Synthesis, Crystal Structure, and Electrochemical Properties of an Isopolyoxovanadate Compound Modified Transition-Metal Complex Based on [V4O12]4−
Abstract
:1. Introduction
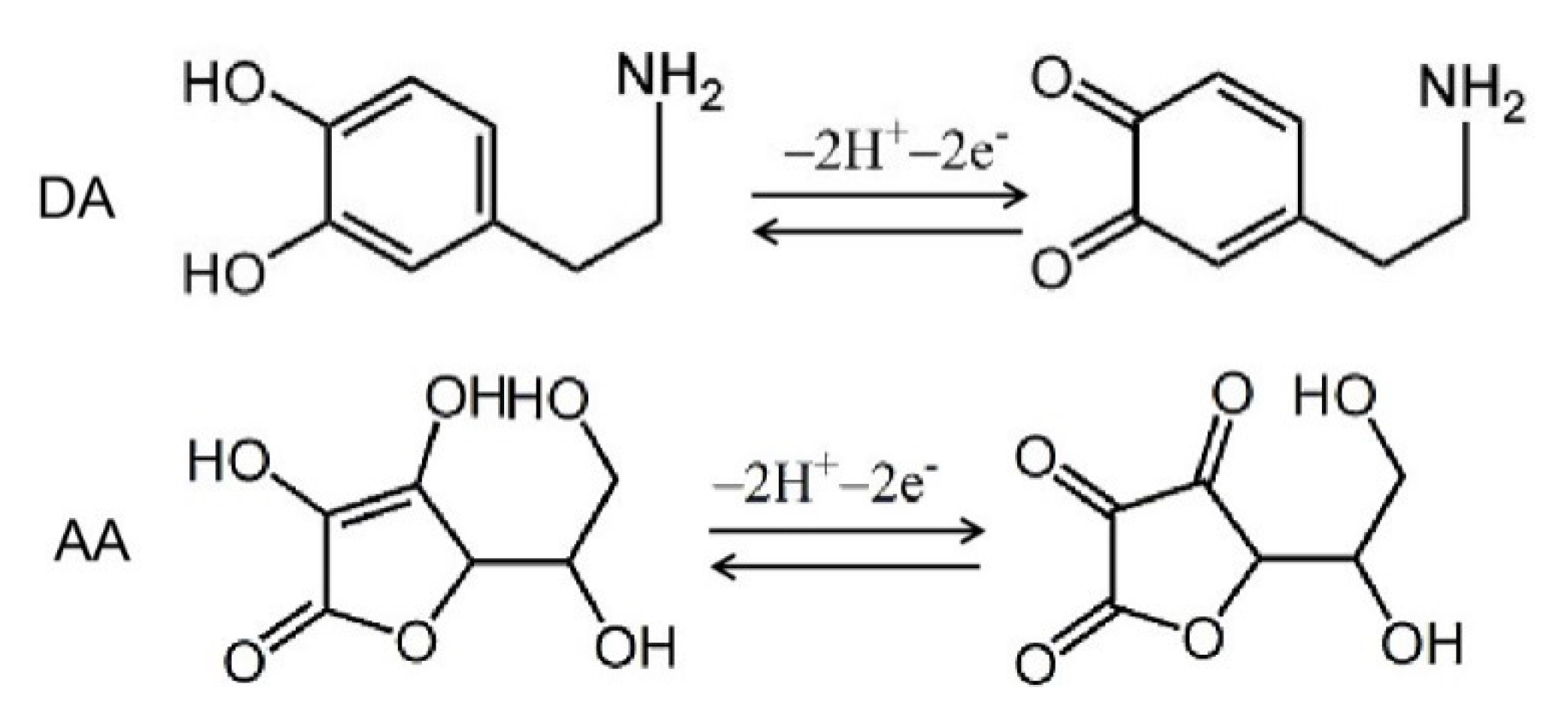
2. Results and Discussion
- First step: [Zn(phen)3]2·(V4O12)·phen·20H2O [Zn(phen)3]2·(V4O12)·phen
- Second step: [Zn(phen)3]2·(V4O12)·phen 2Zn2+ + [V4O12]4−
- Last step: 2Zn2+ + [V4O12]4− → 4VO2 + 2ZnO
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulkapuri, S.; Kurapati, S.K.; Das, S.K. Carbonate encapsulation from dissolved atmospheric CO2 into a polyoxovanadate capsule. Dalton Trans. 2019, 48, 8773–8781. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.K.; Wang, H.; Huang, B.; Li, N.; Hu, K.H.; Wu, B.L.; Xiao, Z.C.; Wei, Y.H.; Wu, P.F. A new scheme for rational design and synthesis of polyoxovanadate hybrids with high antitumor activities. J. Inorg. Biochem. 2019, 193, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Tian, X.R.; Zhang, H.; Gao, Y.Z.; Ma, Y.Y.; Han, Z.G. Synthesis and characterization of Ag-ligand modified polyoxovanadates with three-dimensional structures. J. Solid State Chem. 2019, 269, 278–284. [Google Scholar] [CrossRef]
- Batrice, R.J.; Wacker, J.N.; Glass, E.N.; Jilani, S.Z.; Tong, Y.Y.J.; Nyman, M.; Knope, K.E. Template-free cyclic hexavanadate: Synthesis, characterization, solid-state structure, and solution-state dynamics. Polyhedron 2019, 169, 266–277. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Sun, D.; Men, L.L.; Sun, B.; Li, X.; An, Q.B.; Liu, F.B.; Su, Z.M. Self-assembly of bimetallic polyoxometalates and dicyandiamide to form co/wc@nc for efficient electrochemical hydrogen generation. New J. Chem. 2022, 46, 178–184. [Google Scholar] [CrossRef]
- Chen, B.K.; Huang, X.Q.; Wang, B.; Lin, Z.G.; Hu, J.F.; Chi, Y.N.; Hu, C.W. Three New Imidazole-Functionalized Hexanuclear Oxidovanadium Clusters with Exceptional Catalytic Oxidation Properties for Alcohols. Chem. Eur. J. 2013, 19, 4408–4413. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.W.; Wei, Q.; Zhang, J.; Yang, G.Y. Two Tetra-CdII-Substituted Vanadogermanate Frameworks. J. Am. Chem. Soc. 2014, 136, 5065–5071. [Google Scholar] [CrossRef]
- Cao, J.P.; Xue, Y.S.; Hu, Z.B.; Luo, X.M.; Cui, C.H.; Song, Y.; Xu, Y. Exploring the Magnetic Interaction of Asymmetric Structures Based on Chiral VIII8 Clusters. Inorg. Chem. 2019, 58, 2637–2644. [Google Scholar] [CrossRef]
- Linnenberg, O.; Kozłowski, P.; Besson, C.; Leusen, J.V.; Englert, U.; Monakhov, K.U. A V16-type Polyoxovanadate Structure with Intricate Electronic Distribution: Insights from Magnetochemistry. Cryst. Growth Des. 2017, 17, 2342–2350. [Google Scholar] [CrossRef]
- Zhang, X.; You, W.S.; Zhu, Z.M.; Dang, L.Q.; Sun, Z.G.; Zheng, X.F. Hydrothermal synthesis and characterization of a novel crystal containing [Co4O4]4+ cubane core: [Co4O4(dpaH)4(CH3COO)2]2V4O12·5H2O. Inorg. Chem. Commun. 2006, 9, 526–528. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Yao, S.; Yan, J.H.; Chen, L.; Wang, T.T.; Wang, C.J.; Zhang, Z.M. Design and synthesis of purely inorganic 3D frameworks composed of reduced vanadium clusters and manganese linkers. Dalton Trans. 2015, 44, 20435–20440. [Google Scholar] [CrossRef]
- Zhang, G.H.; Shi, Z.; Xu, Y.H.; Feng, S.H. An Organically Templated Cobalt−Vanadium Oxide with â Cage Units: Hydrothermal Synthesis and X-ray Structural Characterization of (C2H10N2)[Co2(C2O4)V4O12]. Inorg. Chem. 2003, 42, 1170–1174. [Google Scholar]
- Zhang, S.Y.; Guo, W.B.; Tang, Y.Y.; Xu, J.Q.; He, Z.Z. Observation of Spin Relaxation in a Vanadate Chloride with Quasi One-Dimensional Linear Chain. Cryst. Growth Des. 2019, 19, 2228–2234. [Google Scholar] [CrossRef]
- Qin, J.S.; Du, D.Y.; Li, S.L.; Lan, Y.Q.; Shao, K.Z.; Su, Z.M. pH-Tuned self-assembly of organic–inorganic hybrids based on different vanadate chains, Zn(II) ions and flexible ligands: Crystallizing in polar and centrosymmetric space group. CrystEngComm 2011, 13, 779–786. [Google Scholar] [CrossRef]
- Paredes-García, V.; Gaune, S.; Saldías, M.; Garland, M.T.; Baggio, R.; Vega, A.; Salah El Fallah, M.; Escuer, A.; Fur, L.E.; Yazigi, D.V.; et al. Solvatomorphs of dimeric transition metal complexes based on the V4O12 cyclic anion as building block: Crystalline packing and magnetic properties. Inorg. Chim. Acta 2008, 361, 3681–3689. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Q.F.; Ma, P.T.; Zhang, C.; Niu, J.Y.; Wang, J.P. Polyoxovanadate catalysts for oxidation of 1-phenyl ethanol: From the discrete [V4O12]4− and [V10O28]6−anions, to the anionic [V6O17]n4n− coordination polymer. CrystEngComm 2018, 20, 6273–6279. [Google Scholar] [CrossRef]
- Roman, P.; Jose, A.S.; Luque, A.; Gutierrez-Zorrilla, J.M. Observation of a Novel Cyclic Tetrametavanadate Anion Isolated from Aqueous Solution. Inorg. Chem. 1993, 32, 775–776. [Google Scholar] [CrossRef]
- Fuchs, J.; Mahjour, S.; Pickardt, J. Structure of the “True” Metavanadate Ion. Angew. Chem. Int. Ed. 1976, 15, 374–375. [Google Scholar] [CrossRef]
- Tang, Q.L.; Zhou, J.; Liu, X.; Xiao, H.; Li, W.B.; Fu, L.S.; Dong, T.T. A novel 2-D heterometallic polymer containing two types of 1-D cuprous polymeric chains and circle [V4O12]4− clusters. J. Alloys Compd. 2017, 713, 46–50. [Google Scholar] [CrossRef]
- Qi, Y.F.; Lv, C.P.; Li, Y.G.; Wang, E.B.; Li, J.; Song, X.L. A new three-dimensional 4,6-connected self-catenated net constructed from the [V8O23]6-polyoxoanions and metal–organic polymer. Inorg. Chem. Commun. 2010, 13, 384–387. [Google Scholar]
- Kastner, K.; Puscher, B.; Streb, C. Self-assembly of a tetrahedral 58-nuclear barium vanadium oxide cluster. Chem. Commun. 2013, 49, 140–142. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, F.L.; Lin, Z.Z.; Zhou, Y.F.; Yue, C.Y.; Hong, M.C. A Basket Tetradecavanadate Cluster with Blue Luminescence. J. Am. Chem. Soc. 2005, 127, 8588–8589. [Google Scholar] [CrossRef]
- Karet, G.B.; Sun, Z.; Streib, W.E.; Bollinger, J.C.; Hendrickson, D.N.; Christou, G. Stepwise assembly of a polyoxovanadate from mononuclear units in an organic solvent: Carboxylate-stabilised fragments in the conversion of [VOCl4]22− to [V15O36]52−. Chem. Commun. 1999, 2249–2250. [Google Scholar] [CrossRef]
- Wang, K.; Niu, Y.J.; Zhao, D.Y.; Zhao, Y.X.; Ma, P.T.; Zhang, D.D.; Wang, J.P.; Niu, J.Y. The Polyoxovanadate-Based Carboxylate Derivative K6H[VV17VIV12(OH)4O60(OOC(CH2)4COO)8]·nH2O: Synthesis, CrystalStructure, and Catalysis for Oxidation of Sulfifides. Inorg. Chem. 2017, 56, 14053–14059. [Google Scholar] [CrossRef]
- Zhang, P.P.; Peng, J.; Pang, H.J.; Chen, Y.; Zhu, M.; Wang, D.D.; Liu, M.G.; Wang, Y.H. A Cu coordination polymer-modified [V4O12]4− polyanion with interdigitated architecture. Inorg. Chem. Commun. 2010, 13, 1414–1417. [Google Scholar] [CrossRef]
- Zurkova, L.; Kucsera, R.; Gyepes, R.; Sivák, M. Synthesis and X-Ray Crystal Structure of Two Novel Complexes:[MII(phen)3]2V4O12 phen 22H2O (MII = Co,Ni; phen = phenanthroline). Mon. Chem. 2003, 134, 1071–1079. [Google Scholar] [CrossRef]
- Joniakova, D.; Gyepes, R.; Rakovsky, E.; Schwendt, P.; Zurkova, L.; Marek, J.; Micka, Z. Structural variability of copper-1,10-phenanthroline–oxovanadate hybrid inorganic–organic compounds. Polyhedron 2006, 25, 2491–2502. [Google Scholar] [CrossRef]
- Xu, X.H.; Cao, Q.L.; Luo, F.; Wang, G. Synthesis and Crystal Structure of the Bimetallic Complex [Fe(phen)3]2[phen][V4O12]·19H2O. Z. Nat. 2008, 63, 1352–1356. [Google Scholar] [CrossRef]
- Gu, W.; Bian, H.D.; Xu, J.Y.; Yan, S.P.; Liao, D.Z.; Jiang, Z.H. Hydrothermal synthesis, structure, spectroscopic and magnetic properties of a hexanuclear cluster: [{Mn(2,2′-bipy)2}2V4O12]. Inorg. Chem. Commun. 2003, 6, 217–220. [Google Scholar] [CrossRef]
- Dang, D.B.; Zheng, Y.N.; Bai, Y.; Guo, X.Y.; Ma, P.T.; Niu, J.Y. Assembly of Polyoxometalate-Based Metal−Organic Frameworks with Silver(I)-Schiffff Base Coordination Polymeric Chains as Building Blocks. Cryst. Growth Des. 2012, 12, 3856–3867. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, G.Q.; Dang, D.B.; Ma, P.T.; Gao, H.; Niu, J.Y. Assembly of polyoxometalate-based inorganic–organic compounds from silver–Schiff base building blocks: Synthesis, crystal structures and luminescent properties. CrystEngComm 2011, 13, 4181–4187. [Google Scholar] [CrossRef]
- Dang, D.B.; An, B.; Bai, Y.; Niu, J.Y. Assembly of a phospho-molybdic Wells–Dawson-based silver coordination polymer derived from Keggin polyoxoanion cluster. Dalton Trans. 2012, 41, 13856–13861. [Google Scholar] [CrossRef]
- Dang, D.B.; Zheng, G.S.; Bai, Y.; Yang, F.; Gao, H.; Ma, P.T.; Niu, J.Y. Construction of Polyoxometalate-Based Inorganic Organic Compounds Using Silver(I) Double Helicates as Secondary Building Blocks. Inorg. Chem. 2011, 50, 7907–7909. [Google Scholar] [CrossRef]
- Li, L.; Cheng, M.; Bai, Y.; An, B.; Dang, D.B. A polyoxometalate-based inorganic–organic hybrid polymer constructed from silver-Schiff base building block and Keggin-type cluster: Synthesis, crystal structure and photocatalytic performance for the degradation of rhodamine B. Spectrochim. Acta A 2015, 150, 846–854. [Google Scholar] [CrossRef]
- Shi, S.K.; Guo, Z.; Feng, R.; Jin, L.Y.; Bai, Y.; Dang, D.B. Hydrothermal synthesis and crystal structure of a bisupporting Keggin-polyoxometalate hybrid compound decorated with a copper(II) complex unit. Z. Nat. 2018, 73, 197–202. [Google Scholar] [CrossRef]
- Chen, W.H.; Hu, Z.B.; Zhang, Z.S.; Qiu, Z.H.; Zhao, J.H.; Lai, Y.Z.; Mi, J.X. Synthesis, Structure, Characterizations and Photocatalytic Degradation of a New POM-Based Hybrid Compound: (Hdma)4[Cd(phen)3][W18O54(PO4)2]2H2O. J. Clust. Sci. 2017, 28, 1113–1123. [Google Scholar] [CrossRef]
- Xiao, L.N.; Hu, Y.Y.; Wang, L.M.; Wang, Y.; Xu, J.N.; Ding, H.; Cui, X.B.; Xu, J.Q. New compounds based on polyoxometalates and metal halide clusters. CrystEngComm 2012, 14, 8589–8598. [Google Scholar] [CrossRef]
- Guo, H.Y.; Zhang, T.T.; Lin, P.H.; Zhang, X.; Cui, X.B.; Huo, Q.S.; Xu, J.Q. Preparation, structure and characterization of a series of vanadates. CrystEngComm 2017, 19, 265–275. [Google Scholar] [CrossRef]
- Yu, Z.F.; Ke, D.G.; Huang, B.; Zhang, Y.T.; Luo, Z.H.; Wang, H.; Xiao, Z.C. Spectroscopic Studies of a Novel Inorganic–Organic Hybrid Based on Polyoxovanadates under a Wide Range of Wavelengths. J. Clust. Sci. 2019, 30, 5–10. [Google Scholar] [CrossRef]
- Li, C.X.; Zhang, Y.; O’Halloran, K.P.; Zhang, J.W.; Ma, H.Y. Electrochemical behavior of vanadium-substituted Keggin-type polyoxometalates in aqueous solution. J. Appl. Electrochem. 2009, 39, 421–427. [Google Scholar] [CrossRef]
- Rajamani, A.R.; Peter, S.C. Novel nanostructured Pt/CeO2 @Cu2O carbon-based electrode to magnify the electrochemical detection of theneurotransmitter dopamine and analgesic paracetamol. ACS Appl. Nano Mater. 2018, 1, 5148–5157. [Google Scholar] [CrossRef]
- Wang, Q.W.; Jessie, K.; Li, L.; Liu, Y.; Wang, X.H.; Wang, S.T. Fabrication of polyoxometalate/GO/PDDA hybrid nanocomposite modifified electrode and electrocatalysis for nitrite ion, ascorbic acid and dopamine. J. Electroanal. Chem. 2018, 824, 91–98. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Barbosa, A.D.S.; Pires, J.; Balula, S.S.; Cunha-Silva, L.; Freire, C. Novel composite material polyoxovanadate@mil-101(cr): A highly efficient electrocatalyst for ascorbic acid oxidation. ACS Appl. Mater. Interfaces 2013, 5, 13382–13390. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS−97, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]






| D–H⋯A | d(D–H) | d(H⋯A) | d(D⋯A) | ∠(DHA) | Symmetry Codes of Atom A |
|---|---|---|---|---|---|
| C3–H3A⋯O10 | 0.93 | 2.57 | 3.3082 | 137 | |
| C6–H6A⋯O5 | 0.93 | 2.57 | 3.4115 | 151 | |
| C8–H8A⋯O3 | 0.93 | 2.59 | 3.4398 | 152 | |
| C18–H18A⋯O5f | 0.93 | 2.30 | 3.1732 | 157 | 1 + x, y, z |
| C51–H51A⋯O2 | 0.930 | 2.48 | 3.3133 | 149 | |
| C56–H56A⋯O9 | 0.93 | 2.56 | 3.4092 | 152 | |
| C61–H61A⋯O6a | 0.93 | 2.44 | 3.2299 | 143 | 1 − x, 1 − y |
| C66–H66A⋯O7i | 0.93 | 2.35 | 3.2063 | 154 | −1 + x, y, z |
| C68–H68A⋯O8i | 0.93 | 2.58 | 3.4166 | 150 | −1 + x, y, z |
| Chemical Formula | C84N14H96Zn2V4O32 |
|---|---|
| Formula weight | 2148.24 |
| Crystal system | Triclinic |
| Space group | P¯1P¯1 |
| a, Å | 12.9427(8) |
| b, Å | 17.6899(11) |
| c, Å | 23.5108(14) |
| α/° | 106.4910(10) |
| β/° | 91.0390(10) |
| γ/° | 106.8800(10) |
| V, Å3 | 4909.4(5) |
| Z | 2 |
| T, K | 296(2) |
| Dcalc’g cm−3 | 1.453 |
| F(000) | 2212 |
| θ range for data collection/° | 1.262−25.499 |
| Refinement method | Full-matrix least-squares on F2 |
| Goodness of fit on F2 | 1.019 |
| Final R indices [I > 2s(I)] | R1 = 0.0530, wR2 = 0.1058 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, B.; Guo, H.; Fan, Y.; Li, H.; Pang, J.; Dang, D.; Bai, Y. Synthesis, Crystal Structure, and Electrochemical Properties of an Isopolyoxovanadate Compound Modified Transition-Metal Complex Based on [V4O12]4−. Catalysts 2022, 12, 108. https://doi.org/10.3390/catal12020108
Wang Y, Zhang B, Guo H, Fan Y, Li H, Pang J, Dang D, Bai Y. Synthesis, Crystal Structure, and Electrochemical Properties of an Isopolyoxovanadate Compound Modified Transition-Metal Complex Based on [V4O12]4−. Catalysts. 2022; 12(2):108. https://doi.org/10.3390/catal12020108
Chicago/Turabian StyleWang, Yongxiu, Bingjie Zhang, Huili Guo, Yanhua Fan, Haiyan Li, Jingyu Pang, Dongbin Dang, and Yan Bai. 2022. "Synthesis, Crystal Structure, and Electrochemical Properties of an Isopolyoxovanadate Compound Modified Transition-Metal Complex Based on [V4O12]4−" Catalysts 12, no. 2: 108. https://doi.org/10.3390/catal12020108
APA StyleWang, Y., Zhang, B., Guo, H., Fan, Y., Li, H., Pang, J., Dang, D., & Bai, Y. (2022). Synthesis, Crystal Structure, and Electrochemical Properties of an Isopolyoxovanadate Compound Modified Transition-Metal Complex Based on [V4O12]4−. Catalysts, 12(2), 108. https://doi.org/10.3390/catal12020108





