Abstract
Different metathesis catalysts were evaluated regarding their activity for propene production from ethene and trans-butene feedstocks. Nickel, molybdenum, rhenium and tungsten, along with bimetallic nickel-rhenium systems were applied with commercial supports and self-synthesized MCM-41. For the latter support the Si/Al ratio was adjusted as an additional optimization parameter (Si/Al = 60). Attractive activities were observed using Re and NiRe based catalysts at moderate temperatures of 200–250 °C. In contrast, the tungsten-based catalysts were only active above 450 °C. Three catalysts, namely Re/AlMCM-41(60), NiRe/mix (1:1) and W/SiO2 offered propene selectivity’s exceeding 40% at attractive conversion rates. These catalysts were characterized by BET, powder XRD, NH3-TPD and TPR-TPO-TPR cycles. At specific reaction temperatures, reaction-regeneration cycles were performed, which revealed that for the Re and W catalysts the initial reactant conversions and propene selectivity can be recovered. In contrast, for the NiRe catalyst, a continuous, gradual and irreversible decrease of activity was observed. Even though the tungsten catalyst was operated at the highest temperature, no irreversible decrease in conversion and propene selectivity occurred. Therefore, this catalyst has potential as a promising candidate for the synthesis of propene.
1. Introduction
In 2005, Chauvin, Grubbs and Schrock were awarded with the Nobel Prize for their research regarding the metathesis for organic synthesis [1,2,3]. Before Herisson and Chauvin have developed the now generally accepted underlying catalytic cycle of the metathesis [4]. Grubbs developed catalysts based on ruthenium for specialized ring-opening-metathesis-polymerization (ROMP) and ring-closing-metathesis (RCM) [5,6]. To optimize the production of desired components the catalysts were functionalized with organic groups [7]. A promising field is pharmaceutical production [8], where Grubbs catalysts of the second generation were utilized to produce drugs and specialty chemicals [9,10]. Schrock [11,12] achieved more stable functional groups for molybdenum and tungsten imido alkylidene complexes for specialty applications.
For the production of propene from ethene or butene feedstocks numerous publications focused specifically on selecting the type of catalyst. Li [13] utilized MoH-beta-Al2O3 and achieved a conversion of 80% for butene, while the propene selectivity exceeded 95%. The catalyst, however, observed deactivation by oligomerization that resulted in multiple coke species. These species formed at the beginning of the reaction, leading to blockage of active catalyst surface and resulting in high initial loss of activity [14]. The addition of Mg suppressed the oligomerization significantly and enhanced the activity of the catalysts [15]. Liu employed the same Mo-H-beta Al2O3 for the metathesis of ethene and trans-butene, giving propene in sufficient amounts [16]. Further, Mo-MCM-22-Al2O3 was evaluated, with a highly interesting propene selectivity of 95%. Less Al resulted in a more stable catalytic system, though without Al, no activity could be observed [17].
Huang [18] accomplished a conversion of 60–63% of 2-butene with maximum propene selectivity of 88% for WO3-Al2O3-HY. A slight deactivation over 50 h on stream, beginning at 30 h [19] was observed. Zuo [20] found that H2O resulted in irreversible deactivation via reduction of the active WOx species. It was concluded, that sintering and subsequently structural changes were caused. Behr and Schüller found similar effects of deactivation for Re2O7 on alumina supports [21] when employing a pentene feed. The conversion decreased from 40 to 20% at room temperature and under increased pressure. By increasing the temperature above 80 °C, the effect of deactivation significantly increased. Furthermore, water showed an extraordinary influence as an impurity. It acted as a poison in the catalytic system used and thus significantly impeded its functionality.
The highly active and selective ruthenium Grubbs catalysts face deactivation as well. Engel found that the deactivation of Ru alkylidenes suppresses metathesis and only isomerization occurs [22]. In the investigation of Lafaye for N-heteroaromatics metathesis [23], the deactivation is initiated by nitrogen, resulting in the decomposition of Ru Grubbs catalysts of the second generation via steric effects and electron-drawing by ligand species. Further, the deactivation increases with increased Brönsted basicity of the amine. When increasing the stability of Grubbs catalysts, the activity decreases via Π-acidic group interaction with the Ru center. This leads to steric hinders of the active surface, as Poater [24] concluded for Ru-benzylidene Grubbs catalysts. This finding illustrates the necessary trade-off that the catalyst researchers face.
In the present paper, the authors evaluated the activity of several potential metathesis catalysts for the production of propene using a wide range of combinations of varying catalytic metals and the employed supports. Promising candidates are characterized and their stability and long-time operation are evaluated. The overall objective is the selection of a catalyst suitable for application in a mini-plant setup.
2. Results and Discussion
This chapter presents the results of the activity tests for the selected metathesis catalysts for the direct propene production from ethene/trans-butene feedstocks (Section 2.1). The three most promising candidates are selected characterized in detail (Section 2.2) and afterwards submitted to a reaction-regeneration cycle (Section 2.3), where the resulting conversions and selectivity’s are analyzed and discussed.
2.1. Performance Parameters
Suitable performance parameters must be selected to evaluate the respective catalytic processes. Analogous to the mentioned previous studies the conversion (Equation (1)), the selectivity (Equation (2)) and the yield (Equation (3)) are chosen for comparative purposes. In the case of metathesis, conversion must be specified for each of the two reactants used (ethene and trans-butene). For selectivity, the molar flow of a product is related to the entity of all measured products. The equation of yield is adapted to replace the stoichiometric factors. Metathesis is a reversible reaction and all alkenes available can participate as a product and reactant. In the present case, individual reactions were not decoupled and the yield of a product has to be related to the two carbon sources used. Correspondingly, as in (Equation (3)), the total quantity of the reactants is used, and stoichiometry is replaced by the ratio of carbon numbers of the reactants to the product.
2.2. Activity Test
Numerous catalysts were investigated for the direct conversion of ethene to propene [25]. The results of these studies are used to identify a suitable candidate for propene production via cross-metathesis from ethene and trans-butene at atmospheric pressure.
Commercially available materials such as SiO2, γ-Al2O3 and TiO2 were used as supports, as well as self-synthesized AlMCM-41 with different silica to aluminum ratios. In addition, mixed catalysts consisting of MCM-41 and γ-Al2O3 were subsequently impregnated with a selected metathesis metal. The number of all tested catalysts comprised of 101. Their combinations are depicted in Table A1 (Appendix A).
From these prepared catalysts, examples based on their maxima in propene selectivity with varying temperature for an equimolar feed of 2.5% ethene and 2.5% trans-butene are presented here. These selected 16 catalytic systems are summarized in Table 1 with the corresponding temperature for maximum propene selectivity and the obtained conversions of the fed reactants.

Table 1.
Activity results of selected suitable metathesis catalysts for varying temperatures with an equimolar feed of 2.5% of ethene and 2.5% of trans-butene.
Based on the previously performed investigations for the direct conversion of ethene to propene [25,26,27], a nickel incorporated AlMCM-41 was evaluated for cross-metathesis. This unusual active metathesis metal was first concluded by Iwamoto [27], to occur as one reaction step in the overall reaction network of Ni/AlMCM-41. For the two possible preparation methods according to Noreña Franco [28] and Iwamoto [27], the selectivity to propene was always below 30%. Ethene was converted with more than 35 and 50%, trans-butene with more than 85% in both cases. The high temperature of 477 °C allows for the conclusion that not metathesis is the dominant effect, but rather catalytic cracking. As already concluded in [26]. Accordingly, the stability of the catalyst under desired reaction conditions is doubtful [29], as severe deactivation was observed above 400 °C.
Considering Table 1, the heterogeneously catalyzed metathesis with rhenium under atmospheric pressure shifts the maximum in propene generation to significantly lower temperatures in comparison to nickel [25,26]. The highest formation rate is at around 200 °C, except for Re/SiO2. The commercial support with rhenium presented the lowest selectivity to propene of the here presented catalysts, which is associated with low trans-butene conversion. In contrast, the self-synthesized MCM-41 support material in combination with rhenium provides more than 30% and for the interesting cases even more than 42% in propene selectivity. At the point of maximum selectivity, more than 60% of the trans-butene is converted, but only 4.5 to 8% of ethene. This observation indicates that isomerization is the dominant reaction, as supported by the high selectivity to the butene-isomers. In conclusion, propene is generated rather by self-metathesis. Accordingly, ethene is formed in this process, which further reduces its conversion.
For the rhenium catalysts, it can be concluded that an optimum acidity of the MCM-41 support material exists. This optimum is dependent on the preparation method. For the template ion exchange with a Si/Al ratio of 16 with 45.5% propene and the incipient wetness impregnation at a Si/Al ratio of 60 with 42.6% were achieved.
The maximum in propene selectivity (Figure 1a) corresponds to a reduction of the butene isomers. The conversion of ethene and trans-butene increased slightly in this range. Above 400 °C, the conversion of the reactants increases more significantly as a result of coking. In parallel, the butene selectivity decreases, indicating a loss in catalytic activity by coking, which is evident after evaluation of the carbon balances.
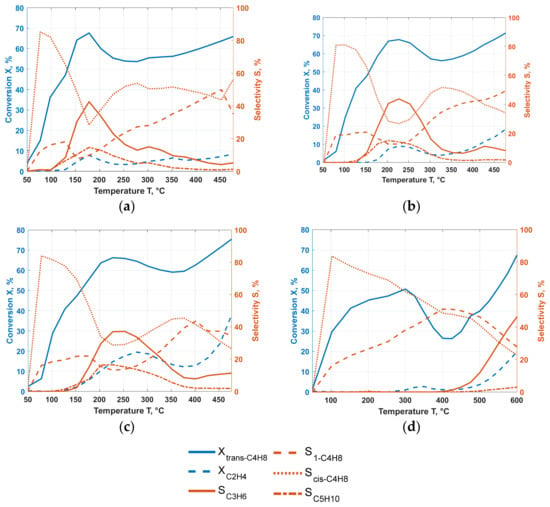
Figure 1.
Conversion Xi of the reactants (ethene and trans-butene) on the left axis and selectivity of the analyzable production Sj (propene, 1-butene, cis-butene and pentene) on the right axis for varying reaction temperature for (a) Re/AlMCM-41 (60), (b) NiRe/mix (1:1), (c) NiRe/AlMCM-41 (60) pretreated under nitrogen for 1 h at 500 °C and (d) W/SiO2.
Similar results are obtained for the bimetallic nickel-rhenium catalysts (Figure 1b,c). This type of catalyst was designed to combine the advantages of both active components, namely nickel for dimerization of ethene and rhenium for the metathesis. Here, an improvement was achieved with trans-butene conversion of 68% and ethene with 9% as the highest values. At the conversion maxima, the propene selectivity increased to 43.9%. The active rhenium metal site in combination with the added Al2O3 converts the reactants more effectively. At 425 °C a second, significantly smaller peak occurs with 10% of propene selectivity achieved, see Figure 1b. This second peak is assumed to correspond to the active nickel site.
Using an optimized catalyst support [25] with a Si/Al ratio of 60, the temperature for the maximum propene selectivity could be significantly reduced for the bimetallic catalyst. This corresponds with a reduced selectivity (16.9%) and lower conversion rates (2.5 and 50.6%). To further improve the performance, a pre-treatment procedure (pT) at 500 °C for 1 h under nitrogen was performed [18,19,30]. This procedure increased the maximum reaction rates to slightly higher temperatures of 250 °C but led to 37.2% propene selectivity, with 17.6% and 65.9% in conversion. Figure 1c shows that propene with over 20% selectivity can be obtained over a wide temperature range. The conversions decreased slightly with increasing temperature. Above 400 °C, the conversion of the reactants increased significantly but did not result in a second maximum in propene selectivity. Accordingly, only the rhenium site seems to be affected by the pre-treatment.
In contrast to rhenium, molybdenum revealed little to no activity under the reaction conditions used and without a pre-treatment procedure. In addition to the examples shown in Table 1, a wide variety of synthesized catalysts was tested, as shown in Table A1. For all catalysts containing Mo, a maximum propene selectivity of 11% was achieved. The conversion of trans-butene was above 10%, whereas ethene conversion varied significantly, but was at a low level. Due to reduced propene formation, molybdenum is excluded in this paper.
The catalysts incorporated with tungsten indicated no propene formation in the range of 50–300 °C range and only the isomerization products were analyzed. Above 300 °C, conversions and propene formation increased significantly. For W/AlMCM-41 with a Si/Al = 60 at 475 °C 34.3% selectivity of propene were detected. Simultaneously, conversions of 12.1% for ethene and 76.8% for trans-butene were measured. The necessary temperature increase for the activation of the tungsten active sites is a common property of these reaction systems, which was already discussed in the introduction. Although an increase in reaction temperature is not desirable, interesting results for W/SiO2 are given (Figure 1d), indicating potential for application. This configuration on a commercial SiO2 support material was heated to 600 °C and managed to achieve a propene selectivity of 46%. Furthermore, there are promising conversion levels of ethene with about 20% and trans-butene with 67.4%.
Tungsten is known to be more robust to reaction conditions and possible impurities than rhenium and molybdenum. Therefore, an increase in temperature is not detrimental, especially given the absence of byproducts. Only minimal amounts of pentene were formed, shown in (Figure 1d). This observation and the high conversion of ethene indicate that predominantly the desired reaction for the formation of propene from ethene and trans-butene takes place.
Another behavior was obvious regarding the catalysts using MCM-41. On the one hand, ethane and hexene are formed here in addition to pentene, and on the other hand, the temperature for the maximum propene formation rate is not equivalent to the highest conversion rates. The aluminized MCM-41 supports have specific acidic Brönsted- and Lewis centers that extend the product spectrum [25]. However, the maximum propene selectivity for the Re impregnated catalysts corresponds to a sink in butene selectivity. At the same time, conversions and pentene selectivity reach a maximum. This observation allows for the assumption that the self-metathesis of butenes is dominant, with the conversion of ethene not reaching the same level as trans-butene.
As mentioned above, not all systems necessarily achieve maximum conversion at the point of highest propene selectivity. For the catalysts with nickel or mixed support of MCM-41 with γ-Al2O3, the highest conversion is found at the highest temperature. This effect can be explained by the activation of nickel and the resulting increase in byproducts. Under these conditions coking on the acidic sites is severe.
Based on the activity tests, three candidates are selected for a more detailed investigation—Re/AlMCM-41(60) with Si/Al = 60, NiRe/mix(1:1) and W/SiO2. For these, a detailed characterization and stability analysis with deactivation and regeneration cycles was performed. For rhenium, the relevant operating temperature was at 200 and 175 °C with selectivities to propene of over 40% and simultaneously high conversions for both trans-butene and ethene. In comparison to other support materials with varying aluminum content, the interesting areas for propene formation are somewhat broader, for the bimetallic catalyst. Furthermore, W/SiO2 was chosen as a catalyst that is active at significantly higher temperatures of 600 °C, but a commercial support provides large quantities at a low cost.
These catalysts were first characterized in the form of fresh powder and a powder used after the reaction. Cyclic experiments for each catalyst system with intermediate regenerations treatments are presented thereafter.
2.3. Characterization
In this section, characteristics of the catalytic materials are summarized. It should be noted, that detailed comparisons are difficult for the rather different materials considered. It was not possible and also not intended to identify the active sites with the analytical methods available. It was the goal to use the data obtained to (i) prepare more detailed characterization studies and in particular to (ii) evaluate reaction engineering aspects regarding the application of these catalysts.
2.3.1. N2 Physisorption (BET)
The adsorption isotherms for the different support materials (Figure 2a) and the chosen catalysts are shown (in Figure 2b). The mesoporous character of the specific supports is evident by the type IV adsorption isotherms present. For MCM-41, the typical course of capillary condensation with a narrow hysteresis (H1) between 0.2 and 0.35 of relative pressure is recognizable. This observation is consistent with the pore sizes of 4.1 nm presented in Table 2 and the total pore volume of around 1.1 cm3/g.
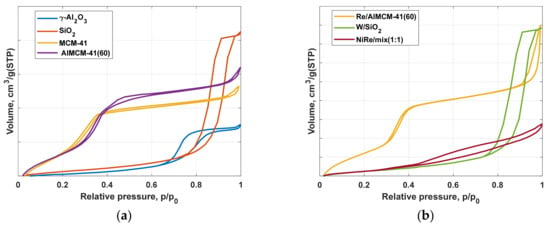
Figure 2.
N2-physisorption isotherms of the used support materials (a), as well as for the fresh and tested catalysts for the metathesis of ethene and trans-butene towards propene (b).

Table 2.
N2-physisorption data for the applied support and catalyst materials as fresh and tested systems after reaction under the specific metathesis reaction conditions.
The hysteresis of the AlMCM-41 compared to the pure MCM-41 showed a deformed hysteresis combination of types H1 and H3 because of mono- and multilayered adsorption on the mesoporous material and changed pore sizes (5.2 nm) by the alumization. The values lie between 0.25 and 0.6 of relative pressure.
The hysteresis of γ-Al2O3 compared to AlMCM-41(60) support starts at a higher relative pressure of about 0.6. This shift is due to the larger pore diameter of this material, as shown in Table 2 with 11 nm and a total pore volume of 0.58 cm3/g.
Silicon dioxide exhibits capillary condensation between relative pressures (p/p0) of 0.65 and 0.97. The corresponding hysteresis size can be explained by the larger pores with a diameter greater than 20 nm, resulting in a total pore volume of 0.97 cm3/g.
The physisorption profiles of the catalysts largely correspond to the respective supports. The rhenium-loaded catalyst (Re/AlMCM-41(60)) shows similar hysteresis curves such as the AlMCM-41 but is deformed in the H3 hysteresis section because of larger pore size. The adsorption isotherm of the tungsten catalyst is very similar to silicon dioxide with a slightly changed pore system (Figure 2b, Table 2). The bimetallic NiRe/mix (1:1) catalyst presents a different form. This hysteresis between 0.43–0.99 of relative pressure is a combination of two hysteresis (MCM-41 and γ-Al2O3) in an overlapped region.
Table 2 provides an overview of the parameters obtained from the BET method for supports and catalysts. Further, the specific surface areas of the tested materials increase in the following order:
2.3.2. Powder X-ray Diffraction (XRD)
XRD measurement results for the supports (a) and catalysts (b) are plotted in Figure 3. The x-ray diffractograms of MCM-41 material are characterized by the characteristic peaks in a narrow region at 2Θ = 2.5°, 4° and 5°. The uniform structure of the MCM-41 consists of hexagonal arrays of non-intersecting tubular nanopores of controlled diameter (4.1 nm).
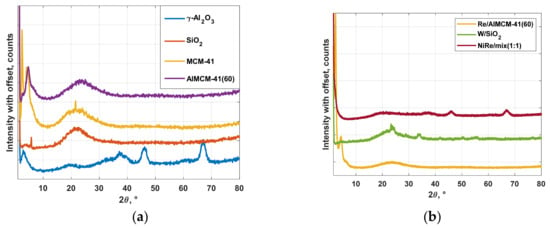
Figure 3.
XRD patterns for (a) the applied support materials and (b) catalysts for the metathesis of ethene and trans-butene in the range of = 0–80°.
The detection up to 2Θ = 80° corresponds to the amorphous structure of the silica. In all materials, no newly formed crystalline structures are recognizable. Except for the tungsten catalyst, here the crystalline structure presents values between 2Θ = 20–60°. The deformation due to different aluminum contents in the support is well defined in the region up to 2Θ = 10°.
The typical progressions of the XRD spectra of the amorphous γ-Al2O3 can be recognized between 2Θ = 25–70° and of the amorphous SiO2 at 2Θ = 15–30°. This characteristic behavior of the γ-alumina is recognizable in the diffractograms of the mix catalysts, as a combination of attenuated peaks in the small-angle range below 2Θ = 10°, shown in Figure 3b. These are characteristics of the AlMCM-41 support fractions. The W/SiO2 diffractogram showed a combination of typical silica support and crystalline phase of impregnated tungsten at 2Θ = 22–40° as a crystalline phase of WO3.
2.3.3. Ammonia Temperature Programmed Desorption (NH3-TPD)
The results of the NH3-TPD measurements to characterize the surface acidity of the investigated catalysts are shown in Figure 4. The Re catalysts on aluminized MCM-41 and NiRe on mixed support present stronger acidity compared to the W/SiO2. The reason is the non-acidity of the silica support [30,31], the low-temperature peak corresponded to weak acid sites caused by the containing tungsten oxide. Additionally, a signal emerges above 500 °C in Figure 4 for the blue and green curves. which could be an indication of increased catalytic activity.
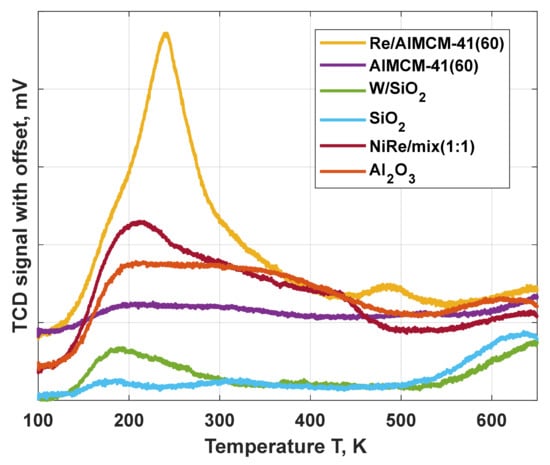
Figure 4.
NH3-TPD profiles of the applied catalysts for the metathesis of ethene and trans-butene.
Both, Re/AlMCM 41 (60) and NiRe/mix (1:1) catalysts indicated not only low temperature peaks or shoulders at 200 °C but also a broad peak up to 400 °C. Further, an ammonia desorption peak above 450 °C, indicating the presence of strong acidic sites [31], which is dependent on the used support and loaded metal. The amount of NH3 molecules desorbed per gram of catalyst at the end of the TPD experiment were calculated (W/SiO2—0.16 mmol/g, NiRe/mix (1:1)—0.71 mmol/g and Re/AlMCM 41—1.02 mmol/g). Herewith the total acidity of the studied catalysts was confirmed, which is an indicator for the occurring deactivation effects of the catalysts.
2.3.4. Temperature-Programmed Reduction/Oxidation/Reduction Cycles
The results of the H2-TPR followed by O2-TPO and a second H2-TPR measurements for the investigated catalysts are shown in Figure 5. The shift in the temperature regions of the TPR/O/R cycle of the selected catalysts among each other is due to the different support materials, the loaded metal and the interactions between the active component and support material.
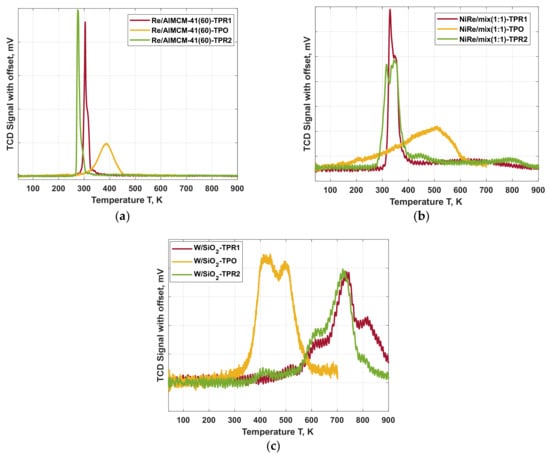
Figure 5.
TPR/O/R cycles of the catalysts (a) Re/AlMCM-41(60), (b) NiRe/mix (1:1) and (c) W/SiO2 applied for the metathesis of ethene and trans-butene towards propene.
The rhenium catalyst (Figure 5a) presents signals in the range of 290 °C to 350 °C. At these temperatures, rhenium appears to be reduced. After the oxidation of the catalyst, the TPR peak is slightly shifted to a higher temperature. This indicates an insignificant change of the active catalyst phase.
The bimetallic nickel rhenium catalysts mixed support has a broad double peak maximum between 270 °C and 480 °C. This observation is rather constant for both TPR measurements, but the intensity of the signal is reduced for the second TPR. The presence of both γ-alumina and MCM-41 supports can explain the high-temperature broad peak at about 750 °C as a reduction of metal species interacting with alumina and incorporated into the support framework by the ion exchange.
The tungsten catalyst shows a wide reduction range between 530 °C and 850 °C, correlating to the range, in which the highest activity was observed. For this catalyst the TPR profiles are identical. Only the occurring shoulder at 800 °C disappears in the second measurement, indicating that a crystalline form of the system cannot be regained after oxidation. Though, this appears to have no detrimental effect on the catalytic performance, as the reaction temperature was only 600 °C and is illustrated further in the stability (Section 2.3). The oxidation behavior of this studied catalyst system confirmed their reactivation/re-oxidation results. The tungsten on SiO2 is the only catalyst with an oxidation region (350–550 °C) at a lower temperature than the reduction profile.
It was determined that the analyzed areas of increased activity correspond to the temperature peaks of the TPR and thus indicate a reduction to an active catalyst state. Accordingly, the three selected catalysts are suitable for further consideration for stability evaluation.
2.4. Stability
The three selected catalysts are analyzed for their stability and regenerability. First, the conversion of the fed reactants is evaluated over a measurement cycle of three reactions each lasting 24 h with intermediate regeneration. The resulting product spectrum is then analyzed and, based on this data, a catalyst can be selected providing sufficient long-time stability.
Initially, the catalyst with aluminized MCM-41 and a rhenium phase on the surface is examined (Figure 6). Initially, 75 and 12% conversions for trans-butene and ethene were achieved, respectively. Within the first 9 h, deactivating effects lower the respective conversions to 68 and 5% for the two reactants. After that, the profiles of the second and third experiment follow the same courses. After the regeneration, the activity of the catalyst could be restored and a constant value for the trans-butene conversion with 70% was obtained. This regeneration of the catalyst is a positive result, beneficial for a possible application, even though a loss in activity with time-on-stream is apparent.
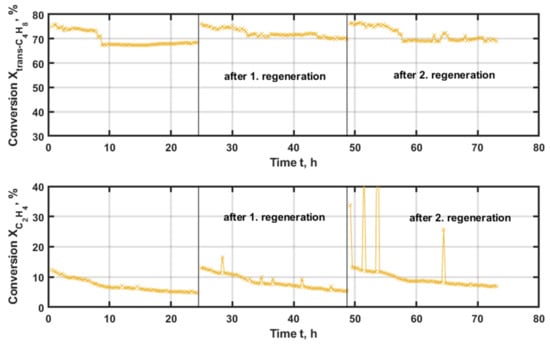
Figure 6.
Conversion of the applied reactants trans-butene (upper graph) and ethene (lower graph) with time-on-stream (TOS) applying Re/AlMCM-41(60) as the catalyst at 200 °C and 500 kgcat∙s∙m−3, with regeneration at 500 °C and 20% oxygen for 3 h.
In contrast, the conversion for NiRe/mix (1:1) (Figure 7), both for trans-butene and for ethene, undergoes an initial decay lasting 5 h. Afterward, the conversion rates remain constant with time-on-stream (TOS). For trans-butene, a maximum of 55% is obtained and reaches 47% of conversion. Ethene conversion is initiated at 3% and is close to zero afterward, which is detrimental as the desired cross-metathesis is not taking place. After the first regeneration, the conversion has the same initial values for trans-butene and ethene, respectively. These values decrease in the first 5 h to 49 and 0%, which are kept constant for the remaining time of the experimental run. With the second regeneration procedure, the conversion of ethene is initially zero, afterwards it slightly increases to 3% within the first 2 h of the experiment and is zero in the following again. In the case of trans-butene, the conversion drops further to 45% and remains constant afterward. The decreasing activity is an indication of the non-ideal regeneration of the catalysts, which reduces the available catalyst surface. As a result, the catalyst activity is reduced. This observation is a negative feature. As deactivation is a dominant effect, even in this lower temperature range. The applicability of the catalyst is limited.
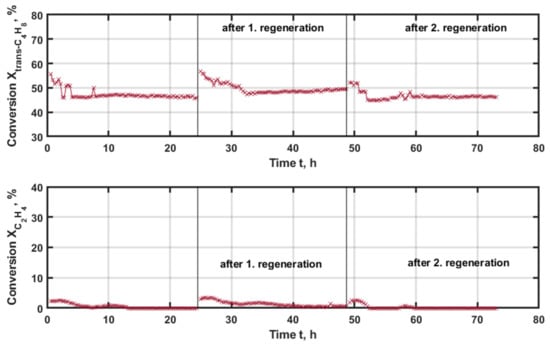
Figure 7.
Conversion of the applied reactants trans-butene (upper graph) and ethene (lower graph) with time-on-stream (TOS) applying NiRe/mix (1:1) as the catalyst at 225 °C and 500 kgcat∙s∙m−3, with regeneration at 500 °C and 20% oxygen for 3 h.
Finally, the catalyst with tungsten on the commercial SiO2 support is tested. Regarding the conversion of trans-butene, shown in Figure 8, it can be seen that this value only undergoes marginal changes over time and that regeneration has no influence on this value, at values higher than 60%. In contrast, the conversion of ethene starts at 10% and rises to 17% during the first measurement. The regeneration with 20% of oxygen at 500 °C does not deteriorate the activity of the catalyst. Contrary, during the second experiment, the catalyst shows an increasing conversion profile over time. The value rises to 20% for ethene, which remains constant after the second regeneration. Such an increased conversion rate is a highly positive result, which indicates an intensification of the desired cross-metathesis towards propene.
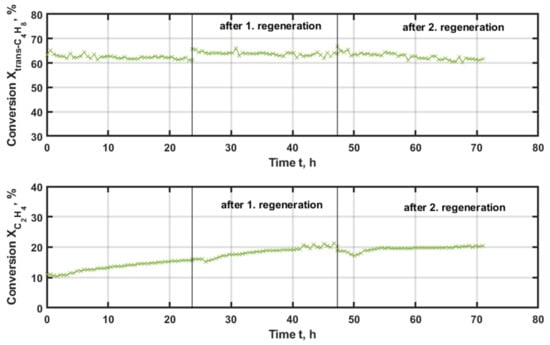
Figure 8.
Conversion of the applied reactants trans-butene (upper graph) and ethene (lower graph) with time-on-stream (TOS) applying W/SiO2 as the catalyst at 600 °C and 500 kgcat∙s∙m−3, with regeneration at 500 °C and 20% oxygen for 3 h.
In comparison, the W/SiO2 conversion profile is stable and ethene even shows increasing values. The other two candidates reveal a decreasing activity with time-on-stream but approach a constant value after the second regeneration. Whereby, NiRe/mix (1:1) presents no reasonable conversion rates of ethene.
Based on this information, the selectivities of the catalysts throughout the three experiments can now be examined under steady-state reaction conditions and with respective regeneration treatments. First, the selectivity distribution of the products for the Re/AlMCM-41(60) catalyst is analyzed (Figure 9).
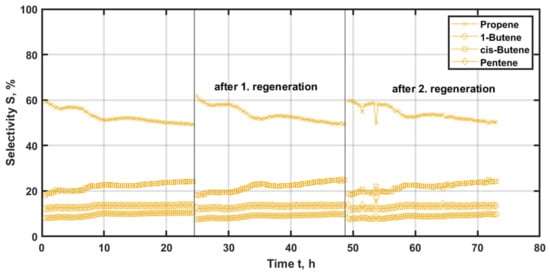
Figure 9.
Selectivity of the main reaction products with time-on-stream (TOS) applying Re/AlMCM-41(60) as the catalyst at 200 °C and 500 kgcat∙s∙m−3, with regeneration at 500 °C and 20% oxygen for 3 h.
In this case, propene is the main product for the entire measurement at around 60% of selectivity, but it decreases to 50% for each experimental run. The measured profiles are reproducible. The byproducts achieve 18% for cis-butene and 8% for 1-butene, initially. The butene isomers increase continuously, reaching 24% and 10% for cis- and 1-butene at the end of the experiment, respectively.
For the second and third deactivation/regeneration cycles, the same profiles can be observed, which are in good agreement with the decreasing ethene conversion. The catalytic activity can be regained, but the catalyst faces constant loss in metathesis rate with TOS. The decreased productivity by metathesis productivity is accompanied by an enhanced, but still less significant, isomerization. This appears to be due to self-metathesis of the butene, as indicated by increasing pentene selectivity.
In the case of NiRe/mix (1:1) catalyst (Figure 10), the experimental results are much different. Initially, cis-butene is the primary product with 42% of selectivity. Subsequently, the selectivity increases to 58% over the course of the experiment. An equivalent course is observed for 1-butene, from 19% to 27%. In contrast, the selectivity for propene and pentene are reversed. Propene starts at 20% and falls to 10%. This observation is in accordance with the loss in ethene conversion. Thus, the isomerization becomes more pronounced, while the propene generation via cross-metathesis is not present and the only source for propene is a self-metathesis of the butene isomers.
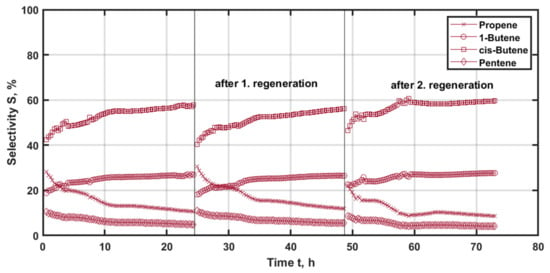
Figure 10.
Selectivity of the main reaction products with time-on-stream (TOS) applying NiRe/mix (1:1) as the catalyst at 225 °C and 500 kgcat∙s∙m−3, with regeneration at 500 °C and 20% oxygen for 3 h.
After the first regeneration, cis-butene is initially formed with 40% of selectivity and increases to 56%. 1-butene follows an equivalent course from a value of 18 to 26%. The desired product propene starts at 30% and decreases continuously to about 12%. The selectivity of hexene and pentene increases by about 3%. The analyzed results support the conclusion that there is an enhancement regarding isomerization and cross-metathesis.
With the subsequent regeneration, the selectivity for propene decreases from 22% to 8% for the third experiment. Accordingly, the isomerization is the dominant effect, which is in accordance with no ethene being converted. Thus, only cis-butene and 1-butene with 60 and 28% selectivity, respectively, are present.
These significantly decreasing profiles of the desired product with TOS are a negative aspect of this catalytic material. Additionally, propene is never the main reaction product, which is due to the dominant effect of isomerization and reduced metathesis, as pentene decreases as well.
The W/SiO2 system is analyzed analogously (Figure 11). This catalyst forms 1-butene as the main product, followed by propene, cis-butene and traces of pentene. The selectivity of propene drops from 32% to 28%. Cis-butene isomers increase analogously from 37% and 40%. The selectivity of the isomers then drops to 30% for 1-butene and 23% for cis-butene. In parallel, the selectivity for pentene remains constant at about 3% and the propene selectivity rises to about 44%. After the subsequent regeneration, the initial propene selectivity is still at 40%. In the following, the profile approaches a local minimum of 38% and afterwards, increases to 50% of propene selectivity. The enhanced conversion of ethene is beneficial for the generation of propene via the cross-metathesis, while less butene is agglomerated by conversion in this reaction. As a result, the selectivity with respect to the butene isomer gradually decreases. This is related to the fact, that the thermodynamic equilibrium shifts towards trans-butene, which is converted in the metathesis.
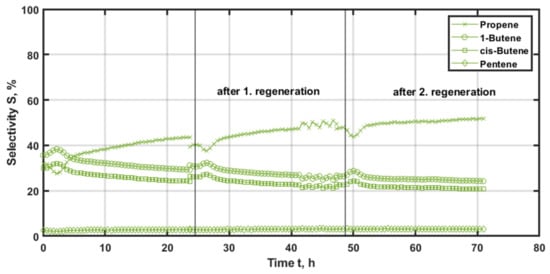
Figure 11.
Selectivity of the main reaction products with time-on-stream (TOS) applying W/SiO2 as the catalyst at 600 °C and 500 kgcat∙s∙m−3, with regeneration at 500 °C and 20% oxygen for 3 h.
In contrast, the selectivity of the byproducts 1-butene, cis-butene and pentene are not subject to significant differences, due to deactivation, within an experimental run. Pentene is constant and remains at the low level for the entire time-on-stream. As for the butene isomers, the slight decrease of 5% over 72 h of operation follows a steady and parallel path from the initial values of 30% for 1-butene and 26% for cis-butene. Therefore, with increasing ethene conversion the reaction rate for propene generation is enhanced to explain the observed phenomena.
Based on the results for the conversion of the reactants used and the resulting product spectra, the selection of a catalyst for the use in a segregated reactor setup is possible. For this specific configuration, W/SiO2 was chosen. For three experiments, each with a subsequent regeneration, a high and constant conversion for trans-butene and a steadily increasing value for ethene were determined. On this basis, the targeted formation of propene from the two reactants is promoted, thus improving selectivity. This catalyst is suitable for operation if the reaction temperature is not of primary interest and the necessary energy for heating can be provided. For setups with a limit regarding the maximum temperature the Re/AlMCM-41 (Si/Al = 60) catalyst would be an interesting alternative candidate, offering high propene selectivity and simple regeneration, even though a decreasing conversion is observed with time-on-stream.
3. Methods
3.1. Catalyst Preparation
The (Al)MCM-41 based catalysts were synthesized by the template ion exchange (TIE) method proposed by Alvarado-Perea et al. [26] via tetrabutylammonium silicate (as a mixture of tetrabutylammonium hydroxide and fumed silica (Sigma–Aldrich, Taufkirchen, Germany) and cetyltrimethylammonium bromide (Merck, Darmstadt, Germany) in deionized water. Finally, sodium aluminate was added for preparation of AlMCM 41 supports. The resultant mixture was with a molar composition of 1 SiO2: 0.35 CTABr: 0.31 TBAOH: 0.000 0.2 NaAlO2: 55H2O. This mixture was aged 48 h at 100 °C in an oven. The resulting white solid was recovered by vacuum filtration, washed and dried at 80 °C. The support was at 600 °C for 6h calcinated only for IWI preparation.
The preparation of Re/AlMCM-41(60) is based on the above-mentioned TIE method, with a solution of ammonium perrhenate (NH4ReO4).
For the NiRe/mix (1:1) catalyst a suspension of MCM-41 and γ-Al2O3 (Sasol, Hamburg, Germany) in deionized water was used, with a solution of ammonium perrhenate (NH4ReO4) and nickel-(II)-nitrate (Ni(NO3)2∙6H2O) under continuous mixing the ionic exchange (IE) was performed. The mixture was aged for 20 h at 80 °C without stirring, then washed, dried for 24 h at 80 °C and was finally calcinated.
The W/SiO2 catalyst was prepared by incipient wetness impregnation (IWI) of the silicon dioxide (Grace, Düren, Germany) with a solution of ammonium metatungstate hydrate ((H26N6O41W12)∙H2O) and continuously stirred for 15 min. The material was dried at room temperature for 24 h and finally calcinated. The calcination of all catalysts in a muffle furnace with a blowing fan at 600 °C for 6 h with a heating rate of 10 K/min in air was carried out.
3.2. Catalyst Characterization
For the determination of the nitrogen adsorption-desorption isotherms and calculation of BET surface areas, pore volumes and pore sizes a NOVA2000e (Quantachrome®, Boynton Beach, FL, USA) was used. The sample was pretreated in a vacuum at 120 °C for 24 h. An X’Pert PRO (PANalytical, Kassel, Germany) using Ni-filter Cu Kα radiation was used for determination of XRD patterns in a 2Θ range from 1.3 to 80° of the catalysts. The reducibility of the catalysts was determined by using ChemStarTPx+ (Quantachrome, Boynton Beach, FL, USA). The catalysts were loaded in a quartz U-tube reactor. Before H2-TPR and O2-TPO experiments, the sample was pretreated in a He flow of 50 cm3/min at 150 °C for 30 min. Afterwards, the sample was cooled to 50 °C. Then followed the TPR measurement with 10% H2 in argon up to 900 °C. TPO measurements with 10% O2 in helium up to 700 °C. The temperature was changed at a rate of 10 K/min. The difference in the hydrogen/oxygen flow was determined by a thermal conductivity detector (TCD).
3.3. Experimental Evaluation
The evaluation of the catalytic activity was carried out in a laboratory fixed-bed reactor. The catalyst was placed in an inert quartz glass tube with an inner diameter of 0.6 cm and a length of 40 cm. This setup further includes a catalytic afterburner and a gas chromatograph. All experiments were performed at atmospheric pressure, with a sample mass of 0.2 g of catalyst. The feed gases from Westfalen AG (Münster, Germany) consisted of ethene (99.95%), trans-butene (5% in N2) and nitrogen (99.9993%). For the regenerative process steps, synthetically air was introduced. The catalyst bed was encased with inert particulate material (-Al2O3-Spheres 1.0/160, Sasol) to ensure an ideally mixed gaseous flow and for pre-heating of the fed gases. The reactor was embedded in an electrical oven (HTM Reetz, Berlin, Germany). The quantitative analysis of the gaseous components was realized by GC6890/MSD5973 (Agilent, Santa Clara, CA, USA), a combination of HP PlotQ and HP Molsieve5A columns with thermal conductivity detection.
During the experimental study, the space velocity (weight of catalyst/flow rate, W/F) in the range of 500–2600 kgcat∙s∙m−3 was varied. The molar fraction of ethene and trans-butene in the feed was kept at an equimolar composition with 2.5% each. For validation of the deactivating behavior, the catalyst bed was kept under constant conditions for 24 h. The catalyst was heated under nitrogen to reach the desired reaction temperature. After each experimental run, the catalyst was regenerated at 500 °C with 20% of oxygen for three hours to ensure complete coke removal from the catalyst bed. After the catalyst regeneration, the reactor was cooled to 50 °C under nitrogen. Each experimental point was measured at least two times, to obtain reliable and reproducible experimental results.
4. Conclusions and Outlook
The objective of this study was the identification of an active and stable catalyst for the desired cross-metathesis of ethene and trans-butene to propene. An intensified process is developed by decoupling the reaction network of the direct conversion of ethene to propene. A wide range of 100 catalysts, with 16 presented here, was validated, exploiting the typical metathesis transition metals rhenium, molybdenum and tungsten with different support materials.
The catalysts incorporated with molybdenum showed little to no activity with no pre-treatment for propene formation from ethene and trans-butene under atmospheric pressure. A similar result can also be observed for tungsten for temperatures below 400 °C. At higher temperatures, high conversion rates and significant propene selectivity were observed for W/SiO2.
Contrary to molybdenum and tungsten, the catalysts with rhenium achieved promising activity and a propene maximum at a temperature of about 200 °C. In all experiments, trans-butene is converted at significantly higher rates than ethene. The use of aluminized MCM-41 as the support material with an optimized Si/Al ratio is particularly suitable for metathesis. An alternative is the use of a bimetallic catalyst using both nickel and a metathesis metal. This approach increases the complexity of the reaction network as another active site is added.
Three promising candidates could be identified for an investigation of their stability. NiRe/mix (1:1) showed a decrease in conversions with increasing reaction time. The activity of ethene conversion drops to zero and cannot be recovered. In contrast, the trans-butene conversion remains constant. After the regeneration procedure, propene is not formed and only isomerization takes place.
For Re/AlMCM-41(60) a similar situation is present, but regeneration is possible. Though deactivation is apparent and reduces the generation of the desired product propene with time-on-stream, propene is still the main reaction product. The most promising results were observed for a W/SiO2 catalyst. The conversion of trans-butene remains at the same level and increases for ethene. At the same time, the selectivity to propene increases as well. In addition, no byproducts besides the butene isomers occur as there are hardly any acidic sites that can catalyze a possible cracking as a side reaction.
In the case of W/SiO2 long time operation was found to be possible. Both the possibility to regain the activity and the satisfying conversion rate of ethene are seen as promising indicators. In combination with the attractive selectivity with respect to propene the catalysts W/SiO2 and Re/AlMCM-41(60) are suitable candidates for the application in small scale setups. Dependent on the available apparatus and desired operating conditions.
Future work will focus on applying a suitable catalytic system within a two-step segregated reactor cascade for improved propene production compared to a single reactor operation. Since an increased operating temperature is not a limiting factor, the tungsten catalyst is seen as a promising candidate for the application considered.
Author Contributions
Conceptualization, A.S.-M., C.H.; software, M.F.; validation, M.F. and T.W.; formal analysis, T.W.; investigation, T.W. and M.F.; resources, A.S.-M. and C.H.; writing—original draft preparation, M.F.; writing—review and editing, C.H. and A.S.-M.; visualization, M.F. and T.W.; project administration, A.S.-M. and C.H.; funding acquisition, C.H., L.A.P. and A.S.-M. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to acknowledge and thank the DAAD (Germany) and CONACYT (Mexico) for their funding of the international exchange of knowledge.
Data Availability Statement
The data presented in this study are available in chapter 2 of the present publication and can be made available upon request.
Acknowledgments
We would especially like to thank Jutta Wilke for supporting the experimental realization and the colleagues at the OvGU for helpful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Entity of prepared metathesis catalysts for highest propene selectivity and the corresponding temperature, conversions and product selectivity’s.
Table A1.
Entity of prepared metathesis catalysts for highest propene selectivity and the corresponding temperature, conversions and product selectivity’s.
| Metal | TIE | IWI | Mix | Commercial | Si/Al | cMetal |
|---|---|---|---|---|---|---|
| Ni | x | x | x | |||
| Re | x | x | x | x | x | x |
| Mo | x | x | x | x | x | x |
| W | x | x | x | x | x | x |
| NiRe | x | x | x |
References
- Chauvin, Y. Olefin metathesis: The early days (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2006, 45, 3740–3747. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, R.H. Olefin-metathesis catalysts for the preparation of molecules and materials (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2006, 45, 3760–3765. [Google Scholar] [CrossRef] [PubMed]
- Schrock, R.R. Multiple metal-carbon bonds for catalytic metathesis reactions (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2006, 45, 3748–3759. [Google Scholar] [CrossRef] [PubMed]
- Herisson, P.J.-L.; Chauvin, Y. Catalyse de transformation des oléfines par les complexes du tungstène. II. Télomérisation des oléfines cycliques en présence d’oléfines acycliques. Die Makromol. Chem. 1971, 141, 161–176. [Google Scholar] [CrossRef]
- Grubbs, R.H.; Chang, S. Recent Advances in Olefin Metathesis and Its Application in Organic Synthesis. Tetrahedron 1998, 54, 4413–4450. [Google Scholar] [CrossRef]
- Grubbs, R.H. Olefin metathesis. Tetrahedron 2004, 60, 7117–7140. [Google Scholar] [CrossRef]
- Trnka, T.M.; Grubbs, R.H. The Development of L 2 X 2 RuCHR Olefin Metathesis Catalysts: An Organometallic Success Story. Acc. Chem. Res. 2001, 34, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Franzen, R.G. Metathesis Reactions on Solid-Phase: Towards New Synthesis Challenges. Top. Catal. 2016, 59, 1143–1150. [Google Scholar] [CrossRef]
- Higman, C.S.; Lummiss, J.A.M.; Fogg, D.E. Olefin Metathesis at the Dawn of Implementation in Pharmaceutical and Specialty-Chemicals Manufacturing. Angew. Chem. Int. Ed. Engl. 2016, 55, 3552–3565. [Google Scholar] [CrossRef]
- Higman, C.S.; Plais, L.; Fogg, D.E. Isomerization During Olefin Metathesis: An Assessment of Potential Catalyst Culprits. ChemCatChem 2013, 5, 3548–3551. [Google Scholar] [CrossRef]
- Schrock, R.R. Olefin Metathesis by Molybdenum Imido Alkylidene Catalysts. Tetrahedron 1999, 55, 8141–8153. [Google Scholar] [CrossRef]
- Schrock, R.R.; Hoveyda, A.H. Molybdenum and tungsten imido alkylidene complexes as efficient olefin-metathesis catalysts. Angew. Chem. Int. Ed. Engl. 2003, 42, 4592–4633. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, W.; Liu, S.; Xu, L.; Han, X.; Bao, X. The role of alumina in the supported Mo/Hβ–Al2O3 catalyst for olefin metathesis: A high-resolution solid-state NMR and electron microscopy study. J. Catal. 2007, 250, 55–66. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Liu, S.; Xie, S.; Zhu, X.; Bao, X.; Xu, L. Promoting effect of Mg in supported Mo/ Hβ–Al2O3 catalyst for cross-metathesis of ethene and butene-2 to propene. J. Mol. Catal. A Chem. 2009, 313, 38–43. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Li, X.; Liu, S.; Huang, H.; Han, X.; Xu, L.; Bao, X. Insights into the Deactivation Mechanism of Heterogeneous Mo/Hβ-Al2O3 Catalysts for Olefin Metathesis. J. Phys. Chem. C 2009, 113, 8228–8233. [Google Scholar] [CrossRef]
- Liu, S.; Huang, S.; Xin, W.; Bai, J.; Xie, S.; Xu, L. Metathesis of ethylene and butylene-2 to propylene with Mo on Hβ-Al2O3 catalysts. Catal. Today 2004, 93-95, 471–476. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Xin, W.; Xie, S.; Zeng, P.; Zhang, L.; Xu, L. Cross metathesis of butene-2 and ethene to propene over Mo/MCM-22-Al2O3 catalysts with different Al2O3 contents. J. Nat. Gas Chem. 2010, 19, 482–486. [Google Scholar] [CrossRef]
- Huang, S.; Liu, S.; Xin, W.; Bai, J.; Xie, S.; Wang, Q.; Xu, L. Metathesis of ethene and 2-butene to propene on W/Al2O3–HY catalysts with different HY contents. J. Mol. Catal. A Chem. 2005, 226, 61–68. [Google Scholar] [CrossRef]
- Huang, S.; Liu, S.; Xin, W.; Xie, S.; Wang, Q.; Xu, L. Effect of Reaction Temperature and Pressure on the Metathesis Reaction between Ethene and 2-Butene to Propene on the WO3/Al2O3-HY Catalyst. J. Nat. Gas Chem. 2006, 15, 93–99. [Google Scholar] [CrossRef]
- Zuo, G.; Xu, Y.; Zheng, J.; Jiang, F.; Liu, X. Investigation on converting 1-butene and ethylene into propene via metathesis reaction over W-based catalysts. RSC Adv. 2018, 8, 8372–8384. [Google Scholar] [CrossRef] [Green Version]
- Behr, A.; Schüller, U. Kinetische Untersuchungen zur heterogen katalysierten Metathese von 1-Penten: Entwicklung einer optimierten Reaktorfahrweise. Chem. Ing. Tech. 2009, 81, 429–439. [Google Scholar] [CrossRef]
- Engel, J.; Smit, W.; Foscato, M.; Occhipinti, G.; Törnroos, K.W.; Jensen, V.R. Loss and Reformation of Ruthenium Alkylidene: Connecting Olefin Metathesis, Catalyst Deactivation, Regeneration, and Isomerization. J. Am. Chem. Soc. 2017, 139, 16609–16619. [Google Scholar] [CrossRef] [PubMed]
- Lafaye, K.; Bosset, C.; Nicolas, L.; Guérinot, A.; Cossy, J. Beyond catalyst deactivation: Cross-metathesis involving olefins containing N-heteroaromatics. Beilstein J. Org. Chem. 2015, 11, 2223–2241. [Google Scholar] [CrossRef] [Green Version]
- Poater, A.; Ragone, F.; Garrido, M.; Pérez, S.; Poch, M.; Correa, A.; Cavallo, L. Deactivation of Ru-benzylidene Grubbs catalysts active in olefin metathesis. Procedia Comput. Sci. 2011, 4, 1222–1229. [Google Scholar] [CrossRef] [Green Version]
- Alvarado Perea, L.; Wolff, T.; Veit, P.; Hilfert, L.; Edelmann, F.T.; Hamel, C.; Seidel-Morgenstern, A. Alumino-mesostructured Ni catalysts for the direct conversion of ethene to propene. J. Catal. 2013, 305, 154–168. [Google Scholar] [CrossRef]
- Felischak, M.; Wolff, T.; Alvarado Perea, L.; Seidel-Morgenstern, A.; Hamel, C. Influence of process parameters on single bed Ni/(Al)MCM-41 for the production of propene from ethene feedstock. Chem. Eng. Sci. 2019, 210, 115246. [Google Scholar] [CrossRef]
- Iwamoto, M. Conversion of Ethene to Propene on Nickel Ion-loaded Mesoporous Silica Prepared by the Template Ion Exchange Method. Catal. Surv. Asia 2008, 12, 28–37. [Google Scholar] [CrossRef]
- Noreña-Franco, L.; Hernandez-Perez, I.; Aguilar-Pliego, J.; Maubert-Franco, A. Selective hydroxylation of phenol employing Cu–MCM-41 catalysts. Catal. Today 2002, 75, 189–195. [Google Scholar] [CrossRef]
- Alvarado Perea, L.; Wolff, T.; Hamel, C.; Seidel-Morgenstern, A. Experimental study of the deactivation of Ni/AlMCM-41 catalyst in the direct conversion of ethene to propene. Appl. Catal. A Gen. 2017, 533, 121–131. [Google Scholar] [CrossRef]
- Maksasithorn, S.; Debecker, D.P.; Praserthdam, P.; Panpranot, J.; Suriye, K.; Ayudhya, S.K.N. NaOH modified WO3/SiO2 catalysts for propylene production from 2-butene and ethylene metathesis. Chin. J. Catal. 2014, 35, 232–241. [Google Scholar] [CrossRef]
- Bouchmella, K.; Hubert Mutin, P.; Stoyanova, M.; Poleunis, C.; Eloy, P.; Rodemerck, U.; Gaigneaux, E.M.; Debecker, D.P. Olefin metathesis with mesoporous rhenium–silicium–aluminum mixed oxides obtained via a one-step non-hydrolytic sol–gel route. J. Catal. 2013, 301, 233–241. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).