Immobilization of Lipases on Modified Silica Clay for Bio-Diesel Production: The Effect of Surface Hydrophobicity on Performance
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Loading Efficiency and Hydrolytic Activity of Immobilized Enzyme
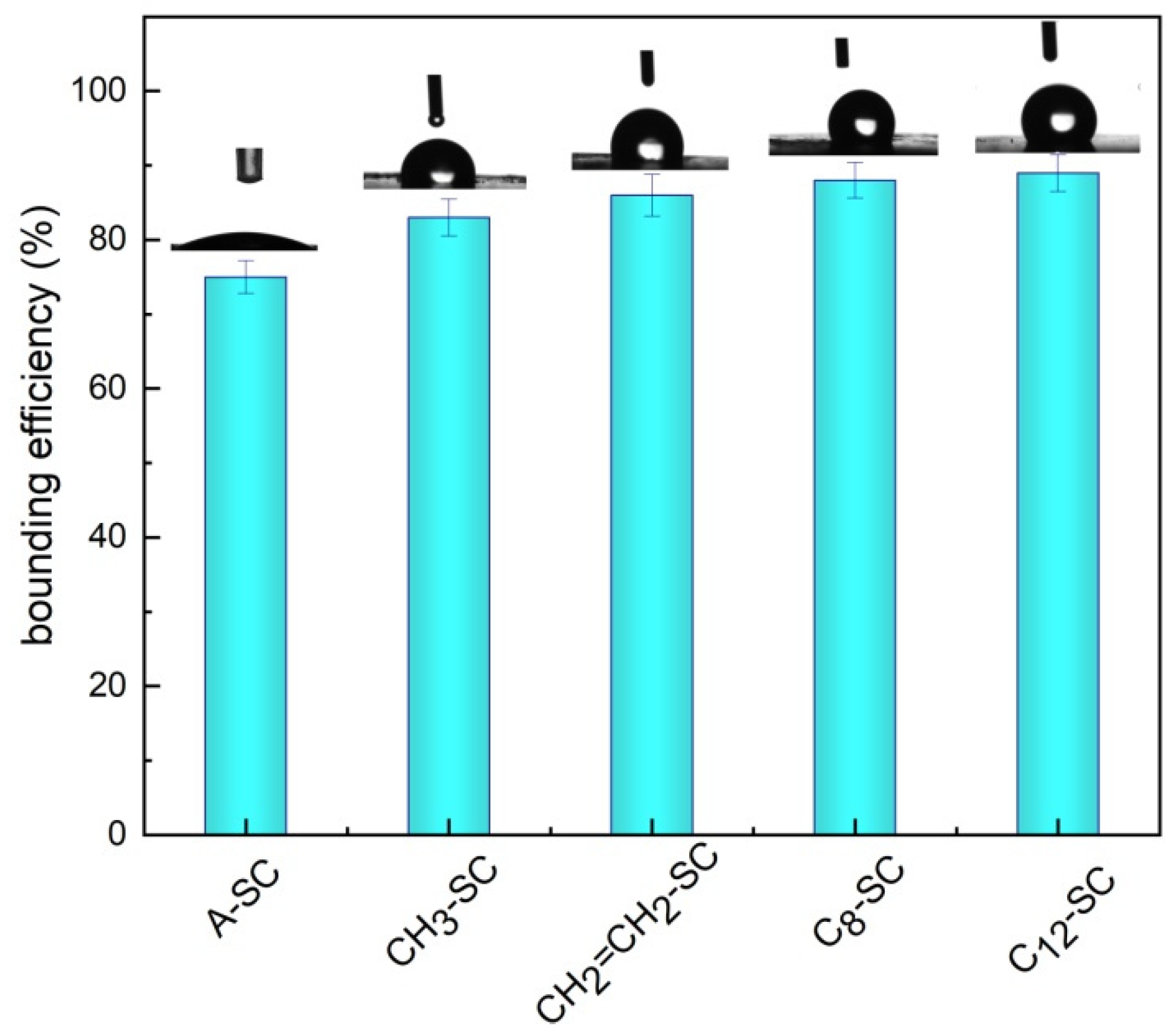
2.2.1. Loading Efficiency of Immobilization
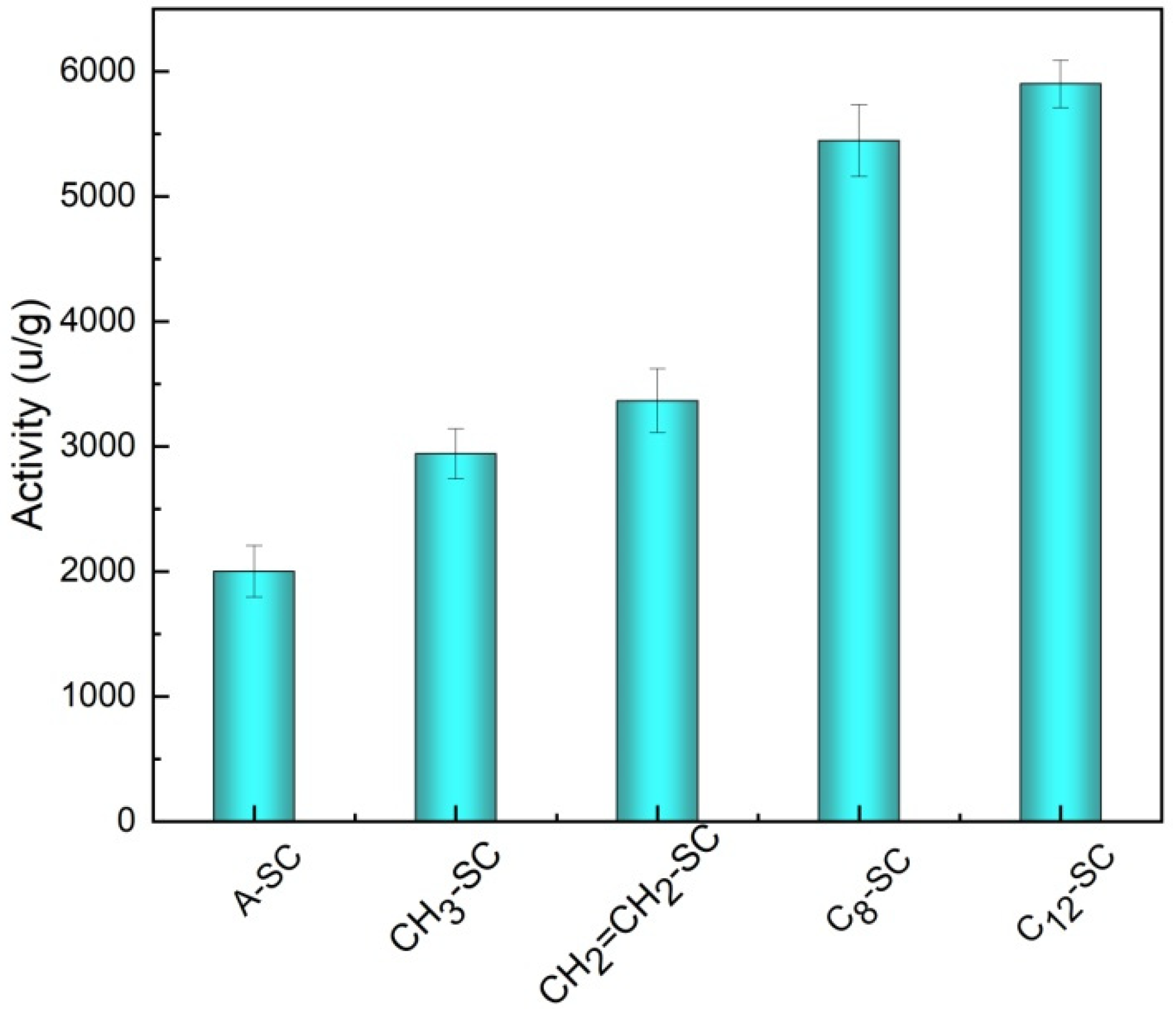
2.2.2. Hydrolytic Activity of Immobilized Enzyme
2.3. Enzymatic Properties of Immobilized Lipase
2.3.1. Effect of Temperature
2.3.2. Effect of pH
2.3.3. Effect on Thermal Stability
2.4. Biodiesel Synthesis
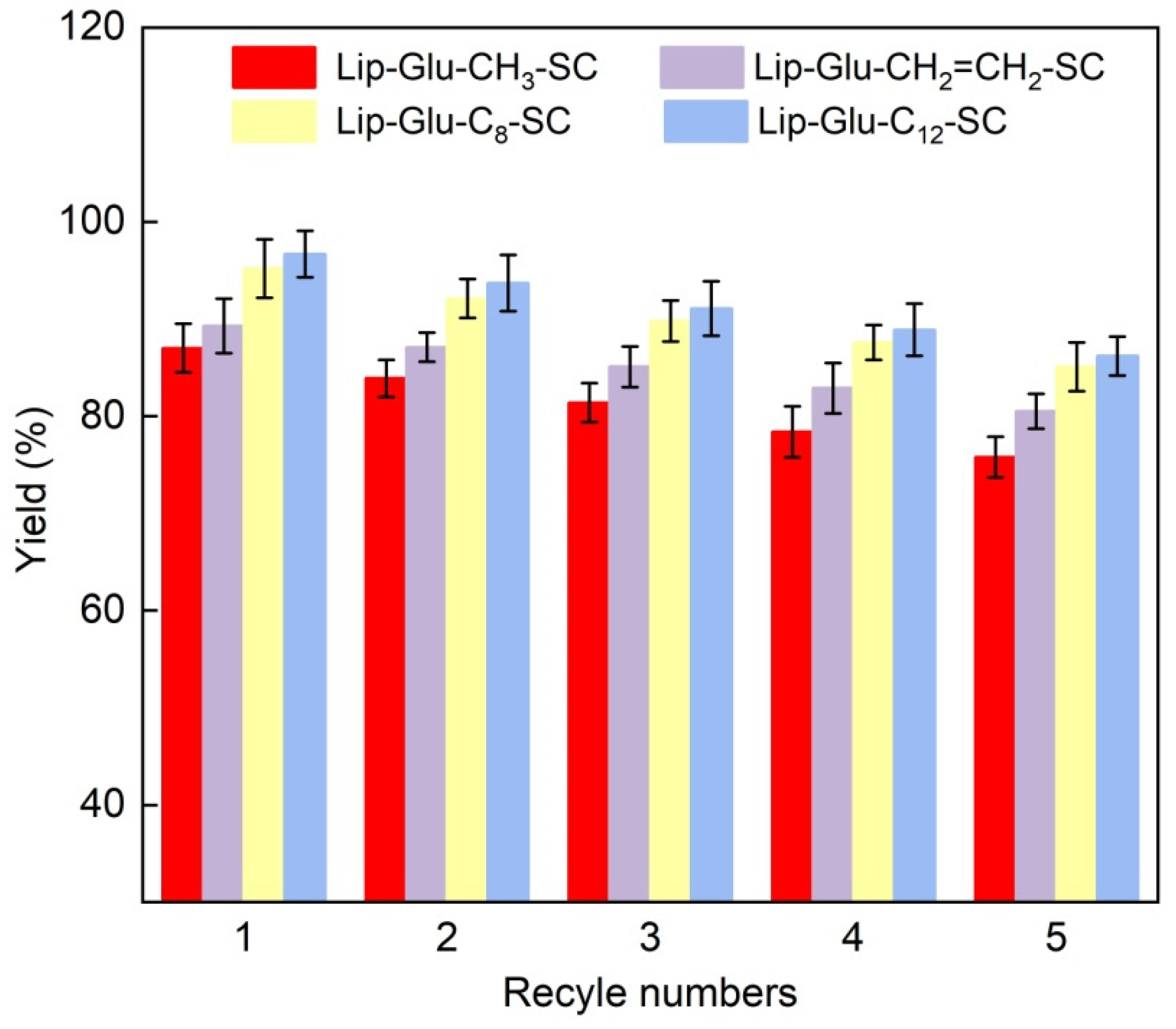
2.5. Reusability of Immobilized Lipase
3. Materials and Methods
3.1. Materials
3.2. Preparation of Hydrophobic Silica Clay and Immobilization of Lipase
3.2.1. Hydrophobic Modification on Silica Clay
3.2.2. Immobilization of Lipase
3.3. Characterization
3.4. Determination of Immobilized Enzyme Loading
3.5. Hydrolytic Activity Assay
3.6. Biodiesel Production
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, C.; Tian, Y.; Jiang, Y.L. Elucidation of lid open and orientation of lipase activated in interfacial activation by amphiphilic environment. Inter. J. Biol. Macromol. 2018, 119, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhu, W.; Matyjaszewski, K. Protection of opening lids: Very high catalytic activity of lipase immobilized on core-shell nanoparticles. Macromolecules 2018, 51, 289–296. [Google Scholar] [CrossRef] [PubMed]
- De, T.; Sikder, J.; Narayanan, C.M. Biodiesel synthesis using immobilised lipase enzyme in semi-fluidised bed bioreactors—bioreactor design and performance analysis. Environ. Prog. Sustain. Energ. 2017, 36, 1537–1545. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Hu, H.; Xu, J. Enhanced enzymatic performance of immobilized lipase on metal organic frameworks with superhydrophobic coating for biodiesel production. J. Colloid Interf. Sci. 2021, 602, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Tacin, M.V.; Costa-Silva, T.A.; Paula, A.V. Microbial lipase: A new approach for a heterogeneous biocatalyst. Prep. Biochem. Biotechnol. 2021, 51, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.E.; Zhang, Z.A.; Tang, A.X. Modification of magnetic graphene oxide with covalent A to fix lipase CRL by surfactants. Chem. Ind. Eng. Prog. 2019, 38, 4255–4263. [Google Scholar]
- Ying, M.; Chen, G. Study on the production of biodiesel by magnetic cell biocatalyst based on lipase-producing bacillus subtilis. Appl. Biochem. Biotechnol. 2007, 137, 793–803. [Google Scholar] [PubMed]
- Lima, L.N.; Oliveira, G.C.; Rojas, M.J. Immobilization of Pseudomonas fluorescens lipase on hydrophobic supports and application in biodiesel synthesis by transesterification of vegetable oils in solvent-free systems. J. Ind. Microb. Biotechnol. 2015, 42, 523–535. [Google Scholar] [CrossRef]
- Amirkhani, L.; Moghaddas, J.; Jafarizadeh, H. Optimization of biodiesel production using immobilized Candida rugosa lipase on magnetic Fe3O4-silica aerogel. Iran. J. Chem. Chem. Eng. 2019, 38, 193–201. [Google Scholar]
- Kalantari, M.; Yu, M.; Liu, Y.; Huang, X.; Yu, C. Engineering mesoporous silica microspheres as hyper-activation supports for continuous enzymatic biodiesel production. Mater. Chem. Front. 2019, 3, 1816–1822. [Google Scholar] [CrossRef]
- Payá-Tormo, L.; Rodríguez-Salarichs, J. Improvement of the activity of a fungal versatile-lipase toward triglycerides: An in silico mechanistic description. Front. Bioeng. Biotechnol. 2019, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, T.R.B.; Ashton, T.D.; Marshall, S.N. Effect of Triton X-100 on the activity and selectivity of lipase immobilized on chemically reduced graphene oxides. Langmuir 2021, 37, 9202–9214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zou, Y.; Shen, Y.; Gao, X.; Zheng, X.; Zhang, X. Dominated effect analysis of the channel size of silica support materials on the catalytic performance of immobilized lipase catalysts in the transformation of unrefined waste cooking oil to biodiesel. BioEnerg. Res. 2014, 7, 1541–1549. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Han, D.; Zhang, H.; Liu, P.; Li, C. An efficient resolution of racemic secondary alcohols on magnetically separable biocatalyst. Biochem. Biophys. Res. Commun. 2008, 365, 609–613. [Google Scholar] [CrossRef]
- Palla, C.A.; Pacheco, C.; Carrín, M.E. Preparation and modification of chitosan particles for Rhizomucor miehei lipase immobilization. Biochem. Eng. J. 2011, 55, 199–207. [Google Scholar] [CrossRef]
- Deng, H.T.; Wang, J.J.; Ma, M.; Liu, Z.Y.; Zheng, F. Hydrophobic surface modification of chitosan gels by stearyl for improving the activity of immobilized lipase. Chinese Chem. Lett. 2009, 20, 995–999. [Google Scholar] [CrossRef]
- Aucoin, M.G.; Erhardt, F.A. Hyperactivation of Rhizomucor miehei lipase by hydrophobic xerogels. Biotechnol. Bioeng. 2004, 85, 647–655. [Google Scholar] [CrossRef]
- Lu, S.; He, J.; Guo, X. Architecture and performance of mesoporous silica-lipase hybrids via non-covalent interfacial adsorption. AIChE J. 2009, 56, 506–514. [Google Scholar] [CrossRef]
- Jin, Q.; Li, X.; Deng, C.; Zhang, Q.; Yi, D.; Wang, X. Silica nanowires with tunable hydrophobicity for lipase immobilization and biocatalytic membrane assembly. J. Colloid Inter. Sci. 2018, 531, 555–563. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, B.; Lv, K.; Kumissay, L.; Wu, B.; Chu, J. Specific immobilization of lipase on functionalized 3D printing scaffolds via enhanced hydrophobic interaction for efficient resolution of racemic 1-indanol. Biochem. Biophys. Res. Commun. 2021, 546, 111–117. [Google Scholar] [CrossRef]
- Rodrigues, C.R.; Virgen-Ortiz, J.J.; dos Santos, C.S.J.; Berenguer-Murcia, A.; Alcantara, R.A.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octylglyoxyl agarose beads. RSC Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, P.; Lassalle, V.L.; Ferreira, M.L. About the role of typical spacer/crosslinker on the design of efficient magnetic biocatalysts based on nanosized magnetite. J. Mol. Catal. B Enzym. 2015, 122, 296–304. [Google Scholar] [CrossRef]
- Weinberger, S.; Pellis, A.; Comerford, J.W.; Farmer, T.J.; Guebitz, G.M. Efficient physisorption of candida antarctica lipase B on polypropylene beads and application for polyester synthesis. Catalysts 2018, 8, 369. [Google Scholar] [CrossRef] [Green Version]
- Arana-Peña, S.; Lokha, Y.; Fernández-Lafuente, R. Immobilization of eversa lipase on octyl agarose beads and preliminary characterization of stability and activity features. Catalysts 2018, 8, 511. [Google Scholar] [CrossRef] [Green Version]
- Zou, T.; Duan, Y.D.; Wang, Q.E.; Cheng, H.M. Preparation of immobilized lipase on silica clay as a potential biocatalyst on synthesis of biodiesel. Catalysts 2020, 10, 1266. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si (OR) (4) and R″Si (OR′) (3) precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Urrutia, P.; Arrieta, R.; Alvarez, L.; Cardenas, C. Immobilization of lipases in hydrophobic chitosan for selective hydrolysis of fish oil: The impact of support functionalization on lipase activity, selectivity and stability. Inter. J. Biol. Macromol. 2018, 108, 674–686. [Google Scholar] [CrossRef]
- Wang, J.; Meng, G.; Tao, K.; Feng, M.; Zhao, X.; Li, Z.; Xu, H. Immobilization of lipases on alkyl silane modified magnetic nanoparticles: Effect of alkyl chain length on enzyme activity. PLoS ONE 2012, 7, e43478. [Google Scholar] [CrossRef]
- Machado, N.B.; Miguez, J.P.; Bolina, I.C.A.; Salviano, A.B.; Gomes, R.A.B.; Tavano, O.L.; Luiz, J.H.H.; Tardioli, P.W.; Cren, E.C. Preparation, functionalization and characterization of rice husk silica for lipase immobilization via adsorption. Enzym. Microb. Technol. 2018, 128, 9–21. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, G.Z.; Xu, Y.; Odoom-Wubah, T.; Jia, L.S.; Huang, J.L.; Sun, D.H.; Li, Q.B. Diatomite supported Pt nanoparticles as efficient catalyst for benzene removal. Ind. Eng. Chem. Res. 2019, 58, 14008–14015. [Google Scholar] [CrossRef]
- No, D.S.; Zhao, T.T.; Lee, J.; Lee, J.S. Synthesis of phytosteryl ester containing pinolenic acid in a solvent-free system using immobilized Candida rugosa lipase. J. Agr. Food Chem. 2013, 61, 8934–8940. [Google Scholar] [CrossRef] [PubMed]
- Deon, M.; Ricardi, N.C.; Andrade, R.C.D. Designing a support for lipase immobilization based on magnetic, hydrophobic, and mesoporous silica. Langmuir 2020, 36, 10147–10155. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.C.S.; Rueda, N.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Tuning the catalytic properties of lipases immobilized on divinylsulfone activated agarose by altering its nanoenvironment. Enzym. Microb. Technol. 2015, 77, 1–7. [Google Scholar] [CrossRef]
- He, S.S.; Song, D.W.; Chen, M.; Cheng, H.M. Immobilization of lipases on magnetic collagen fibers and its applications for short-chain ester synthesis. Catalysts 2017, 7, 178. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, J.C.S.; Rueda, N.; Sanchez, A.; Villalonga, R.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Versatility of divinylsulfone supports permits the tuning of CALB properties during its immobilization. RSC Adv. 2015, 5, 35801–35810. [Google Scholar] [CrossRef]
- Remonatto, D.; de Oliveira, J.V.; Guisan, J.M.; de Oliveira, D.; Ninow, J.; Fernandez-Lorente, G. Production of FAME and FAEE via alcoholysis of sunflower oil by eversa lipases immobilized on hydrophobic supports. Appl. Biochem. Biotechnol. 2018, 185, 705–716. [Google Scholar] [CrossRef]
- Zhang, W.W.; Yang, X.L.; Jia, J.Q.; Wang, N.; Hu, C.L. Surfactant-activated magnetic cross-linked enzyme aggregates (magnetic CLEAs) of Thermomyces lanuginosus lipase for biodiesel production. J. Mol. Catalysis B Enzym. 2015, 115, 83–89. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, M.Y.; Shi, J.; Zheng, M.M.; Huang, F.H. Preparation of immobilized lipase based on hollow mesoporous silica spheres and its application in ester synthesis. Molecules 2019, 24, 395. [Google Scholar] [CrossRef] [Green Version]
- GB/T 2013-2010; Density Determination Method for Liquid Petrochemical Products. Available online: http://www.gb688.cn/bzgk/gb/newGbInfo?hcno=AE3625C61D714EBF721B10A83BA8CB93 (accessed on 8 January 2022).
- GB/T 265-1988; Petroleum Products Kinematic Viscosity Determination Method and Dynamic Viscosity Calculation Method. Available online: http://www.gb688.cn/bzgk/gb/newGbInfo?hcno=AD148CF5E97E23EEE5BC82FB60098519 (accessed on 8 January 2022).
- GB 264-1983; Determination of Acid Value of Petroleum Products. Available online: http://www.gb688.cn/bzgk/gb/newGbInfo?hcno=1CD5381B75A393C0213F549FF79016B1 (accessed on 8 January 2022).
- GB/T 17144-1997; Determination of Residual Carbon in Petroleum Products (Micro Method). Available online: http://www.gb688.cn/bzgk/gb/newGbInfo?hcno=CAB7C5B4326B2A08F50ECC2C7B926F77 (accessed on 8 January 2022).
- You, Q.; Yin, X.; Zhao, Y.; Zhang, Y. Biodiesel production from jatropha oil catalyzed by immobilized Burkholderia cepacia lipase on modified attapulgite. Bioresour. Technol. 2013, 148, 202–207. [Google Scholar] [CrossRef]


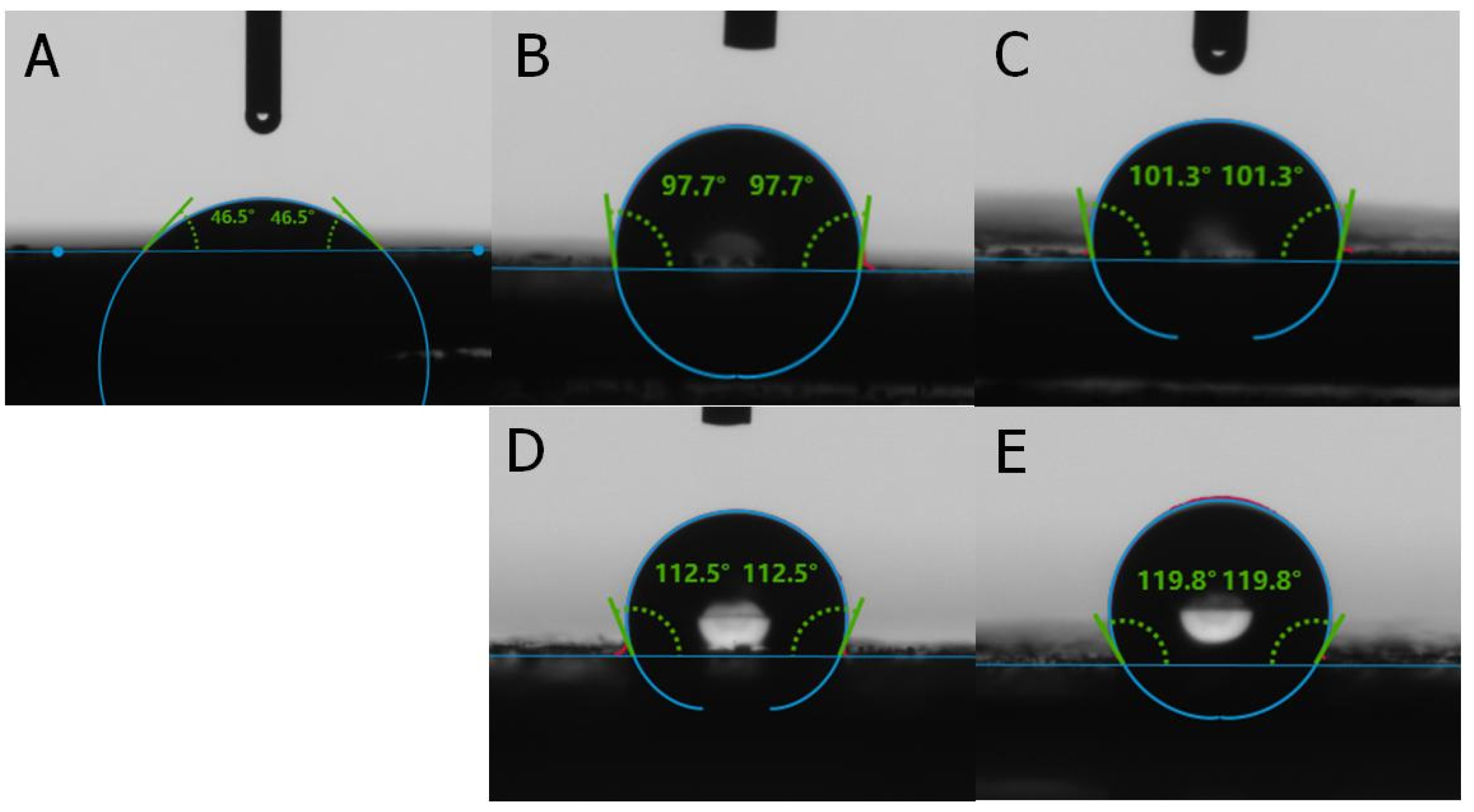



| Samples | N (%) | C (%) | H (%) | S (%) |
|---|---|---|---|---|
| CH3-SC | 0.42 | 2.47 | 0.94 | 0 |
| CH2=CH2-SC | 0.39 | 3.12 | 0.98 | 0 |
| C8-SC | 0.40 | 3.22 | 1.11 | 0 |
| C12-SC | 0.42 | 3.71 | 1.09 | 0 |
| Samples | A-SC | CH3-SC | CH2=CH2-SC | C8-SC | C12-SC |
|---|---|---|---|---|---|
| Contact angle (°) | 64.3 | 119.5 | 122.1 | 132.1 | 143.2 |
| Property | Result | Standard Range | Standard/ Method |
|---|---|---|---|
| Density (20 °C /kg m−3) | 873 | 820–900 | GB/T 2013-2010/ Densimeter |
| Kinematic viscosity (40 °C /mm2 s–1) | 4.5 | 1.9–6.0 | GB/T 265-1988/ Capillary viscometer |
| Acid value (mg KOH g–1) | 0.10 | ≤0.8 | GB 264-1983/ KOH titration |
| Residual carbon content (%) | 0.03 | <0.3 | GB/T 17144-1997/ Muffle furnace |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Zou, T.; Wu, S.; Cheng, H. Immobilization of Lipases on Modified Silica Clay for Bio-Diesel Production: The Effect of Surface Hydrophobicity on Performance. Catalysts 2022, 12, 242. https://doi.org/10.3390/catal12020242
Duan Y, Zou T, Wu S, Cheng H. Immobilization of Lipases on Modified Silica Clay for Bio-Diesel Production: The Effect of Surface Hydrophobicity on Performance. Catalysts. 2022; 12(2):242. https://doi.org/10.3390/catal12020242
Chicago/Turabian StyleDuan, Youdan, Ting Zou, Sijin Wu, and Haiming Cheng. 2022. "Immobilization of Lipases on Modified Silica Clay for Bio-Diesel Production: The Effect of Surface Hydrophobicity on Performance" Catalysts 12, no. 2: 242. https://doi.org/10.3390/catal12020242
APA StyleDuan, Y., Zou, T., Wu, S., & Cheng, H. (2022). Immobilization of Lipases on Modified Silica Clay for Bio-Diesel Production: The Effect of Surface Hydrophobicity on Performance. Catalysts, 12(2), 242. https://doi.org/10.3390/catal12020242






