Lipozyme® TL IM Biocatalyst for Castor Oil FAME and Triacetin Production by Interesterification: Activity, Stability, and Kinetics
Abstract
:1. Introduction
2. Results
2.1. Effect of Operational Conditions on Yield and Activity
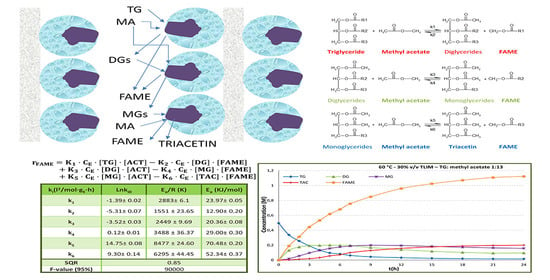
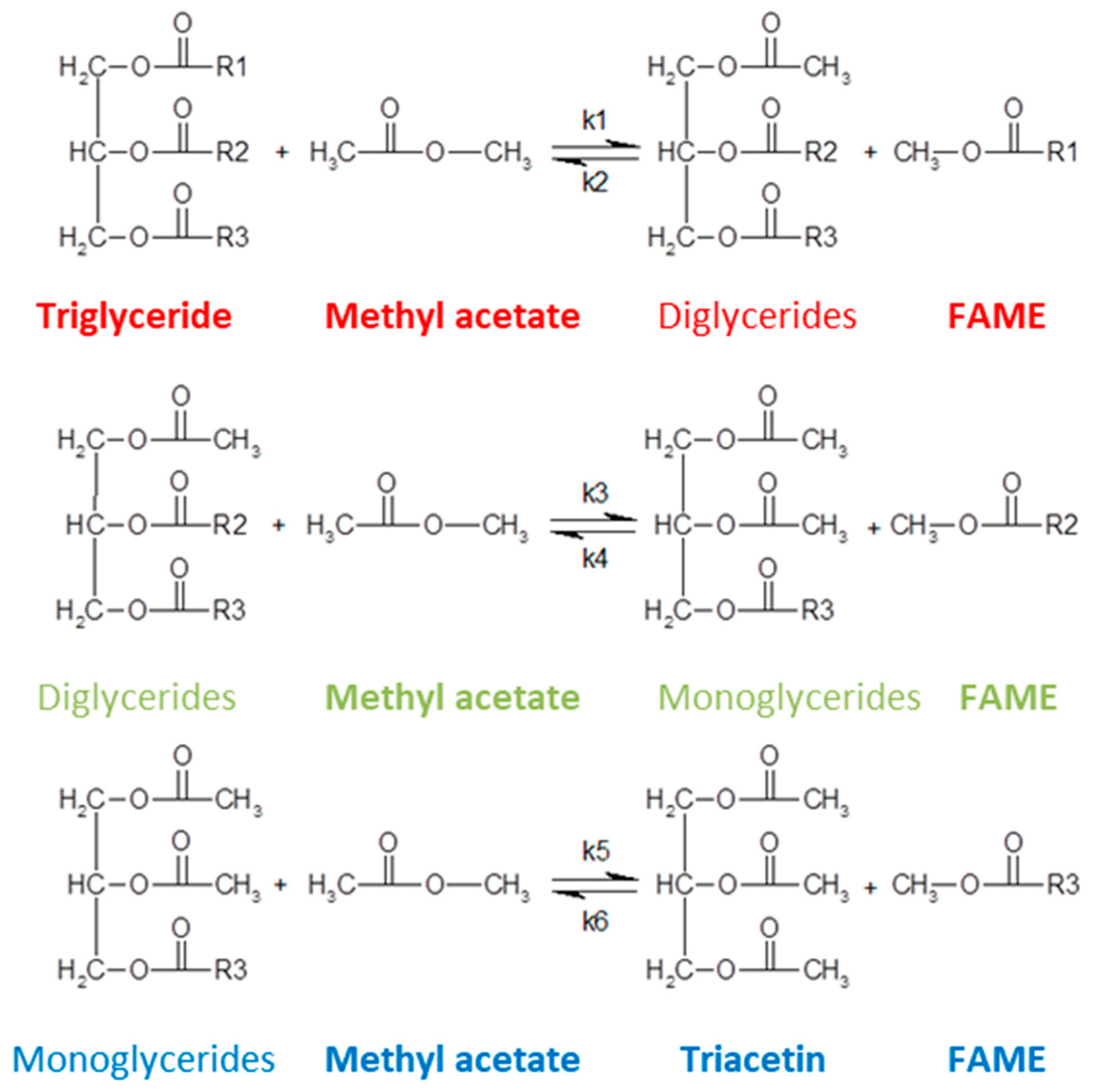
2.2. Kinetic Modelling
2.3. Biocatalyst Operational Stability
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Enzymatic Interesterification of Castor Oil and Methyl Acetate
3.2.2. Analytical Methods
- At zero time the eluent is: 30% (A) and 70% (B).
- From 0 to 10 min the eluent progressively goes to: 100% (B).
- From 10 to 20 min the eluent progressively changes to: 50% (B) and 50% (C).
- From 20 to 30 min the eluent is: 50% (B) and 50% (C).
- From 30 to 32 min the eluent changes progressively to: 100% (B).
- From 32 to 34 min the eluent progressively changes to: 30% (A) and 70% (B).
3.2.3. Statistical Non-Linear Regression Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Y.M.; Han, R.; Wang, C.; Yu, B.; Liang, Q.M.; Yuan, X.C.; Chang, J.; Zhao, Q.; Liao, H.; Tang, B.; et al. Self-preservation strategy for approaching global warming targets in the post-Paris Agreement era. Nat. Commun. 2020, 11, 1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desing, H.; Widmer, R.; Beloin-Saint-Pierre, D.; Hischier, R.; Wäger, P. Powering a sustainable and circular economy—An engineering approach to estimating renewable energy potentials within earth system boundaries. Energies 2019, 12, 4723. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Li, J. Emerging anthropogenic circularity science: Principles, practices, and challenges. iScience 2021, 24, 102237. [Google Scholar] [CrossRef] [PubMed]
- Mandari, V.; Devarai, S.K. Biodiesel Production Using Homogeneous, Heterogeneous, and Enzyme Catalysts via Transesterification and Esterification Reactions: A Critical Review. Bioenergy Res. 2022, 15, 935–961. [Google Scholar] [CrossRef]
- Shokravi, H.; Shokravi, Z.; Heidarrezaei, M.; Ong, H.C.; Rahimian Koloor, S.S.; Petrů, M.; Lau, W.J.; Ismail, A.F. Fourth generation biofuel from genetically modified algal biomass: Challenges and future directions. Chemosphere 2021, 285, 131535. [Google Scholar] [CrossRef]
- Aamer Mehmood, M.; Shahid, A.; Malik, S.; Wang, N.; Rizwan Javed, M.; Nabeel Haider, M.; Verma, P.; Umer Farooq Ashraf, M.; Habib, N.; Syafiuddin, A.; et al. Advances in developing metabolically engineered microbial platforms to produce fourth-generation biofuels and high-value biochemicals. Bioresour. Technol. 2021, 337, 125510. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, R.; Li, Q.; Li, M.; Liu, C.; Jia, P. Castor oil as a platform for preparing bio-based chemicals and polymer materials. Green Mater. 2021, 40, 99–109. [Google Scholar] [CrossRef]
- Pasha, M.K.; Dai, L.; Liu, D.; Du, W.; Guo, M. Biodiesel production with enzymatic technology: Progress and perspectives. Biofuels Bioprod. Biorefining 2021, 15, 1526–1548. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Arana-Peña, S.; da Rocha, T.N.; Miranda, L.P.; Berenguer-Murcia, Á.; Tardioli, P.W.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Liquid lipase preparations designed for industrial production of biodiesel. Is it really an optimal solution? Renew. Energy 2021, 164, 1566–1587. [Google Scholar] [CrossRef]
- Naveenkumar, R.; Baskar, G. Process optimization, green chemistry balance and technoeconomic analysis of biodiesel production from castor oil using heterogeneous nanocatalyst. Bioresour. Technol. 2021, 320, 124347. [Google Scholar] [CrossRef]
- Andrade, T.A.; Errico, M.; Christensen, K.V. Evaluation of Reaction Mechanisms and Kinetic Parameters for the Transesterification of Castor Oil by Liquid Enzymes. Ind. Eng. Chem. Res. 2017, 56, 9478–9488. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, X.; Jiang, Y.; Liu, P.; Zhou, L.; Ma, L.; He, Y.; Li, H.; Gao, J. Silica nanoflowers-stabilized pickering emulsion as a robust biocatalysis platform for enzymatic production of biodiesel. Catalysts 2019, 9, 1026. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, X.; Jiang, Y.; Zhou, L.; Ma, L.; He, Y.; Gao, J. Biocatalytic pickering emulsions stabilized by lipase-immobilized carbon nanotubes for biodiesel production. Catalysts 2018, 8, 587. [Google Scholar] [CrossRef] [Green Version]
- Hajar, M.; Vahabzadeh, F. Biolubricant production from castor oil in a magnetically stabilized fluidized bed reactor using lipase immobilized on Fe3O4 nanoparticles. Ind. Crops Prod. 2016, 94, 544–556. [Google Scholar] [CrossRef]
- Goswami, D.; Sen, R.; Basu, J.K.; De, S. Surfactant enhanced ricinoleic acid production using Candida rugosa lipase. Bioresour. Technol. 2010, 101, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, P.; Marie, J.M.; Maria Xavier, A.J. Castor oil-derived monomer ricinoleic acid based biodegradable unsaturated polyesters. Polym. Degrad. Stab. 2019, 170, 109016. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, F.; Fu, Q.; Zhang, X.; Zhu, X. Mechanical properties of renewable plasticizer based on ricinoleic acid for PVC. Polym. Test. 2019, 76, 199–206. [Google Scholar] [CrossRef]
- Sun, S.; Guo, J. Enhanced ricinoleic acid preparation using lipozyme TLIM as a novel biocatalyst: Optimized by response surface methodology. Catalysts 2018, 8, 486. [Google Scholar] [CrossRef] [Green Version]
- Zada, B.; Kwon, M.; Kim, S.W. Current Trends in Acetins Production: Green versus Non-Green Synthesis. Molecules 2022, 27, 2255. [Google Scholar] [CrossRef]
- Maddikeri, G.L.; Pandit, A.B.; Gogate, P.R. Ultrasound assisted interesterification of waste cooking oil and methyl acetate for biodiesel and triacetin production. Fuel Process. Technol. 2013, 116, 241–249. [Google Scholar] [CrossRef]
- Patel, V.R.; Dumancas, G.G.; Viswanath, L.C.K.; Maples, R.; Subong, B.J.J. Castor oil: Properties, uses, and optimization of processing parameters in commercial production. Lipid Insights 2016, 9, LPI-S40233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabiś, S.; Kula, J. Synthesis and Bioactivity of (R)-Ricinoleic Acid Derivatives: A Review. Curr. Med. Chem. 2016, 23, 4037–4056. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.S.; Piovesan, L.A.; D’Oca, C.R.M.; Hack, C.R.L.; Treptow, T.G.M.; Rodrigues, M.O.; Vendramini-Costa, D.B.; Ruiz, A.L.T.G.; De Carvalho, J.E.; D’Oca, M.G.M. Antiproliferative activity of synthetic fatty acid amides from renewable resources. Bioorganic Med. Chem. 2015, 23, 340–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matysiak, S.; Chmiel, A.; Skolimowski, J.; Kula, J.; Pasternak, B.; Blaszczyk, A. Synthesis and cytotoxicity of (R)- and (S)-ricinoleic acid amides and their acetates. Chirality 2017, 29, 616–622. [Google Scholar] [CrossRef]
- Park, C.G.; Kim, J.J.; Kim, H.K. Lipase-mediated synthesis of ricinoleic acid vanillyl ester and evaluation of antioxidant and antibacterial activity. Enzyme Microb. Technol. 2020, 133, 109454. [Google Scholar] [CrossRef]
- Pemha, R.; Kuete, V.; Pagès, J.M.; Pegnyemb, D.E.; Mosset, P. Synthesis and biological evaluation of four new ricinoleic acid-derived 1-o-alkylglycerols. Mar. Drugs 2020, 18, 113. [Google Scholar]
- Mohini, Y.; Prasad, R.B.N.; Karuna, M.S.L.; Poornachandra, Y.; Ganesh Kumar, C. Synthesis and biological evaluation of ricinoleic acid-based lipoamino acid derivatives. Bioorganic Med. Chem. Lett. 2016, 26, 5198–5202. [Google Scholar] [CrossRef]
- Geesala, R.; Bar, N.; Dhoke, N.R.; Basak, P.; Das, A. Porous polymer scaffold for on-site delivery of stem cells—Protects from oxidative stress and potentiates wound tissue repair. Biomaterials 2016, 77, 1–13. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Kundu, S.C.; Reis, R.L.; Correlo, V.M. Electric Phenomenon: A Disregarded Tool in Tissue Engineering and Regenerative Medicine. Trends Biotechnol. 2020, 38, 24–49. [Google Scholar] [CrossRef]
- Xu, Y.; Du, W.; Liu, D. Study on the kinetics of enzymatic interesterification of triglycerides for biodiesel production with methyl acetate as the acyl acceptor. J. Mol. Catal. B Enzym. 2005, 32, 241–245. [Google Scholar] [CrossRef]
- Sun, S.; Li, K. Biodiesel production from phoenix tree seed oil catalyzed by liquid lipozyme TL100L. Renew. Energy 2020, 151, 152–160. [Google Scholar] [CrossRef]
- Malani, R.S.; Umriwad, S.B.; Kumar, K.; Goyal, A.; Moholkar, V.S. Ultrasound–assisted enzymatic biodiesel production using blended feedstock of non–edible oils: Kinetic analysis. Energy Convers. Manag. 2019, 188, 142–150. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, Y.; Hu, F.; Yao, A.; Wang, Z.; Wei, F. Transesterification for biodiesel production catalyzed by combined lipases: Optimization and kinetics. AIChE J. 2010, 56, 1659–1665. [Google Scholar] [CrossRef]
- Chang, M.Y.; Chan, E.S.; Song, C.P. Biodiesel production catalysed by low-cost liquid enzyme Eversa® Transform 2.0: Effect of free fatty acid content on lipase methanol tolerance and kinetic model. Fuel 2021, 283, 119266. [Google Scholar] [CrossRef]
- Holčapek, M.; Jandera, P.; Fischer, J.; Prokeš, B. Analytical monitoring of the production of biodiesel by high-performance liquid chromatography with various detection methods. J. Chromatogr. A 1999, 858, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Molinero, L.; Ladero, M.; Tamayo, J.J.; García-Ochoa, F. Homogeneous catalytic esterification of glycerol with cinnamic and methoxycinnamic acids to cinnamate glycerides in solventless medium: Kinetic modeling. Chem. Eng. J. 2014, 247, 174–182. [Google Scholar] [CrossRef]


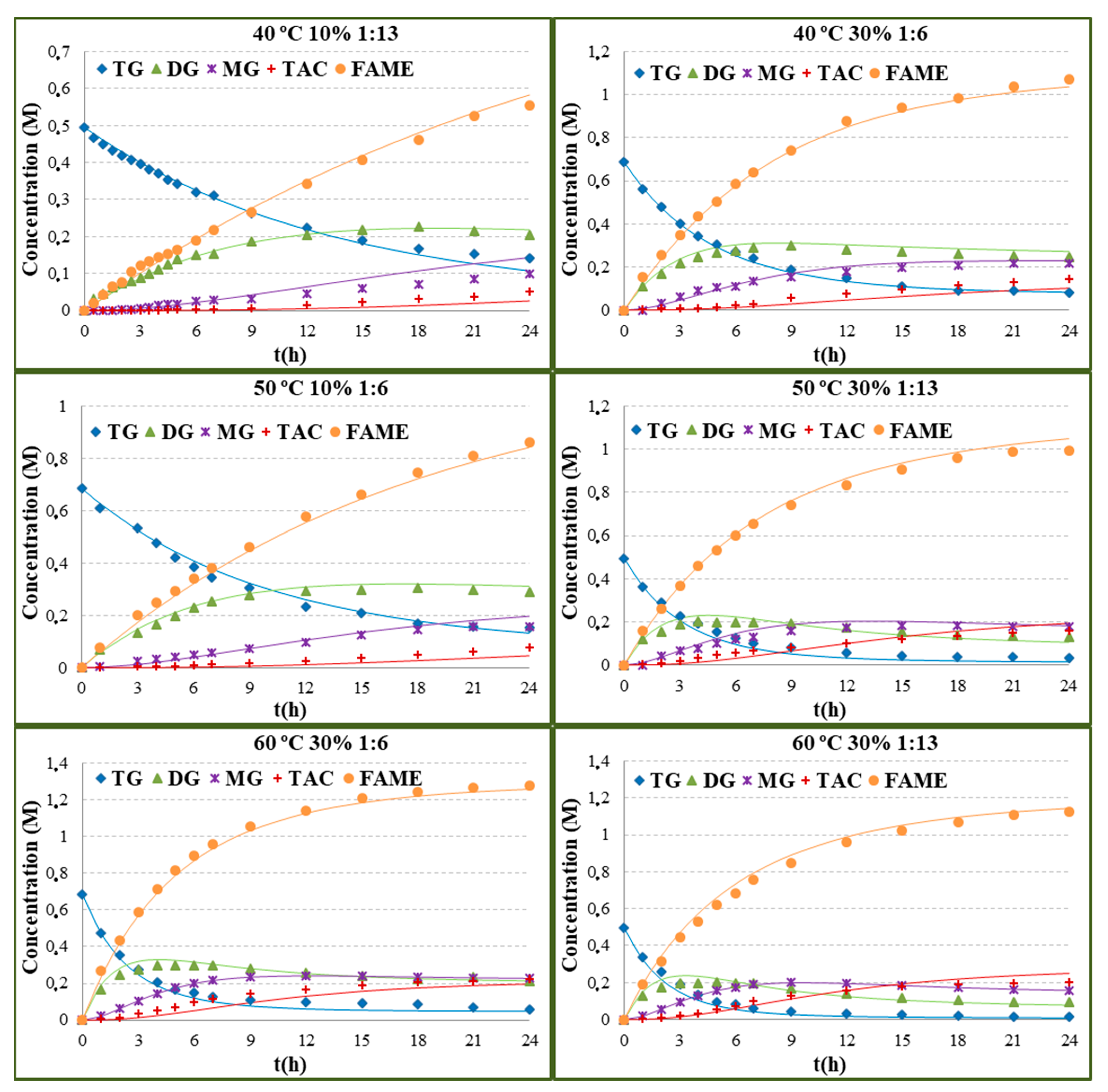

| Run | Enzyme Concentration (% w/w of Oil) | Temperature (°C) | Substrate Molar Ratio | TG Conversion (24 h) | FAME Yield (24 h) | r0 × 106 (mol/(L·s)) |
|---|---|---|---|---|---|---|
| E1 | 10 | 40 | 1:06 | 0.67 | 0.35 | 5.72 |
| E2 | 10 | 40 | 1:13 | 0.71 | 0.37 | 4.13 |
| E3 | 10 | 50 | 1:06 | 0.77 | 0.42 | 7.62 |
| E4 | 10 | 50 | 1:13 | 0.89 | 0.55 | 6.19 |
| E5 | 10 | 60 | 1:06 | 0.85 | 0.5 | 8.58 |
| E6 | 10 | 60 | 1:13 | 0.87 | 0.52 | 8.25 |
| E7 | 30 | 40 | 1:06 | 0.88 | 0.54 | 19.06 |
| E8 | 30 | 40 | 1:13 | 0.88 | 0.59 | 17.88 |
| E9 | 30 | 50 | 1:06 | 0.91 | 0.6 | 24.77 |
| E10 | 30 | 50 | 1:13 | 0.93 | 0.64 | 20.63 |
| E11 | 30 | 60 | 1:06 | 0.91 | 0.64 | 30.49 |
| E12 | 30 | 60 | 1:13 | 0.97 | 0.71 | 23.38 |
| T (°C) | k1 (×104) (l2/mol·gE·h) | k2 (×104) (l2/mol·gE·h) | k3 (×104) (l2/mol·gE·h) | k4 (×104) (l2/mol·gE·h) | k5 (×104) (l2/mol·gE·h) | k6 (×104) (l2/mol·gE·h) | SQR | F |
|---|---|---|---|---|---|---|---|---|
| 40 | 2.36 ± 0.03 | 2.30 ± 0.27 | 1.49 ± 0.06 | 4.39 ± 0.50 | 0.57 ± 0.07 | 2.87 ± 0.61 | 0.023 | 4.93 × 105 |
| 50 | 3.69 ± 0.07 | 2.58 ± 0.64 | 2.33 ± 0.11 | 5.72 ± 1.26 | 1.69 ± 0.22 | 3.14 ± 0.91 | 0.020 | 2.58 × 105 |
| 60 | 4.57 ± 0.09 | 3.11 ± 0.40 | 2.65 ± 0.11 | 5.88 ± 0.69 | 2.07 ± 0.14 | 3.71 ± 0.70 | 0.035 | 1.82 × 105 |
| ki (l2/(mol·gE·h)) | Ln ki0 | Ea/R (K) | Ea (kJ/mol) |
|---|---|---|---|
| k1 | 3.21 ± 0.01 | 3618.0 ± 3.23 | 30.08 ± 0.03 |
| k2 | −4.72 ± 0.10 | 1200.0 ± 33.63 | 9.98 ± 0.28 |
| k3 | −0.07 ± 0.02 | 2724.16 ± 7.77 | 22.65 ± 0.06 |
| k4 | −6.09 ± 0.07 | 483.19 ± 22.83 | 4.02 ± 0.19 |
| k5 | 3.86 ± 0.06 | 4244.81 ± 19.67 | 35.29 ± 0.16 |
| k6 | −4.76 ± 0.16 | 1100.0 ± 53.90 | 9.15 ± 0.45 |
| SQR | 0.028 | ||
| F | 753,000 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Calvo, A.; Gallardo, M.E.; Ladero, M. Lipozyme® TL IM Biocatalyst for Castor Oil FAME and Triacetin Production by Interesterification: Activity, Stability, and Kinetics. Catalysts 2022, 12, 1673. https://doi.org/10.3390/catal12121673
Gómez-Calvo A, Gallardo ME, Ladero M. Lipozyme® TL IM Biocatalyst for Castor Oil FAME and Triacetin Production by Interesterification: Activity, Stability, and Kinetics. Catalysts. 2022; 12(12):1673. https://doi.org/10.3390/catal12121673
Chicago/Turabian StyleGómez-Calvo, Alba, M. Esther Gallardo, and Miguel Ladero. 2022. "Lipozyme® TL IM Biocatalyst for Castor Oil FAME and Triacetin Production by Interesterification: Activity, Stability, and Kinetics" Catalysts 12, no. 12: 1673. https://doi.org/10.3390/catal12121673