Highly Efficient Hierarchical Porous Carbon Supported Pd-Based Catalysts for Additive-Free Dehydrogenation of Formic Acid
Abstract
:1. Introduction
2. Results and Discussion
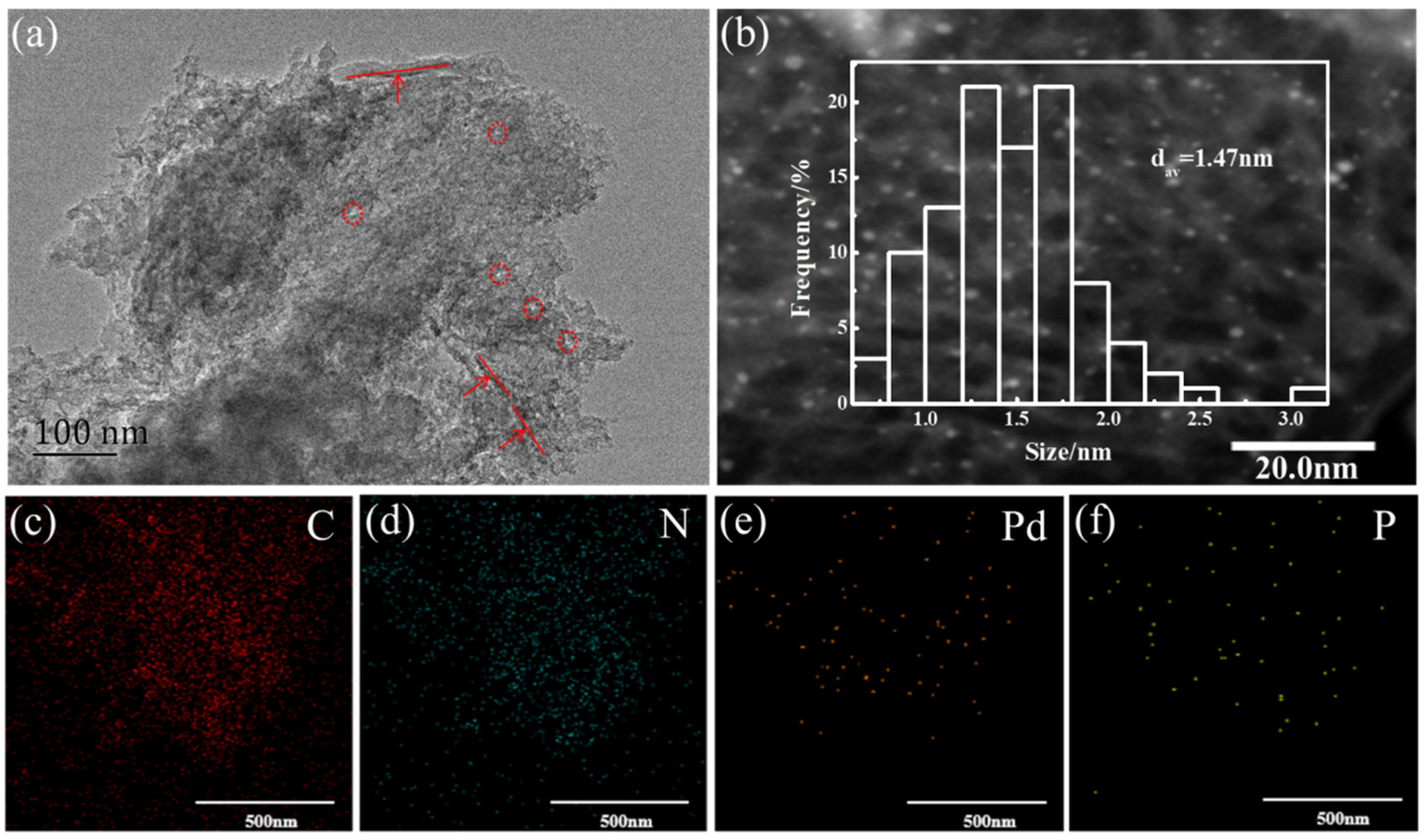
2.1. Preparation and Characterization of Catalysts
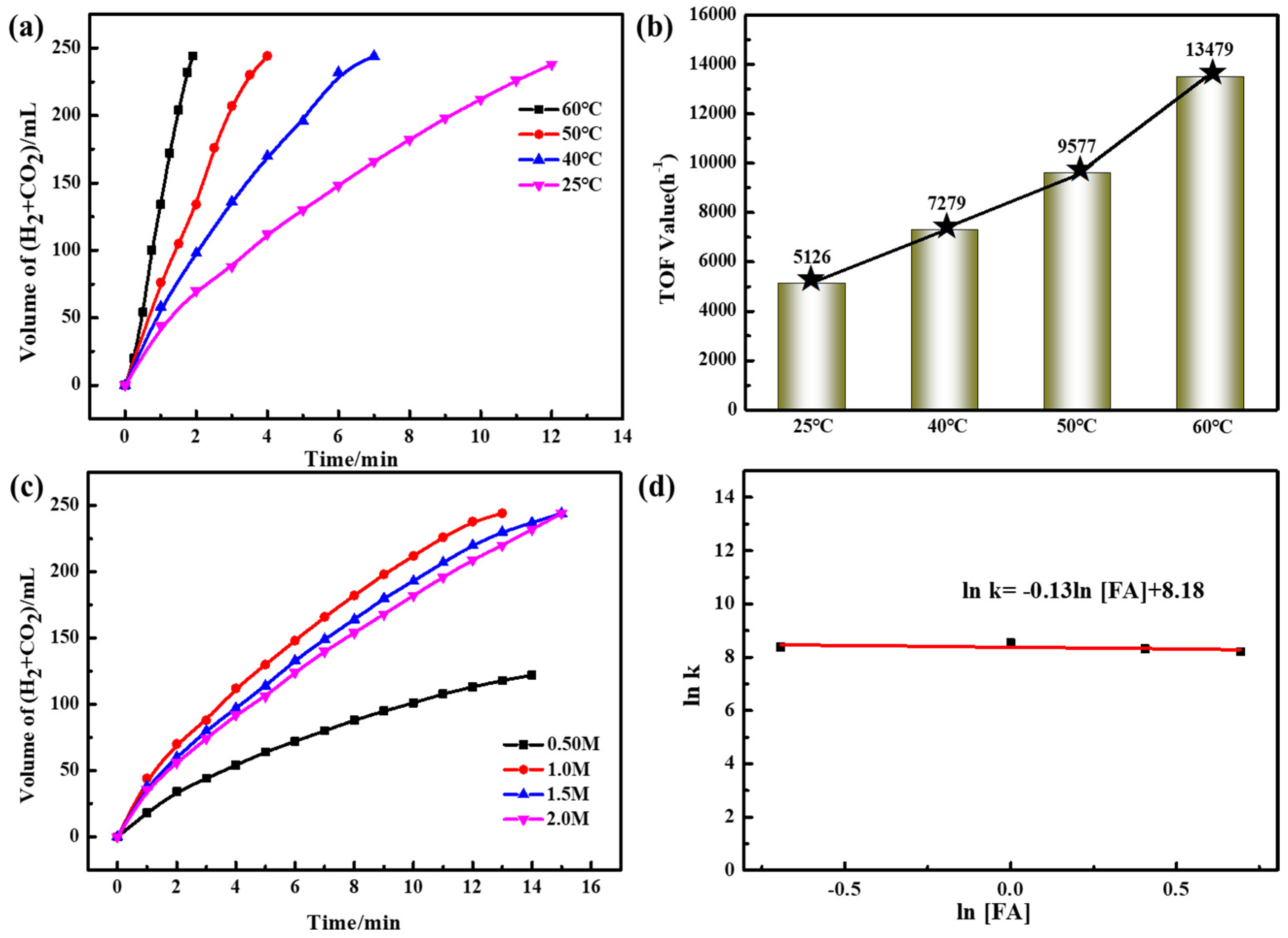
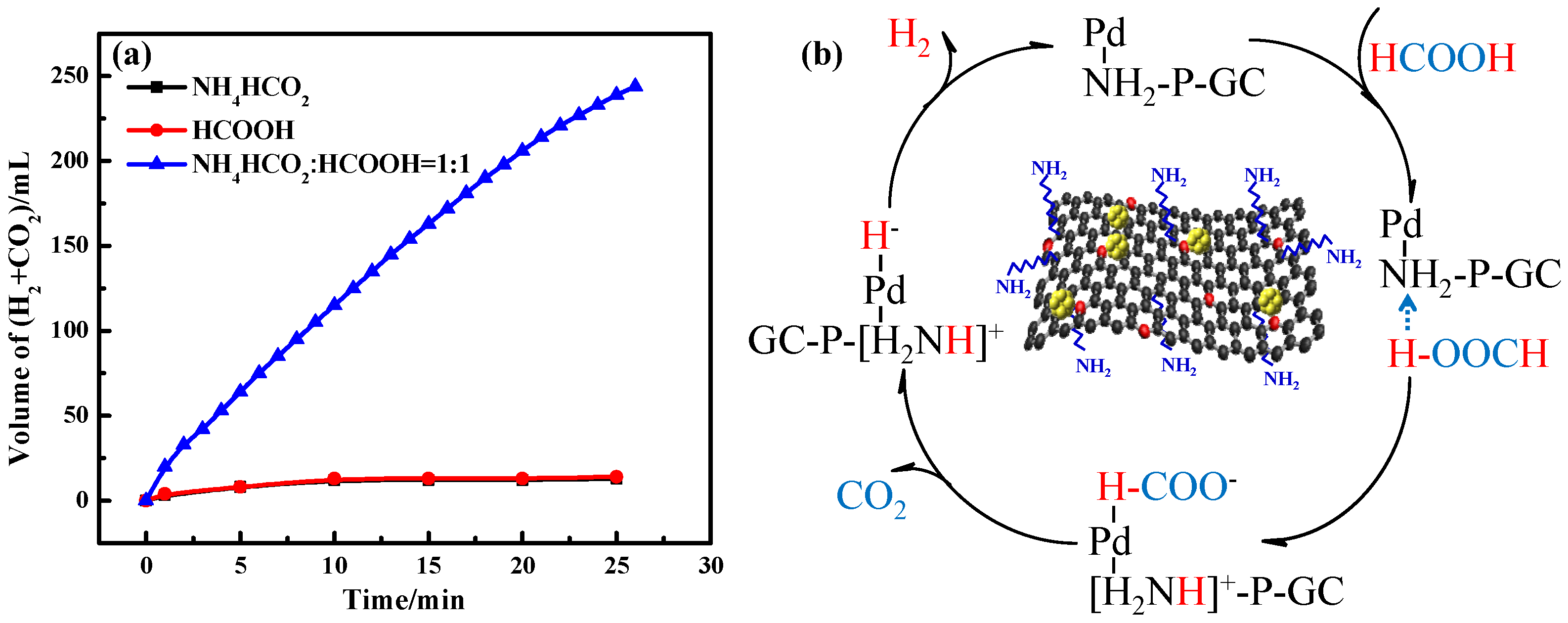
2.2. Catalytic Performance for FA Dehydrogenation
2.3. Catalytic Mechanism and Stability
3. Materials and Methods
3.1. Chemicals and Characterization Techniques
3.2. Preparation of Phosphorus-Doped Porous Carbon
3.3. Preparation of Pd-Based Catalysts
3.4. Catalytic Activities and Recycling Stability for Dehydrogenation of FA
3.5. Calculation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Höök, M.; Tang, X. Depletion of Fossil Fuels and Anthropogenic Climate Change—A Review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Trends in Atmospheric Carbon Dioxide. Global Monitoring Laboratory. Available online: http://www.esrl.noaa.gov/gmd/ccgg/trends/ (accessed on 26 April 2020).
- Butler, C.D. Climate change, health and existential risks to civilization: A comprehensive review (1989–2013). Int. J. Environ. Res. Public Health 2018, 15, 2266. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Brandon, N.P.; Kurban, Z. Clean energy and the hydrogen economy. Phil. Trans. R. Soc. A 2017, 375, 20160400. [Google Scholar] [CrossRef] [PubMed]
- Bolatkhan, K.; Kossalbayev, B.D.; Zayadan, B.K.; Tomo, T.; Veziroglu, T.N.; Allakhverdiev, S.I. Hydrogen production from phototrophic microorganisms: Reality and perspectives. Int. J. Hydrog. Energy 2019, 44, 5799e811. [Google Scholar] [CrossRef]
- He, T.; Pachfule, P.; Wu, H.; Xu, Q.; Chen, P. Hydrogen carriers. Nat. Rev. Mater. 2016, 1, 16067–16083. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid organic hydrogen carriers (LOHCs): Toward a hydrogen-free hydrogen economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef]
- Niermann, M.; Drünert, S.; Kaltschmitt, M.; Bonhoff, K. Liquid organic hydrogen carriers (LOHCs)—Techno-economic analysis of LOHCs in a defined process chain. Energy Environ. Sci. 2019, 12, 290–307. [Google Scholar] [CrossRef]
- Eppinger, J.; Huang, K.W. Formic acid as a hydrogen energy carrier. ACS Energy Lett. 2017, 2, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Van Putten, R.; Wissink, T.; Swinkels, T.; Pidko, E.A. Fuelling the hydrogen economy: Scale-up of an integrated formic acid-to-power system. Int. J. Hydrog. Energy 2019, 44, 28533–28541. [Google Scholar] [CrossRef]
- Getman, R.B.; Bae, Y.S.; Wilmer, C.E.; Snurr, R.Q. Review and analysis of molecular simulations of methane, hydrogen, and acetylene storage in metal–organic frameworks. Chem. Rev. 2012, 112, 703–723. [Google Scholar] [CrossRef] [PubMed]
- Grasemann, M.; Laurenczy, G. Formic acid as a hydrogen source–recent developments and future trends. Energy Environ. Sci. 2012, 5, 8171–8181. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.; Kumar, A. Hydrogen energy future with formic acid: A renewable chemical hydrogen storage system. Catal. Sci. Technol. 2016, 6, 12–40. [Google Scholar] [CrossRef]
- Ding, T.Y.; Zhao, Z.G.; Ran, M.F.; Yang, Y.Y. Superior activity of Pd nanoparticles confined in carbon nanotubes for hydrogen production from formic acid decomposition at ambient temperature. J. Colloid Interface Sci. 2019, 538, 474–480. [Google Scholar] [CrossRef] [PubMed]
- El-Nagar, G.A.; Dawood, K.M.; El-Deab, M.S.; Al-Andouli, B.E. Efficient direct formic acid fuel cell (DFAFC) anode of nano-sized palladium complex: High durability and activity origin. Appl. Catal. B Environ. 2017, 213, 118–126. [Google Scholar] [CrossRef]
- Mellmann, D.; Sponholz, P.; Junge, H.; Beller, M. Formic acid as a hydrogen storage material—Development of homogeneous catalysts for selective hydrogen release. Chem. Soc. Rev. 2016, 45, 3954–3988. [Google Scholar] [CrossRef] [PubMed]
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous catalysis for sustainable hydrogen storage in formic acid and alcohols. Chem. Rev. 2017, 118, 372–433. [Google Scholar] [CrossRef]
- Onishi, N.; Iguchi, M.; Yang, X.; Kanega, R.; Kawanami, H.; Xu, Q.; Himeda, Y. Development of effective catalysts for hydrogen storage technology using formic acid. Adv. Energy Mater. 2019, 9, 1801275. [Google Scholar] [CrossRef]
- Guan, C.; Pan, Y.; Zhang, T.; Ajitha, M.J.; Huang, K.W. An update on formic acid dehydrogenation by homogeneous catalysis. Chem. Asian J. 2020, 15, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Zell, T.; Langer, R. CO2-based hydrogen storage—Formic acid dehydrogenation. Phys. Sci. Rev. 2018, 3, 20170012. [Google Scholar]
- Jiang, K.; Xu, K.; Zou, S.; Cai, W.B. B-Doped Pd catalyst: Boosting room-temperature hydrogen production from formic acid–formate solutions. J. Am. Chem. Soc. 2014, 136, 4861–4864. [Google Scholar] [CrossRef]
- Wang, N.; Sun, Q.; Bai, R.; Li, X.; Guo, G.; Yu, J. In situ confinement of ultrasmall Pd clusters within nanosized silicalite-1 zeolite for highly efficient catalysis of hydrogen generation. J. Am. Chem. Soc. 2016, 138, 7484–7487. [Google Scholar] [CrossRef]
- Bi, Q.Y.; Du, X.L.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Efficient subnanometric gold-catalyzed hydrogen generation via formic acid decomposition under ambient conditions. J. Am. Chem. Soc. 2012, 134, 8926–8933. [Google Scholar] [CrossRef]
- Zhong, H.; Iguchi, M.; Chatterjee, M.; Himeda, Y.; Xu, Q.; Kawanami, H. Formic acid-based liquid organic hydrogen carrier system with heterogeneous catalysts. Adv. Sustain. Syst. 2018, 2, 1700161. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, W.; Jiang, Z.; Fang, T. A review on liquid-phase heterogeneous dehydrogenation of formic acid: Recent advances and perspectives. Chem. Pap. 2018, 72, 2121–2135. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Bulusheva, L.G.; Beloshapkin, S.; O’Connor, T.; Okotrub, A.V.; Ryan, K.M. Pd clusters supported on amorphous, low-porosity carbon spheres for hydrogen production from formic acid. ACS Appl. Mater. Interfaces 2015, 7, 8719–8726. [Google Scholar] [CrossRef]
- García-Aguilar, J.; Navlani-García, M.; Berenguer-Murcia, Á.; Mori, K.; Kuwahara, Y.; Yamashita, H.; Cazorla-Amorós, D. Evolution of the PVP-Pd surface interaction in nanoparticles through the case study of formic acid decomposition. Langmuir 2016, 32, 12110–12118. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, M.; Navlani-García, M.; Kuwahara, Y.; Mori, K.; Yamashita, H. Palladium nanoparticles supported on titanium doped graphitic carbon nitride for formic acid dehydrogenation. Chem.-An. Asian J. 2017, 12, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Navlani-García, M.; Mori, K.; Salinas-Torres, D.; Kuwahara, Y.; Yamashita, H. New approaches toward the hydrogen production from formic acid dehydrogenation over Pd-based heterogeneous catalysts. Front. Mater. 2019, 6, 44. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Qiu, H.; Cao, W.; Fu, H.; Wan, H.; Xu, Z.; Zheng, S. Ultrafine Pd particles embedded in nitrogen-enriched mesoporous carbon for efficient H2 production from formic acid decomposition. ACS Sustain. Chem. Eng. 2019, 7, 1963–1972. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Mao, S.; Gong, Y.; Chen, Y.; Wang, Y. Pd nanoparticles anchored on amino-functionalized hierarchically porous carbon for efficient dehydrogenation of formic acid under ambient conditions. J. Mater. Chem. A 2019, 7, 25791–25795. [Google Scholar] [CrossRef]
- Wang, X.; Meng, Q.; Gao, L.; Jin, Z.; Ge, J.; Liu, C.; Xing, W. Recent progress in hydrogen production from formic acid decomposition. Int. J. Hydrog. Energy 2018, 43, 7055–7071. [Google Scholar] [CrossRef]
- Karatas, Y.; Bulut, A.; Yurderi, M.; Ertas, I.E.; Alal, O.; Gulcan, M.; Celebi, M.; Kivrak, H.; Kaya, M.; Zahmakiran, M. PdAu-MnOx nanoparticles supported on amine-functionalized SiO2 for the room temperature dehydrogenation of formic acid in the absence of additives. Appl. Catal. B 2016, 180, 586–595. [Google Scholar] [CrossRef]
- Yan, J.M.; Li, S.J.; Yi, S.S.; Wulan, B.R.; Zheng, W.T.; Jiang, Q. Anchoring and upgrading ultrafine NiPd on room-temperature-synthesized bifunctional NH2-N-rGO toward low-cost and highly efficient catalysts for selective formic acid dehydrogenation. Adv. Mater. 2018, 30, 1703038. [Google Scholar] [CrossRef]
- Ye, W.; Pei, W.; Zhou, S.; Huang, H.; Li, Q.; Zhao, J.; Lu, R.; Ge, Y.; Zhang, S. Controlling the synthesis of uniform electron-deficient Pd clusters for superior hydrogen production from formic acid. J. Mater. Chem. A 2019, 7, 10363–10371. [Google Scholar] [CrossRef]
- Luo, Y.X.; Yang, Q.F.; Nie, W.D.; Yao, Q.L.; Zhang, Z.J.; Lu, Z.H. Anchoring IrPdAu nanoparticles on NH2-SBA-15 for fast hydrogen production from formic acid at room temperature. ACS Appl. Mater. Interfaces 2020, 12, 8082–8090. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, A.; Bhowmik, K.; De, G.; Mukherjee, A. Synthesis of amine functionalized graphite nanosheets and their water-soluble derivative for drug loading and controlled release. New J. Chem. 2015, 39, 2451–2458. [Google Scholar] [CrossRef]
- Ramanathan, T.; Fisher, F.T.; Ruoff, R.S.; Brinson, L.C. Amino-functionalized carbon nanotubes for binding to polymers and biological systems. Chem. Mater. 2005, 17, 1290–1295. [Google Scholar] [CrossRef]
- Kitamura, H.; Sekido, M.; Takeuchi, H.; Ohno, M. The method for surface functionalization of single-walled carbon nanotubes with fuming nitric acid. Carbon 2011, 49, 3851–3856. [Google Scholar] [CrossRef]
- Masuda, S.; Mori, K.; Futamura, Y.; Yamashita, H. PdAg nanoparticles supported on functionalized mesoporous carbon: Promotional effect of surface amine groups in reversible hydrogen delivery/storage mediated by formic acid/CO2. ACS Catal. 2018, 8, 2277–2285. [Google Scholar] [CrossRef]
- Luo, Y.X.; Nie, W.; Ding, Y.; Yao, Q.; Feng, G.; Lu, Z.H. Robust hydrogen production from additive-Free formic acid via mesoporous silica-confined Pd-ZrO2 nanoparticles at room temperature. ACS Appl. Energy Mater. 2021, 4, 4945–4954. [Google Scholar] [CrossRef]
- Yurderi, M.; Bulut, A.; Caner, N.; Celebi, M.; Kaya, M.; Zahmakiran, M. Amine grafted silica supported CrAuPd alloy nanoparticles: Superb heterogeneous catalysts for the room temperature dehydrogenation of formic acid. Chem. Commun. 2015, 51, 11417–11420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tsumori, N.; Kitta, M.; Xu, Q. Fast dehydrogenation of formic acid over palladium nanoparticles immobilized in nitrogen-doped hierarchically porous carbon. ACS Catal. 2018, 8, 12041–12045. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, L.; Liu, Z.; Tsumori, N.; Kitta, M.; Xu, Q. Phosphate-mediated immobilization of high-performance AuPd nanoparticles for dehydrogenation of formic acid at room temperature. Adv. Funct. Mater. 2019, 1903341. [Google Scholar] [CrossRef]
- Chaparro-Garnica, J.; Navlani-García, M.; Salinas-Torres, D.; Morallon, E.; Cazorla-Amorós, D. Highly stable N-doped carbon-supported Pd-based catalysts prepared from biomass waste for H2 production from formic acid. ACS Sustain. Chem. Eng. 2020, 8, 15030–15043. [Google Scholar] [CrossRef]
- Santos, J.; Megias-Sayago, C.; Ivanova, S.; Centeno, M.; Odriozola, J. Functionalized biochars as supports for Pd/C catalysts for efficient hydrogen production from formic acid. Appl. Catal. B Environ. 2021, 282, 119615. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, S.; Meng, X.; Mao, S.; Lian, X.; Wang, Y. Ultrasmall PdAu alloy nanoparticles anchored on amine-functionalized hierarchically porous carbon as additive-free catalysts for highly efficient dehydrogenation of formic acid. Appl. Catal. B Environ. 2021, 291, 120140. [Google Scholar] [CrossRef]
- Yang, S.; Peng, L.; Huang, P.; Wang, X.; Sun, Y.; Cao, C.; Song, W. Nitrogen, phosphorus, and sulfur co-doped hollow carbon shell as superior metal-free catalyst for selective oxidation of aromatic alkanes. Angew. Chem. Int. Ed. 2016, 128, 4084–4088. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Zacharska, M.; Shlyakhova, E.V.; Chuvilin, A.L.; Guo, Y.; Beloshapkin, S.; Okotrub, A.V.; Bulusheva, L.G. Single isolated Pd2+ cations supported on N-doped carbon as active sites for hydrogen production from formic acid decomposition. ACS Catal. 2016, 6, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, E.; Althahban, S.M.; Luo, Y.; Kriegel, R.; Shaw, G.; Morgan, D.J.; He, Q.; Watanabe, M.; Armbrüster, M.; Kiely, C.J.; et al. Highly selective PdZn/ZnO catalysts for the methanol steam reforming reaction. Catal. Sci. Technol. 2018, 8, 5848–5857. [Google Scholar] [CrossRef]
- Nie, W.; Luo, Y.; Yang, Q.; Feng, G.; Yao, Q.; Lu, Z.H. An amine-functionalized mesoporous silica-supported PdIr catalyst: Boosting room-temperature hydrogen generation from formic acid. Inorg. Chem. Front. 2020, 7, 709–717. [Google Scholar] [CrossRef]
- Javaid, R.; Kawasaki, S.I.; Ookawara, R.; Sato, K.; Nishioka, M.; Suzuki, A.; Suzuki, T.M. Continuous dehydrogenation of aqueous formic acid under sub-critical conditions by use of hollow tubular reactor coated with thin palladium oxide layer. J. Chem. Eng. Jpn. 2013, 46, 751–758. [Google Scholar] [CrossRef]
- Yang, F.; Fan, X.; Wang, C.; Yang, W.; Hou, L.; Xu, X.; Feng, A.; Dong, S.; Chen, K.; Wang, Y.; et al. P-doped nanomesh graphene with high-surface-area as an efficient metal-free catalyst for aerobic oxidative coupling of amines. Carbon 2017, 121, 443–451. [Google Scholar] [CrossRef]
- Yadav, M.; Akita, T.; Tsumori, N.; Xu, Q. Strong Metal-Molecular Support Interaction (SMMSI): Amine-functionalized gold nanoparticles encapsulated in silica nanospheres highly active for catalytic decomposition of formic acid. J. Mater. Chem. 2012, 22, 12582–12586. [Google Scholar] [CrossRef]
- Zou, L.; Chen, M.; Zhang, Q.; Mao, Q.; Huang, Y.; Liang, Z. Pd/UIO-66/sepiolite: Toward highly efficient dual-supported Pd-based catalyst for dehydrogenation of formic acid at room temperature. J. Catal. 2020, 388, 66–76. [Google Scholar] [CrossRef]
- Zhang, S.L.; Li, S.J.; Wang, J.Y.; Shang, H.N.; Bai, Y.X.; Liang, J.S. Amine-functionalized carbon nanotubes supported NiAuPd nanoparticles as an efficient in-situ prepared catalyst for formic acid dehydrogenation. Int. J. Hydrog. Energy 2021, 46, 34727–34736. [Google Scholar] [CrossRef]
- Koh, K.; Jeon, M.; Yoon, C.W.; Asefa, T. Formic acid dehydrogenation over Pd NPs supported on amine-functionalized SBA-15 catalysts: Structure–activity relationships. J. Mater. Chem. A 2017, 5, 16150–16161. [Google Scholar] [CrossRef]
- Ding, R.D.; Li, Y.L.; Leng, F.; Jia, M.J.; Yu, J.H.; Hao, X.F.; Xu, J.Q. PdAu nanoparticles supported by diamine-containing UiO-66 for formic acid dehydrogenation. ACS Appl. Nano Mater. 2021, 4, 9790–9798. [Google Scholar] [CrossRef]

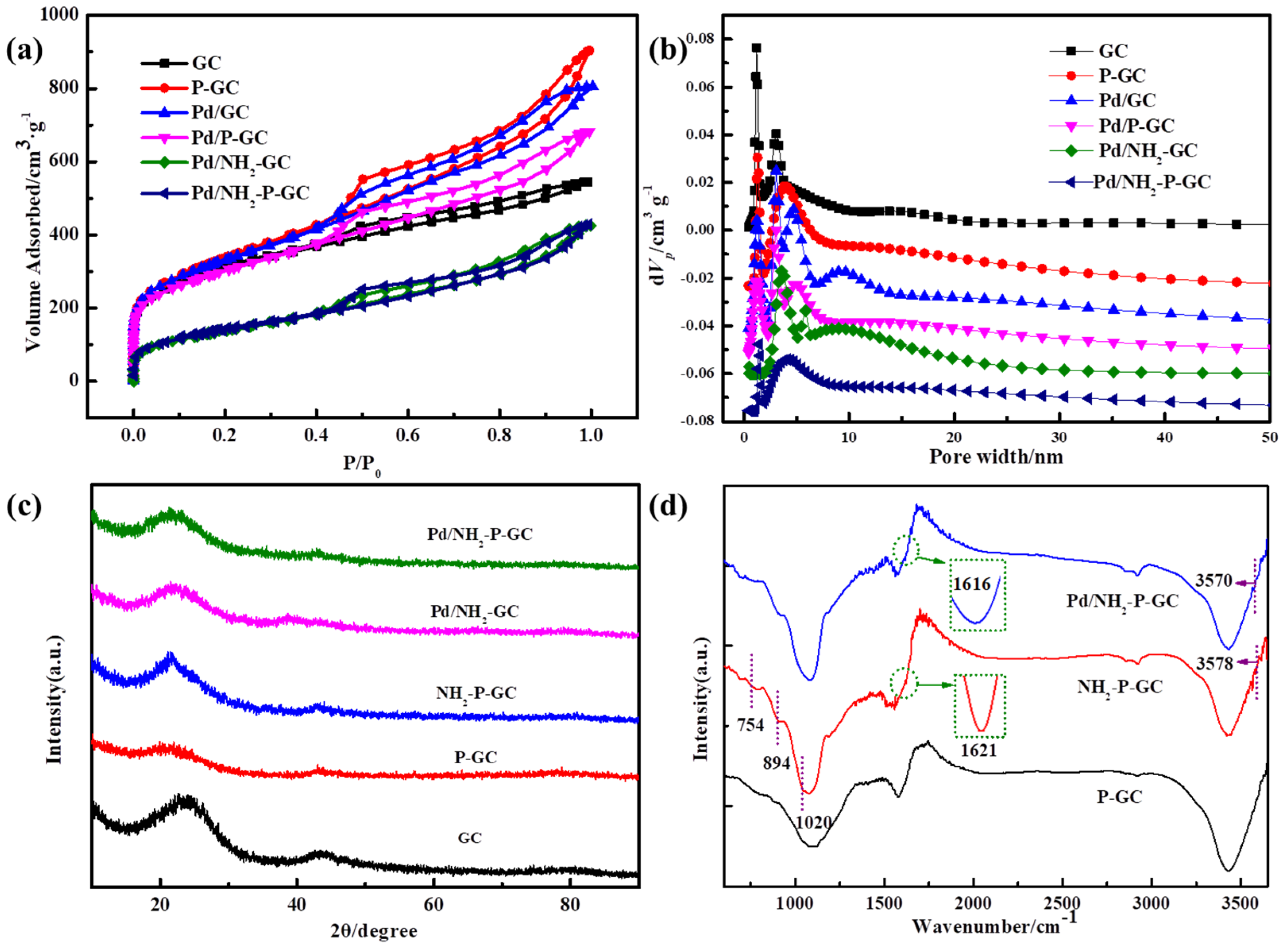

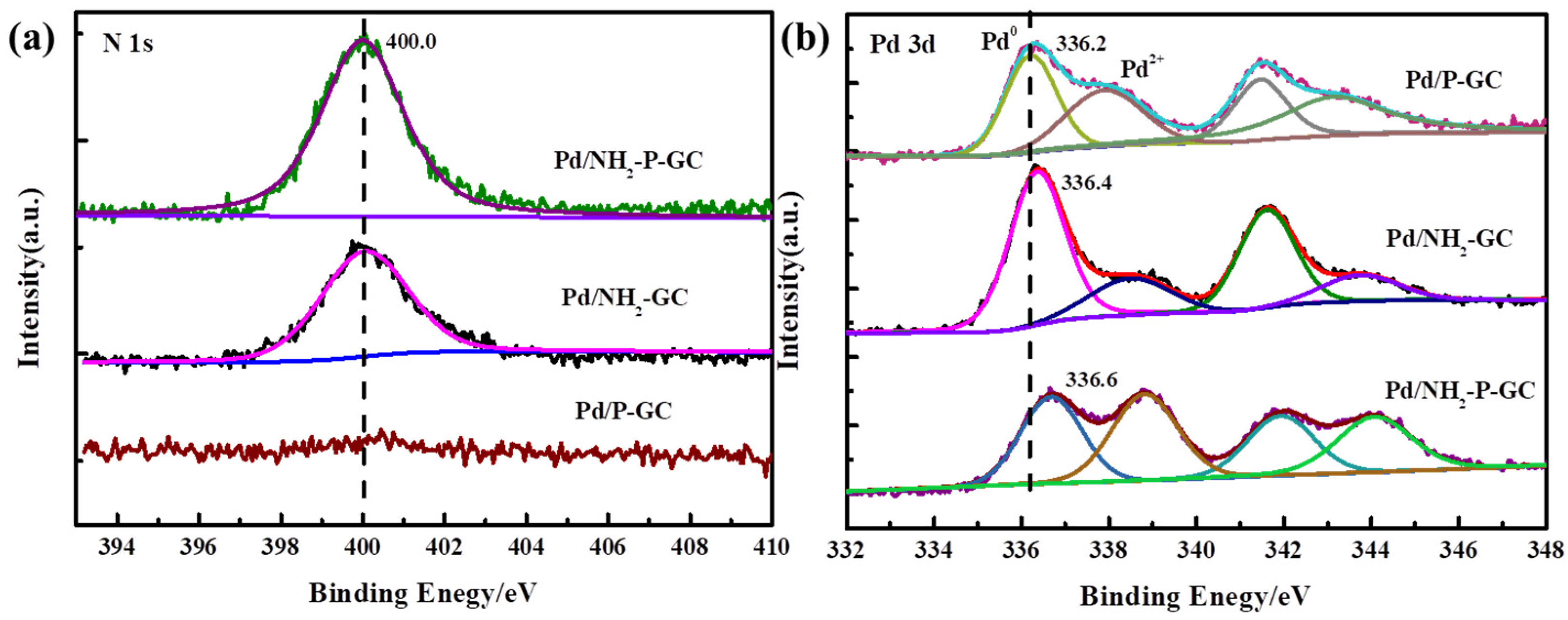
| Sample | SBET a (m2g−1) | V0 b (cm3g−1) | Vt c (cm3g−1) | Dpore d (<2 nm) | Dpore (>2 nm) |
|---|---|---|---|---|---|
| GC | 1070 | 0.84 | 0.65 | 1.2 | 3.2 |
| P-GC | 1191 | 1.37 | 0.74 | 1.3 | 3.8 |
| Pd/GC | 1143 | 1.23 | 0.76 | 1.2 | 3.2, 4.6, 9.1 |
| Pd/P-GC | 1055 | 1.04 | 0.64 | 1.2 | 3.2, 4.9 |
| Pd/NH2-GC | 482 | 0.65 | 0.33 | - | 3.5, 5.8 |
| Pd/NH2-P-GC | 495 | 0.66 | 0.31 | 1.3 | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, X.; Tian, F.; Bai, M.; Zhang, Y.; Wang, W.; Zhao, Z.; Shao, X.; Ji, X. Highly Efficient Hierarchical Porous Carbon Supported Pd-Based Catalysts for Additive-Free Dehydrogenation of Formic Acid. Catalysts 2022, 12, 240. https://doi.org/10.3390/catal12020240
Miao X, Tian F, Bai M, Zhang Y, Wang W, Zhao Z, Shao X, Ji X. Highly Efficient Hierarchical Porous Carbon Supported Pd-Based Catalysts for Additive-Free Dehydrogenation of Formic Acid. Catalysts. 2022; 12(2):240. https://doi.org/10.3390/catal12020240
Chicago/Turabian StyleMiao, Xinyi, Fengwu Tian, Miaomiao Bai, Yujia Zhang, Wei Wang, Zuoping Zhao, Xianzhao Shao, and Xiaohui Ji. 2022. "Highly Efficient Hierarchical Porous Carbon Supported Pd-Based Catalysts for Additive-Free Dehydrogenation of Formic Acid" Catalysts 12, no. 2: 240. https://doi.org/10.3390/catal12020240
APA StyleMiao, X., Tian, F., Bai, M., Zhang, Y., Wang, W., Zhao, Z., Shao, X., & Ji, X. (2022). Highly Efficient Hierarchical Porous Carbon Supported Pd-Based Catalysts for Additive-Free Dehydrogenation of Formic Acid. Catalysts, 12(2), 240. https://doi.org/10.3390/catal12020240