The Impact of Alternative Fuels on Ship Engine Emissions and Aftertreatment Systems: A Review
Abstract
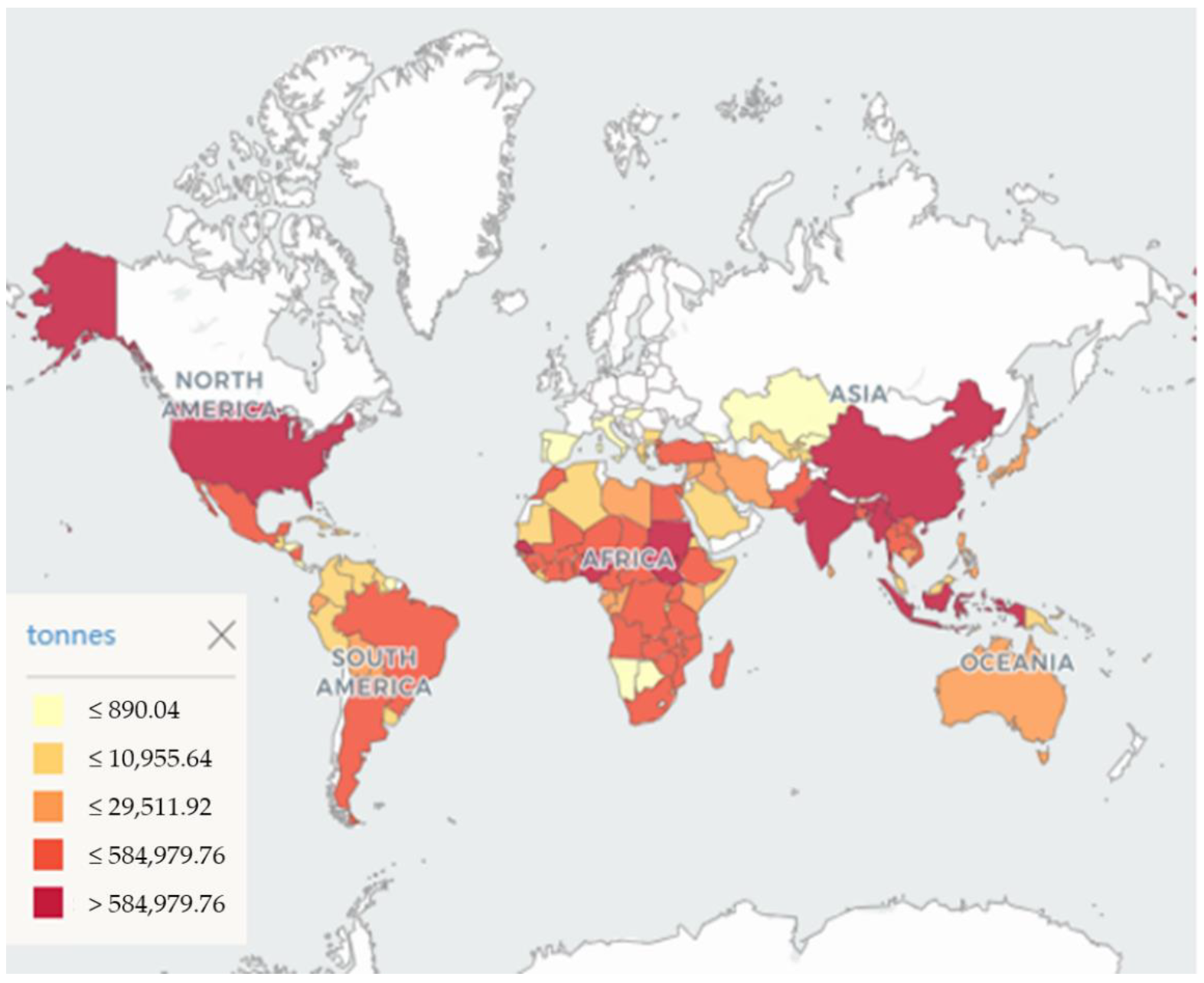
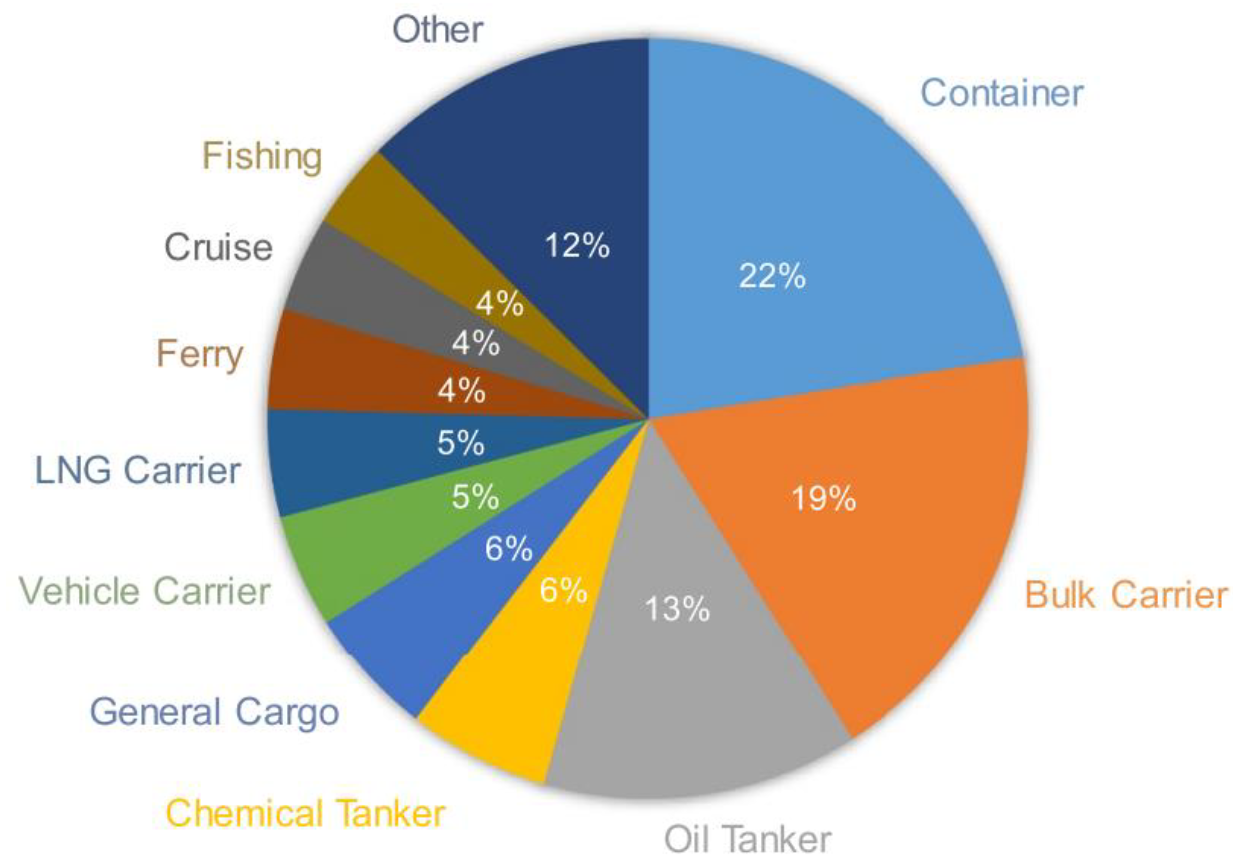
:1. Introduction
2. Waste Oil
2.1. Waste Plastic Oil
2.2. Waste Lubricating Oil
3. Biodiesel
3.1. Vegetable Oil
3.2. Waste Edible Oil
4. Alcohol Fuels
5. Natural Gas
6. Emission Reduction Technology
6.1. Fuel Optimization
6.1.1. Reduce the Viscosity of Biodiesel
6.1.2. Excess Hydrogen Method
6.1.3. Transesterification
6.1.4. Microemulsion Technology
6.1.5. Catalytic Cracking Method
6.1.6. Adjust the Injection Timing
6.2. After Treatment Technology
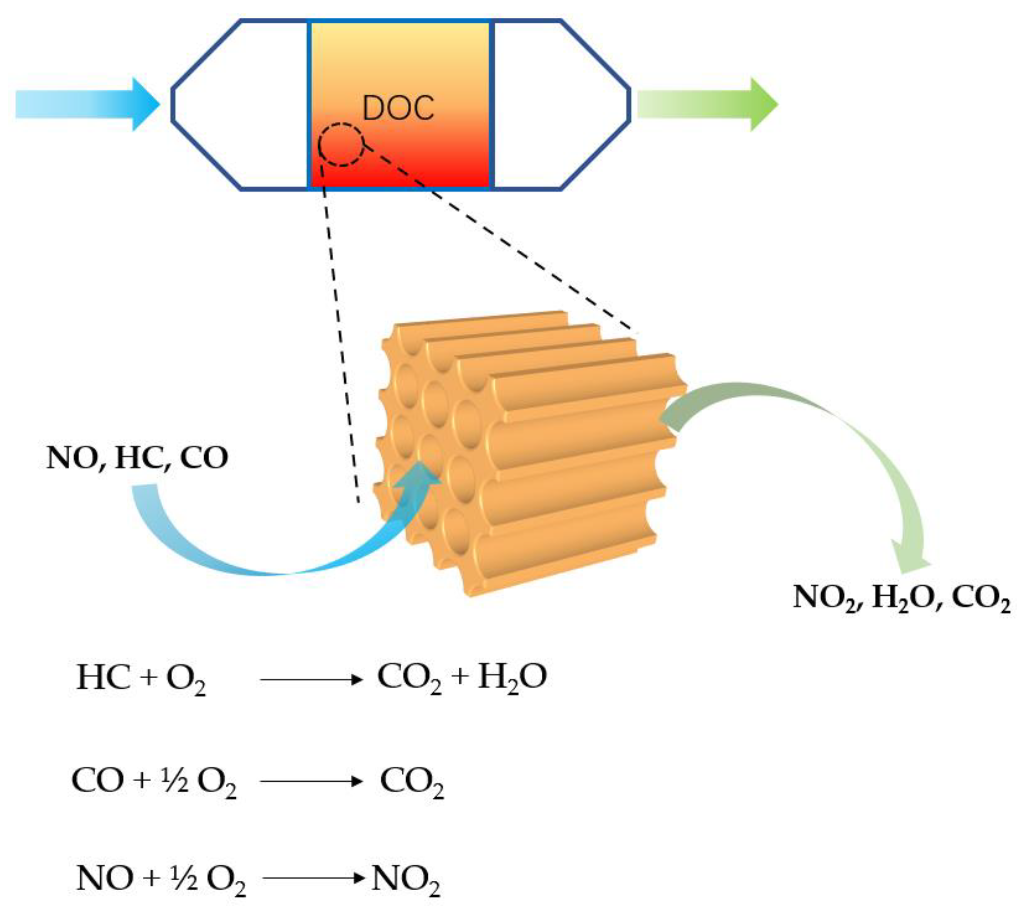
6.2.1. DOC Technology
6.2.2. Low Temperature Plasma Technology
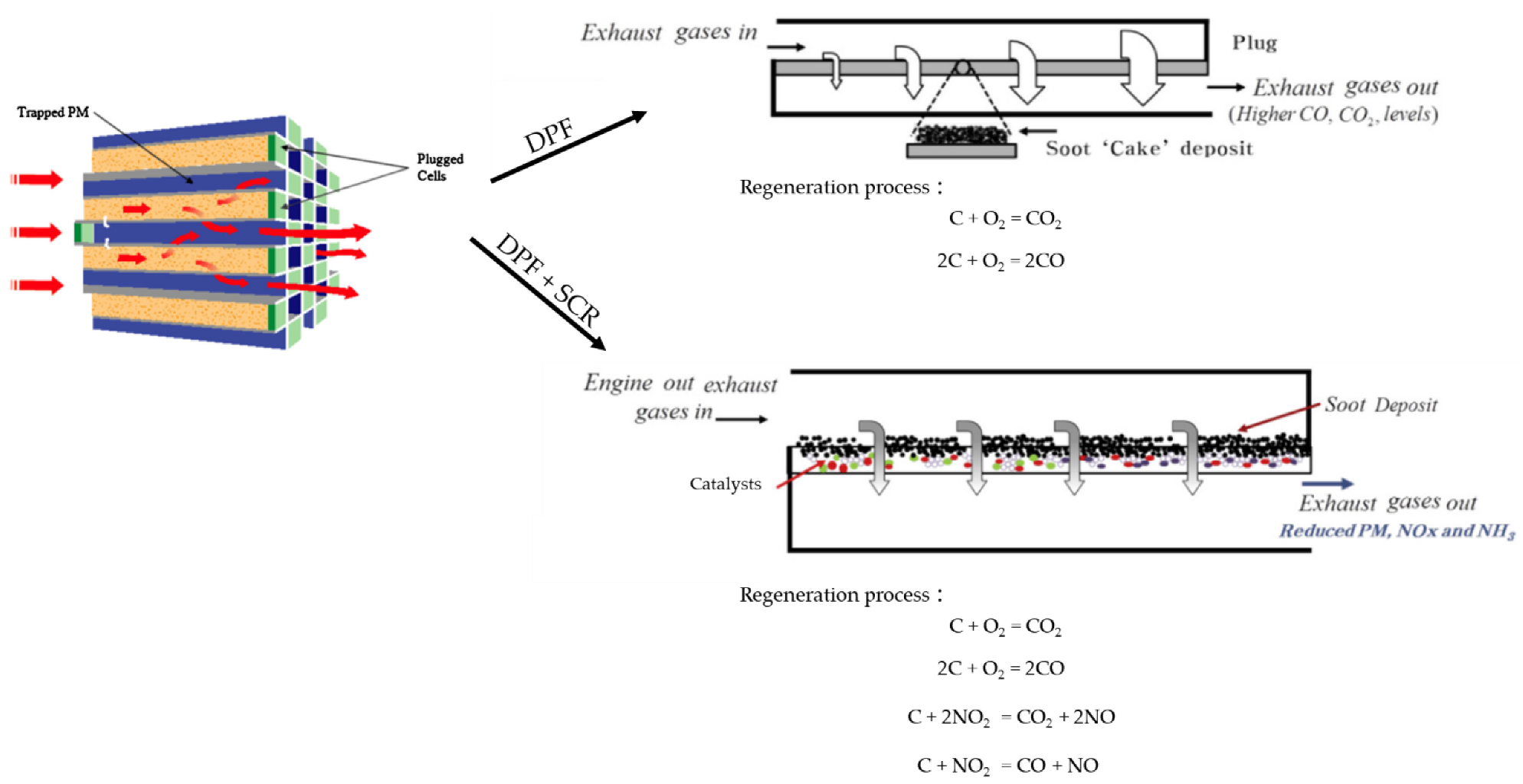
6.2.3. DPF Technology
7. Summary and Prospect
- Diesel alternative fuel based on waste oil was prepared by a series of purification processes of waste plastic oil or waste lubricating oil. The effect of alternative fuel prepared from waste oil on engine emission was studied through the actual test of engine. It is found that the use of waste plastic oil will reduce the content of PM, which could reduce the negative impact on SCR catalyst [36]; however, the waste plastic oil as an alternative fuel for diesel engines will increase the emission of NOX, which is due to the high heat release rate and high combustion temperature. In contrast, the alternative fuel prepared from waste lubricating oil will increase the emissions of PM and HC, and it was mainly due to the incomplete combustion of the fuel, which cause by the high viscosity of the waste lubricating oil; however, the incomplete combustion of fuel could reduce the combustion temperature, which is best for the decrease of NOX emission. Controlling the viscosity of alternative fuels within a reasonable range can effectively improve the utilization of alternative fuels, which will be a hot issue in recent years.
- Biodiesel is a diesel alternative fuel prepared by transesterification, pyrolysis and microemulsion of vegetable oil, animal fat and waste edible oil. The sulfur content in biodiesel is very low, which can effectively reduce the concentration of SO2 in exhaust gas. Biodiesel is rich in oxygen-containing functional groups, which can effectively improve fuel utilization and reduce PM and HC emissions; but the biodiesel will increase the emissions of NOX, which due to the high combustion temperature. The biodiesel can effectively reduce the adverse effect of SO2 on SCR catalyst and the blockage of SCR catalyst by PM and HC. Biodiesel is a potential alternative fuel to diesel.
- Alcohol fuel is usually mixed with biodiesel and commercial diesel to prepare engine alternative fuel. It is found that higher alcohols have good blending ability and cetane number, so it is an excellent fuel improver. The addition of higher alcohol will effectively reduce the NOX emission of the engine and reduce the working load of SCR catalyst; however, with the increase of alcohol concentration, HC emission will also increase. Therefore, in the future research, we should systematically study the content of alcohols to understand the impact of different alcohols on engine emission law at different concentrations.
- Natural gas is an efficient and clean energy with wide distribution and rich reserves. Since it does not contain sulfur elements, it can effectively reduce SO2 emissions. Moreover, the use of natural gas can also effectively reduce the content of PM in exhaust gas. In addition, the lean combustion of natural gas engines will also reduce the content of NOX in emissions.
- The use of technical means to improve fuel characteristics or to treat exhaust gas can also effectively solve the pollution problem of emissions. We can achieve the effect of reducing emissions by improving the physical properties of the fuel, such as reducing the viscosity, or improving the chemical properties, such as adding excess hydrogen. The use of DOC, DPF, SCR and other technologies to treat the exhaust gas can also greatly reduce the pollutant content in the discharge.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dhal, G.C.; Dey, S.; Mohan, D.; Prasad, R. Simultaneous abatement of diesel soot and NOx emissions by effective catalysts at low temperature: An overview. Catal. Rev. 2018, 60, 437–496. [Google Scholar] [CrossRef]
- Qi, D.; Yang, K.; Zhang, D.; Chen, B. Combustion and emission characteristics of diesel-tung oil-ethanol blended fuels used in a CRDI diesel engine with different injection strategies. Appl. Therm. Eng. 2017, 111, 927–935. [Google Scholar] [CrossRef]
- Vignesh, R.; Ashok, B. Critical interpretative review on current outlook and prospects of selective catalytic reduction system for De-NOx strategy in compression ignition engine. Fuel 2020, 276, 117996. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engines; McGraw-Hill: New York, NY, USA, 1984. [Google Scholar]
- Richter, H.; Howard, J. Formation of polycyclic aromatic hydrocarbons and their growth to soot—A review of chemical reaction pathways. Prog. Energy Combust. Sci. 2000, 26, 565–608. [Google Scholar] [CrossRef]
- Jung, Y.; Pyo, Y.D.; Jang, J.; Kim, G.C.; Cho, C.P.; Yang, C. NO, NO2 and N2O emissions over a SCR using DOC and DPF systems with Pt reduction. Chem. Eng. J. 2019, 369, 1059–1067. [Google Scholar] [CrossRef]
- Tavakoli, M.T.; Amdahl, J.; Leira, B.J. Experimental investigation of oil leakage from damaged ships due to collision and grounding. Ocean Eng. 2011, 38, 1894–1907. [Google Scholar] [CrossRef]
- Jing, D.; Dai, L.; Hu, H.; Ding, W.; Wang, Y.; Zhou, X. CO2 emission projection for Arctic shipping: A system dynamics approach. Ocean Coast. Manag. 2021, 205, 105531. [Google Scholar] [CrossRef]
- Johnson, T.V. Review of diesel emissions and control. Int. J. Engine Res. 2009, 10, 275–285. [Google Scholar] [CrossRef]
- Sun, C.; Liu, H.; Chen, W.; Chen, D.; Yu, S.; Liu, A.; Dong, L.; Feng, S. Insights into the Sm/Zr co-doping effects on N2 selectivity and SO2 resistance of a MnOx-TiO2 catalyst for the NH3-SCR reaction. Chem. Eng. J. 2018, 347, 27–40. [Google Scholar] [CrossRef]
- Wu, S.; Yao, X.; Zhang, L.; Cao, Y.; Zou, W.; Li, L.; Ma, K.; Tang, C.; Gao, F.; Dong, L. Improved low temperature NH3-SCR performance of FeMnTiOx mixed oxide with CTAB-assisted synthesis. Chem. Commun. 2015, 51, 3470–3473. [Google Scholar] [CrossRef]
- Sun, P.; Guo, R.-T.; Liu, S.-M.; Wang, S.-X.; Pan, W.-G.; Li, M.-Y.; Liu, S.-W.; Liu, J.; Sun, X. Enhancement of the low-temperature activity of Ce/TiO2 catalyst by Sm modification for selective catalytic reduction of NOx with NH3. Mol. Catal. 2017, 433, 224–234. [Google Scholar] [CrossRef]
- Lu, X.; Geng, P.; Chen, Y. NOx Emission Reduction Technology for Marine Engine Based on Tier-III: A Review. J. Therm. Sci. 2020, 29, 1242–1268. [Google Scholar] [CrossRef]
- Geng, P.; Mao, H.; Zhang, Y.; Wei, L.; You, K.; Ju, J.; Chen, T. Combustion characteristics and NOx emissions of a waste cooking oil biodiesel blend in a marine auxiliary diesel engine. Appl. Therm. Eng. 2017, 115, 947–954. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, H.; Jiang, D.; Zeng, K.; Liu, B.; Zhang, J.; Wang, X. Combustion behaviors of a compression-ignition engine fuelled with diesel/methanol blends under various fuel delivery advance angles. Bioresour. Technol. 2004, 95, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Ahouissoussi, N.B.; Wetzstein, M.E. A comparative cost analysis of biodiesel, compressed natural gas, methanol, and diesel for transit bus systems. Resour. Energy Econ. 1998, 20, 1–15. [Google Scholar] [CrossRef]
- Kumar, M.S.; Ramesh, A.; Nagalingam, B. An experimental comparison of methods to use methanol and Jatropha oil in a compression ignition engine. Biomass Bioenergy 2003, 25, 309–318. [Google Scholar] [CrossRef]
- Issa, M.; Ibrahim, H.; Ilinca, A.; Hayyani, M.Y. A Review and Economic Analysis of Different Emission Reduction Techniques for Marine Diesel Engines. Open J. Mar. Sci. 2019, 9, 148–171. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Wang, X.; Wei, Z.; Wang, L.; Wang, C.; Chen, Z. A review of NOx and SOx emission reduction technologies for marine diesel engines and the potential evaluation of liquefied natural gas fuelled vessels. Sci. Total Environ. 2020, 766, 144319. [Google Scholar] [CrossRef]
- Elgohary, M.M.; Seddiek, I.S.; Salem, A. Overview of alternative fuels with emphasis on the potential of liquefied natural gas as future marine fuel. Proc. Inst. Mech. Eng. Part M J. Eng. Marit. Environ. 2014, 229, 365–375. [Google Scholar] [CrossRef]
- Yilmaz, N.; Sanchez, T.M. Analysis of operating a diesel engine on biodiesel-ethanol and biodiesel-methanol blends. Energy 2012, 46, 126–129. [Google Scholar] [CrossRef]
- Babu, D.; Anand, R. Effect of biodiesel-diesel-n-pentanol and biodiesel-diesel-n-hexanol blends on diesel engine emission and combustion characteristics. Energy 2017, 133, 761–776. [Google Scholar] [CrossRef]
- Noor, C.W.M.; Noor, M.M.; Mamat, R. Biodiesel as alternative fuel for marine diesel engine applications: A review. Renew. Sustain. Energy Rev. 2018, 94, 127–142. [Google Scholar] [CrossRef]
- McGill, R.; Remley, W.; Winther, K. Alternative Fuels for Marine Applications. A Report from the IEA Advanced Motor Fuels Implementing Agreement. 2013, Volume 54. Available online: https://www.methanol.org/wp-content/uploads/2016/07/AMF_Annex_41-Alt-Fuels-for-Marine-May-2013.pdf (accessed on 11 January 2022).
- Saiyasitpanich, P.; Lu, M.; Keener, T.C.; Liang, F.; Khang, S.-J. The Effect of Diesel Fuel Sulfur Content on Particulate Matter Emissions for a Nonroad Diesel Generator. J. Air Waste Manag. Assoc. 2005, 55, 993–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbett, J.J.; Fischbeck, P. Emissions from ships. Science 1997, 278, 823–824. [Google Scholar] [CrossRef]
- Aramkitphotha, S.; Tanatavikorn, H.; Yenyuak, C.; Vitidsant, T. Low sulfur fuel oil from blends of microalgae pyrolysis oil and used lubricating oil: Properties and economic evaluation. Sustain. Energy Technol. Assess. 2018, 31, 339–346. [Google Scholar] [CrossRef]
- Ampah, J.D.; Yusuf, A.A.; Afrane, S.; Jin, C.; Liu, H. Reviewing two decades of cleaner alternative marine fuels: Towards IMO’s decarbonization of the maritime transport sector. J. Clean. Prod. 2021, 320, 128871. [Google Scholar] [CrossRef]
- Radwan, A.; Morsy, M.; Elbadan, A. Economical and Environmental Advantages of using NG as a Fuel in Inland Water Transport. In Proceedings of the 25th CIMAC conference, Vienna, Austria, 21–24 May 2007. [Google Scholar]
- Maghanaki, M.M.; Ghobadian, B.; Najafi, G.; Galogah, R.J. Potential of biogas production in Iran. Renew. Sustain. Energy Rev. 2013, 28, 702–714. [Google Scholar] [CrossRef]
- Gabiña, G.; Martin, L.; Basurko, O.C.; Clemente, M.; Aldekoa, S.; Uriondo, Z. Performance of marine diesel engine in propulsion mode with a waste oil-based alternative fuel. Fuel 2019, 235, 259–268. [Google Scholar] [CrossRef]
- Sushma, P. Waste Plastic Oil as an Alternative Fuel for Diesel Engine—A Review. In Proceedings of the 2nd International Conference on Advancements in Aeromechanical Materials for Manufacturing, Telangana, India, 13–14 July 2018; IOP Publishing: Bristol, UK, 2018; p. 012066. [Google Scholar]
- Singhabhandhu, A.; Tezuka, T. The waste-to-energy framework for integrated multi-waste utilization: Waste cooking oil, waste lubricating oil, and waste plastics. Energy 2010, 35, 2544–2551. [Google Scholar] [CrossRef]
- Mishra, A.; Siddiqi, H.; Kumari, U.; Behera, I.D.; Mukherjee, S.; Meikap, B. Pyrolysis of waste lubricating oil/waste motor oil to generate high-grade fuel oil: A comprehensive review. Renew. Sustain. Energy Rev. 2021, 150, 111446. [Google Scholar] [CrossRef]
- Eze, W.U.; Umunakwe, R.; Obasi, H.C.; Ugbaja, M.I.; Uche, C.C.; Madufor, I.C. Plastics waste management: A review of pyrolysis technology. Clean Technol. Recycl. 2021, 1, 50–69. [Google Scholar] [CrossRef]
- Rinaldini, C.; Mattarelli, E.; Savioli, T.; Cantore, G.; Garbero, M.; Bologna, A. Performance, emission and combustion characteristics of a IDI engine running on waste plastic oil. Fuel 2016, 183, 292–303. [Google Scholar] [CrossRef]
- Devaraj, J.; Robinson, Y.; Ganapathi, P. Experimental investigation of performance, emission and combustion characteristics of waste plastic pyrolysis oil blended with diethyl ether used as fuel for diesel engine. Energy 2015, 85, 304–309. [Google Scholar] [CrossRef]
- Damodharan, D.; Sathiyagnanam, A.; Rana, D.; Kumar, B.R.; Saravanan, S. Combined influence of injection timing and EGR on combustion, performance and emissions of DI diesel engine fueled with neat waste plastic oil. Energy Convers. Manag. 2018, 161, 294–305. [Google Scholar] [CrossRef]
- Mani, M.; Subash, C.; Nagarajan, G. Performance, emission and combustion characteristics of a DI diesel engine using waste plastic oil. Appl. Therm. Eng. 2009, 29, 2738–2744. [Google Scholar] [CrossRef]
- Qi, D.; Chen, H.; Geng, L.; Bian, Y. Effect of diethyl ether and ethanol additives on the combustion and emission characteristics of biodiesel-diesel blended fuel engine. Renew. Energy 2011, 36, 1252–1258. [Google Scholar] [CrossRef]
- Bhaskar, T.; Uddin, A.; Muto, A.; Sakata, Y.; Omura, Y.; Kimura, K.; Kawakami, Y. Recycling of waste lubricant oil into chemical feedstock or fuel oil over supported iron oxide catalysts. Fuel 2004, 83, 9–15. [Google Scholar] [CrossRef]
- Monier, V.; Labouze, E.; Sofres, T. Critical Review of Existing Studies and Life Cycle Analysis on the Regeneration and Incineration of Waste Oils; European Commission, DG Environment: Brussels, Belgium, 2001. [Google Scholar]
- Li, L.; Wang, J.; Wang, Z.; Xiao, J. Combustion and emission characteristics of diesel engine fueled with diesel/biodiesel/pentanol fuel blends. Fuel 2015, 156, 211–218. [Google Scholar] [CrossRef]
- Tajima, H.; Takasaki, K.; Nakashima, M.; Yanagi, J.; Takaishi, T.; Ishida, H.; Osafune, S.; Iwamoto, K. Combustion of Used Lubricating Oil in a Diesel Engine; SAE Transactions; SAE International: Warrendale, PA, USA, 2001; pp. 1159–1164. [Google Scholar]
- El-Mekkawi, S.A.; El-Ibiari, N.; Attia, N.; El-Diwani, G.I.; El-Ardy, O.A.; El Morsi, A. Reducing the environmental impact of used lubricating oil through the production of fuels by pyrolysis. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100308. [Google Scholar] [CrossRef]
- Rincón, J.; Cañizares, P.; García, M.T. Regeneration of used lubricant oil by ethane extraction. J. Supercrit. Fluids 2007, 39, 315–322. [Google Scholar] [CrossRef]
- Arpa, O.; Yumrutaş, R.; Argunhan, Z. Experimental investigation of the effects of diesel-like fuel obtained from waste lubrication oil on engine performance and exhaust emission. Fuel Process. Technol. 2010, 91, 1241–1249. [Google Scholar] [CrossRef]
- Arpa, O.; Yumrutaş, R.; Kaşka, Ö. Desulfurization of diesel-like fuel produced from waste lubrication oil and its utilization on engine performance and exhaust emission. Appl. Therm. Eng. 2013, 58, 374–381. [Google Scholar] [CrossRef]
- Wang, X.; Ni, P. Combustion and emission characteristics of diesel engine fueled with diesel-like fuel from waste lubrication oil. Energy Convers. Manag. 2017, 133, 275–283. [Google Scholar] [CrossRef]
- Aydın, H. Scrutinizing the combustion, performance and emissions of safflower biodiesel-kerosene fueled diesel engine used as power source for a generator. Energy Convers. Manag. 2016, 117, 400–409. [Google Scholar] [CrossRef]
- Breyer, S.; Mekhitarian, L.; Rimez, B.; Haut, B. Production of an alternative fuel by the co-pyrolysis of landfill recovered plastic wastes and used lubrication oils. Waste Manag. 2017, 60, 363–374. [Google Scholar] [CrossRef]
- Agarwal, A.K. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog. Energy Combust. Sci. 2007, 33, 233–271. [Google Scholar] [CrossRef]
- Wei, L.; Cheung, C.; Ning, Z. Influence of waste cooking oil biodiesel on combustion, unregulated gaseous emissions and particulate emissions of a direct-injection diesel engine. Energy 2017, 127, 175–185. [Google Scholar] [CrossRef]
- Wei, L.; Cheng, R.; Mao, H.; Geng, P.; Zhang, Y.; You, K. Combustion process and NOx emissions of a marine auxiliary diesel engine fuelled with waste cooking oil biodiesel blends. Energy 2018, 144, 73–80. [Google Scholar] [CrossRef]
- Yesilyurt, M.K. The effects of the fuel injection pressure on the performance and emission characteristics of a diesel engine fuelled with waste cooking oil biodiesel-diesel blends. Renew. Energy 2019, 132, 649–666. [Google Scholar] [CrossRef]
- Bora, A.P.; Gupta, D.P.; Durbha, K.S. Sewage sludge to bio-fuel: A review on the sustainable approach of transforming sewage waste to alternative fuel. Fuel 2020, 259, 116262. [Google Scholar] [CrossRef]
- Pokoo-Aikins, G.; Heath, A.; Mentzer, R.A.; Mannan, M.S.; Rogers, W.J.; El-Halwagi, M.M. A multi-criteria approach to screening alternatives for converting sewage sludge to biodiesel. J. Loss Prev. Process. Ind. 2010, 23, 412–420. [Google Scholar] [CrossRef]
- Abrar, I.; Bhaskarwar, A.N. Microemulsion fuels for compression ignition engines: A review on engine performance and emission characteristics. Fuel 2019, 257, 115944. [Google Scholar] [CrossRef]
- Murugesan, A.; Umarani, C.; Subramanian, R.; Nedunchezhian, N. Bio-diesel as an alternative fuel for diesel engines—A review. Renew. Sustain. Energy Rev. 2009, 13, 653–662. [Google Scholar] [CrossRef]
- Kumar, M.S.; Ramesh, A.; Nagalingam, B. Complete Vegetable Oil Fueled Dual Fuel Compression Ignition Engine; The Automotive Research Association of India; SAE International: Warrendale, PA, USA, 2001. [Google Scholar] [CrossRef]
- Balat, M. Potential alternatives to edible oils for biodiesel production—A review of current work. Energy Convers. Manag. 2011, 52, 1479–1492. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Mubashir, M.; Asif, S.; Waseem, A.; Mukhtar, A.; Saqib, S.; Munawaroh, H.S.H.; Lam, M.K.; Khoo, K.S.; et al. A practical approach for synthesis of biodiesel via non-edible seeds oils using trimetallic based montmorillonite nano-catalyst. Bioresour. Technol. 2021, 328, 124859. [Google Scholar] [CrossRef]
- Atmanli, A. Comparative analyses of diesel–waste oil biodiesel and propanol, n-butanol or 1-pentanol blends in a diesel engine. Fuel 2016, 176, 209–215. [Google Scholar] [CrossRef]
- Yilmaz, N.; Vigil, F.M. Potential use of a blend of diesel, biodiesel, alcohols and vegetable oil in compression ignition engines. Fuel 2014, 124, 168–172. [Google Scholar] [CrossRef]
- Shahir, S.; Masjuki, H.; Kalam, A.; Imran, A.; Ashraful, A. Performance and emission assessment of diesel–biodiesel–ethanol/bioethanol blend as a fuel in diesel engines: A review. Renew. Sustain. Energy Rev. 2015, 48, 62–78. [Google Scholar] [CrossRef]
- Atmanli, A. Effects of a cetane improver on fuel properties and engine characteristics of a diesel engine fueled with the blends of diesel, hazelnut oil and higher carbon alcohol. Fuel 2016, 172, 209–217. [Google Scholar] [CrossRef]
- Yesilyurt, M.K. A detailed investigation on the performance, combustion, and exhaust emission characteristics of a diesel engine running on the blend of diesel fuel, biodiesel and 1-heptanol (C7 alcohol) as a next-generation higher alcohol. Fuel 2020, 275, 117893. [Google Scholar] [CrossRef]
- Yilmaz, N.; Donaldson, A.B.; Johns, A. Some Perspectives on Alcohol Utilization in a Compression Ignition Engine. SAE Trans. 2005, 114, 1198–1203. [Google Scholar]
- Morsy, M.H. Assessment of a direct injection diesel engine fumigated with ethanol/water mixtures. Energy Convers. Manag. 2015, 94, 406–414. [Google Scholar] [CrossRef]
- Ali, S.S.; Swaminathan, M. Effective utilization of waste cooking oil in a diesel engine equipped with CRDi system using C8 oxygenates as additives for cleaner emission. Fuel 2020, 275, 118003. [Google Scholar]
- Ashok, B.; Nanthagopal, K.; Anand, V.; Aravind, K.; Jeevanantham, A.; Balusamy, S. Effects of n-octanol as a fuel blend with biodiesel on diesel engine characteristics. Fuel 2019, 235, 363–373. [Google Scholar] [CrossRef]
- Cheung, C.; Zhu, L.; Huang, Z. Regulated and unregulated emissions from a diesel engine fueled with biodiesel and biodiesel blended with methanol. Atmos. Environ. 2009, 43, 4865–4872. [Google Scholar] [CrossRef]
- Myhre, G.; Shindell, D.; Bréon, F.; Collins, W.; Fuglestvedt, J.; Huang, J. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group 1 to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Stenersen, D.; Thonstad, O. GHG and NOx Emissions from Gas Fuelled Engines; Postboks 4762 Sluppen NO-7465 Trondheim NORWAY; SINTEF Ocean AS; Mapping, Verification, Reduction Technologies. 13 June 2017. Available online: https://www.nho.no/siteassets/nox-fondet/rapporter/2018/methane-slip-from-gas-engines-mainreport-1492296.pdf (accessed on 12 January 2022).
- Sharafian, A.; Blomerus, P.; Mérida, W. Natural gas as a ship fuel: Assessment of greenhouse gas and air pollutant reduction potential. Energy Policy 2019, 131, 332–346. [Google Scholar] [CrossRef]
- Chen, H.; He, J.; Zhong, X. Engine combustion and emission fuelled with natural gas: A review. J. Energy Inst. 2019, 92, 1123–1136. [Google Scholar] [CrossRef]
- Milojević, S.; Pesic, R. Theoretical and experimental analysis of a CNG cylinder rack connection to a bus roof. Int. J. Automot. Technol. 2012, 13, 497–503. [Google Scholar] [CrossRef]
- Jahirul, M.; Masjuki, H.; Saidur, R.; Kalam, A.; Jayed, M.; Wazed, E.D.M.A. Comparative engine performance and emission analysis of CNG and gasoline in a retrofitted car engine. Appl. Therm. Eng. 2010, 30, 2219–2226. [Google Scholar] [CrossRef]
- Aslam, M.; Masjuki, H.; Kalam, M.; Abdesselam, H.; Mahlia, T.; Amalina, M. An experimental investigation of CNG as an alternative fuel for a retrofitted gasoline vehicle. Fuel 2006, 85, 717–724. [Google Scholar] [CrossRef]
- Kunwar, B.; Moser, B.; Chandrasekaran, S.R.; Rajagopalan, N.; Sharma, B.K. Catalytic and thermal depolymerization of low value post-consumer high density polyethylene plastic. Energy 2016, 111, 884–892. [Google Scholar] [CrossRef]
- Shah, J.; Jan, M.R.; Mabood, F.; Jabeen, F. Catalytic pyrolysis of LDPE leads to valuable resource recovery and reduction of waste problems. Energy Convers. Manag. 2010, 51, 2791–2801. [Google Scholar] [CrossRef]
- Bow, Y.; Rusdianasari, R.; Pujiastuti, L.S. Pyrolysis of Polypropylene Plastic Waste into Liquid Fuel. In Proceedings of the 6th International Conference on Sustainable Agriculture, Food and Energy, Manila, Philippines, 18–21 October 2018; IOP Publishing: Bristol, UK, 2019; p. 012128. [Google Scholar]
- Honus, S.; Kumagai, S.; Fedorko, G.; Molnár, V.; Yoshioka, T. Pyrolysis gases produced from individual and mixed PE, PP, PS, PVC, and PET—Part I: Production and physical properties. Fuel 2018, 221, 346–360. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Nhuchhen, D.R.; Sit, S.P.; Layzell, D.B. Alternative fuels co-fired with natural gas in the pre-calciner of a cement plant: Energy and material flows. Fuel 2021, 295, 120544. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. An overview of biodiesel oxidation stability. Renew. Sustain. Energy Rev. 2012, 16, 5924–5950. [Google Scholar] [CrossRef]
- Tomić, M.; Durisic-Mladenovic, N.; Mićić, R.; Simikić, M.; Savin, L. Effects of accelerated oxidation on the selected fuel properties and composition of biodiesel. Fuel 2019, 235, 269–276. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.; Lee, P.; Chua, K.J.; Chou, S. Pine oil–biodiesel blends: A double biofuel strategy to completely eliminate the use of diesel in a diesel engine. Appl. Energy 2014, 130, 466–473. [Google Scholar] [CrossRef]
- Duncan, A.M.; Pavlicek, N.; Depcik, C.D.; Scurto, A.M.; Stagg-Williams, S.M. High-Pressure Viscosity of Soybean-Oil-Based Biodiesel Blends with Ultra-Low-Sulfur Diesel Fuel. Energy Fuels 2012, 26, 7023–7036. [Google Scholar] [CrossRef]
- Mahmudul, H.; Hagos, F.; Mamat, R.; Adam, A.A.; Ishak, W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Tamilselvan, P.; Nallusamy, N.; Rajkumar, S. A comprehensive review on performance, combustion and emission characteristics of biodiesel fuelled diesel engines. Renew. Sustain. Energy Rev. 2017, 79, 1134–1159. [Google Scholar] [CrossRef]
- Karabektas, M.; Ergen, G.; Hosoz, M. The effects of preheated cottonseed oil methyl ester on the performance and exhaust emissions of a diesel engine. Appl. Therm. Eng. 2008, 28, 2136–2143. [Google Scholar] [CrossRef]
- Devan, P.; Mahalakshmi, N. A study of the performance, emission and combustion characteristics of a compression ignition engine using methyl ester of paradise oil—Eucalyptus oil blends. Appl. Energy 2009, 86, 675–680. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Z.; Zeng, K.; Chen, H.; Wang, X.; Miao, H.; Jiang, D. Experimental Study on Emissions of a Spark-Ignition Engine Fueled with Natural Gas−Hydrogen Blends. Energy Fuels 2008, 22, 273–277. [Google Scholar] [CrossRef]
- Hoang, A.T. The Performance of Diesel Engine Fueled Diesel Oil in Comparison with Heated Pure Vegetable Oils Available in Vietnam. J. Sustain. Dev. 2017, 10, 93–101. [Google Scholar] [CrossRef]
- Bilgin, A.; Gulum, M. Effects of various transesterification parameters on the some fuel properties of hazelnut oil methyl ester. Energy Procedia 2018, 147, 54–62. [Google Scholar] [CrossRef]
- Schulman, J.H.; Stoeckenius, W.; Prince, L.M. Mechanism of Formation and Structure of Micro Emulsions by Electron Microscopy. J. Phys. Chem. 1959, 63, 1677–1680. [Google Scholar] [CrossRef]
- Goering, C.E.; Schwab, A.W.; Campion, R.M.; Pryde, E.H. Soyoil-Ethanol Microemulsions as Diesel Fuel. Trans. ASAE 1983, 26, 1602–1604. [Google Scholar] [CrossRef]
- Kayali, I.; Karaeen, M.; Ahmed, W.; Qamhieh, K.; Olsson, U. Alternative Diesel Fuel: Microemulsion Phase Behavior and Combustion Properties. J. Dispers. Sci. Technol. 2016, 37, 894–899. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Use of vegetable oils as I.C. engine fuels—A review. Renew. Energy 2004, 29, 727–742. [Google Scholar] [CrossRef]
- Veses, A.; Aznar, M.; López, J.; Callén, M.; Murillo, R.; García, T. Production of upgraded bio-oils by biomass catalytic pyrolysis in an auger reactor using low cost materials. Fuel 2015, 141, 17–22. [Google Scholar] [CrossRef]
- Kar, Y. Catalytic cracking of pyrolytic oil by using bentonite clay for green liquid hydrocarbon fuels production. Biomass-Bioenergy 2018, 119, 473–479. [Google Scholar] [CrossRef]
- Mani, M.; Nagarajan, G. Influence of injection timing on performance, emission and combustion characteristics of a DI diesel engine running on waste plastic oil. Energy 2009, 34, 1617–1623. [Google Scholar] [CrossRef]
- Tsolakis, A. Effects on Particle Size Distribution from the Diesel Engine Operating on RME-Biodiesel with EGR. Energy Fuels 2006, 20, 1418–1424. [Google Scholar] [CrossRef]
- Gabiña, G.; Martin, L.; Basurko, O.C.; Clemente, M.; Aldekoa, S.; Uriondo, Z. Waste oil-based alternative fuels for marine diesel engines. Fuel Process. Technol. 2016, 153, 28–36. [Google Scholar] [CrossRef]
- Pham, V.C.; Rho, B.-S.; Kim, J.-S.; Lee, W.-J.; Choi, J.-H. Effects of Various Fuels on Combustion and Emission Characteristics of a Four-Stroke Dual-Fuel Marine Engine. J. Mar. Sci. Eng. 2021, 9, 1072. [Google Scholar] [CrossRef]
- Li, M.; Wu, H.; Zhang, T.; Shen, B.; Zhang, Q.; Li, Z. A comprehensive review of pilot ignited high pressure direct injection natural gas engines: Factors affecting combustion, emissions and performance. Renew. Sustain. Energy Rev. 2020, 119, 109653. [Google Scholar] [CrossRef]
- Reşitoğlu, I.; Altinisik, K.; Keskin, A.; Yildirimcan, S.; Ocakoglu, K.; Omar, M.A. Development of Fe2O3 based catalysts to control pollutant emissions in diesel engines. Fuel 2017, 208, 111–116. [Google Scholar] [CrossRef]
- Hu, J.; Liao, J.; Hu, Y.; Lei, J.; Zhang, M.; Zhong, J.; Yan, F.; Cai, Z. Experimental investigation on emission characteristics of non-road diesel engine equipped with integrated DOC + CDPF + SCR aftertreatment. Fuel 2021, 305, 121586. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, W.; Guo, Y.; Li, J.; Wei, F. Environmental impact, treatment technology and monitoring system of ship domestic sewage: A review. Sci. Total Environ. 2021, 811, 151410. [Google Scholar] [CrossRef]
- Reddy, V.; Sarah, A.; Rajanikanth, B. Exploring Synergy Effect of Plasma with Lignite Ash on NOx Abatement in a Biofuel Exhaust. Mater. Today Proc. 2018, 5, 23268–23274. [Google Scholar] [CrossRef]
- Jiang, B.; Zhao, S.; Wang, Y.; Wenren, Y.; Zhu, Z.; Harding, J.; Zhang, X.; Tu, X.; Zhang, X. Plasma-enhanced low temperature NH3-SCR of NOx over a Cu-Mn/SAPO-34 catalyst under oxygen-rich conditions. Appl. Catal. B Environ. 2021, 286, 119886. [Google Scholar] [CrossRef]
- Ou, J.; Meng, Z.; Hu, Y.; Du, Y. Experimental investigation on the variation characteristics of soot layer thickness and pressure drop during DPF/CDPF active regeneration. Chem. Eng. Sci. 2021, 241, 116682. [Google Scholar] [CrossRef]
- Hu, S.; Deng, B.; Wu, D.; Hou, K. Energy flow behavior and emission reduction of a turbo-charging and EGR non-road diesel engine equipped with DOC and DPF under NRTC (non-road transient cycle). Fuel 2021, 305, 121571. [Google Scholar] [CrossRef]
- Guo, M.; Fu, Z.; Ma, D.; Ji, N.; Song, C.; Liu, Q. A Short Review of Treatment Methods of Marine Diesel Engine Exhaust Gases. Procedia Eng. 2015, 121, 938–943. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, Y.; Utaki, S.; Zhang, W.; Fukuma, T.; Kusaka, J. Effects of Soot Deposition on NOx Purification Reaction and Mass Transfer in a SCR/DPF Catalyst; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2018. [Google Scholar]
- Johansen, K. Multi-catalytic soot filtration in automotive and marine applications. Catal. Today 2015, 258, 2–10. [Google Scholar] [CrossRef]
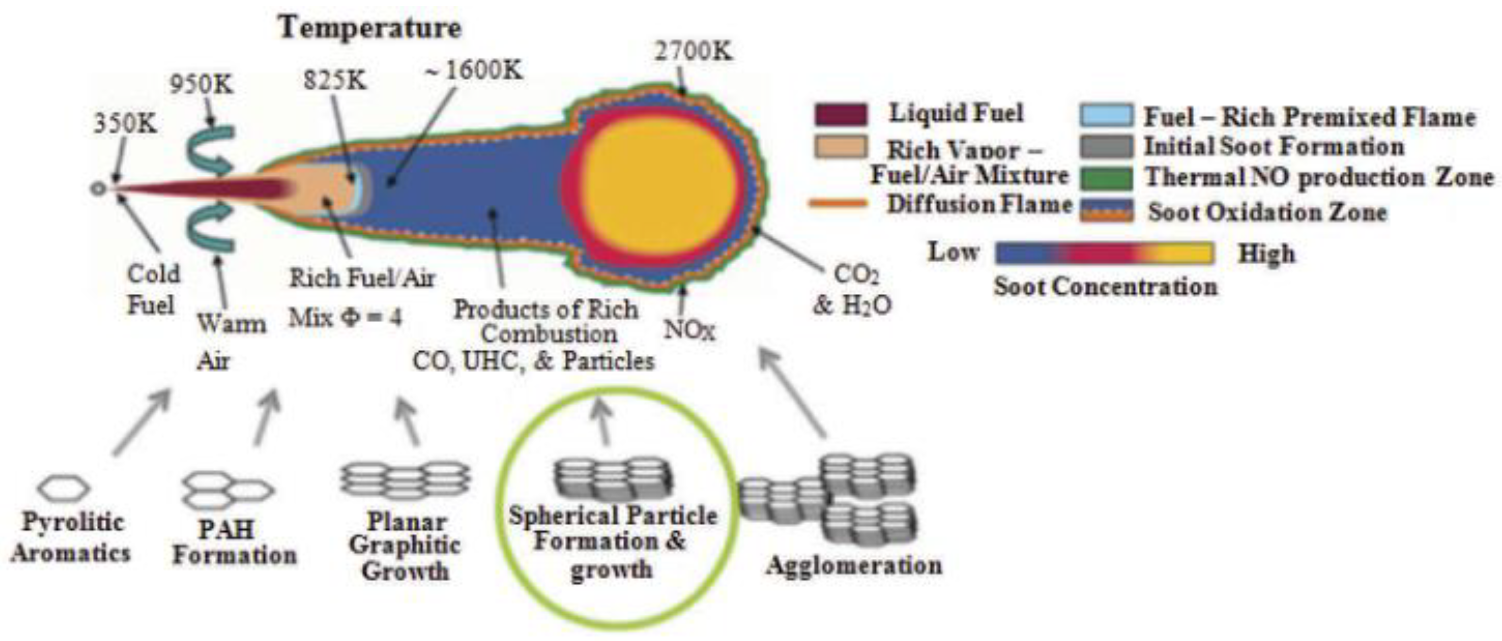
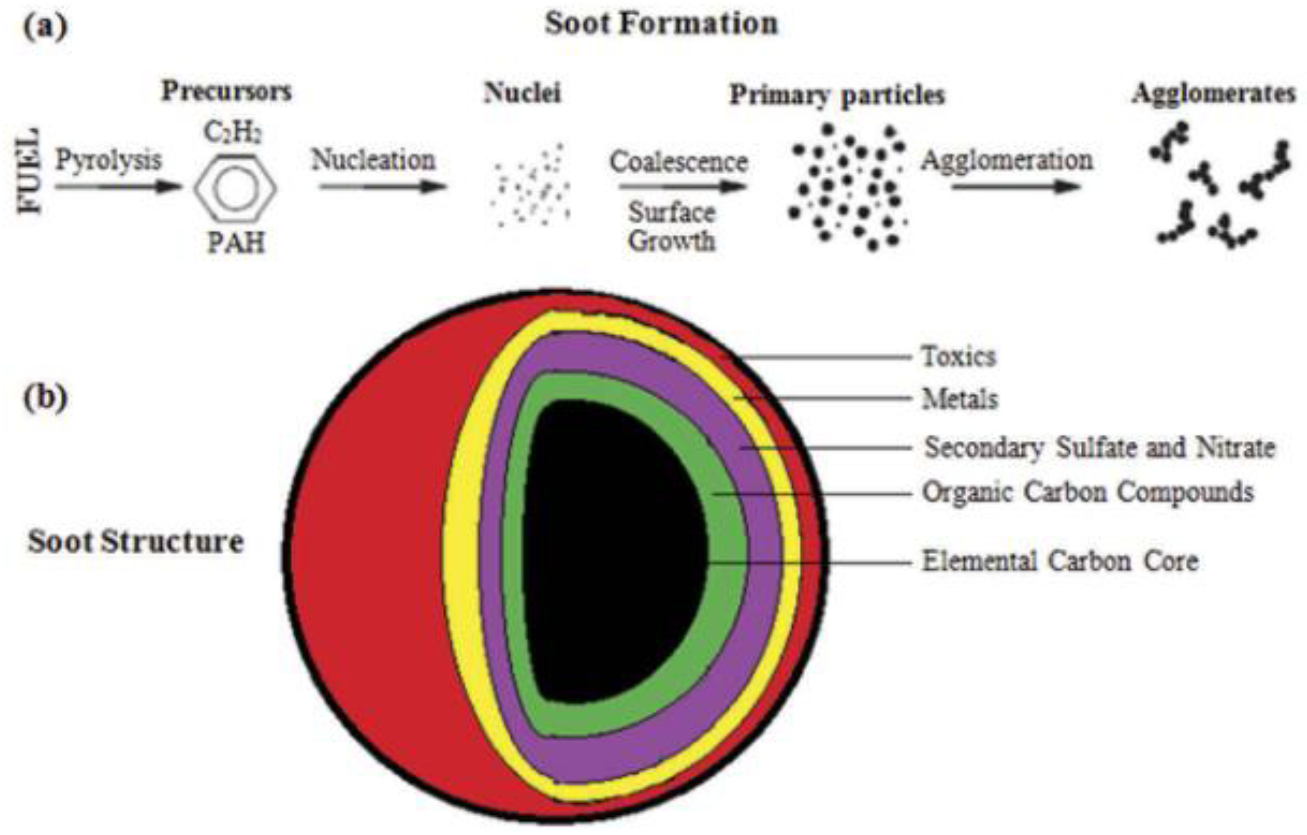
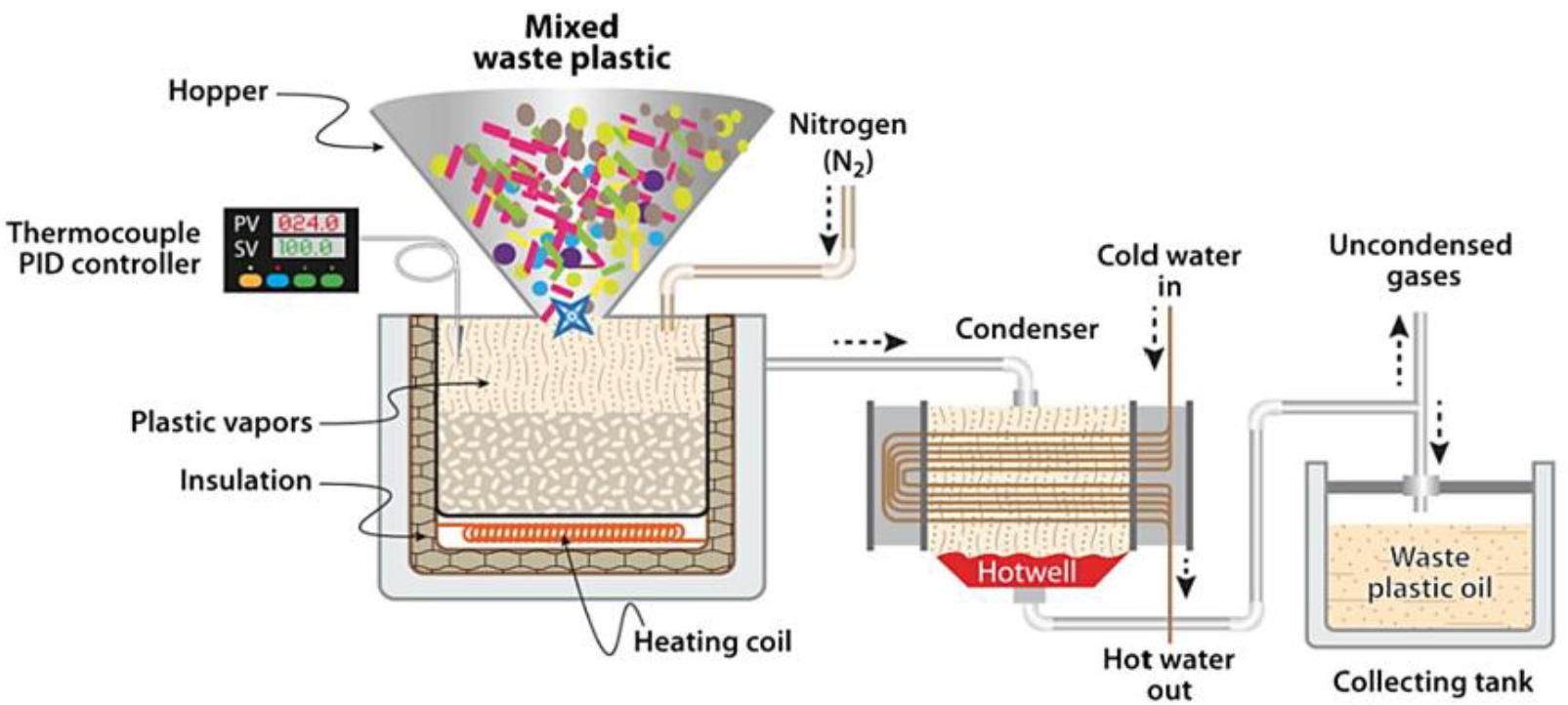
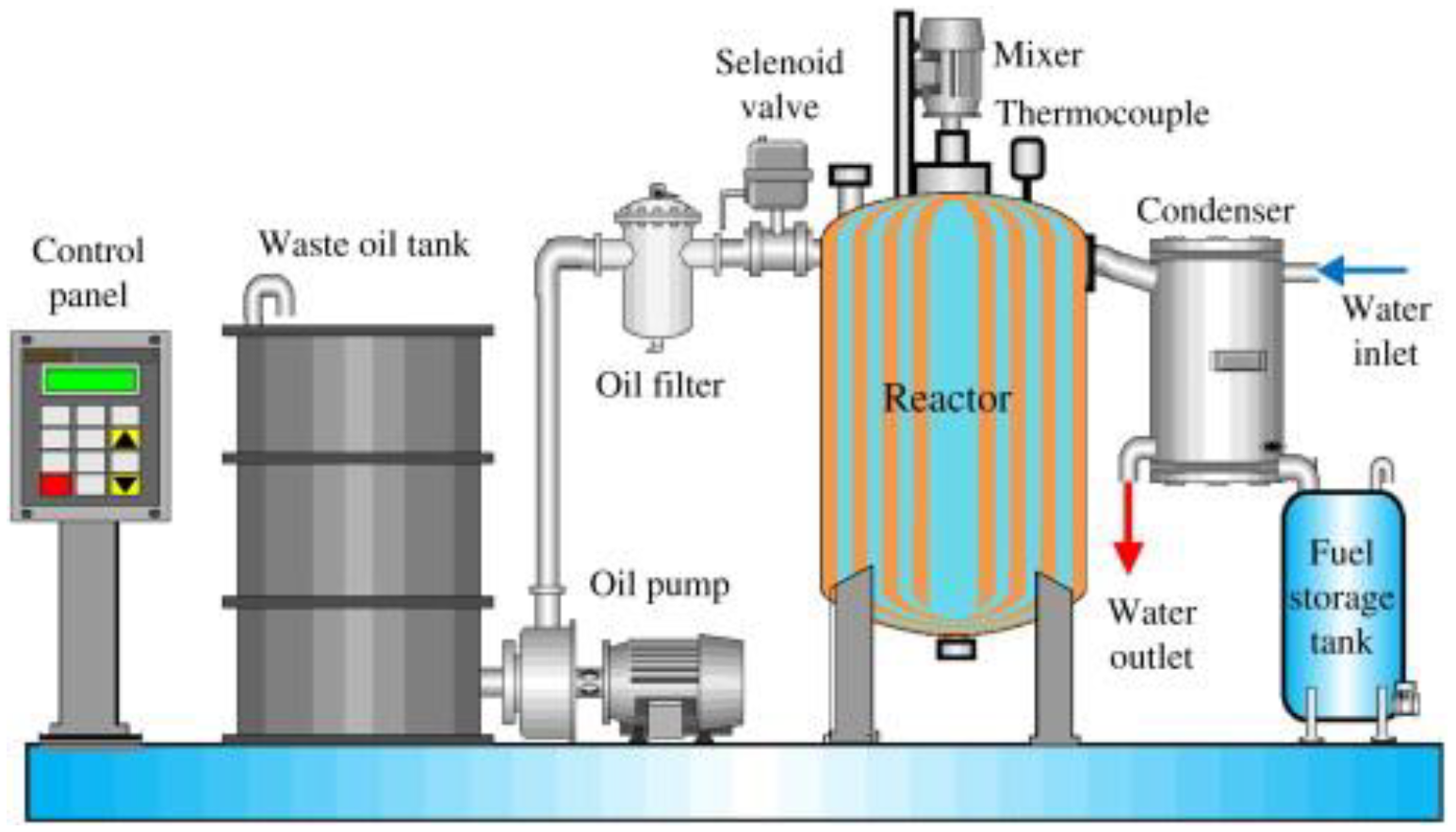
| Criteria | HFO | LSFO | LNG | Biodiesel | Methnol |
|---|---|---|---|---|---|
| Energy density | A | A | B | A | B |
| Technological maturity | B | B | B | A | C |
| Local emissions | D | D | B | D | B |
| GHG emissions | E | E | C | B | C |
| Energy cost | A | B | A | D | C |
| Capital cost | A | A | B | A | B |
| Converter storage | B | A | C | A | B |
| Bunkering availability | A | A | B | D | C |
| Commercial readiness | A | A | A | C | B |
| Flammability | A | A | A | A | C |
| Toxicity | A | A | A | A | C |
| Regulations and guidelines | A | A | A | A | B |
| Global production capacity and locations | A | A | A | D | B |
| Renewability | D | D | D | C | B |
| Safety | A | A | A | A | B |
| Fuel | Density (kg/m3) | Heating Value (MJ/kg) | Sulfur (wt%) | Viscosity (40 °C) (mm2/s) | Cetane Number | Heat of Evaporation (kJ/kg) | Flash Point (°C) | Carbon Content (wt%) | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HFO | 990 | 40.8 | 1.3 | <700 (50 °C) | >20 | - | >60 | - | - | - | [23,44] | |
| ULSD | 840 | 42.5–44.8 | <10 mg/kg | 2.4 | 45–55 | 250–290 | 50–82 | 86.6 | - | - | [14,17] | |
| Biodiesel | WCO | 873.8 | 37.5 | <10 mg/kg | 4.395 | 55.3 | 300 | 182.5 | 77.1 | 1. Could direct substitute for conventional fuels 2. Carbon neutral | 1. High cost 2. High NOx and soot emissions 3. Limited production capacity | [14] |
| Jatropha oil | 918.6 | 39.774 | - | 49.93 | 40–45 | - | 240 | - | [17,28] | |||
| WFO | 916.7 | 38.97 | 0.33 | 42.53 | 56 | - | 327 | 75.03 | [22] | |||
| HVO | 770–790 | - | - | - | >70 | - | - | - | [28] | |||
| Alcohol | Methanol | 790 | 19.674–19.8 | - | 0.59 | 3–5 | 1110 | 11 | 37.5 | 1. Liquid fuel that enables use of upgraded existing 2. Renewable sources | 1. High cost 2. Absence of bunkering infrastructure 3. High greenhouse gas emissions | [15,17,21] |
| Ethanol | 790 | 28.6 | - | 1.1 | 6 | - | 13 | - | [21] | |||
| Butanol | 808 | 33.1 | - | 2.63 | 25 | - | 35 | - | [28,64] | |||
| Waste lubricating oil | 895–986 | 41.8–43.52 | 0.2 | 3.49 | 56.8 | 360 | 71–244 | 84.76 ± 0.75 | 1. High calorific value 2. Beneficial to lubricating fuel injectors 3. Low cost | 1. Contains metallic impurities 2. High greenhouse gas emissions | [27,31,34,44,47,64] | |
| Waste plastic oil | HPDE | 800–920 | 45.4 | - | 2.420–2.52 | - | - | 40–48 | 85.3 | 1. Environmental protection 2. Sufficient raw materials | 1. Increase the emission of NOx 2. High greenhouse gas emissions 3. Lack of policies and infrastructure | [80] |
| LDPE | 768–802 | 39.1 | - | 1.650–1.801 | - | - | 50 | 85.3 | [81] | |||
| PP | 767–800 | 40 | - | 2.72 | - | - | 31–36 | 85.61 | [82] | |||
| PET | 870–900 | 28.2 | - | - | - | - | - | 92.32 | [28,83] | |||
| PS | 850–860 | 43 | - | 1.4 (50 °C) | - | - | 28 | 62.10 | [84] | |||
| Natural gas | 0.78 | 47.57 | 0 | - | 130 | - | - | 74.15 | 1. Mature technology 2. Eliminates SOx pollution 3. Low cost | 1. High greenhouse gas emissions 2. Mast be stored in insulated tanks | [28,85] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Xu, S.; Yuan, P.; Xing, Y.; Shen, B.; Li, Z.; Zhang, C.; Wang, X.; Wang, Z.; Ma, J.; et al. The Impact of Alternative Fuels on Ship Engine Emissions and Aftertreatment Systems: A Review. Catalysts 2022, 12, 138. https://doi.org/10.3390/catal12020138
Feng S, Xu S, Yuan P, Xing Y, Shen B, Li Z, Zhang C, Wang X, Wang Z, Ma J, et al. The Impact of Alternative Fuels on Ship Engine Emissions and Aftertreatment Systems: A Review. Catalysts. 2022; 12(2):138. https://doi.org/10.3390/catal12020138
Chicago/Turabian StyleFeng, Shuo, Shirui Xu, Peng Yuan, Yuye Xing, Boxiong Shen, Zhaoming Li, Chenguang Zhang, Xiaoqi Wang, Zhuozhi Wang, Jiao Ma, and et al. 2022. "The Impact of Alternative Fuels on Ship Engine Emissions and Aftertreatment Systems: A Review" Catalysts 12, no. 2: 138. https://doi.org/10.3390/catal12020138
APA StyleFeng, S., Xu, S., Yuan, P., Xing, Y., Shen, B., Li, Z., Zhang, C., Wang, X., Wang, Z., Ma, J., & Kong, W. (2022). The Impact of Alternative Fuels on Ship Engine Emissions and Aftertreatment Systems: A Review. Catalysts, 12(2), 138. https://doi.org/10.3390/catal12020138







