Study on the Formaldehyde Oxidation Reaction of Acid-Treated Manganese Dioxide Nanorod Catalysts
Abstract
1. Introduction
2. Results and Discussion
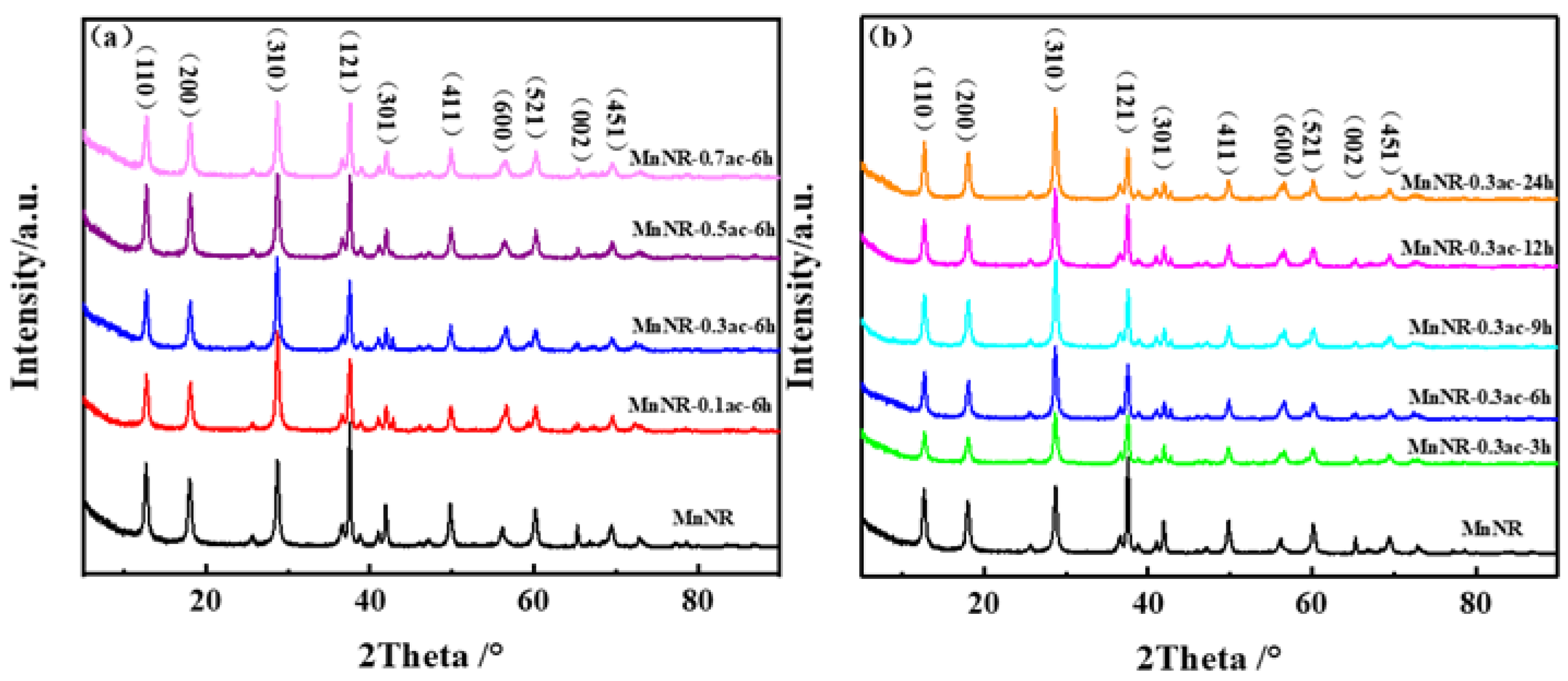
2.1. Microstructure of Catalysts
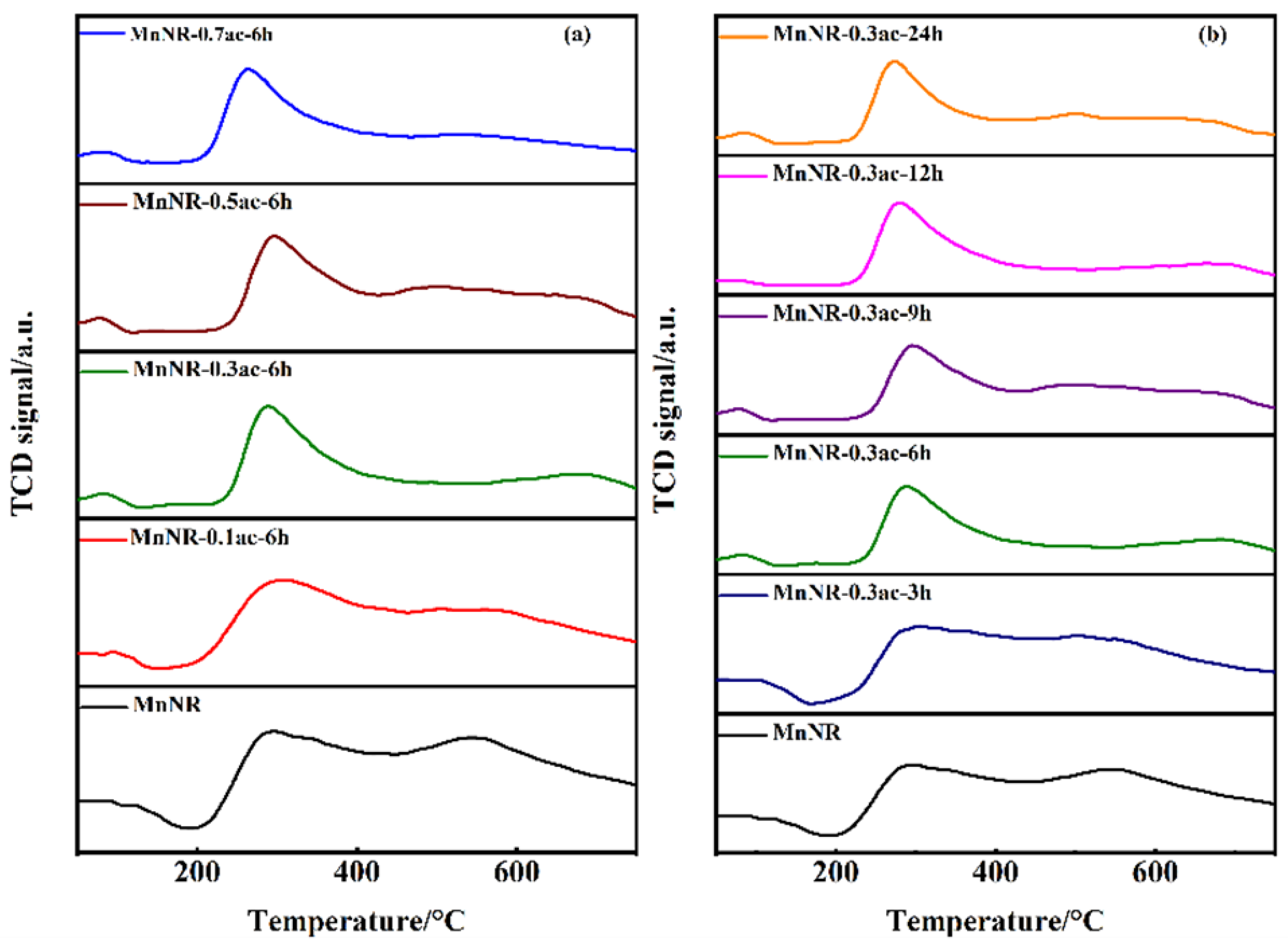
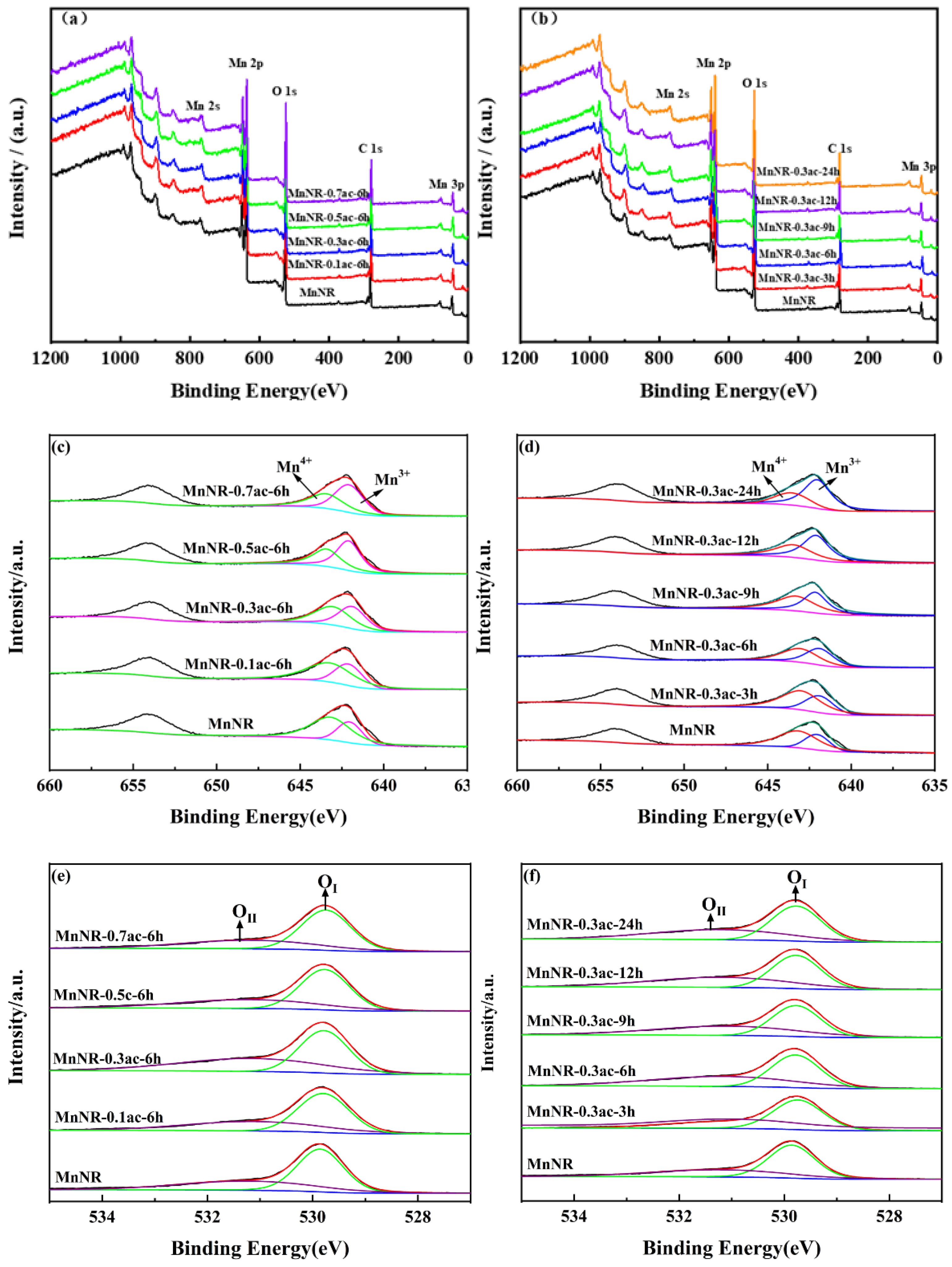
2.2. Chemical Properties of the Catalysts
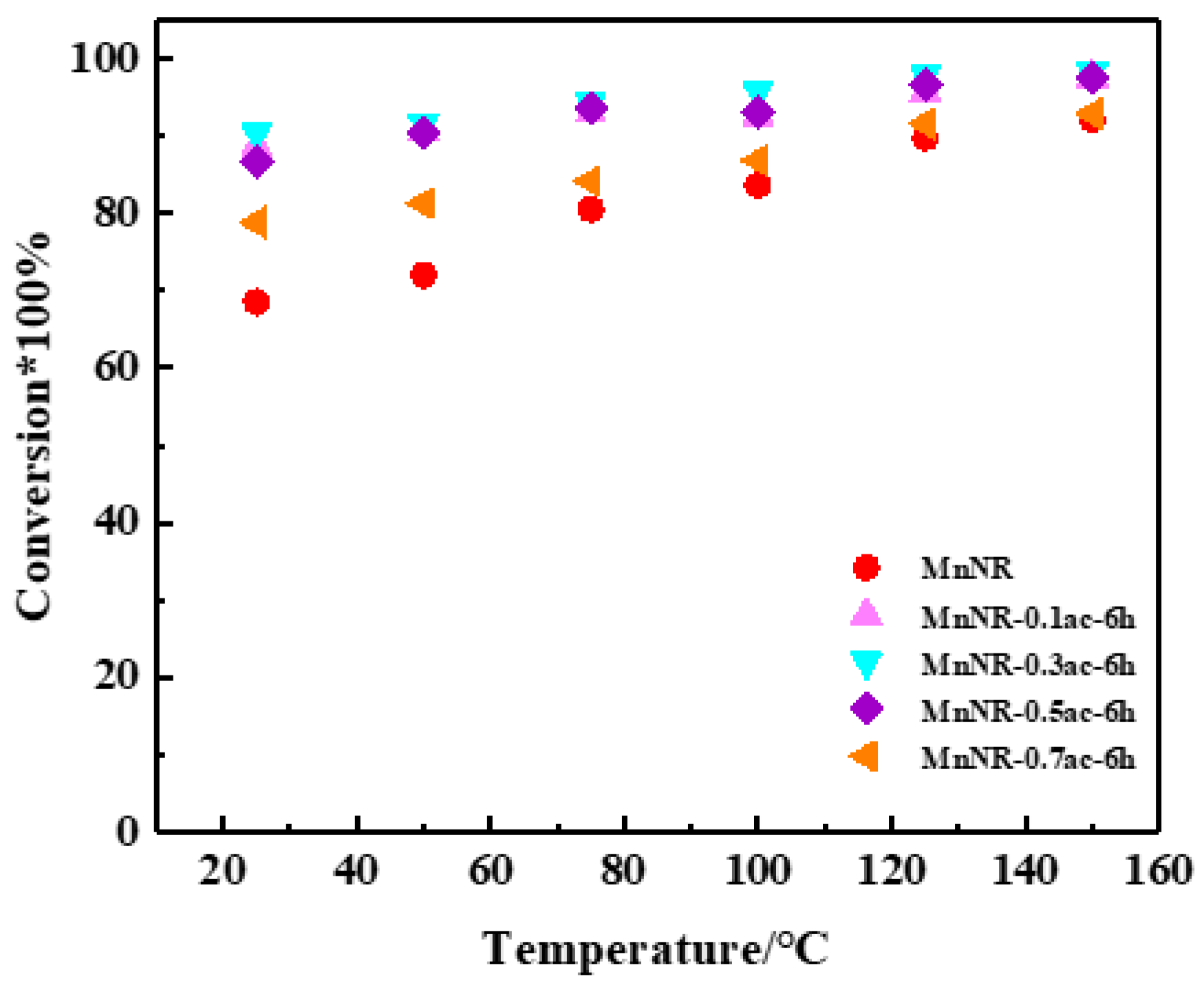
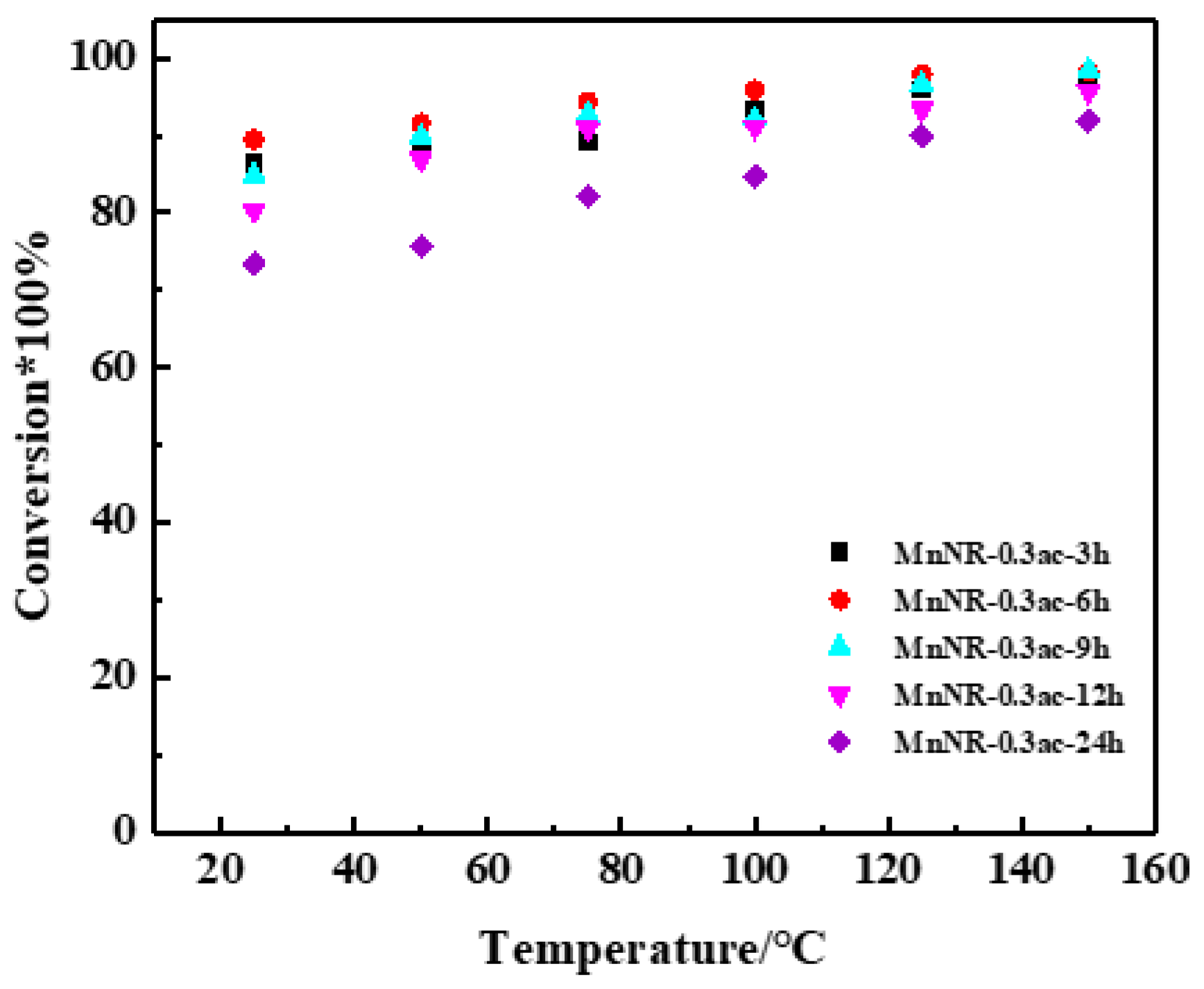
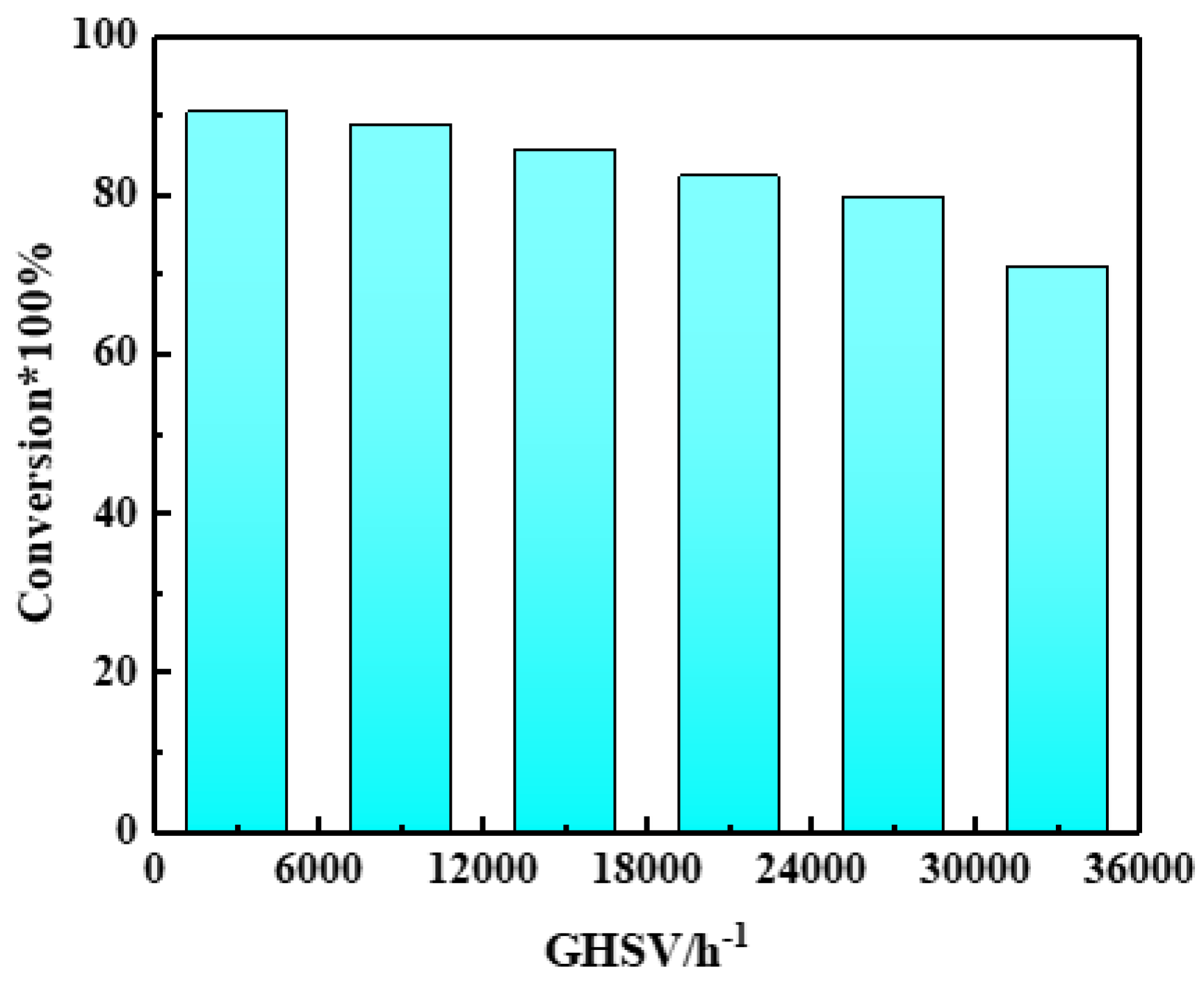
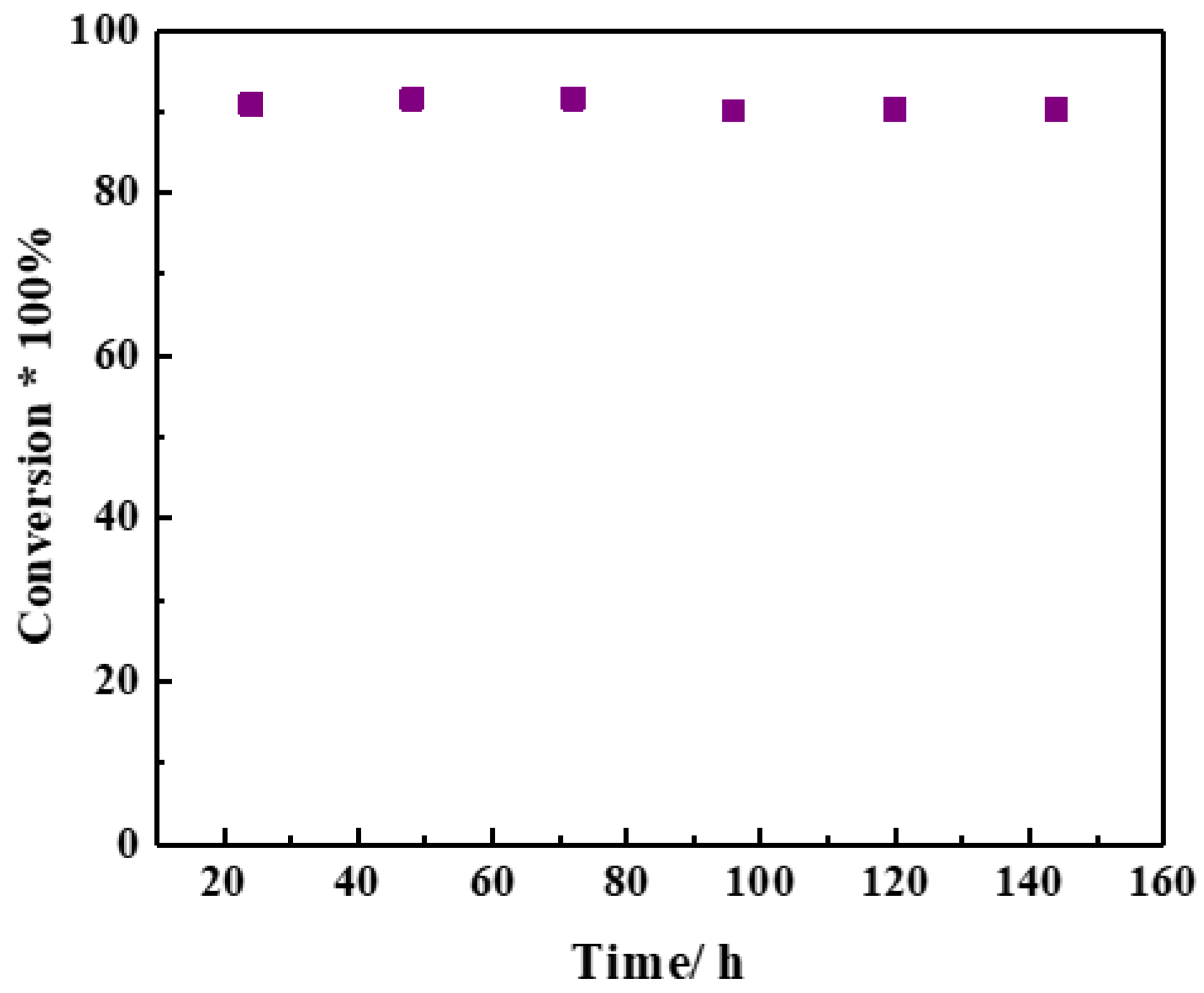
2.3. Reaction Performance of Catalysts
3. Experimental
3.1. Preparation of Catalysts
3.2. Catalyst Characterization
3.3. Catalyst Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahmanpour, A.-M.; Hoadley, A.; Mushrif, S.-H. Hydrogenation of Carbon Monoxide into Formaldehyde in Liquid Media. ACS Sustain. Chem. Eng. 2016, 4, 3970–3977. [Google Scholar] [CrossRef]
- Kaithal, A.; Hölscher, M.; Leitner, W. Carbon monoxide and hydrogen (syngas) as a C1-building block for selective catalytic methylation. Chem. Sci. 2020, 12, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Heim, L.-E.; Konnerth, H.; Prechtl, M.-H.-G. The Prospecting Shortcut to an Old Molecule: Formaldehyde Synthesis at Low Temperature in Solution. ChemSusChem 2016, 9, 2905–2907. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-J.; Li, D.-D.; Zhang, P.-Y.; Li, J.-G.; Wang, J.; Yu, J.G. Formaldehyde and volatile organic compound (VOC) emissions from particleboard: Identification of odorous compounds and effects of heat treatment. Build. Environ. 2017, 117, 118–126. [Google Scholar] [CrossRef]
- Gilbert, N.-L.; Guay, M.; Gauvin, D.; Dietz, R.-N.; Chan, C.-C.; Lévesque, B. Air change rate and concentration of formaldehyde in residential indoor air. Atmos. Environ. 2007, 42, 2424–2428. [Google Scholar] [CrossRef]
- Giese, M.; Bauer-Doranth, U.; Langebartels, C.; Sandermann, H. Detoxification of Formaldehyde by the Spider Plant (Chlorophytum comosum L.) and by Soybean (Glycine max L.) Cell-Suspension Cultures. Plant Physiol. 1994, 104, 1301–1309. [Google Scholar] [CrossRef]
- Suresh, S.; Bandosz, T.-J. Removal of formaldehyde on carbon -based materials: A review of the recent approaches and findings. Carbon 2018, 137, 207–221. [Google Scholar] [CrossRef]
- Ye, J.-W.; Zhu, X.-F.; Cheng, B.; Yu, J.-G.; Jiang, C.-J. Few-Layered Graphene-like Boron Nitride: A Highly Efficient Adsorbent for Indoor Formaldehyde Removal. Environ. Sci. Technol. Lett. 2017, 4, 20–25. [Google Scholar] [CrossRef]
- Liang, W.-J.; Li, J.; Li, J.-X.; Zhu, T.; Jin, Y.-Q. Formaldehyde removal from gas streams by means of NaNO2 dielectric barrier discharge plasma. J. Hazard. Mater. 2009, 175, 1090–1095. [Google Scholar] [CrossRef]
- Liao, Y.-C.; Xie, C.-S.; Liu, Y.; Chen, H.; Li, H.-Y.; Wu, J. Comparison on photocatalytic degradation of gaseous formaldehyde by TiO2, ZnO and their composite. Ceram. Int. 2012, 38, 4437–4444. [Google Scholar] [CrossRef]
- Kehr, E.; Riehl, G.; Hoferichter, E.; Roffael, E.; Dix, B. Feuchtebeständigkeit und Hydrolyseresistenz von Holz-zu-Holz-Bindungen in Spanplatten, hergestellt mit formaldehydarmen modifizierten Harnstoff-Formaldehydharzen unter Einsatz verschiedener Härtungsbeschleunigersysteme. Holz als Roh-und Werkstoff 1993, 52, 253–260. [Google Scholar] [CrossRef]
- Duan, Y.-Y.; Song, S.-Q.; Cheng, B.; Yu, J.-G.; Jiang, C.-J. Effects of hierarchical structure on the performance of tin oxide-supported platinum catalyst for room-temperature formaldehyde oxidation. Chin. J. Catal. 2017, 38, 199–206. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Sun, S.-M.; Lu, H.; Hao, Z.-P.; Yang, C.-G.; Wang, B.; Chen, C.; Song, M.-Y. Remarkable MnO2 structure-dependent H2O promoting effect in HCHO oxidation at room temperature. J. Hazard. Mater. 2021, 414, 125542. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Wang, F.-G. Room temperature HCHO oxidation over the Pt/CeO2 catalysts with different oxygen mobilities by changing ceria shapes. Appl. Catal. A Gen. 2022, 630, 118469. [Google Scholar] [CrossRef]
- Wang, S.; Han, K.-H.; Deng, Z.-Y.; Wang, F.-G. CeO2 Nanorods Decorated with Pt Nanoparticles as Catalysts for Oxidative Elimination of Formaldehyde. ACS Appl. Nano Mater. 2022, 5, 10036–10046. [Google Scholar] [CrossRef]
- Wang, F.-G.; Wang, Y.; Han, K.-H.; Yu, H. Efficient elimination of formaldehyde over Pt/Fe3O4 catalyst at room temperature. J. Environ. Chem. Eng. 2020, 8, 104041. [Google Scholar] [CrossRef]
- Dong, Y.-X.; Su, C.-G.; Liu, K.; Wang, H.-M.; Zheng, Z.; Zhao, W.; Lu, S.-H. The Catalytic Oxidation of Formaldehyde by FeOx-MnO2-CeO2 Catalyst: Effect of Iron Modification. Catalysts 2021, 11, 555. [Google Scholar] [CrossRef]
- Fan, Z.-Y.; Fang, W.-J.; Zhang, Z.-X.; Chen, M.-X.; Shangguan, W.-F. Highly active rod-like Co3O4 catalyst for the formaldehyde oxidation reaction. Catal. Commun. 2018, 103, 10–14. [Google Scholar] [CrossRef]
- Cui, W.-Y.; Wang, C.; Wu, J.; Tan, N.-D. Research progress of formaldehyde oxidation over manganese oxide catalyst. Fine Chem. Ind. 2019, 36, 2353–2363. [Google Scholar] [CrossRef]
- Sekine, Y. Oxidative decomposition of formaldehyde by metal oxides at room temperature. Atmos. Environ. 2002, 36, 5543–5547. [Google Scholar] [CrossRef]
- Li, Y.-B.; Han, S.-H.; Zhang, L.-P.; Yu, Y.-F. Manganese-Based Catalysts for Indoor Volatile Organic Compounds Degradation with Low Energy Consumption and High Efficiency. Trans. Tianjin Univ. 2021, 28, 53–66. [Google Scholar] [CrossRef]
- Li, S.-J.; Chen, L.-L.; Ma, Z.; Li, G.-S.; Zhang, D.-Q. Research Progress on Photocatalytic/Photoelectrocatalytic Oxidation of Nitrogen Oxides. Trans. Tianjin Univ. 2021, 27, 295–312. [Google Scholar] [CrossRef]
- Li, S.-D.; Zheng, D.-S.; Guo, F. Research progress on catalytic oxidation of manganese dioxide. Mod. Chem. Ind. 2020, 40, 52–56. [Google Scholar] [CrossRef]
- Tian, H.; He, J.-H. Research progress of formaldehyde oxidation catalyzed by manganese oxide. Chem. Bull. 2013, 76, 100–106. [Google Scholar] [CrossRef]
- Robinson, D.-M.; Go, Y.-B.; Mui, M.; Gardner, G.; Zhang, Z.-G.; Mastrogiovanni, D.; Garfunkel, E.; Li, J.; Greenblatt, M.; Dismukes, G.C. Photochemical water oxidation by crystalline polymorphs of manganese oxides: Structural requirements for catalysis. J. Am. Chem. Soc. 2013, 135, 3494–3501. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Li, Y.-B.; Wang, L.; Zhang, C.-B.; He, H. Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures. Catal. Sci. Technol. 2015, 5, 2305–2313. [Google Scholar] [CrossRef]
- Rong, S.-P.; Zhang, P.-Y.; Liu, F.; Yang, Y.-J. Engineering Crystal Facet of α-MnO2 Nanowire for Highly Efficient Catalytic Oxidation of Carcinogenic Airborne Formaldehyde. ACS Catal. 2018, 8, 3435–3446. [Google Scholar] [CrossRef]
- Zhou, J.; Qin, L.-F.; Xiao, W.; Zeng, C.; Li, N.; Lv, T.; Zhu, H. Oriented growth of layered-MnO2 nanosheets over α-MnO2 nanotubes for enhanced room-temperature HCHO oxidation. Appl. Catal. B Environ. 2017, 207, 233–243. [Google Scholar] [CrossRef]
- Chen, T.; Dou, H.-Y.; Li, X.-L.; Tang, X.-F.; Li, J.-H.; Hao, J.-M. Tunnel structure effect of manganese oxides in complete oxidation of formaldehyde. Microporous Mesoporous Mater. 2009, 122, 270–274. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.-L.; Cai, J.; Zhan, Y.-K.; He, N.-D. Manganese based catalysts for catalytic oxidation of formaldehyde and their synergistic effects. Adv. Chem. 2019, 31, 311–321. [Google Scholar]
- Bai, B.-Y.; Qiao, Q.; Li, J.-H.; Hao, J.-M. Progress in research on catalysts for catalytic oxidation of formaldehyde. Chin. J. Catal. 2016, 37, 102–122. [Google Scholar] [CrossRef]
- Wei, Y.-Z.; Chen, M.-Y.; Ren, X.-Y.; Wang, Q.-F.; Han, J.-F.; Wu, W.-Z.; Yang, X.-G.; Wang, S.; Yu, J.-H. One-Pot Three-Dimensional Printing Robust Self-Supporting MnOx /Cu-SSZ-13 Zeolite Monolithic Catalysts for NH3-SCR. CCS Chem. 2022, 4, 1708–1719. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Rochard, G.; Giraudon, J.-M.; Liu, J.; Lamonier, J.-F. Mesoporous MnO2 hollow spheres for enhanced catalytic oxidation of formaldehyde. Sustain. Mater. Technol. 2018, 20, e00091. [Google Scholar] [CrossRef]
- Bai, B.-Y.; Qiao, Q.; Li, J.-H.; Hao, J.-M. Synthesis of three-dimensional ordered mesoporous MnO2 and its catalytic performance in formaldehyde oxidation. Chin. J. Catal. 2016, 37, 27–31. [Google Scholar] [CrossRef]
- Wepasnick, K.-A.; Smith, B.-A.; Schrote, K.-E. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Cui, W.-Y.; Xue, D.; Yuan, X.-L.; Zheng, B.; Jia, M.-J.; Zhang, W.-X. Acid-treated TiO2 nanobelt supported platinum nanoparticles for the catalytic oxidation of formaldehyde at ambient conditions. Appl. Surf. Sci. 2017, 411, 105–112. [Google Scholar] [CrossRef]
- Wang, S.-H.; Liu, Q.; Zhao, Z.-Q.; Fan, C.; Chen, X.-P.; Xu, G.; Wu, M.-H.; Chen, J.-J.; Li, J.-H. Enhanced Low-Temperature Activity of Toluene Oxidation over the Rod-like MnO2/LaMnO3 Perovskites with Alkaline Hydrothermal and Acid-Etching Treatment. Ind. Eng. Chem. Res. 2020, 59, 6556–6564. [Google Scholar] [CrossRef]
- Gao, J.-H.; Zhou, H.; Zhang, F.-C.; Ji, K.-M.; Liu, P.; Liu, Z.-H.; Zhang, K. Effect of Preparation Method on the Catalytic Performance of HZSM-5 Zeolite Catalysts in the MTH Reaction. Materials 2022, 15, 2206. [Google Scholar] [CrossRef]
- Fang, R.-M.; Feng, Q.-Y.; Huang, H.-B.; Ji, J.; He, M.; Zhan, Y.-J.; Liu, B.-Y.; Leung, D.-Y.-C. Effect of K + ions on efficient room-temperature degradation of formaldehyde over MnO2 catalysts. Catal. Today 2019, 327, 154–160. [Google Scholar] [CrossRef]
- Sun, D.; Wageh, S.; Al-Ghamdi, A.-A.; Le, Y.; Yu, J.-G.; Jiang, C.-J. Pt/C@MnO2 composite hierarchical hollow microspheres for catalytic formaldehyde decomposition at room temperature. Appl. Surf. Sci. 2018, 466, 301–308. [Google Scholar] [CrossRef]
- Lu, S.-H.; Wang, X.; Zhu, Q.-Y.; Chen, C.-C.; Zhou, X.-F.; Huang, F.-L.; Li, K.-L.; He, L.-L.; Liu, Y.-X.; Pang, F.-J. Ag–K/MnO2 nanorods as highly efficient catalysts for formaldehyde oxidation at low temperature. RSC Adv. 2018, 8, 14221–14228. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-D.; Yang, G.-L.; Li, P.-L.; Wang, J.-L.; Zhang, P.-Y. Promotion of formaldehyde oxidation over Ag catalyst by Fe doped MnOx support at room temperature. Catal. Today 2016, 277, 257–265. [Google Scholar] [CrossRef]
- Huang, H.; Dai, Q.-G.; Wang, X.-Y. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2014, 158–159, 96–105. [Google Scholar] [CrossRef]
- Cai, T.; Huang, H.; Deng, W.; Dai, Q.-G.; Liu, W.; Wang, X.-Y. Catalytic combustion of 1,2-dichlorobenzene at low temperature over Mn-modified Co3O4 catalysts. Appl. Catal. B Environ. 2015, 166–167, 393–405. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, C.-B.; He, H.; Teraoka, Y. Catalytic decomposition of N2O over CeO2 promoted Co3O4 spinel catalyst. Appl. Catal. B Environ. 2007, 75, 167–174. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Wang, D.-H.; Xue, W.-J. Investigation of Formaldehyde Oxidation over Co3O4-CeO2 and Au/ Co3O4-CeO2 Catalysts at Room Temperature: Effective Removal and Determination of Reaction Mechanism. Environ. Sci. Technol. ES&T 2011, 45, 3628–3634. [Google Scholar] [CrossRef]
- Jiang, T.-T.; Wang, X.; Chen, J.-Z.; Mai, Y.-L.; Liao, B.; Hu, W. Hierarchical Ni/Co-LDHs catalyst for catalytic oxidation of indoor formaldehyde at ambient temperature. J. Mater. Sci. Mater. Electron. 2020, 31, 3500–3509. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.-X.; Huang, W.-M.; Xiu, T.-P.; Zhuang, C.-G.; Shi, J.-L. The catalytic oxidation removal of low-concentration HCHO at high space velocity by partially crystallized mesoporous MnOx. Chem. Eng. J. 2017, 320, 667–676. [Google Scholar] [CrossRef]
- Han, Z.; Wang, C.; Zou, X.; Chen, T.; Dong, S.; Zhao, Y.; Xie, J.; Liu, H. Diatomite-supported birnessite-type MnO2 catalytic oxidation of formaldehyde: Preparation, performance and mechanism. Appl. Surf. Sci. 2020, 502, 144201. [Google Scholar] [CrossRef]


| Sample | SBET (m2/g) | Dp (nm) | Vp (cm3/g) |
|---|---|---|---|
| MnNR | 47.6 | 11.6 | 0.14 |
| MnNR-0.1ac-6h | 49.7 | 18.2 | 0.20 |
| MnNR-0.3ac-6h | 51.8 | 20.2 | 0.21 |
| MnNR-0.5ac-6h | 49.6 | 20.3 | 0.24 |
| MnNR-0.7ac-6h | 48.0 | 20.6 | 0.25 |
| MnNR-0.3ac-3h | 48.2 | 17.1 | 0.20 |
| MnNR-0.3ac-9h | 50.9 | 20.9 | 0.20 |
| MnNR-0.3ac-12h | 48.4 | 21.3 | 0.22 |
| MnNR-0.3ac-24h | 45.5 | 22.2 | 0.22 |
| Sample | Mn3+ | Mn4+ | OI | OII | Molar Ratio of Surface Elements | ||
|---|---|---|---|---|---|---|---|
| OII/OI + OII a | Mn3+ a | Mn4+ a | |||||
| MnNR | 641.8 | 643 | 529.9 | 531.1 | 35% | 31% | 69% |
| MnNR-0.1ac-6h | 641.9 | 643.1 | 529.8 | 531.1 | 40% | 35% | 65% |
| MnNR-0.3ac-6h | 641.9 | 643.1 | 529.8 | 531.2 | 48% | 47% | 53% |
| MnNR-0.5ac-6h | 642 | 643.3 | 529.8 | 531.2 | 42% | 56% | 44% |
| MnNR-0.7ac-6h | 642.1 | 643.4 | 529.7 | 531.3 | 41% | 57% | 43% |
| MnNR-0.3ac-3h | 641.9 | 643 | 529.8 | 531.2 | 48% | 33% | 67% |
| MnNR-0.3ac-9h | 641.9 | 643.1 | 529.8 | 531.2 | 48% | 50% | 50% |
| MnNR-0.3ac-12h | 642 | 643.1 | 529.7 | 531.2 | 47% | 52% | 48% |
| MnNR-0.3ac-24h | 642 | 643.5 | 529.7 | 531.2 | 45% | 61% | 39% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Su, Y.; Yang, Y.; Liu, P.; Zhang, K.; Ji, K. Study on the Formaldehyde Oxidation Reaction of Acid-Treated Manganese Dioxide Nanorod Catalysts. Catalysts 2022, 12, 1667. https://doi.org/10.3390/catal12121667
Li Y, Su Y, Yang Y, Liu P, Zhang K, Ji K. Study on the Formaldehyde Oxidation Reaction of Acid-Treated Manganese Dioxide Nanorod Catalysts. Catalysts. 2022; 12(12):1667. https://doi.org/10.3390/catal12121667
Chicago/Turabian StyleLi, Yanqiu, Yuan Su, Yunfeng Yang, Ping Liu, Kan Zhang, and Keming Ji. 2022. "Study on the Formaldehyde Oxidation Reaction of Acid-Treated Manganese Dioxide Nanorod Catalysts" Catalysts 12, no. 12: 1667. https://doi.org/10.3390/catal12121667
APA StyleLi, Y., Su, Y., Yang, Y., Liu, P., Zhang, K., & Ji, K. (2022). Study on the Formaldehyde Oxidation Reaction of Acid-Treated Manganese Dioxide Nanorod Catalysts. Catalysts, 12(12), 1667. https://doi.org/10.3390/catal12121667




