Effect of Pd/Ce Loading and Catalyst Components on the Catalytic Abatement of Toluene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Combustion Performance of Pd/γ-Al2O3 and Pd-Ce/γ-Al2O3 Powder Catalyst
2.2. Catalytic Combustion Performance of the Monolithic Catalyst
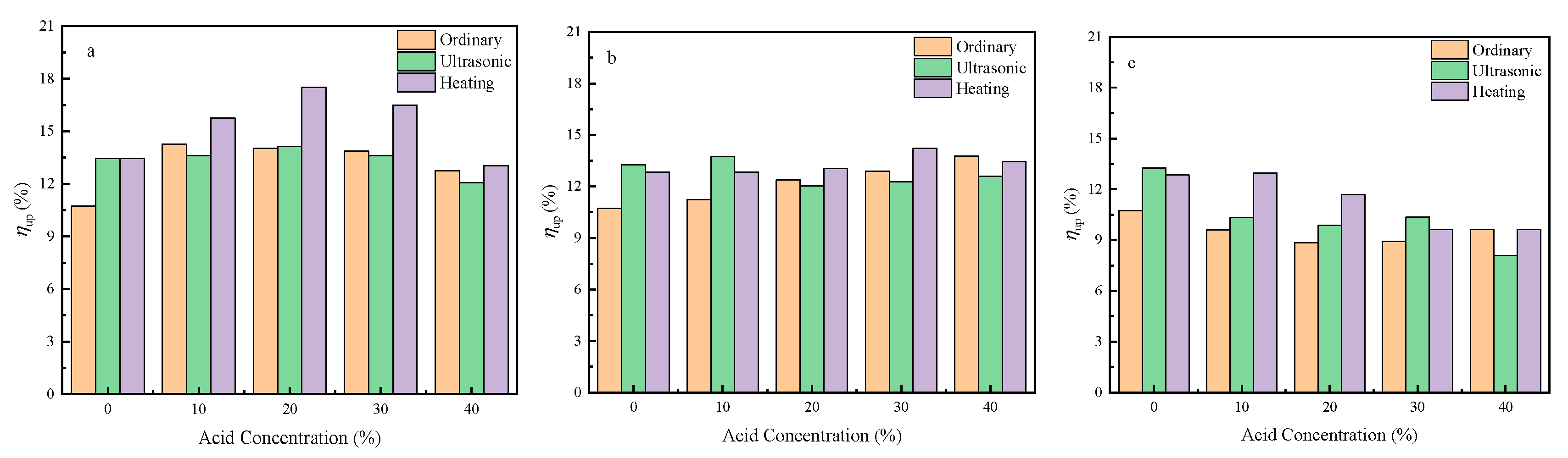
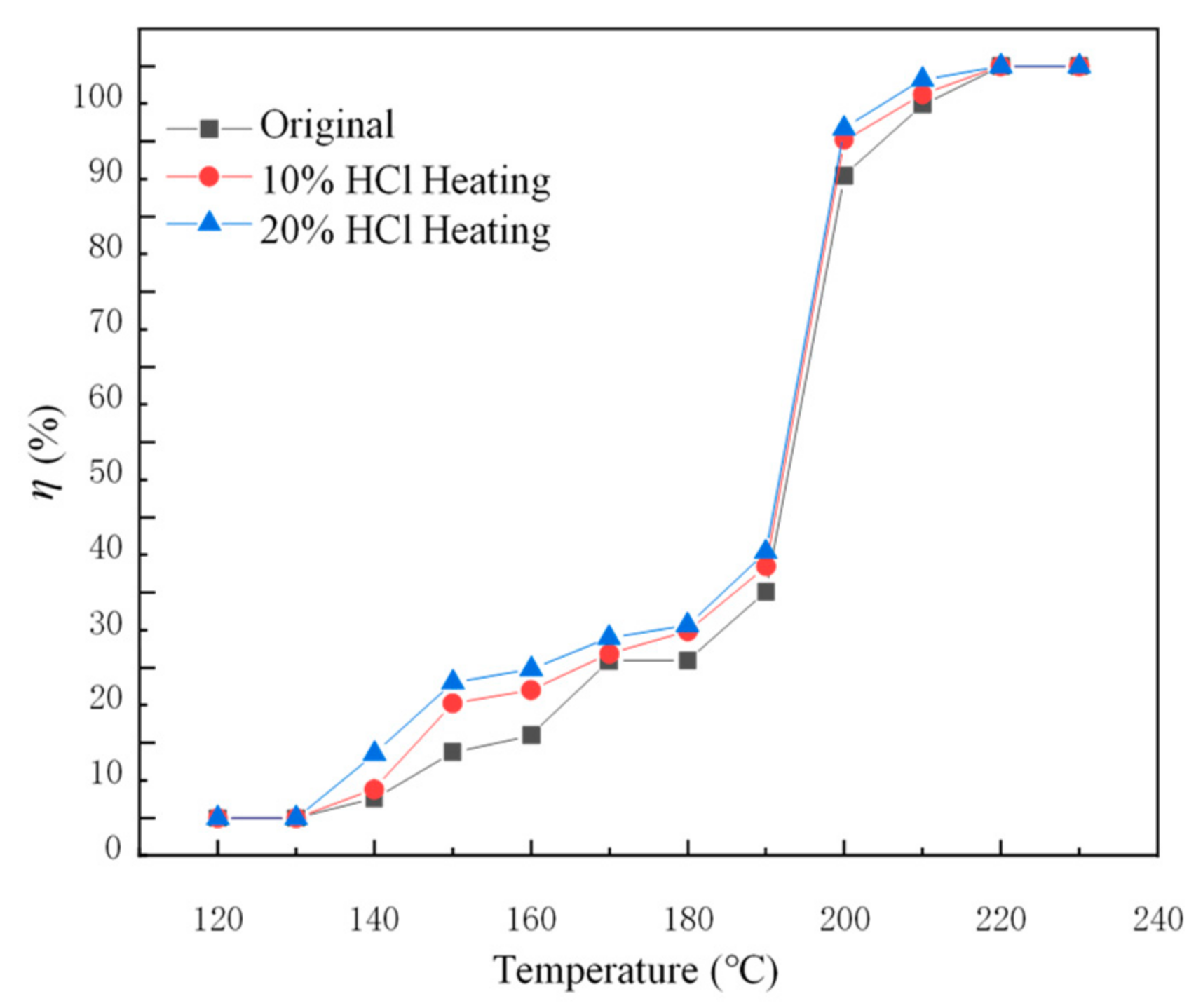
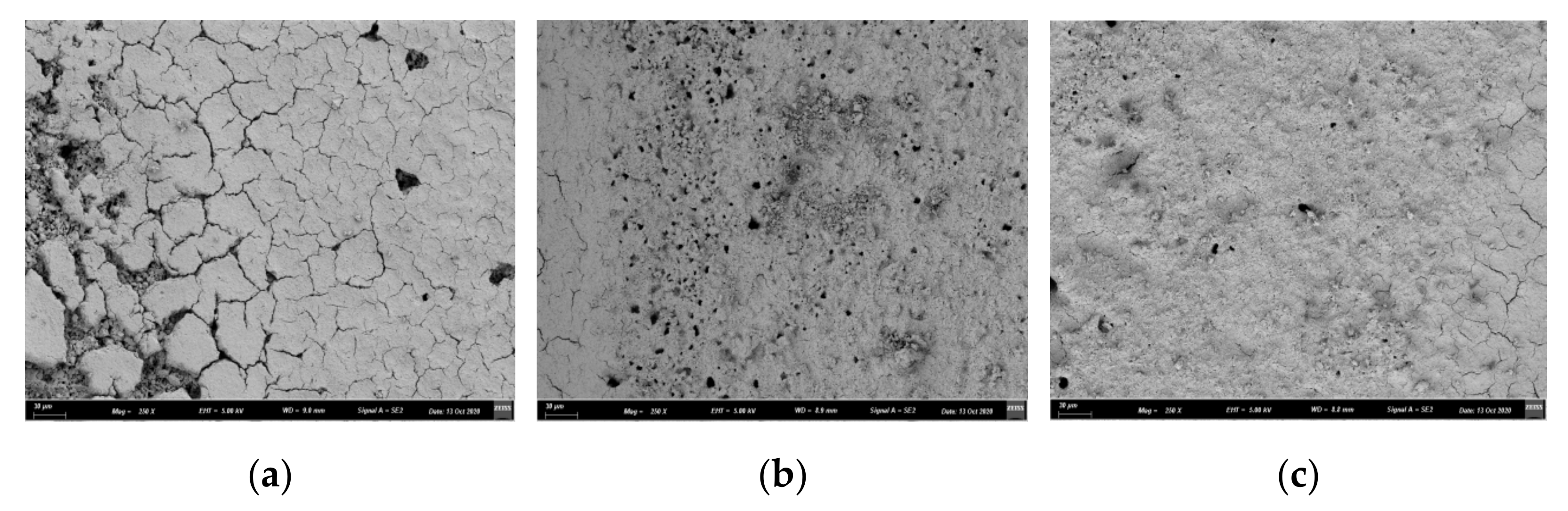
2.2.1. Effect of Substrate Pretreatment on the Performance of the Catalyst
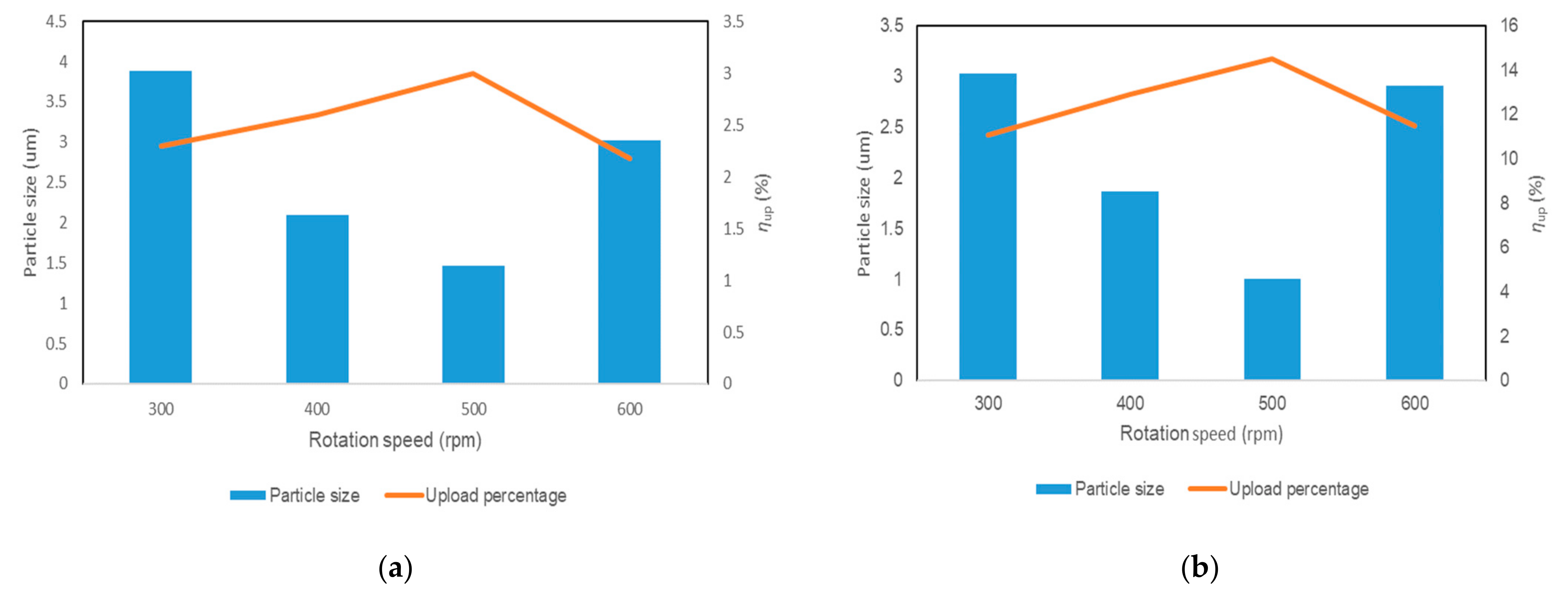
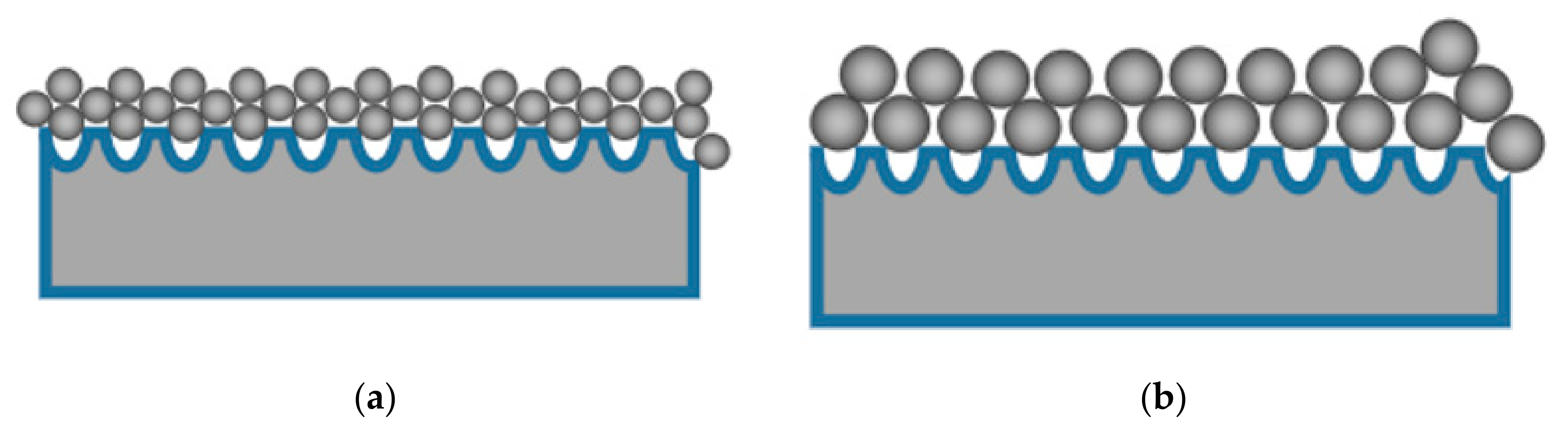
2.2.2. Effect of Particle Size of the Washcoat on the Performance of the Catalyst
2.2.3. Effect of Dispersant on the Performance of the Catalyst
2.2.4. Catalytic Degradation of Toluene with the Optimized Catalyst
3. Materials and Methodology
3.1. Materials
3.2. Experiment and Apparatus
- (1)
- Preparation of the Pd/γ-Al2O3 & Pd-Ce/γ-Al2O3 catalyst
- (2)
- Substrate pretreatment
- (3)
- Preparation of the catalyst washcoat
- (4)
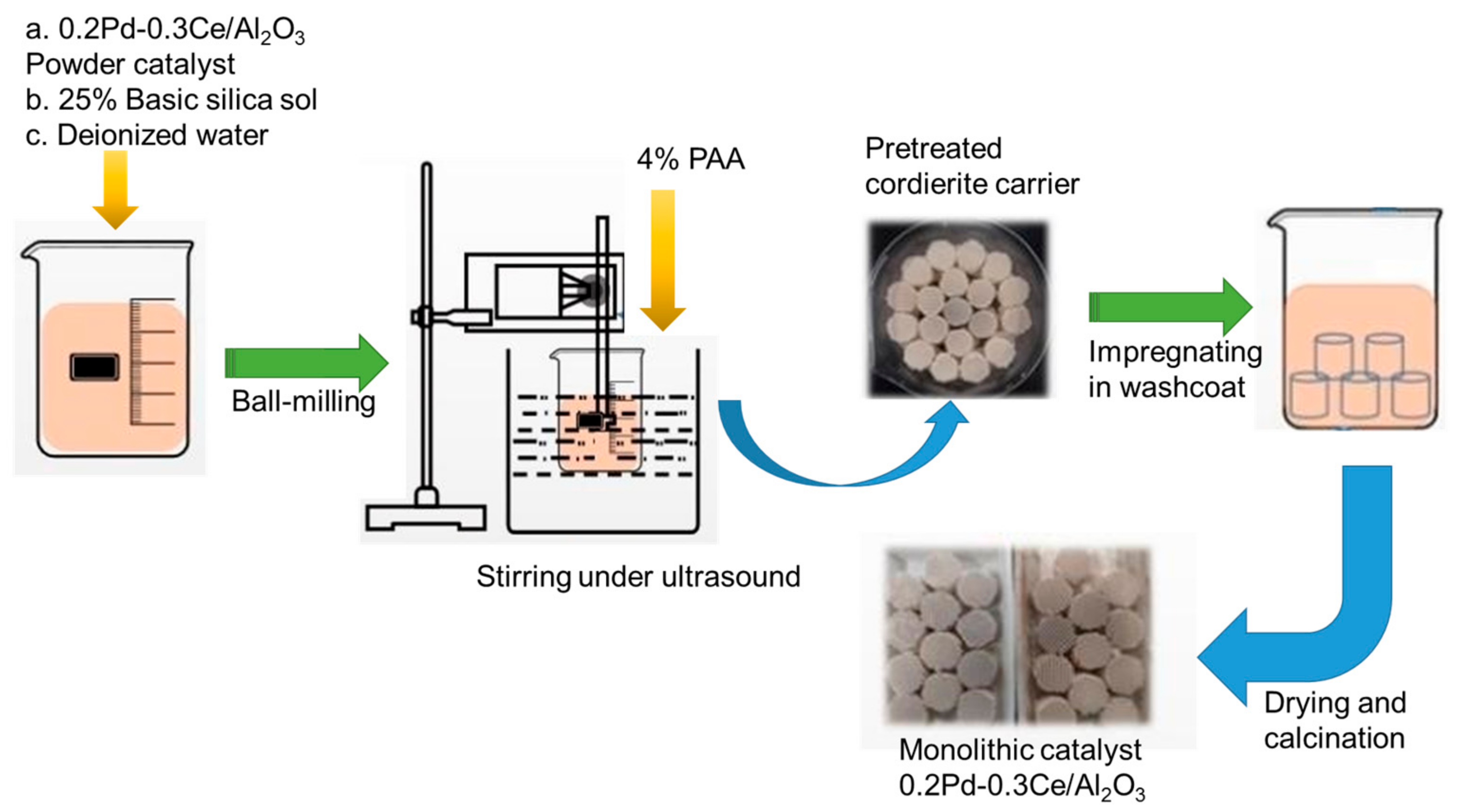
- Preparation of monolithic catalyst
- (5)
- Catalytic properties characterization
- (6)
- Catalyst evaluation
4. Conclusions
- (1)
- The activity of the Pd/γ-Al2O3 catalyst increased with the increasing of the temperature. With the addition of a small amount of the rare earth element Ce, the catalytic activity of the catalyst increased with the increasing of the Ce content. It was found that the 0.2Pd-0.3Ce/γ-Al2O3 catalysts had the best toluene catalytic activity.
- (2)
- The pretreatment of the cordierite with 20% HCl could improve the properties of the cordierite. When the cordierite was pretreated, the monolithic catalytic activity of the catalyst increased. Cordierite pretreated by 20% HCl under heating conditions had better low-temperature activity.
- (3)
- The upload ratio was inversely proportional to the size of the washcoat. Reducing the size of the catalyst particle in the washcoat could increase the loading ratio and the specific surface area. When the particle size of the washcoat decreased from 3.03 micron to 1.01 micron, the loading ratio increased by nearly 3%. At the same time, the T10 and T90 of the catalytic degradation of toluene were reduced by about 10 °C. It was observed that 4% PAA had the best dispersion performance for the washcoat. The catalytic efficiency of the monolithic catalyst increased as a result of the substrate pretreatment, adjusting the washcoat particle size, and improving the washcoat dispersion property.
Author Contributions
Funding
Conflicts of Interest
References
- Ahin, M.; Kutluay, S.; Horoz, S.; Ece, M.A. Fabrication and characterization of 3,4-diaminobenzophenone-functionalized magnetic nanoadsorbent with enhanced VOC adsorption and desorption capacity. Environ. Sci. Pollut. Res. 2020, 28, 5231–5253. [Google Scholar]
- Takeguchi, T.; Aoyama, S.; Ueda, J.; Kikuchi, R.; Eguchi, K. Catalytic Combustion of Volatile Organic Compounds on Supported Precious Metal Catalysts. Top. Catal. 2003, 23, 159–162. [Google Scholar] [CrossRef]
- Herk, D.V.; Castano, P.; Makkee, M.; Moulijn, J.A.; Kreutzer, M.T. Catalyst testing in a multiple-parallel, gas-liquid, powder-packed bed microreactor. Appl. Catal. A Gen. 2009, 365, 199–206. [Google Scholar] [CrossRef]
- Nijhuis, T.A.; Beers, A.E.W.; Vergunst, T.; Hoek, I.; Kapteijn, F.; Moulijn, J.A. Preparation of monolithic catalysts. Catal. Rev. 2001, 43, 345–380. [Google Scholar] [CrossRef]
- Lu, X.; Tang, W.; Li, M.; Dang, Y.; Gao, P.X. Mass transport in nanoarray monolithic catalysts: An experimental-theory study. Chem. Eng. J. 2021, 405, 126906. [Google Scholar] [CrossRef]
- Lyuba, I.; Anna, V.; Petya, P.; Giuseppe, P.; Leonarda, L.; Rodolfo, Z.; Zbigniew, K.; Tatyana, T. Effect of Y Modified Ceria Support in Mono and Bimetallic Pd–Au Catalysts for Complete Benzene Oxidation. Catalysts 2018, 8, 283. [Google Scholar]
- Liu, P.; Zhao, Y.; Qin, R.; Gu, L.; Zhang, P.; Fu, G.; Zheng, N. A vicinal effect for promoting catalysis of Pd/TiO2:supports of atomically dispersed catalysts play more roles than simply serving as ligands. Sci. Bull. 2018, 63, 675–682. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.; Zuo, P.; Liu, M. Study on the correlation between the surface active species of Pd/cordierite monolithic catalyst and its catalytic activity. Mater. Sci. Eng. B-Adv. 2016, 211, 45–52. [Google Scholar] [CrossRef]
- Vergunst, T.; Kapteijn, F.; Moulijn, J.A. Monolithic catalysts-non-uniform active phase distribution by impregnation. Appl. Catal. A.-Gen. 2001, 213, 179–187. [Google Scholar] [CrossRef]
- Wang, T.; Yang, S.; Kai, S.; Fang, X. Preparation of Pt/beta zeolite–Al2O3/cordierite monolith for automobile exhaust purification. Ceram. Int. 2011, 37, 621–626. [Google Scholar] [CrossRef]
- Leonov, A.N.; Smorygo, O.L.; Sheleg, V.K. Monolithic catalyst supports with foam structure. React. Kinet. Catal. Lett. 1997, 60, 259–267. [Google Scholar] [CrossRef]
- Dai, P.; Zhao, X.; Xu, D.; Wang, C.; Tao, X.; Liu, X.; Gao, J. Preparation, characterization, and properties of Pt/Al2O3/cordierite monolith catalyst for hydrogen generation from hydrolysis of sodium borohydride in a flow reactor. Int. J. Hydrog. Energy 2019, 44, 28463–28470. [Google Scholar]
- Tiscornia, I.S.; Lacoste, A.M.; Gómez, L.E.; Boix, A.V. CuO–CeO2/SiO2 coating on ceramic monolith: Effect of the nature of the catalyst support on CO preferential oxidation in a H2-rich stream. Int. J. Hydrog. Energy 2020, 45, 6636–6650. [Google Scholar]
- Yang, Y.; Zhao, D.; Gao, Z.; Tian, Y.; Li, X. Interface interaction induced oxygen activation of cactus-like Co3O4/OMS-2 nanorod catalysts in situ grown on monolithic cordierite for diesel soot combustion. Appl. Catal. B Environ. 2021, 286, 119932. [Google Scholar]
- Zhu, M.P.; Yang, J.C.E.; Duan, X.; Wang, S.; Fu, M.L. Engineered Co2AlO4/CoAl2O4@Al2O3 monolithic catalysts for peroxymonosulfate activation: Co3+/Co2+ and ODefect/OLattice ratios dependence and mechanism. Chem. Eng. J. 2020, 409, 128162. [Google Scholar] [CrossRef]
- Tang, X.; Wa, J.; Ma, Y.; Li, J.; Liu, B. Low-temperature and stable CO oxidation of Co3O4/TiO2 monolithic catalysts. Chin. Chem. Lett. 2020, 32, 48–52. [Google Scholar] [CrossRef]
- Martín, J.C.; Ávila, P.; Suárez, S.; Yates, M.; Martín-Rojo, A.B.; Barthelemy, C.; Martín, J.A. Influence of support acid pretreatment on the behaviour of CoOx/γ-alumina monolithic catalysts in the CH4-SCR reaction. Appl. Catal. B Environ. 2006, 67, 270–278. [Google Scholar] [CrossRef]
- Martín, J.C.; Rasmussen, S.B.; Suárez, S.; Yates, M.; Gil-Llambías, F.J.; Villarroel, M.; Ávila, P. Effect of sulphuric acid pretreatment concentration on the behaviour of CoOX/g-Al2O3-SO4 monolithic catalysts in the lean CH4-SCR process. Appl. Catal. B Environ. 2009, 91, 423–427. [Google Scholar] [CrossRef]
- Jiang, L.; Chu, G.W.; Liu, Y.Z.; Liu, W.; Luo, Y. Preparation of cordierite monolithic catalyst for α-methylstyrene hydrogenation in a rotating packed bed reactor. Chem. Eng. Process. 2020, 150, 107882. [Google Scholar] [CrossRef]
- Lai, X.; Zhou, X.; Zhang, H.; Jiang, X.; Chen, Y. Toluene oxidation over monolithic MnOx/La-Al2O3 catalyst prepared by a CTAB-assisted impregnation method. Appl. Surf. Sci. 2020, 526, 146714. [Google Scholar] [CrossRef]
- Baharudin, L.; Watson, M.J. Monolithic substrate support catalyst design considerations for steam methane reforming operation. Rev. Chem. Eng. 2017, 34, 481–501. [Google Scholar] [CrossRef]
- Lu, S.S.; Zhang, J.; Sun, Y.; Liu, H. Preparation and characterization of CuO-CeO2-ZrO2/cordierite monolith catalysts. Ceram. Int. 2017, 43, 5957–5962. [Google Scholar] [CrossRef]
- Sun, H.C.; Wang, Y.Q.; Zhao, H.C.; Zhang, L.Y. Catalytic performance toward toluene combustion of La0.8Sr0.2MnO3 monolithic catalyst with acid-treated honeycomb-shaped cordierite ceramic support. Mater. Rep. 2016, 30, 32–36. (In Chinese) [Google Scholar]
- Cuo, Z.X.; Wang, D.D.; Gong, Y.; Zhao, F.; Liu, H.D.; Chen, Y.F. A Novel Porous Ceramic Membrane Supported Monolithic Cu-Doped Mn-Ce Catalysts for Benzene Combustion. Catalysts 2019, 9, 652. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.Y.; Ma, Y.X.; Zhao, Z.B.; Hu, X.; Ye, Z.M.; Yao, J.F.; Buckley, C.E.; Dong, D.H. Comparison of fibrous catalysts and monolithic catalysts for catalytic methane partial oxidation. Renew. Energy 2019, 138, 1010–1017. [Google Scholar] [CrossRef]
- Li, G.; Lv, L.; Fan, H.T.; Ma, J.Y.; Li, Y.Q.; Wan, Y.; Zhao, X.S. Effect of the agglomeration of TiO2 nanoparticles on their photocatalytic performance in the aqueous phase. J. Colloid Interf. Sci. 2010, 348, 342–347. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Q.; Chen, W.X.; Wu, F.; Yang, Y.; Werner, D.; Tao, S.; Wang, X.L. A mechanistic study of stable dispersion of titanium oxide nanoparticles by humic acid. Water Res. 2018, 135, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Si, R.; Flytzani-Stephanopoulos, M. Shape and crystal-plane effects of nanoscale ceria on the activity of Au-CeO2 catalysts for the water-gas shift reaction. Angew. Chem. Int. Ed. Engl. 2008, 47, 2884–2887. [Google Scholar] [CrossRef] [PubMed]
- Vayssilov, G.N.; Lykhach, Y.; Migani, A.; Staudt, T.; Petrova, G.P.; Tsud, N.; Skála, T.; Bruix, A.; Illas, F.; Prince, K.C.; et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater. 2011, 10, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Agrafiotis, C.; Ekonomakou, A.T. The effect of particle size on the adhesion properties of oxide washcoats on cordierite honeycombs. J. Mater. Sci. Lett. 1999, 18, 1421–1424. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, L.N.; Wen, L.; Cheng, L.; He, Y.; Xu, B.; Wu, Q.M.; Liu, S.J. Effects of particle size and color on photocuring performance of Si3N4 ceramic slurry by stereolithography. J. Eur. Ceram. Soc. 2021, 41, 2386–2394. [Google Scholar] [CrossRef]
- Sigmund, W.M.; Bell, N.S.; Bergström, L. Novel Powder-Processing Methods for Advanced Ceramics. J. Am. Ceram. Soc. 2010, 83, 1557–1574. [Google Scholar] [CrossRef]
- Maskara, A.; Smith, D.M. Agglomeration during the Drying of Fine Silica Powders, Part II: The Role of Particle Solubility. J. Am. Ceram. Soc. 1997, 80, 1715–1722. [Google Scholar] [CrossRef]
- Engwayu, J.; Pawlik, M. Adsorption of anionic polymers on hematite-a study of zeta potential distributions. Miner. Eng. 2020, 148, 106225. [Google Scholar] [CrossRef]
- Ren, J.; Song, S.; Lopez-Valdivieso, A.; Shen, J.; Lu, S. Dispersion of Silica Fines in Water–Ethanol Suspensions. J. Colloid Interf. Sci. 2001, 238, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.B.; Zhuang, Z.Q.; Liu, Y.; Lin, Z.F. Study on dispersion of BaTiO3 particles by polyacrylic acid and its mechanism. J. Ceram. 2008, 4, 27–30. (In Chinese) [Google Scholar]







| Cordierite Substrate | N2 Adsorption | Mercury Porosimetry | ||
|---|---|---|---|---|
| Surface Area (m2/g) | Pore Volume (×10−3 mL/g) | Surface Area (m2/g) | Pore Volume (mL/g) | |
| Without pretreatment | 1.0 | 1.3 | 0.571 | 0.2906 |
| 10% HNO3—ordinary | 1.7 | 2.0 | 0.777 | 0.3244 |
| 20% HCl—ordinary | 1.3 | 1.9 | 0.648 | 0.3429 |
| 20% HCl—heating | 10.1 | 4.4 | 0.849 | 0.3490 |
| 10% HCl—heating | 10.8 | 3.9 | 0.862 | 0.3436 |
| 10% Citric acid—heating | 1.2 | 1.6 | 0.577 | 0.3470 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Shi, X.; Li, Q.; Ren, S.; Yin, G. Effect of Pd/Ce Loading and Catalyst Components on the Catalytic Abatement of Toluene. Catalysts 2022, 12, 225. https://doi.org/10.3390/catal12020225
Liang W, Shi X, Li Q, Ren S, Yin G. Effect of Pd/Ce Loading and Catalyst Components on the Catalytic Abatement of Toluene. Catalysts. 2022; 12(2):225. https://doi.org/10.3390/catal12020225
Chicago/Turabian StyleLiang, Wenjun, Xiujuan Shi, Qinglei Li, Sida Ren, and Guobin Yin. 2022. "Effect of Pd/Ce Loading and Catalyst Components on the Catalytic Abatement of Toluene" Catalysts 12, no. 2: 225. https://doi.org/10.3390/catal12020225
APA StyleLiang, W., Shi, X., Li, Q., Ren, S., & Yin, G. (2022). Effect of Pd/Ce Loading and Catalyst Components on the Catalytic Abatement of Toluene. Catalysts, 12(2), 225. https://doi.org/10.3390/catal12020225






