of TPA in NiFe-LDH [28]. The groups of metal oxide and metal hydroxide in NiFe-LDH-TPA0 could be deduced from O 1s spectra in Figure 3c, which are located at 529.3 eV and 530.9 eV respectively. In contrast, three divided O 1s peaks (530 eV, 531.8 eV, and 533.4 eV) of NiFe-LDH with TPA are attributed to the metal oxide, O-C=O, molecular water, respectively [28,29]. The group of metal hydroxide is not observed in NiFe-LDH with TPA, which might be owing to the existence of TPA on the NiFe-LDH surface hindering the formation of O 1s signal of hydroxide or metal hydroxides. Additionally, TPA might cause the appearance of molecular water. The peaks of Fe2+ (2p3/2: 710.6 eV; 2p1/2: 724.0 eV) and Fe3+ (2p3/2: 712.9 eV; 2p1/2: 726.1 eV) for NiFe-LDH with TPA exhibit an obvious binding energy blue-shift comparing to Fe2+ (2p3/2: 709.9 eV; 2p1/2: 723.2 eV) and Fe3+ (2p3/2: 712.2 eV; 2p1/2: 725.4 eV) for NiFe-LDH-TPA0 (Figure 3d). As shown in Figure 3e, the peaks of Ni2+ 2p3/2, Ni2+ 2p1/2, Ni3+ 2p3/2, and Ni3+ 2p1/2 for NiFe-LDH-TPA0 are observed at 855.1 eV, 872.5 eV, 856.5 eV, and 874.5 eV, respectively. In contrast, these peaks for both NiFe-LDH-TPA1 and NiFe-LDH-TPA2 are at 856.18 eV, 873.76 eV, 857.5 eV, and 876.0 eV. It is also noticed that a weak Ni signal for NiFe-LDH-TPA0.5 might be due to the low content of surface nickel. The peaks of Ni2+ and Ni3+ for NiFe-LDH with TPA also shift to higher binding energy compared to NiFe-LDH-TPA0, which could be attributed to the strong electron interactions between the metal active centers and carboxylic acid groups of TPA [39,40,41]. It could be observed that the peak area ratios of Ni3+/Ni2+ and Fe3+/Fe2+ are increasing with the increase of the Eq amount of TPA, which means that the percentage of high-valance metals in the catalyst is constantly rising. This increase further suggests that the presence of carboxylic acid groups might promote the conversion of Ni and Fe atoms to a higher valence state. According to the above analysis, it is concluded that TPA can be successfully introduced into NiFe-LDH system by hydrothermal synthesis, which may inhibit the formation of metal hydroxides, enhance electron interactions with Ni or Fe atoms, improve the content of surface Ni, and facilitate the formation of the higher valence state of Fe and Ni.
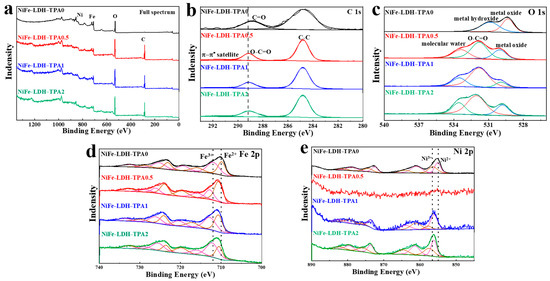
Figure 3.
XPS measurements. (a) XPS full spectrum, high-resolution XPS spectra of (b) C 1s, (c) O 1s, (d) Fe 2p, and (e) Ni 2p for NiFe-LDH-TPA0 (black line), NiFe-LDH-TPA0.5 (red line), NiFe-LDH-TPA1 (blue line), and NiFe-LDH-TPA2 (green line).
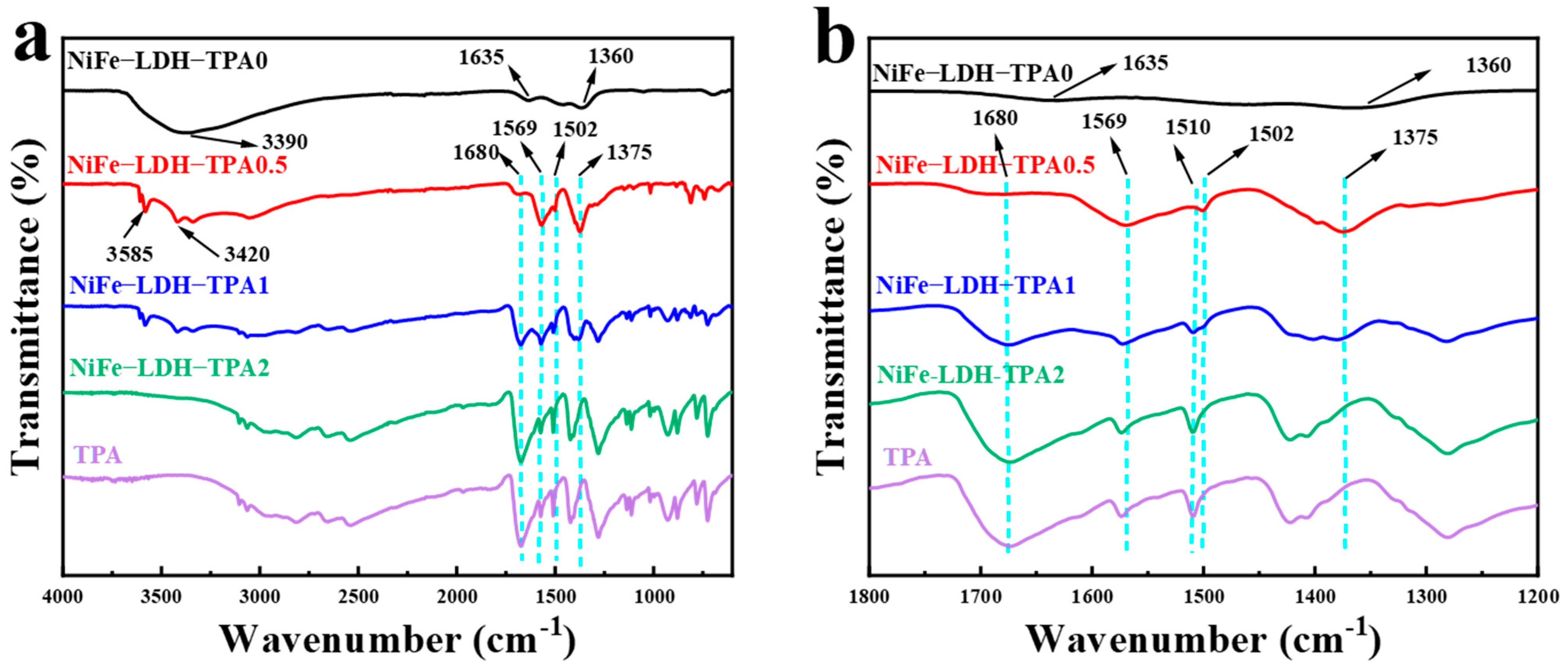
The Fourier transform infrared (FTIR) spectrum was investigated to identify the functional groups of catalysts and TPA, which was used as a reference to distinguish the differences of functional groups between the samples of NiFe-LDH without and with TPA. As shown in Figure 4, NiFe-LDH-TPA0 as a double-layer hydroxide has different characteristic absorption peaks with NiFe-LDH-TPA. The strong broad band at 3390 cm−1 is related to the stretching vibrations of O-H, and two distinct peaks at 1635 and 1365 cm−1 correspond to the bending vibration of H2O and the stretching vibration of NO3 groups in the LDH interlayer respectively [38,42]. Compared with the infrared absorption spectra of NiF-LDH-TPA0 and pure TPA, the peak at 1680 cm−1 can be observed in the samples of NiFe-LDH with TPA, corresponding to the carboxyl groups in TPA. The FTIR spectra of NiFe-LDH-TPA0.5, NiFe-LDH-TPA1 with three different peaks at 3585 cm−1, 3420 cm−1, and 1502 cm−1 could be attributed to the stretching vibrations of OH-, stretching vibrations of O-H and the para-aromatic CH groups, respectively [43]. In contrast, these peaks cannot be observed in the NiFe-LDH-TPA2 spectra. With the increase of TPA, a new peak at 1510 cm−1 appears and gradually grows up, leading to the disappearance of the peak at 1502 cm−1. Another two distinct peaks around 1569 cm−1 and 1375 cm−1 in FTIR spectra might be ascribed to asymmetric and symmetric vibrations of the carboxylate group, respectively. Moreover, with the increase of the molar amount of TPA from 0.5 Eq to 2 Eq, the vibrational absorption intensity of carboxylic groups decreases. Therefore, in the case of excessive TPA, the spectrum shows a similar absorption to that of pure TPA, which can be considered as a mixture of excessive TPA and metal atoms, and excessive TPA will also conceal the absorption of coordination compounds. From the infrared absorption spectrum, it can be concluded that the addition of TPA produces the coordination between carboxylate and metal center, accompanied by free carboxylic acid groups, which regulate the chemical environment of the metal atomic center to a certain extent.
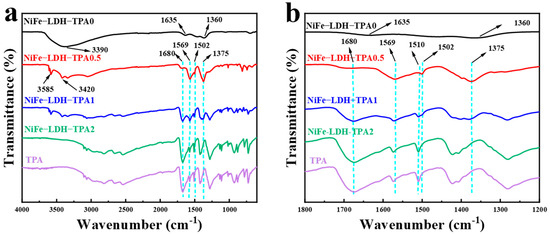
Figure 4.
Fourier transform infrared (FTIR) spectra. (a) FTIR spectra of NiFe-LDH-TPA0, NiFe-LDH-TPA0.5, NiFe-LDH-TPA1, NiFe-LDH-TPA2 powder, and TPA as a reference. (b) Zoom in (a) on the range of 1200–1800 cm−1 in order to display more details of infrared absorption peaks.
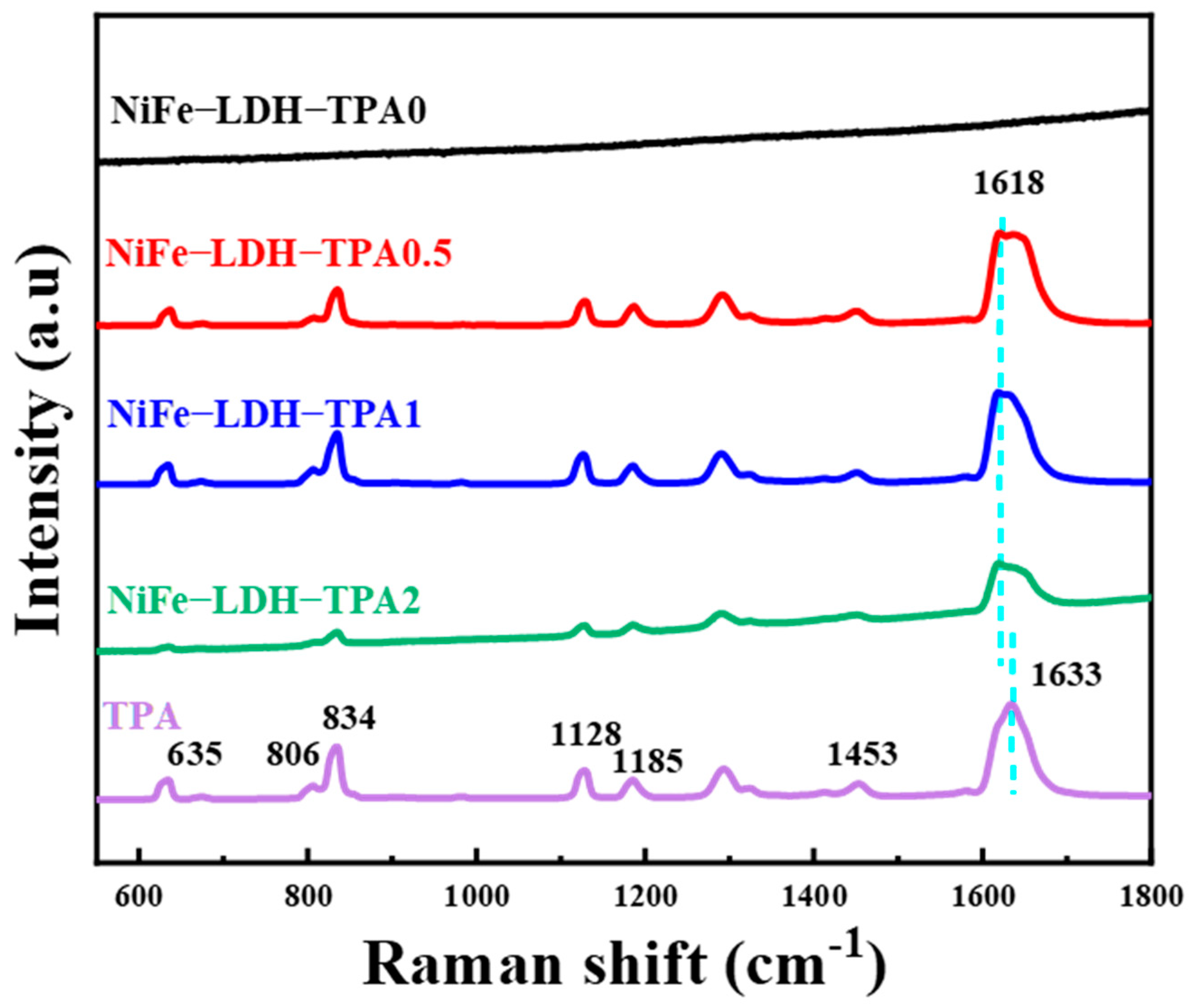
Raman spectra are given in Figure 5. XRD confirmed that the sample we synthesized has a double-layer hydroxide structure, so there is no obvious Raman vibration mode in the range of 600–1800 cm−1, which give agreement with previous publication [39]. The addition of TPA will introduce more Raman modes similar to pure TPA, which has more complex vibrational fingerprints such as in-plane (1128, 1185, 1453, and 1633 cm−1) and out of plane (635, 806, and 834 cm−1) Raman modes [28,43]. In particular, the samples of NiFe-LDH with TPA have a special Raman mode around 1618 cm−1, which could be attributed to out-of-phase stretching modes of the carboxylate group. The vibrational mode of the in-plane phase of the carboxylate group was not observed by Raman spectroscopy, which is inconsistent with previous reports [28,43]. The absence of a Raman mode may be due to the intermolecular affinity in hydrothermal synthesis, which is different from the previously reported electrodeposition and solvothermal methods [28,32,43]. From the Raman spectra, the addition of TPA could adjust the chemical state of metal ions in the complex, and there are a certain proportion of coordinated carboxylates and free uncoordinated carboxylic acid groups in the catalysts of NiFe-LDH with TPA, which is consistent with the results of the FTIR spectra.
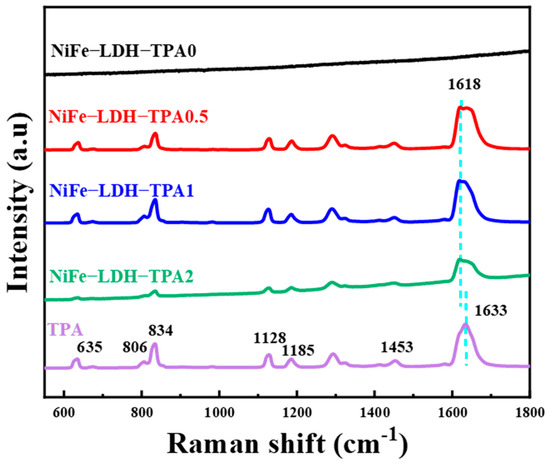
Figure 5.
Raman spectra of NiFe-LDH-TPA0, NiFe-LDH-TPA0.5, NiFe-LDH-TPA1, NiFe-LDH-TPA2, and TPA as a reference.
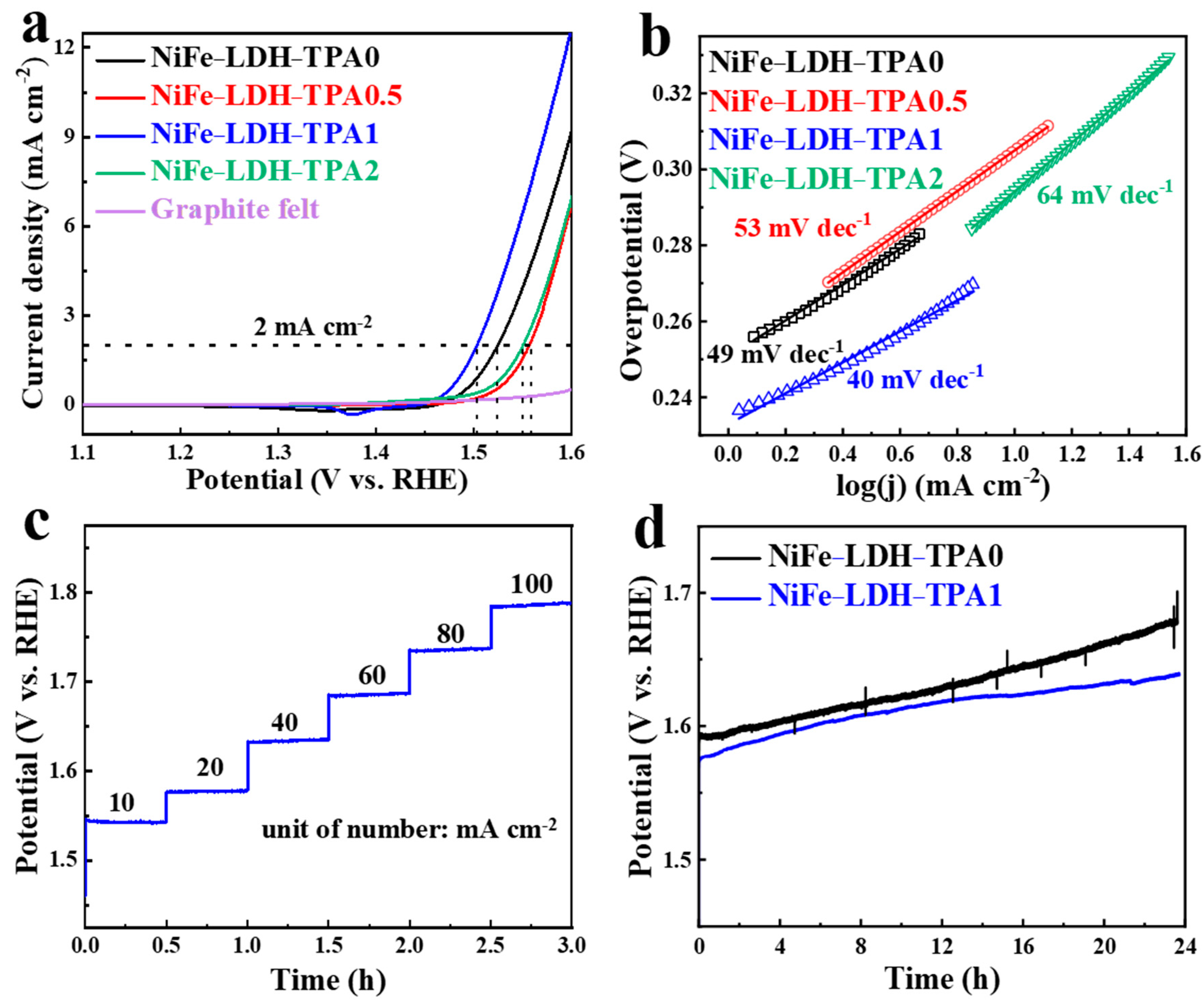
The electrocatalytic performance was evaluated by LSV curves, Tafel slopes, multi-current chronopotentiometry, and extended chronopotentiometry, as shown in Figure 6. Please notice that the LSV curves were normalized with electrochemical surface area (ECSA) for an accurate comparison. The ECSA was measured by the double-layer capacitance method and the specific handling method could be found in Li’s paper [32]. In Figure 6a, the peaks in the range of 1.3–1.45 V (vs. RHE) could be attributed to the change of valance from metal active center atoms, while the sharply rising current in the range of 1.45–1.6 V represents the oxidation of hydroxide together with the catalytic reaction of OER. The potential corresponding to the current density at 0.1 mA cm−2 could be defined as the onset potential [27], which are 1.47 V, 1.48 V, 1.45 V, and 1.42 V (vs. RHE) for NiFe-LDH-TPA0, NiFe-LDH-TPA0.5, NiFe-LDH-TPA1, and NiFe-LDH-TPA2), respectively. All the catalysts with the presence of TPA show lower onset potentials than NiFe-LDH, which might be attributed to the presence of the carboxylic group as demonstrated by the results of XRD, FTIR, Raman, and XPS. In particular, the XPS data in Figure 3d,e exhibit the strong electron interactions between the metal active centers and carboxylic acid groups of TPA, which might lead to the regulation of d-band electronic structure of Ni and Fe atoms. Moreover, the potentials of the samples at the current density of 2mA cm−2 are 1.52 V, 1.55 V, 1.50 V, and 1.55 V (vs. RHE), which correspond to overpotentials of 0.29 V, 0.32 V, 0.27 V, and 0.32 V, respectively. Therefore, NiFe-LDH-TPA1 shows the lowest overpotential among the four catalysts. In order to further verify the catalytic performance of these catalysts, the Tafel slopes were calculated and the results are shown in Figure 6b. The Tafel slopes of NiFe-LDH-TPA0, NiFe-LDH-TPA0.5, NiFe-LDH-TPA1, and NiFe-LDH-TPA2 are 49 mV dec−1, 53 mV dec−1, 40 mV dec−1, and 64 mV dec−1, respectively. It is well known that a small Tafel slope corresponds to a rapid rise of the electrocatalytic current density [44], which indicates NiFe-LDH-TPA1 shows the best OER performance due to its smallest Tafel slope and lowest overpotential among the others. In contrast, NiFe-LDH-TPA2 shows the largest overpotential (0.32 V) at 2 mA cm−2 and Tafel slope (64 mV dec−1), which may be due to the ratio of Fe to Ni (58.33 in Table 1). The different ratios of Fe to Ni have shown a great influence on the electrocatalytic performance [15]. It is also worth noting that NiFe-LDH-TPA0.5 shows a worse catalytic performance than NiFe-LDH-TPA1. Although the ratio of Fe to Ni for both NiFe-LDH-TPA0.5 (4.09 in Table 1) and NiFe-LDH-TPA1 (4.92 in Table 1) is very similar, XPS data indicate the weak intensity of Ni signal (Figure 3e) resulting from a small amount of Ni on the surface of the catalyst. Thus, the addition of TPA influences the distribution and content of Ni and Fe in NiFe-LDH catalyst and consequently influences catalyst performance. In other words, just the right amount of TPA can boost catalytic activity.
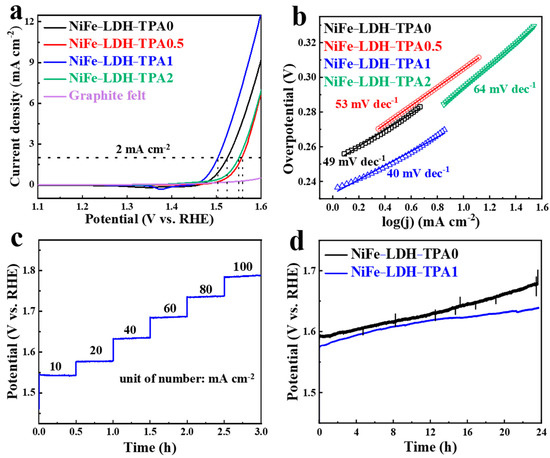
Figure 6.
Electrochemical catalytic performance test. (a) Linear sweep voltammetry (LSV) curves and (b) Tafel slopes of NiFe-LDH-TPA0, NiFe-LDH-TPA0.5, NiFe-LDH-TPA1, NiFe-LDH-TPA2, and graphite felt in 1.0 M KOH. (c) Multi-current chronopotentiometric process of NiFe-LDH-TPA1 in 1.0 M KOH. The current density started at 10 mA cm−2 and finished at 100 mA cm−2, and each step lasted for 30 min in 1 M KOH. (d) Extended chronopotentiometric curves of NiFe-LDH-TPA1 and NiFe-LDH-TPA0 at 20 mA cm−2 current density for 24 h of electrolysis in 1.0 M KOH.
In addition to efficient catalytic ability, catalytic stability is also an important indicator to measure the catalytic performance of a catalyst. Here, a 1 × 1 cm2 of NiFe-LDH-TPA1 sample was used to test the electrochemical stability of chronopotentiometry. As shown in the multi-current chronopotentiometry (Figure 6c), the electrolysis was continuously conducted at the step currents of 10, 20, 40, 60, 80, and 100 mA cm−2 for 30 min every step. The flat steeped line segment suggests the catalytic stability of NiFe-LDH-TPA1. Figure 6d shows the extended chronopotentiometric curves of NiFe-LDH-TPA0 and NiFe-LDH-TPA1 under continuous electrolysis at a current of 20 mA cm−2 for 24 h. It is shown that the potential of NiFe-LDH-TPA0 is higher than that of NiFe-LDH-TPA1 during 24 h, and especially the potential difference between two catalysts is further increasing after 14 h, which indicates that NiFe-LDH-TPA1 has better catalytic stability than NiFe-LDH-TPA0. In another word, this is confirmed that the NiFe-LDH catalyst with the moderate portions of TPA could give a significant improvement on catalytic performance.
Based on the above experimental results, we could see that the addition of TPA can improve the oxygen evolution efficiency of NiFe-LDH during the OER process. One of the important reasons is that the carboxyl groups around the metal hydroxide can effectively induce the redistribution of electrons in the outer shell of the active center [30]. It will optimize the d-band centers of Ni and Fe metal atoms in NiFe-LDH. Moreover, this adjustment could be found in XPS results. Therefore, the energy required for the metal active center M to be converted from a low valence state to a high valence state during the oxygen evolution reaction is reduced, thereby realizing the oxygen evolution reaction at a lower overpotential. At the same time, due to the existence of carboxylic acid, this can play a role in transporting protons to a certain extent, thereby accelerating the speed of OER [32,33,45].
3. Materials and Methods
3.1. Materials
All chemicals were used as received without further purification: Ferric nitrate nonahydrate (Fe(NO3)3·9H2O, 98%, Adamas-beta, Shanghai, China), nickel nitrate hexahydrate (Ni(NO3)2·6H2O, 98%, Adamas-beta, Shanghai, China), terephthalic Acid (TPA) (C8H6O4, 98%, Adamas-beta, Shanghai, China), Urea (CH4N2O, 99%, Solarbio, Beijing, China), and deionized water.
3.2. Preparation of NiFe-LDH-TPA
Firstly, four precursor solutions were prepared by mixing 0.5 mmol of (Fe(NO3)3·9H2O, 98%), 0.5 mmol of (Ni(NO3)2·6H2O, 98%), and 1 mmol of Urea with the addition of 0, 0.5, 1 or 2 mmol of TPA in 36 mL water. The graphite felt was ultrasonicated in 1 M HCl for 10 min, washed with deionized water, continued to ultrasonicate in ethanol for 10 min, and followed by drying with nitrogen flow. The cleaned graphite felt (the thickness of 1 mm and area of 1 cm × 2 cm) was transferred in an autoclave, which filled the precursor solution to ensure the graphite felt to be immersed. The hydrothermal reaction in the autoclave was carried out under 120 °C for 12 h. After the reaction, the catalyst-deposited graphite felt was taken out, rinsed with water and absolute ethanol three times each. The washed felt was dried in the oven under 60 °C for 6 h. The as-synthesized catalysts deposited onto graphite felts are named NiFe-LDH-TPA0, NiFe-LDH-TPA0.5, NiFe-LDH-TPA1, and NiFe-LDH-TPA2, corresponding to the above precursor solutions with 0, 0.5, 1, and 2 equivalent (Eq) of TPA to the total amount of nickel and iron, respectively.
3.3. Structural Characterizations
The morphology was observed with JSM-6010PLUS/LA scanning electron microscopy (SEM) (JEOL, Akishima, Japan) and JEM 2100 Plus transmission electron microscopy (TEM) (JEOL, Akishima, Japan) with an acceleration voltage of 200 kV. Energy-dispersive X-ray spectrometry (EDS) was also operated on JEM 2100PLUS equipped with HAADF Detector and Oxford X-MAXN 100TLE (JEOL, Akishima, Japan). The Fourier Transform infrared (FTIR) spectra were acquired on V70 (Bruker, Germany) at room temperature. The crystal structure of the samples was determined by X-ray diffraction (XRD) with a Bruker D8 Advance powder diffractometer (Bruker, Germany). Raman modes were acquired on Raman Spectrometer (SR-500I-D2-1F1) (TechnoSpex Pte Ltd., Singapore) with 500 mm focal length, motorized Czerny-Turner Spectrograph. The X-ray photoelectron spectra (XPS) was performed by Thermo-Fisher ESCALAB 250Xi (Thermo Fisher Scientific, Shanghai, China) with monochromatic Al Kα as the X-ray source. The diameter of the point area selected for the sample test is 500 μm, and the charge neutralization gun is used to supplement the charge. Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) was measured by NexION 2000 (Thermo Fisher Scientific, Shanghai, China).
3.4. Electrochemical Characterizations
Electrochemical data, including linear sweep voltammetry (LSV), cyclic Voltammetry (CV), electrochemical active surface area (ECSA), and chronopotentiometry measurements, were measured by Bio-Logic VMP3 FlexP 0160 (Bio-Logic, France). In the process of measurements, the catalysts were tested in the water bath at a constant temperature of 298 K. All the electrochemical data were carried out by using a three-electrode system: the prepared catalyst-deposited graphite felt as the working electrode, a Pt mesh as the counter electrode, and a Hg/HgO electrode as the reference electrode. The LSV or CV curves were measured with the scan rate of 5 mV s−1 and IR compensated of 85%. Tafel slope is the log function of the current density (j) in the LSV curves. Using log(j) and the corresponding voltage constitute the relationship diagram between log(j) and potential. Here, the chosen overpotentials are in the range between 0.22 V to 0.34 V. Finally, the value of the slope is calculated by fitting the plot.
4. Conclusions
In conclusion, we report a new type of catalyst by incorporating TPA with NiFe-LDH to enhance electrocatalytic performances. These catalysts were prepared through one-step hydrothermal synthesis on the precursor solutions. Among prepared catalysts, NiFe-LDH-TPA1 containing one equivalent of TPA shows the best performance with the current density of 2 mA cm−2 at an overpotential of 270 mV, the Tafel slope of 40 mV dec−1, and excellent stable electrocatalytic performance for OER.
These catalysts were characterized using a variety of methods. XRD, XPS, FTIR, and Raman spectra showed that the NiFe-LDH with TPA had both coordinated and uncoordinated carboxylate groups, which might offer the adjustment of the d orbital of the active metal center (Ni and Fe) through the strong electron interaction between carboxylate groups and NiFe metal center. This adjustment could influence the oxidation process and improve the catalytic activity. In addition, the ICP-OES results prove the amount of TPA added may affect the deposition ratio of Ni and Fe, which might also lead to different catalytic activities. Moreover, this study provides an approach for regulating the coordination environment of the active metal centers in NiFe-LDH catalysts toward improving the catalytic performances.
Author Contributions
Conceptualization, G.L., J.Z., L.L. and C.Y.; investigation, G.L. and J.Z.; writing—original draft preparation, G.L. and J.Z.; writing—review and editing, G.L., J.Z., L.L. and C.Y.; supervision, L.L. and T.-C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key Research and Development Program of Ministry of Science and Technology of China (2021YFA1500604), National Natural Science Foundation of China for the financial support (NSFC- 21727801), and ShanghaiTech University start-up funding.
Acknowledgments
We are grateful for the support from the Analytical Instrumentation Center (#SPST-AIC 10112914), SPST, ShanghaiTech University. We thank the Centre for High-resolution Electron Microscopy (CћEM) and Analytical Instrumentation Center, supported by SPST of ShanghaiTech University under contract No. EM02161943. We appreciate Jihong Cheng at ShanghaiTech University for the help with XPS measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, H.S.; Kim, H.; Flores, M.C.; Jung, G.-S.; In, S.-I. Surface Modification of Electrocatalyst for Optimal Adsorption of Reactants in Oxygen Evolution Reaction. Catalysts 2021, 11, 717. [Google Scholar] [CrossRef]
- Ibn Shamsah, S.M. Earth-Abundant Electrocatalysts for Water Splitting: Current and Future Directions. Catalysts 2021, 11, 429. [Google Scholar] [CrossRef]
- Aghabarari, B.; Luque-Centeno, J.M.; Capel-Sánchez, M.; Lázaro Elorri, M.J.; Martínez-Huerta, M.V. Ni-Based Composites from Chitosan Biopolymer a One-Step Synthesis for Oxygen Evolution Reaction. Catalysts 2019, 9, 471. [Google Scholar] [CrossRef] [Green Version]
- Bao, J.; Xie, J.; Lei, F.; Wang, Z.; Liu, W.; Xu, L.; Guan, M.; Zhao, Y.; Li, H. Two-Dimensional Mn-Co LDH/Graphene Composite towards High-Performance Water Splitting. Catalysts 2018, 8, 350. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Chen, Y.; Gong, C.; Sui, L.; Sun, Q.; Lv, K.; Dong, L. N, S, P-Codoped Graphene-Supported Ag-MnFe2O4 Heterojunction Nanoparticles as Bifunctional Oxygen Electrocatalyst with High Efficiency. Catalysts 2021, 11, 1550. [Google Scholar] [CrossRef]
- Pedersen, A.F.; Escudero-Escribano, M.; Sebok, B.; Bodin, A.; Paoli, E.; Frydendal, R.; Friebel, D.; Stephens, I.E.L.; Rossmeisl, J.; Chorkendorff, I.; et al. Operando XAS Study of the Surface Oxidation State on a Monolayer IrOx on RuOx and Ru Oxide Based Nanoparticles for Oxygen Evolution in Acidic Media. J. Phys. Chem. B 2018, 122, 878–887. [Google Scholar] [CrossRef] [Green Version]
- Escudero-Escribano, M.; Pedersen, A.F.; Paoli, E.A.; Frydendal, R.; Friebel, D.; Malacrida, P.; Rossmeisl, J.; Stephens, I.E.L.; Chorkendorff, I. Importance of Surface IrOx in Stabilizing RuO2 for Oxygen Evolution. J. Phys. Chem. B 2018, 122, 947–955. [Google Scholar] [CrossRef] [Green Version]
- Paoli, E.A.; Masini, F.; Frydendal, R.; Deiana, D.; Schlaup, C.; Malizia, M.; Hansen, T.W.; Horch, S.; Stephens, I.E.L.; Chorkendorff, I. Oxygen evolution on well-characterized mass-selected Ru and RuO2 nanoparticles. Chem. Sci. 2015, 6, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Q.; Zhang, B.; Tian, L.; Li, K.; Zhang, X. Recent Advances in Transition Metal Carbide Electrocatalysts for Oxygen Evolution Reaction. Catalysts 2020, 10, 1164. [Google Scholar] [CrossRef]
- Wang, M.; Chen, K.; Liu, J.; He, Q.; Li, G.; Li, F. Efficiently Enhancing Electrocatalytic Activity of α-MnO2 Nanorods/N-Doped Ketjenblack Carbon for Oxygen Reduction Reaction and Oxygen Evolution Reaction Using Facile Regulated Hydrothermal Treatment. Catalysts 2018, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Kuai, C.; Xi, C.; Hu, A.; Zhang, Y.; Xu, Z.; Nordlund, D.; Sun, C.J.; Cadigan, C.A.; Richards, R.M.; Li, L.; et al. Revealing the Dynamics and Roles of Iron Incorporation in Nickel Hydroxide Water Oxidation Catalysts. J. Am. Chem. Soc. 2021, 143, 18519–18526. [Google Scholar] [CrossRef]
- Landon, J.; Demeter, E.; İnoğlu, N.; Keturakis, C.; Wachs, I.E.; Vasić, R.; Frenkel, A.I.; Kitchin, J.R. Spectroscopic Characterization of Mixed Fe–Ni Oxide Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Electrolytes. ACS Catal. 2012, 2, 1793–1801. [Google Scholar] [CrossRef]
- Louie, M.W.; Bell, A.T. An investigation of thin-film Ni-Fe oxide catalysts for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337. [Google Scholar] [CrossRef] [Green Version]
- Corrigan, D.A. The Catalysis of the Oxygen Evolution Reaction by Iron Impurities in Thin Film Nickel Oxide Electrodes. J. Electrochem. Soc. 1987, 134, 377–384. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J. A review on NiFe-based electrocatalysts for efficient alkaline oxygen evolution reaction. J. Power Sources 2020, 448, 227375. [Google Scholar] [CrossRef]
- Gao, R.; Yan, D. Recent Development of Ni/Fe-Based Micro/Nanostructures toward Photo/Electrochemical Water Oxidation. Adv. Energy Mater. 2019, 10, 1900954. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An advanced Ni-Fe layered double hydroxide electrocatalyst for water oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel-iron oxyhydroxide oxygen-evolution electrocatalysts: The role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef]
- Ahn, H.S.; Bard, A.J. Surface Interrogation Scanning Electrochemical Microscopy of Ni1–xFexOOH (0 < x < 0.27) Oxygen Evolving Catalyst: Kinetics of the “fast” Iron Sites. J. Am. Chem. Soc. 2016, 138, 313–318. [Google Scholar] [PubMed]
- Wang, J.; Kong, H.; Zhong, H.; Jiang, Y.; Guo, F.; Alonso-Vante, N.; Feng, Y. Recent Progress on Transition Metal Based Layered Double Hydroxides Tailored for Oxygen Electrode Reactions. Catalysts 2021, 11, 1394. [Google Scholar] [CrossRef]
- Li, J.; Jiang, S.; Shao, M.; Wei, M. Host-Guest Engineering of Layered Double Hydroxides towards Efficient Oxygen Evolution Reaction: Recent Advances and Perspectives. Catalysts 2018, 8, 214. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, A.; Yu, Q.; Luo, Y.; Zhang, Z.; Zhang, C.; Qiu, L.; Liu, B. Controllable structure reconstruction of nickel-iron compounds toward highly efficient oxygen evolution. Nanoscale 2020, 12, 10751–10759. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Zhu, Q.-L. Metal-Organic Frameworks for Electrocatalysis. In Methods for Electrocatalysis; Inamuddin, B.R., Asiri, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 29–66. [Google Scholar]
- Zhou, Y.; Abazari, R.; Chen, J.; Tahir, M.; Kumar, A.; Ikreedeegh, R.R.; Rani, E.; Singh, H.; Kirillov, A.M. Bimetallic metal–organic frameworks and MOF-derived composites: Recent progress on electro- and photoelectrocatalytic applications. Coord. Chem. Rev. 2022, 451, 214264. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.-T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 13946–13952. [Google Scholar] [CrossRef]
- Sun, F.; Wang, G.; Ding, Y.; Wang, C.; Yuan, B.; Lin, Y. NiFe-Based Metal-Organic Framework Nanosheets Directly Supported on Nickel Foam Acting as Robust Electrodes for Electrochemical Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1800584. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, X.; Zhang, Y.; Zhou, Y.; Ostrikov, K.K. Bimetal–Organic Frameworks from In Situ-Activated NiFe Foam for Highly Efficient Water Splitting. ACS Sustain. Chem. Eng. 2021, 9, 1826–1836. [Google Scholar] [CrossRef]
- Li, C.F.; Zhao, J.W.; Xie, L.J.; Wu, J.Q.; Ren, Q.; Wang, Y.; Li, G.R. Surface-Adsorbed Carboxylate Ligands on Layered Double Hydroxides/Metal-Organic Frameworks Promote the Electrocatalytic Oxygen Evolution Reaction. Angew Chem. Int. Ed. Engl. 2021, 60, 18129–18137. [Google Scholar] [CrossRef]
- Fu, Y.; Li, T.; Zhou, G.; Guo, J.; Ao, Y.; Hu, Y.; Shen, J.; Liu, L.; Wu, X. Dual-metal-driven Selective Pathway of Nitrogen Reduction in Orderly Atomic-hybridized Re2MnS6 Ultrathin Nanosheets. Nano Lett. 2020, 20, 4960–4967. [Google Scholar] [CrossRef]
- Li, W.; Li, F.; Yang, H.; Wu, X.; Zhang, P.; Shan, Y.; Sun, L. A bio-inspired coordination polymer as outstanding water oxidation catalyst via second coordination sphere engineering. Nat. Commun. 2019, 10, 5074. [Google Scholar] [CrossRef] [PubMed]
- Tyburski, R.; Liu, T.; Glover, S.D.; Hammarstrom, L. Proton-Coupled Electron Transfer Guidelines, Fair and Square. J. Am. Chem. Soc. 2021, 143, 560–576. [Google Scholar] [CrossRef] [PubMed]
- Hanaor, D.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J. Eur. Ceram. 2012, 32, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Li, K.; Xie, Z.; Yang, G.; Yu, S.; Wang, W.; Yu, H.; Baxter, J.; Meyer, H.M.; Cullen, D.A.; et al. Constructing Ultrathin W-Doped NiFe Nanosheets via Facile Electrosynthesis as Bifunctional Electrocatalysts for Efficient Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 20070–20080. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, K.; Xie, Z.; Yang, G.; Yu, S.; Wang, W.; Cullen, D.A.; Yu, H.; Zhang, F. W-induced morphological modification of NiFe layered double hydroxides as efficient electrocatalysts for overall water splitting. Electrochim. Acta 2021, 395, 139199. [Google Scholar] [CrossRef]
- Ding, P.; Meng, C.; Liang, J.; Li, T.; Wang, Y.; Liu, Q.; Luo, Y.; Cui, G.; Asiri, A.M.; Lu, S.; et al. NiFe Layered-Double-Hydroxide Nanosheet Arrays on Graphite Felt: A 3D Electrocatalyst for Highly Efficient Water Oxidation in Alkaline Media. Inorg. Chem. 2021, 60, 12703–12708. [Google Scholar] [CrossRef]
- Abo El-Reesh, G.Y.; Farghali, A.A.; Taha, M.; Mahmoud, R.K. Novel synthesis of Ni/Fe layered double hydroxides using urea and glycerol and their enhanced adsorption behavior for Cr(VI) removal. Sci. Rep. 2020, 10, 587. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zheng, D.; Deng, T.; Chen, Q.; Zhu, C.; Pei, C.; Li, H.; Wu, F.; Shi, W.; Yang, S.W.; et al. Boosting Electrocatalytic Activity of 3d-Block Metal (Hydro)oxides by Ligand-Induced Conversion. Angew. Chem. Int. Ed. Engl. 2021, 60, 10614–10619. [Google Scholar] [CrossRef]
- Yu, M.; Zhou, S.; Wang, Z.; Zhao, J.; Qiu, J. Boosting electrocatalytic oxygen evolution by synergistically coupling layered double hydroxide with MXene. Nano Energy 2018, 44, 181–190. [Google Scholar] [CrossRef]
- Wang, A.L.; Xu, H.; Feng, J.X.; Ding, L.X.; Tong, Y.X.; Li, G.R. Design of Pd/PANI/Pd sandwich-structured nanotube array catalysts with special shape effects and synergistic effects for ethanol electrooxidation. J. Am. Chem. Soc. 2013, 135, 10703–10709. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Peng, W.; Liu, L.; Li, M. Electrocatalytic oxidation of methanol at Ni–Al layered double hydroxide film modified electrode in alkaline medium. Electrochim. Acta 2011, 56, 5754–5758. [Google Scholar] [CrossRef]
- Yuan, B.; Li, C.; Liu, Y.; Wang, C.; Guan, L.; Li, K.; Lin, Y. Nanocubic bimetallic organic framework self-templated from Ni precursor as efficient electrocatalysts for oxygen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 11705–11716. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkne, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Hammes-Schiffer, S. Proton-Coupled Electron Transfer: Moving Together and Charging Forward. J. Am. Chem. Soc. 2015, 137, 8860–8871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).