Preparation, Property Characterization of Gd2YSbO7/ZnBiNbO5 Heterojunction Photocatalyst for Photocatalytic Degradation of Benzotriazole under Visible Light Irradiation
Abstract
:1. Introduction
2. Result and Discussion
2.1. XRD Analysis
2.2. UV-Vis Diffuse Reflectance Spectra
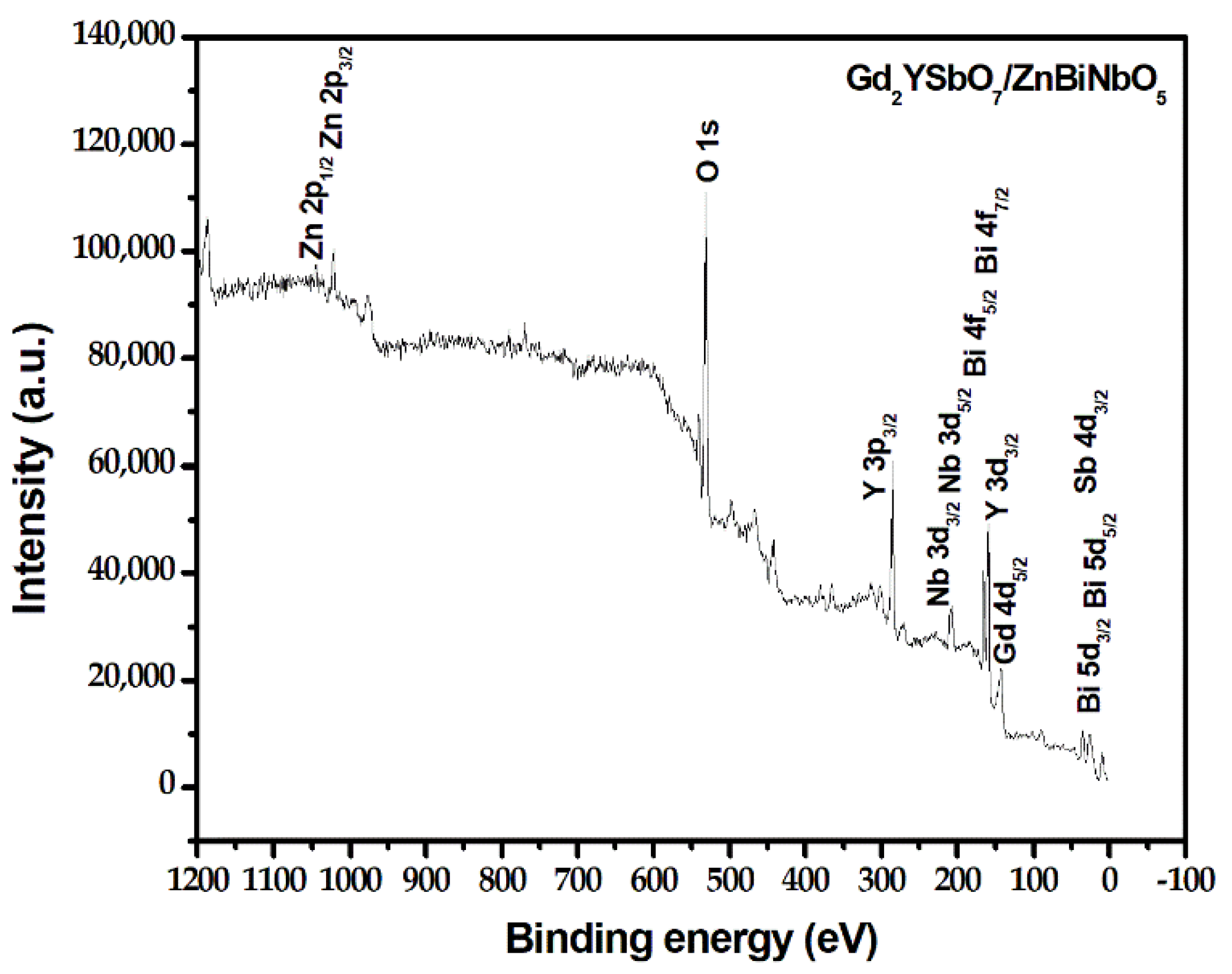
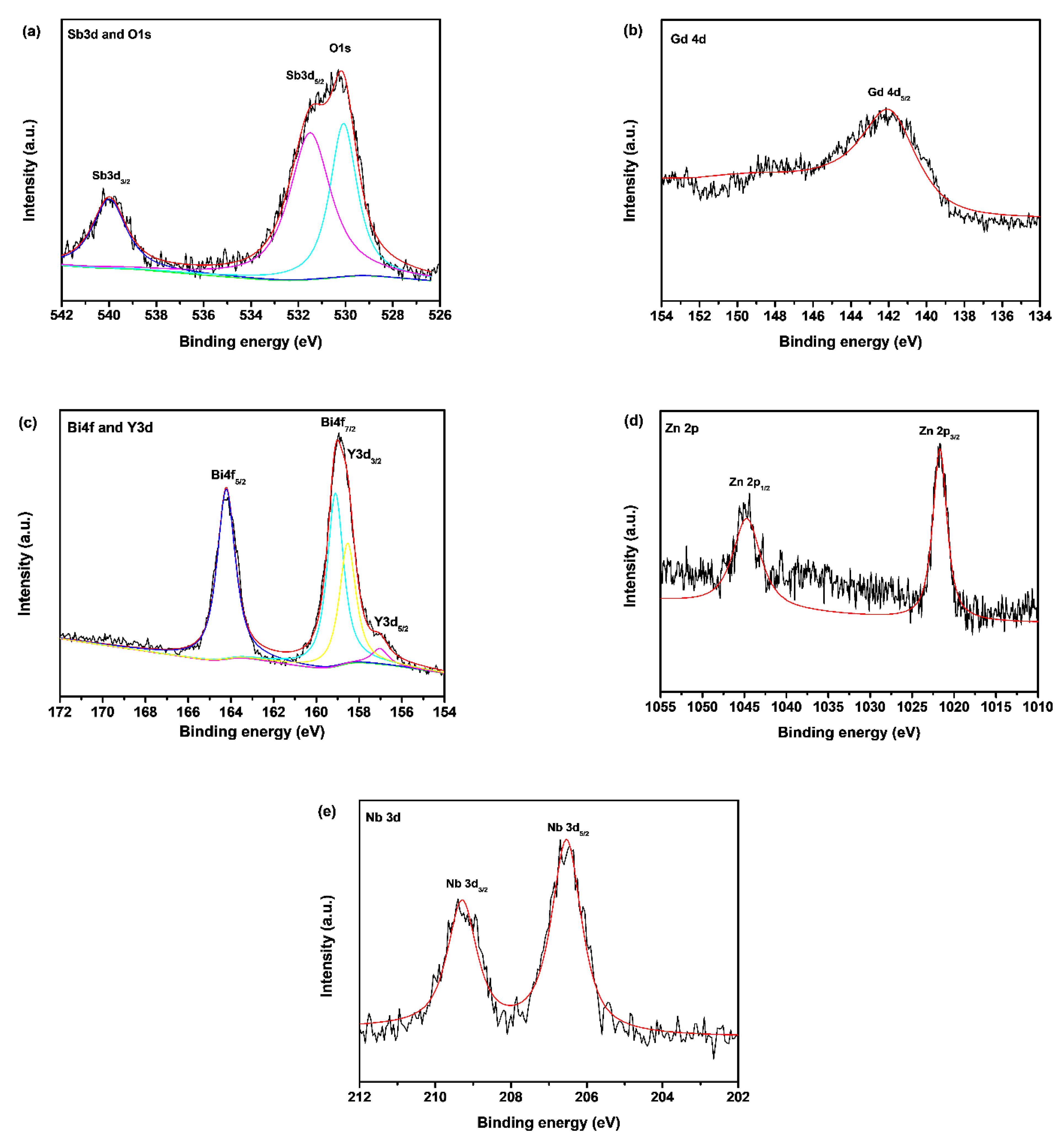
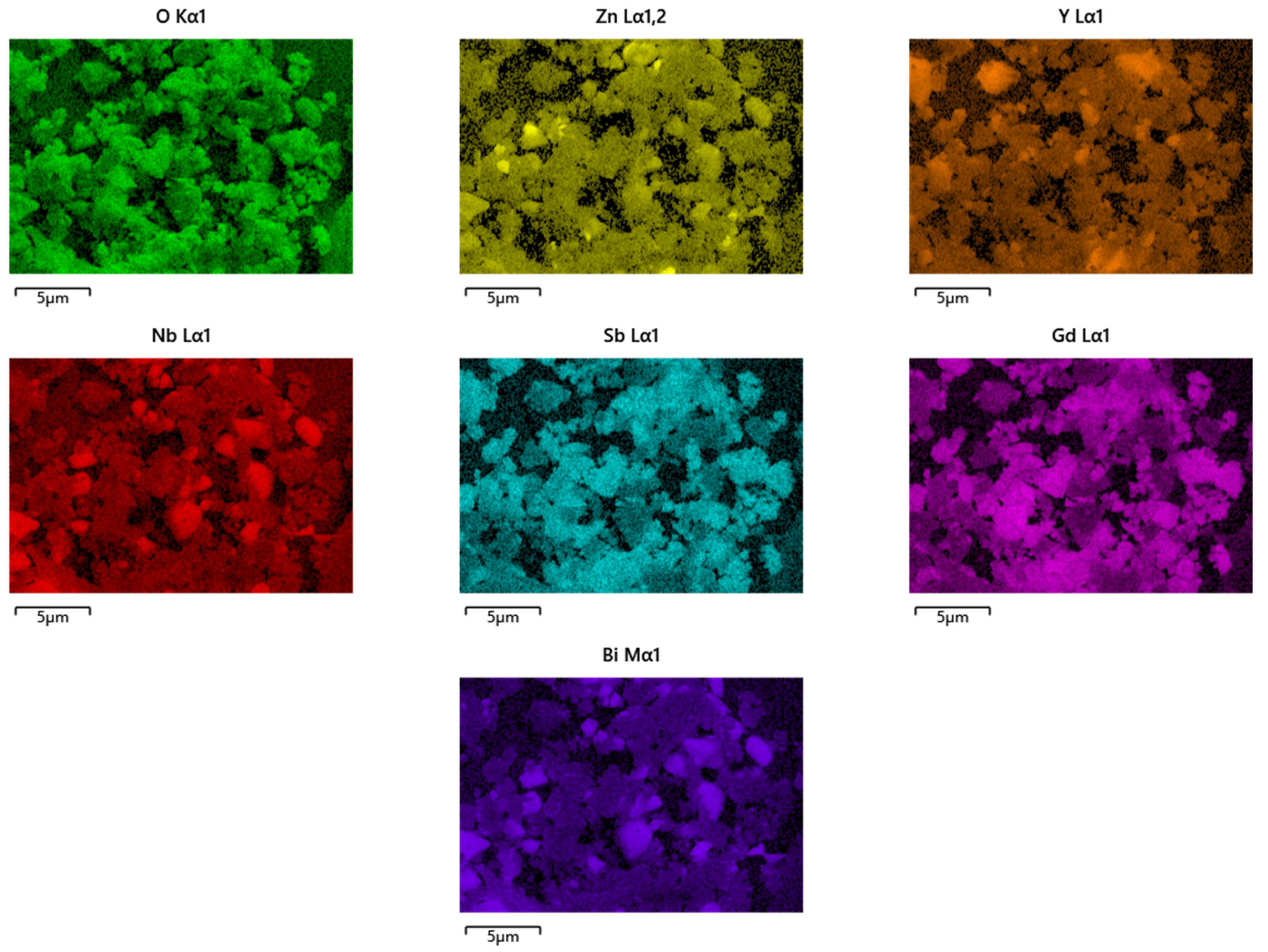
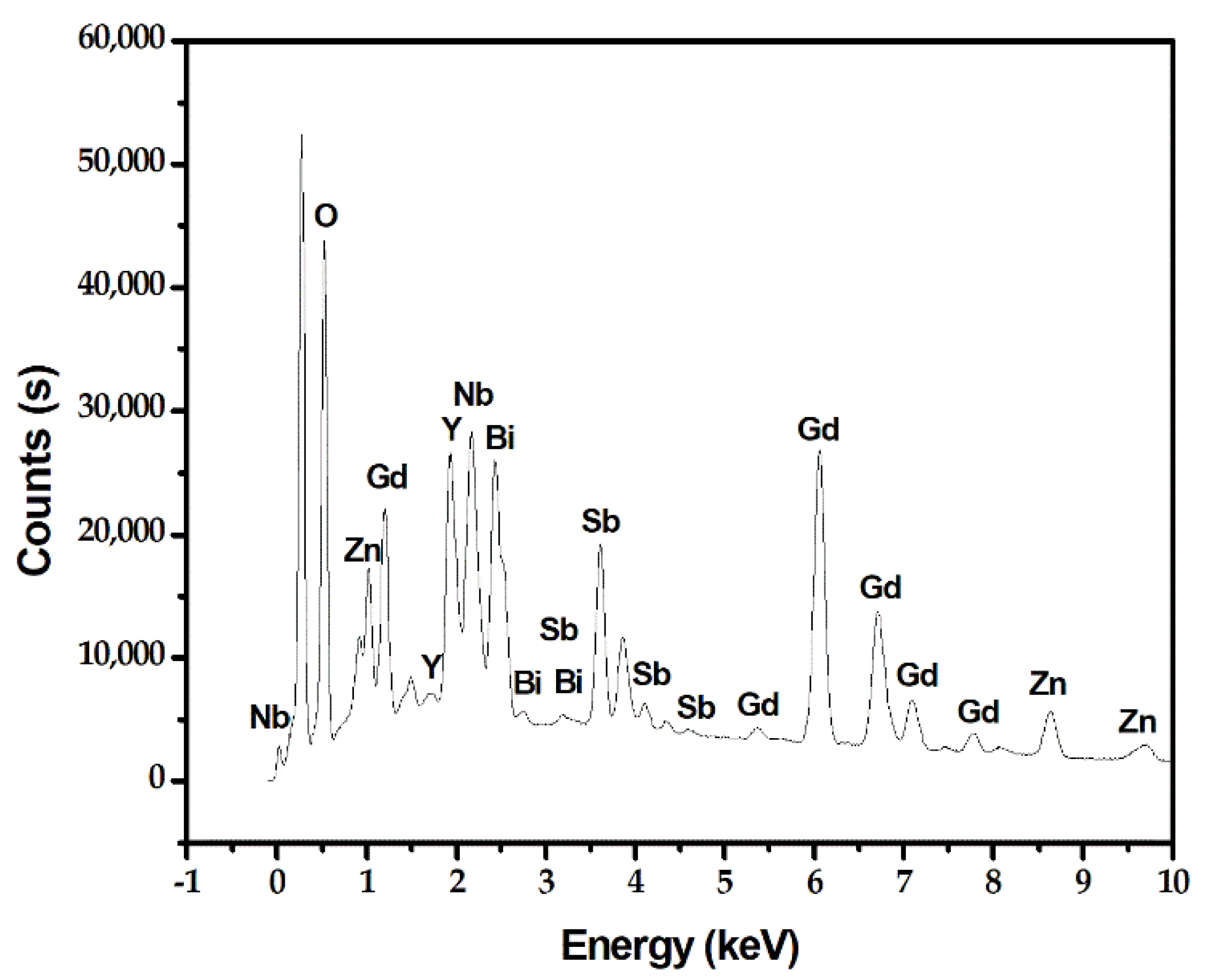
2.3. Property Characterization of Gd2YSbO7/ZnBiNbO5 Heterojunction Photocatalyst
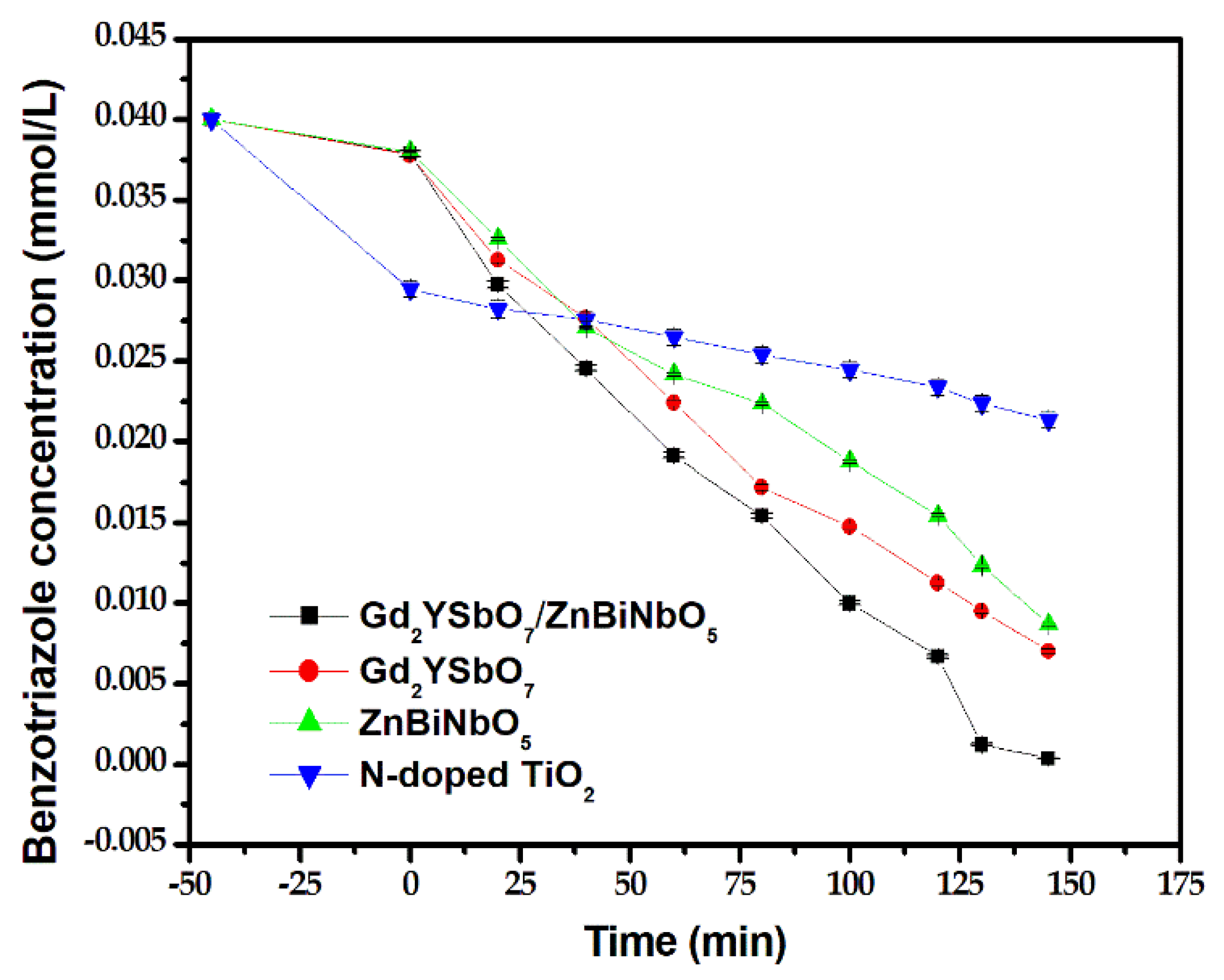
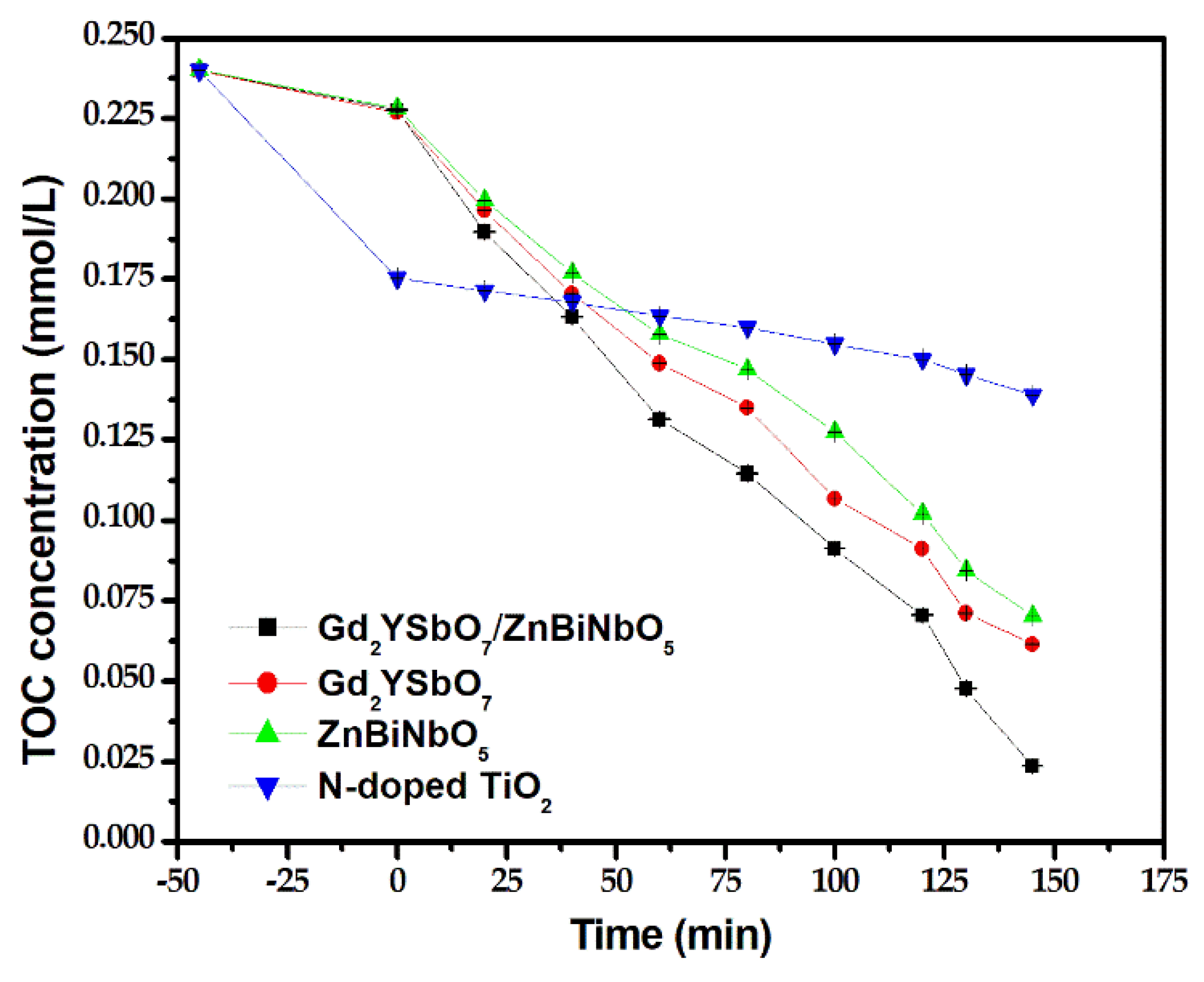
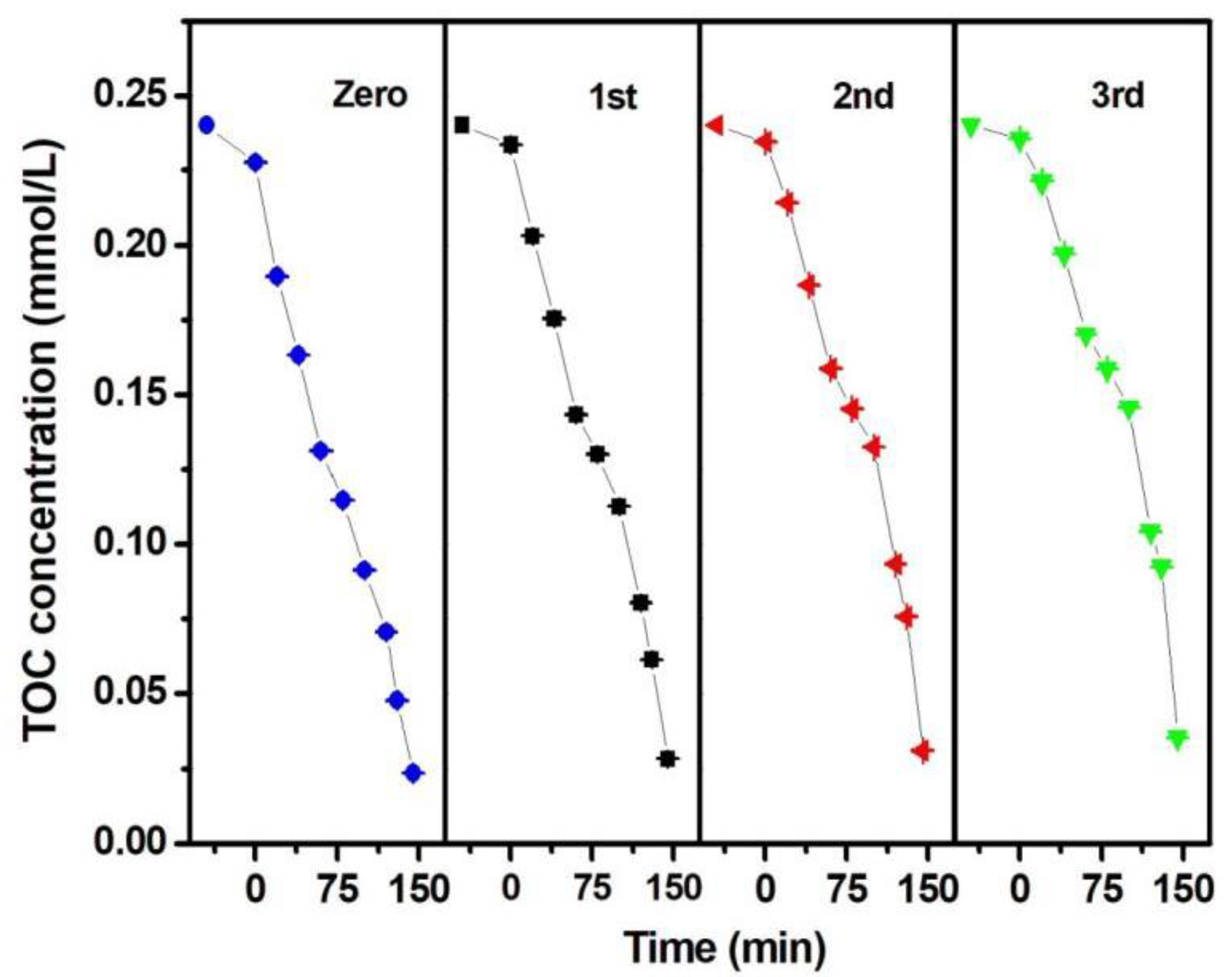
2.4. Photocatalytic Activity
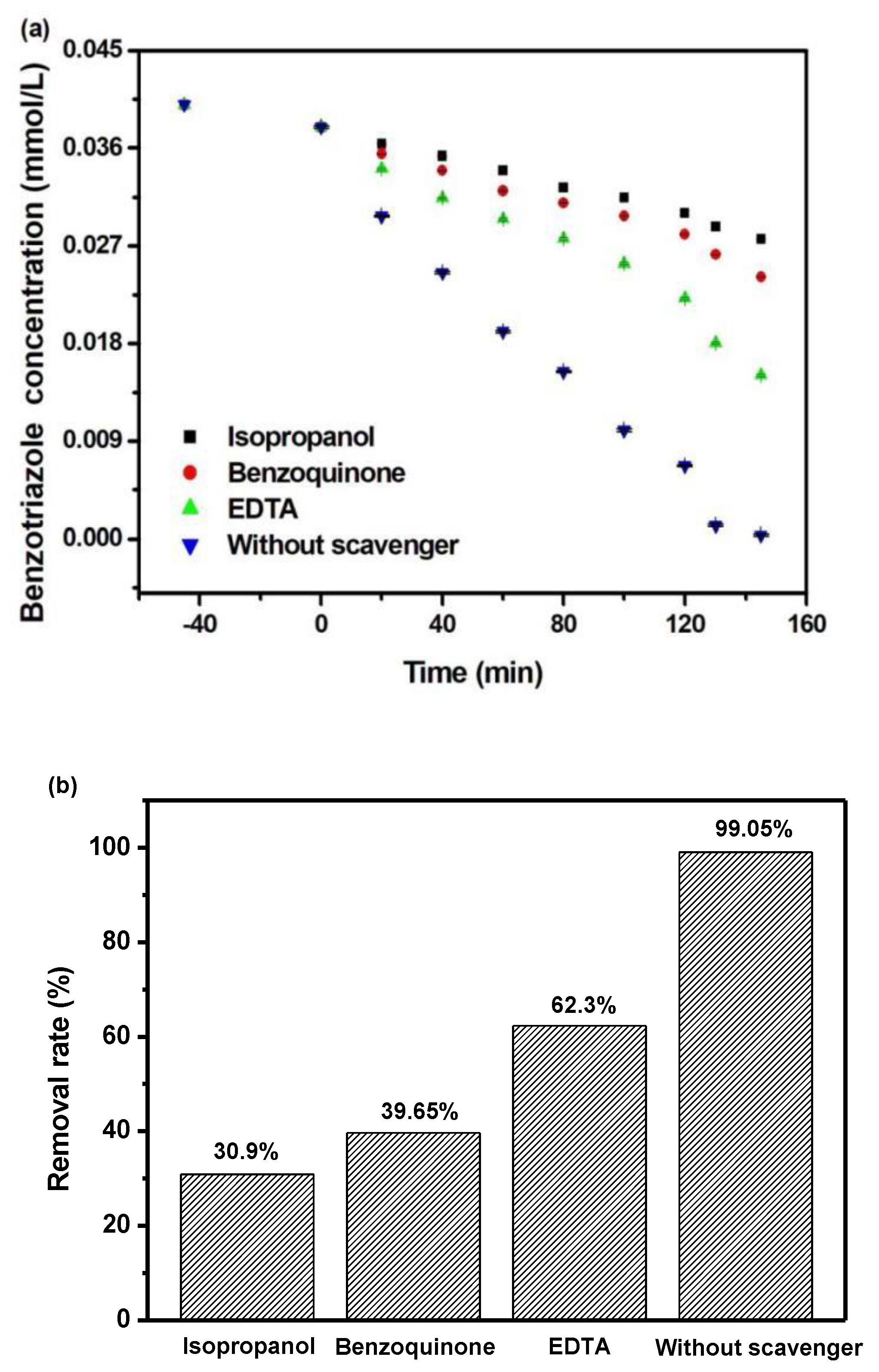
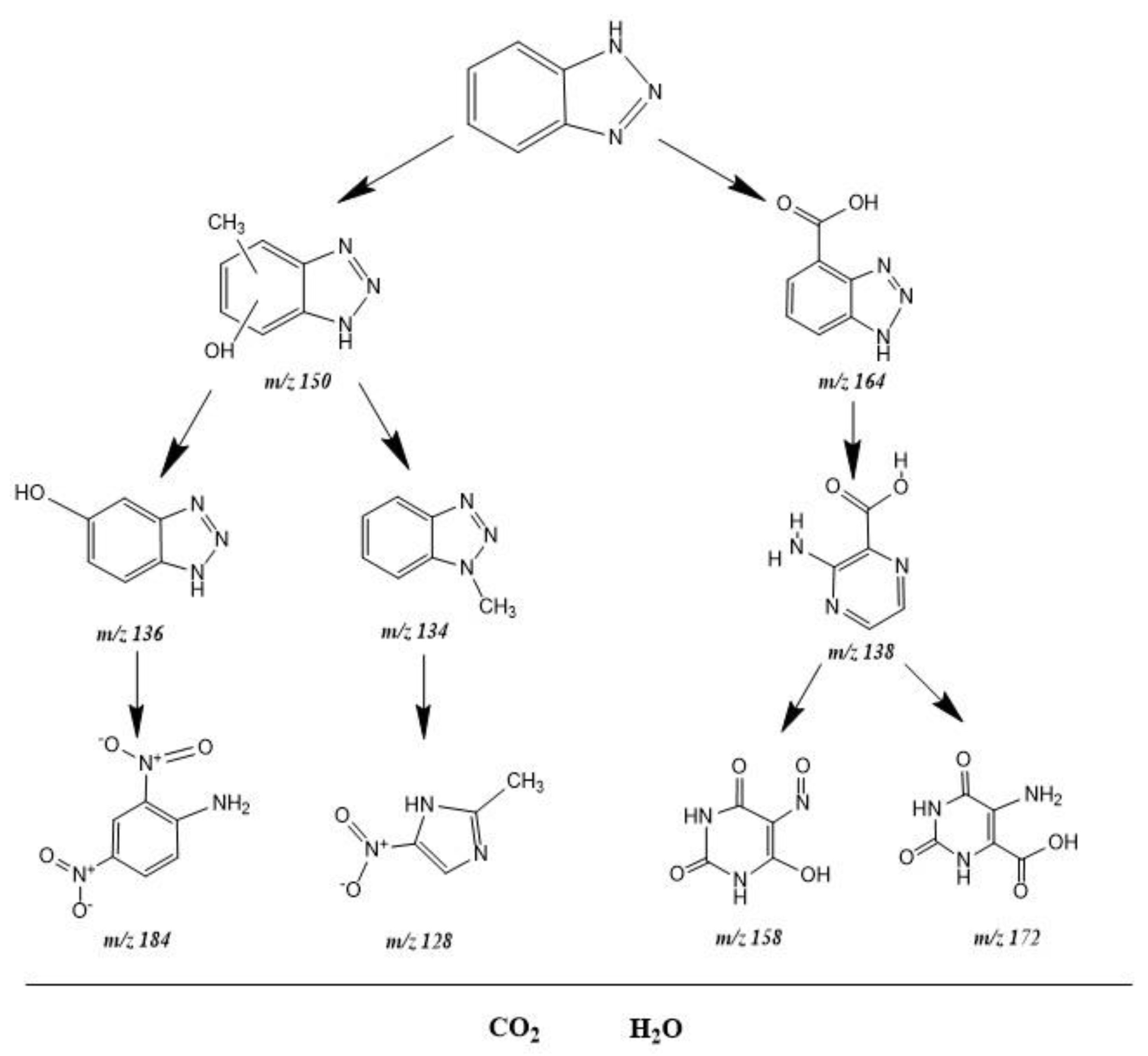
2.5. Possible Degradation Mechanism Analysis
3. Experimental Section
3.1. Materials and Reagents
3.2. Preparation Method of Gd2YSbO7
3.3. Preparation Method of ZnBiNbO5
3.4. Synthesis of N-Doped TiO2
3.5. Synthesis of Gd2YSbO7/ZnBiNbO5 Heterojunction Photocatalyst
3.6. Characterizations
3.7. Photoelectrochemical Experiments
3.8. Experimental Setup and Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huntscha, S.; Hofstetter, T.B.; Schymanski, E.L.; Spahr, S.; Hollender, J. Biotransformation of benzotriazoles: Insights from transformation product identification and compound-specific isotope analysis. Environ. Sci. Technol. 2014, 48, 4435–4443. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Huang, P.Q. Photophysical and photocatalytic properties of BiSnSbO6 under visible light irradiation. Materials 2018, 11, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voutsa, D.; Hartmann, P.; Schaffner, C.; Giger, W. Benzotriazoles, alkylphenols and bisphenol A in bunicipal wastewaters and in the Glatt River, Switzerland. Environ. Sci. Pollut. Res. 2006, 13, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Ruan, T.; Wang, T.; Song, S.; Guo, F.; Jiang, G. Determination of nine benzotriazole UV stabilizers in environmental water samples by automated on-line solid phase extraction coupled with high-performance liquid chromatography−tandem mass spectrometry. Talanta 2014, 120, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Lai, H.; Ying, G.; Ma, Y.; Chen, Z.; Chen, F.; Liu, Y. Occurrence and dissipation of benzotriazoles and benzotriazole ultraviolet stabilizers in biosolid-amended soils. Environ. Toxicol. Chem. 2014, 33, 761–767. [Google Scholar] [CrossRef]
- Jia, Y.; Bakken, L.R.; Breedveld, G.D.; Aagaard, P.; Frostegård, Å. Organic compounds that reach subsoil may threaten groundwater quality: Effect of benzotriazole on degradation kinetics and microbial community composition. Soil Biol. Biochem. 2006, 38, 2543–2556. [Google Scholar] [CrossRef]
- Nakata, H.; Murata, S.; Filatreau, J. Occurrence and concentrations of benzotriazole UV stabilizers in marine organisms and sediments from the Ariake Sea, Japan. Environ. Sci. Technol. 2009, 43, 6920–6926. [Google Scholar] [CrossRef]
- Kim, J.; Ramaswamy, B.R.; Chang, K.; Isobe, T.; Tanabe, S. Multiresidue analytical method for the determination of antimicrobials, preservatives, benzotriazole UV stabilizers, flame retardants and plasticizers in fish using ultra high performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3511–3520. [Google Scholar] [CrossRef]
- Hem, L.J.; Hartnik, T.; Roseth, R.; Breedveld, G.D. Photochemical degradation of benzotriazole. J. Environ. Sci. Health A 2003, 38, 471–481. [Google Scholar] [CrossRef]
- Hollingsworth, J.; Sierra-Alvarez, R.; Zhou, M.; Ogden, K.L.; Field, J.A. Anaerobic biodegradability and methanogenic toxicity of key constituents in copper chemical mechanical planarization effluents of the semiconductor industry. Chemosphere 2005, 59, 1219–1228. [Google Scholar] [CrossRef]
- Xu, J.; Li, L.; Guo, C.S.; Zhang, Y.; Wang, S.F. Removal of benzotriazole from solution by BiOBr photocatalysis under simulated solar irradiation. Chem. Eng. J. 2013, 221, 230–237. [Google Scholar] [CrossRef]
- Zhe, L.; Shirley, A.S.; Thomas, E.P.; Amila, O.D.S. Occurrence and fate of substituted diphenylamine antioxidants and benzotriazole UV stabilizers in various Canadian wastewater treatment processes. Water Res. 2017, 124, 158–166. [Google Scholar]
- Zhe, L.; Amila, O.D.S.; Thomas, E.P.; Cyril, J.C.; Gerald, R.T.; Mark, R.S.; Derek, C.G.M. Distribution, partitioning and bioaccumulation of substituted diphenylamine antioxidants and benzotriazole UV stabilizers in an Urban Creek in Canada. Environ. Sci. Technol. 2016, 50, 9089–9097. [Google Scholar]
- Javaid, R.; Qazi, U.Y. Catalytic oxidation process for the degradation of synthetic dyes: An overview. Inter. J. Env. Res. Pub. Heal. 2019, 16, 2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aarthi, T.; Narahari, P.; Madras, G. Photocatalytic degradation of azure and sudan dyes using nano TiO2. J. Hazard. Mater. 2007, 149, 725–734. [Google Scholar] [CrossRef]
- Rahman, M.U.; Qazi, U.Y.; Hussain, T.; Nadeem, N.; Zahid, M.; Bhatti, H.N.; Shahid, I. Solar driven photocatalytic degradation potential of novel graphitic carbon nitride based nano zero-valent iron doped bismuth ferrite ternary composite. Opt. Mater. 2021, 120, 111408. [Google Scholar] [CrossRef]
- Tahmasebi, N.; Esmaeilpour, H.; Movahedifard, F.; Hakimyfard, A.; Moayeri, H. Fabrication of a novel MoO3/Cs3PMo12O40 composite for photocatalytic decolorization of rhodamine B. Mat. Sci. Semicon. Proc. 2021, 131, 105876. [Google Scholar] [CrossRef]
- Mokhtari, F.; Tahmasebi, N. Hydrothermal synthesis of W-doped BiOCl nanoplates for photocatalytic degradation of rhodamine B under visible light. J. Phys. Chem. Solids 2021, 149, 109804. [Google Scholar] [CrossRef]
- Subhan, M.A.; Rifat, T.P.; Saha, P.C.; Alam, M.M.; Asiri, A.M.; Rahman, M.M.; Akter, S.; Raihan, T.; Azad, A.K.; Uddin, J. Enhanced visible light-mediated photocatalysis, antibacterial functions and fabrication of a 3-chlorophenol sensor based on ternary Ag2O·SrO·CaO. RSC Adv. 2020, 10, 11274–11291. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, L.; Della Sala, A.; Fiorentino, A.; Li Puma, G. Disinfection of urban wastewater by solar driven and UV lamp—TiO2 photocatalysis: Effect on a multi drug resistant Escherichia coli strain. Water Res. 2014, 53, 145–152. [Google Scholar] [CrossRef]
- Sakai, T.; Da Loves, A.; Okada, T.; Mishima, S. Titania/CnTAB nanoskeleton as adsorbent and photocatalyst for removal of alkylphenols dissolved in water. J. Hazard. Mater. 2013, 248, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, P.N.; Uma, S.; Rodriguez, S.; Klabunde, K.J. Aerogel processing of MTi2O5 (M = Mg, Mn, Fe, Co, Zn, Sn) compositions using single source precursors: Synthesis, characterization and photocatalytic behavior. J. Mol. Catal. A Chem. 2005, 229, 145–150. [Google Scholar] [CrossRef]
- Zhang, H.S.; Wen, Z.Y.; Zhao, Y.D.; Li, G.; Li, Z.J. Preparation, characterization of A2Ce2O7 (A = La and Gd) and their photo-catalytic properties. Energy Environ. Focus. 2015, 4, 324–329. [Google Scholar]
- Ladan, R.; Alireza, H.; Mohammad, S. High efficient solar light photocatalytic degradation of malachite green by solid state synthesized Bi2Sn2O7 and Bi2MxSn2O7 (M = Y3+, Eu3+, Gd3+ and Yb3+) nanomaterials. J. Nanoanal. 2020, 7, 1–17. [Google Scholar]
- Hebeish, A.A.; Abdelhady, M.M.; Youssef, A.M. TiO2 nanowire and TiO2 nanowire doped Ag-PVP nanocomposite for antimicrobial and self-cleaning cotton textile. Carbohyd. Polym. 2013, 91, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, X.J.; Yin, L.; Li, B.; Hong, X.; Fan, Z.; Chen, B.; Xue, C.; Zhang, H. One-pot synthesis of CdS nanocrystals hybridized with single-layer transition-metal dichalcogenide nanosheets for efficient photocatalytic hydrogen evolution. Angew. ChemInt. Ed. Engl. 2015, 54, 1210–1214. [Google Scholar]
- Zhang, Z.; Li, A.; Cao, S.W.; Bosman, M.; Li, S.; Xue, C. Direct evidence of plasmon enhancement on photocatalytic hydrogen generation over Au/Pt-decorated TiO2 nanofibers. Nanoscale 2014, 6, 5217–5222. [Google Scholar] [CrossRef]
- Zhang, N.; Shi, J.; Mao, S.S.; Guo, L. Co3O4 quantum dots: Reverse micelle synthesis and visible-light-driven photocatalytic overall water splitting. Chem. Commun. 2014, 50, 2002–2004. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Li, M.; Shen, S.; Wang, Y.; Feng, Z.; Shi, J.; Han, H.; Li, C. Photocatalytic overall water splitting promoted by an alpha-beta phase junction on Ga2O3. Angew. Chem. Int. 2012, 51, 13089–13092. [Google Scholar] [CrossRef]
- Huang, J.G.; Zhao, X.G.; Zheng, M.Y.; Li, S.; Wang, Y.; Liu, X.J. Preparation of N-doped TiO2 by oxidizing TiN and its application on phenol degradation. Water Sci. Technol. 2013, 68, 934–939. [Google Scholar] [CrossRef]
- Mohammadi, K.; Sadeghi, M.; Azimirad, R. Facile synthesis of SrFe12O19 nanoparticles and its photocatalyst application. J. Mater. Sci. Mater. EL 2017, 28, 10042–10047. [Google Scholar] [CrossRef]
- Mohammadi, K.; Sadeghi, M.; Azimirad, R.; Ebrahimi, M. Barium hexaferrite nanoparticles: Morphology-controlled preparation, characterization and investigation of magnetic and photocatalytic properties. J. Mater. Sci. Mater. EL 2017, 28, 9983–9988. [Google Scholar] [CrossRef]
- Shieh, D.L.; Lin, Y.S.; Yeh, J.H.; Chen, S.C.; Lin, B.C.; Lin, J.L. N-doped, porous TiO2 with rutile phase and visible light sensitive photocatalytic activity. Chem. Commun. 2012, 48, 2528–2530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lu, J.; Jiang, J.; Li, X.; Lu, M.; Yuan, G.; Wang, Z.; Zheng, M.; Seo, H.J. Simple fabrication of N-doped mesoporous TiO2 nanorods with the enhanced visible light photocatalytic activity. Nanoscale Res. Lett. 2014, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Burda, C. The electronic origin of the visible-light absorption properties of C-, N- and S-doped TiO2 nanomaterials. J. Am. Chem. Soc. 2008, 130, 5018–5019. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Huo, Y.; Zhu, J. Supercritical preparation of a highly active S-doped TiO2 photocatalyst for methylene blue mineralization. Environ. Sci. Technol. 2007, 41, 4410–4414. [Google Scholar] [CrossRef]
- Li, Z.; Ding, D.; Ning, C. P-Type hydrogen sensing with Al-and V-doped TiO2 nanostructures. Nanoscale Res. Lett. 2013, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Wang, S.; Zou, J.J.; Huang, Z.F.; Wang, L.; Zhang, X. Ti3+-defected and V-doped TiO2 quantum dots loaded on MCM-41. Chem. Commun. 2014, 50, 988–990. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, C.; Zhang, L.; Li, X.; Liu, Y. Electrospun nanofibers of V-doped TiO2 with high photocatalytic activity. J. Colloid. Interf. Sci. 2010, 351, 57–62. [Google Scholar] [CrossRef]
- Liu, G.; Han, C.; Pelaez, M.; Zhu, D.; Liao, S.; Likodimos, V.; Ioannidis, N.; Kontos, A.G.; Falaras, P.; Dunlop, P.S.; et al. Synthesis, characterization and photocatalytic evaluation of visible light activated C-doped TiO2 nanoparticles. Nanotechnology 2012, 23, 294003. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, P.; Li, Q. New insight into the enhanced visible-light photocatalytic activities of B-, C- and B/C-doped anatase TiO2 by first-principles. Phys. Chem. Chem. Phys. 2013, 15, 12040–12047. [Google Scholar] [CrossRef]
- Zhang, W.J.; Ma, Z.; Du, L.; Li, H. Role of PEG4000 in sol-gel synthesis of Sm2Ti2O7 photocatalyst for enhanced activity. J. Alloy. Compd. 2017, 704, 26–31. [Google Scholar] [CrossRef]
- Wang, S.X.; Li, W.; Wang, S.; Jiang, J.M.; Chen, Z.H. Synthesis of well-defined hierarchical porous La2Zr2O7 monoliths via non-alkoxidesolegel process accompanied by phase separation. Micropor. Mesopor. Mat. 2016, 221, 32–39. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Arakawa, H. Preparation, structural and photophysical properties of Bi2InNbO7 compound. J. Mater. Sci. 2000, 19, 1909–1911. [Google Scholar]
- Shin, H.; Byun, T.H. Effect of Pt loading onto ball-milled TiO2 on the visible-light photocatalytic activity to decompose rhodamine B. Res. J. Chem. Environ. 2013, 17, 41–46. [Google Scholar]
- Kanhere, P.; Shenai, P.; Chakraborty, S.; Ahuja, R.; Zheng, J.; Chen, Z. Mono- and co-doped NaTaO3 for visible light photocatalysis. Phys. Chem. Chem. Phys. 2014, 16, 16085–16094. [Google Scholar] [CrossRef] [Green Version]
- Daskalaki, V.M.; Antoniadou, M.; Li Puma, G.; Kondarides, D.I.; Lianos, P. Solar light-responsive Pt/CdS/TiO2 photocatalysts for hydrogen production and simultaneous degradation of inorganic or organic sacrificial agents in wastewater. Environ. Sci. Technol. 2010, 44, 7200–7205. [Google Scholar] [CrossRef]
- Luan, J.F.; Zhao, W.; Feng, J.W.; Cai, H.L.; Zheng, Z.; Pan, B.C.; Wu, X.S.; Zou, Z.G.; Li, Y.M. Structural, photophysical and photocatalytic properties of novel Bi2AlVO7. J. Hazard. Mater. 2009, 164, 781–789. [Google Scholar] [CrossRef]
- Xing, C.C.; Zhang, Y.; Liu, Y.P.; Wang, X.; Li, J.S.; Martinez-Alanis, P.R.; Spadaro, M.C.; Guardia, P.; Arbiol, J.; Llorca, J.; et al. Photodehydrogenation of ethanol over Cu2O/TiO2 heterostructures. Nanomaterials 2021, 11, 1399. [Google Scholar] [CrossRef]
- Stathi, P.; Solakidou, M.; Deligiannakis, Y. Lattice defects engineering in W-, Zr-doped BiVO4 by flame spray pyrolysis: Enhancing photocatalytic O2 evolution. Nanomaterials 2021, 11, 501. [Google Scholar] [CrossRef]
- Thomas, A.M.; Peter, J.; Nagappan, S.; Mohan, A.; Ha, C.S. Dual stimuli-responsive copper nanoparticles decorated SBA-15: A highly efficient catalyst for the oxidation of alcohols in water. Nanomaterials 2020, 10, 2051. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, J.W.; Ma, D.; Fan, Z.; Lu, L.; Niu, C. In situ synthesis of C-doped TiO2@g-C3N4 core-shell hollow nanospheres with enhanced visible-light photocatalytic activity for H2 evolution. Chem. Eng. J. 2017, 322, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Cao, C.Y.; Liu, H.; Li, P.; Wei, F.F.; Jiang, Y.; Song, W.G. A Bi/BiOCl heterojunction photocatalyst with enhanced electron-hole separation and excellent visible light photodegrading activity. J. Mater. Chem. A 2014, 2, 1677–1681. [Google Scholar] [CrossRef]
- Wang, P.Q.; Bai, Y.; Luo, P.Y.; Liu, J.Y. Ag2O/Ag3PO4 heterostructures: Highly efficient and stable visible-light-induced photocatalyst for degradation of methyl orange and phenol. Micro Nano Lett. 2013, 8, 340–344. [Google Scholar] [CrossRef]
- Tang, Y.; Tao, Y.; Zhou, T.; Yang, B.; Wang, Q.; Zhu, Z.; Xie, A.; Luo, S.; Yao, C.; Li, X. Direct Z-scheme La1-xCexMnO3 catalyst for photothermal degradation of toluene. Environ. Sci. Pollut. R 2019, 26, 36832–36844. [Google Scholar] [CrossRef]
- Wang, J.H.; Zou, Z.G.; Ye, J.H. Synthesis, structure and photocatalytic property of a new hydrogen evolving photocatalyst Bi2InTaO7. Mater. Sci. Forum. 2003, 423, 485–490. [Google Scholar] [CrossRef]
- Kohno, M.; Ogura, S.; Sato, K.; Inoue, Y. Properties of photocatalysts with tunnel structures: Formation of a surface lattice O− radical by the UV irradiation of BaTi4O9 with a pentagonal-prism tunnel structure. Chem. Phys. Lett. 1997, 267, 72–76. [Google Scholar] [CrossRef]
- Kudo, A.; Kato, H.; Nakagawa, S. Water splitting into H2 and O2 on new Sr2M2O7 (M = Nb and Ta) photocatalysts with layered perovskite structures: Factors affecting the photocatalytic activity. J. Phys. Chem. B 2000, 104, 571–575. [Google Scholar] [CrossRef]
- Nowak, M.; Kauch, B.; Szperlich, P. Determination of energy band gap of nanocrystalline SbSI. Rev. Sci. Instrum. 2009, 80, 046107. [Google Scholar] [CrossRef]
- Zhou, F.; Kang, K.; Maxisch, T.; Ceder, G.; Morgan, D. The electronic structure and band gap of LiFePO4 and LiMnPO4. Solid State Commun. 2004, 132, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Tauc, J.; Grigorov, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi. 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis with YFeO3 electrodes. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Cui, B.Y.; Cui, H.T.; Li, Z.R.; Dong, H.Y.; Li, X.; Zhao, L.F.; Wang, J.W. Novel Bi3O5I2 hollow microsphere and its enhanced photocatalytic activity. Catalysts 2019, 9, 709. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, W.; Cantillo, A.; Salazar, B.; Diaz-Uribe, C.; Ramos, W.; Romero, E.; Hurtado, M. Comparative study of ZnO thin films doped with transition metals (Cu and Co) for methylene blue photodegradation under visible irradiation. Catalysts. 2020, 10, 528. [Google Scholar] [CrossRef]
- Qazi, U.Y.; Javaid, R.; Zahid, M.; Tahir, N.; Afzal, A.; Lin, X.M. Bimetallic NiCo-NiCoO2 nano-heterostructures embedded on copper foam as a self-supported bifunctional electrode for water oxidation and hydrogen production in alkaline media. Int. J. Hydrogen Energ. 2021, 46, 18936–18948. [Google Scholar] [CrossRef]
- Qazi, U.Y.; Javaid, R.; Tahir, N.; Jamil, A.; Afzal, A. Design of advanced self-supported electrode by surface modification of copper foam with transition metals for efficient hydrogen evolution reaction. Int. J. Hydrogen Energ. 2020, 45, 33396–33406. [Google Scholar] [CrossRef]
- Deonikar, V.G.; Patil, S.S.; Tamboli, M.S.; Ambekar, J.D.; Kulkarni, M.V.; Panmand, R.P.; Umarji, G.G.; Shinde, M.D.; Rane, S.B.; Munirathnam, N.R.; et al. Growth study of hierarchical Ag3PO4/LaCO3OH heterostructures. Phys. Chem. Chem. Phys. 2017, 19, 20541–20550. [Google Scholar] [CrossRef]
- Patil, S.S.; Tamboli, M.S.; Deonikar, V.G.; Umarji, G.G.; Ambekar, J.D.; Kulkarni, M.V.; Kolekar, S.S.; Kale, B.B.; Patil, D.R. Magnetically separable Ag3PO4/NiFe2O4 composites with enhanced photocatalytic activity. Dalton Trans. 2015, 44, 20426–20434. [Google Scholar] [CrossRef]
- Jiang, L.B.; Yuan, X.Z.; Zeng, G.M.; Liang, J.; Chen, X.H.; Yu, H.B.; Wang, H.; Wu, Z.B.; Zhang, J.; Xiong, T. In-situ synthesis of direct solid-state dual Z-scheme WO3/g-C3N4/Bi2O3 photocatalyst for the degradation of refractory pollutant. Appl. Catal. B 2018, 227, 376–385. [Google Scholar] [CrossRef]
- Cao, W.; Jiang, C.Y.; Chen, C.; Zhou, H.F.; Wang, Y.P. A novel Z-scheme CdS/Bi4O5Br2 heterostructure with mechanism analysis: Enhanced photocatalytic performance. J. Alloys Compd. 2021, 861, 158554. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhou, M.; Gu, J.; Li, X. Spectrophotometric and high performance liquid chromatographic methods for sensitive determination of bisphenol A. Spectrochim. Acta A 2013, 122, 153–157. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Luan, J. Preparation, Property Characterization of Gd2YSbO7/ZnBiNbO5 Heterojunction Photocatalyst for Photocatalytic Degradation of Benzotriazole under Visible Light Irradiation. Catalysts 2022, 12, 159. https://doi.org/10.3390/catal12020159
Yao Y, Luan J. Preparation, Property Characterization of Gd2YSbO7/ZnBiNbO5 Heterojunction Photocatalyst for Photocatalytic Degradation of Benzotriazole under Visible Light Irradiation. Catalysts. 2022; 12(2):159. https://doi.org/10.3390/catal12020159
Chicago/Turabian StyleYao, Ye, and Jingfei Luan. 2022. "Preparation, Property Characterization of Gd2YSbO7/ZnBiNbO5 Heterojunction Photocatalyst for Photocatalytic Degradation of Benzotriazole under Visible Light Irradiation" Catalysts 12, no. 2: 159. https://doi.org/10.3390/catal12020159
APA StyleYao, Y., & Luan, J. (2022). Preparation, Property Characterization of Gd2YSbO7/ZnBiNbO5 Heterojunction Photocatalyst for Photocatalytic Degradation of Benzotriazole under Visible Light Irradiation. Catalysts, 12(2), 159. https://doi.org/10.3390/catal12020159






