Photocatalytic Degradation of Ciprofloxacin by UV Light Using N-Doped TiO2 in Suspension and Coated Forms
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Characteristics of the N-Doped TiO2 Catalyst
2.1.1. Energy-Dispersive Spectroscopy (EDS)
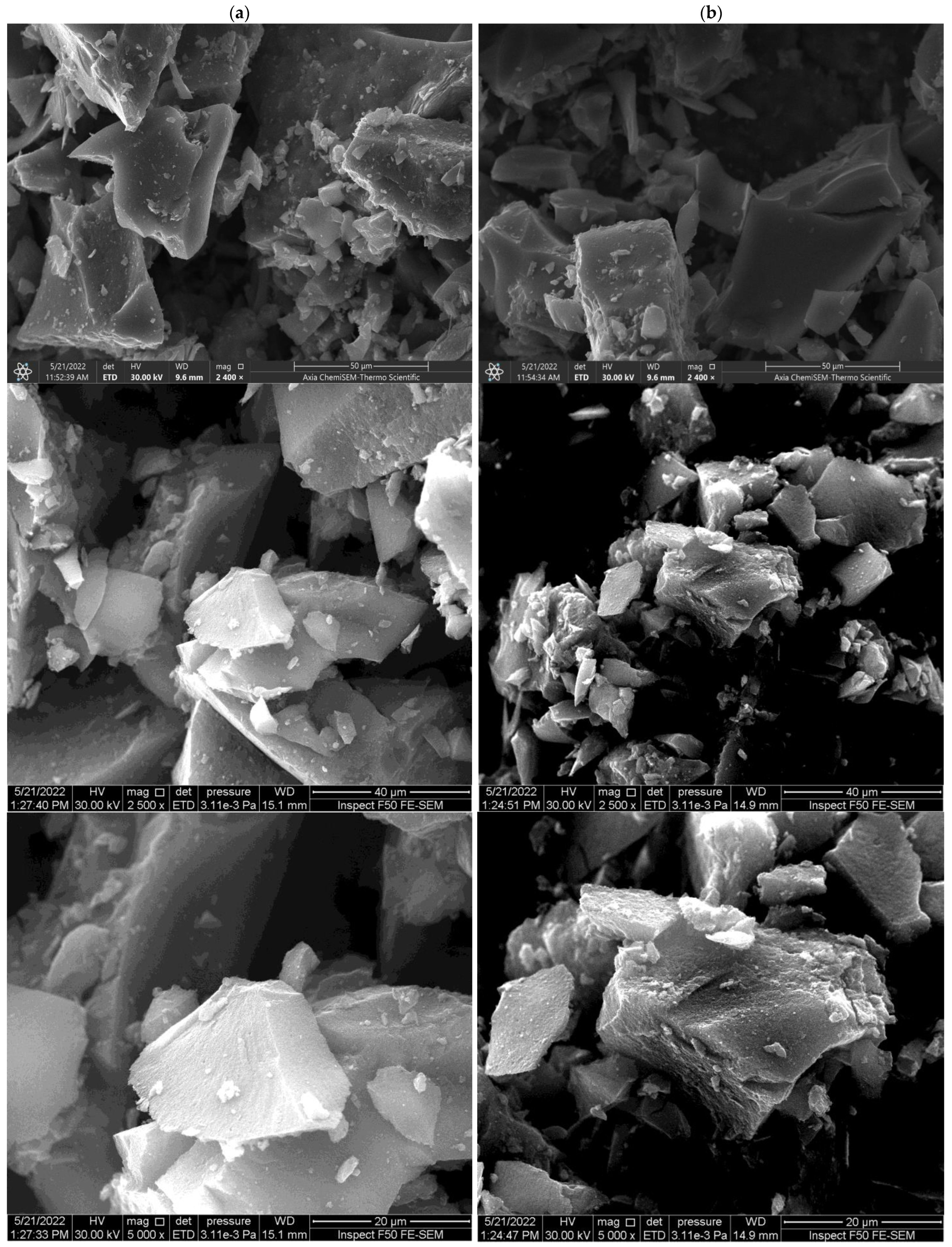
2.1.2. Scanning Electron Microscopy (SEM)
2.1.3. Fourier-Transform Infrared (FTIR) Spectroscopy
2.2. Photocatalytic Degradation of CIP with Nano-TiO2
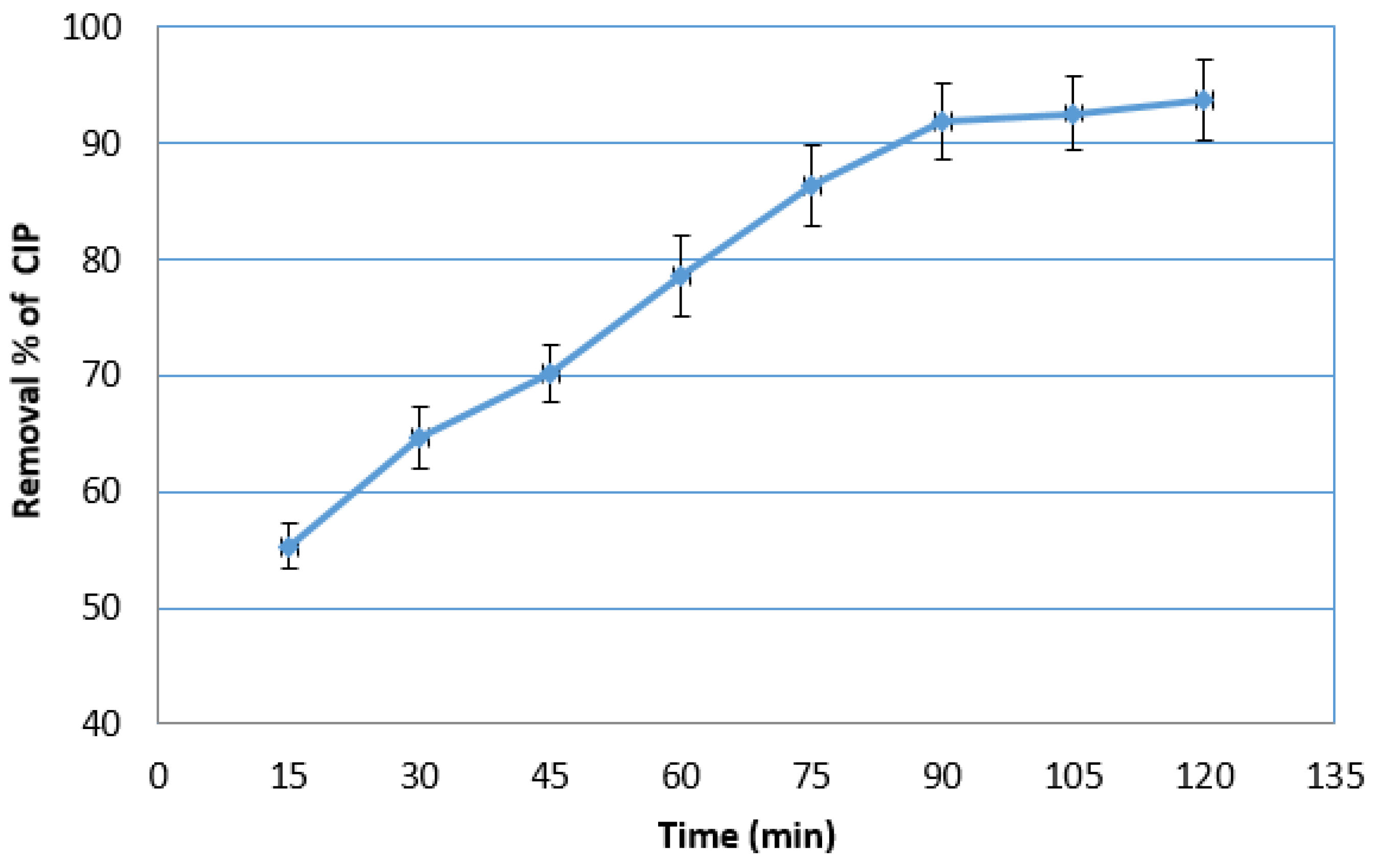
2.2.1. Photocatalytic Degradation with the Suspension of Net TiO2
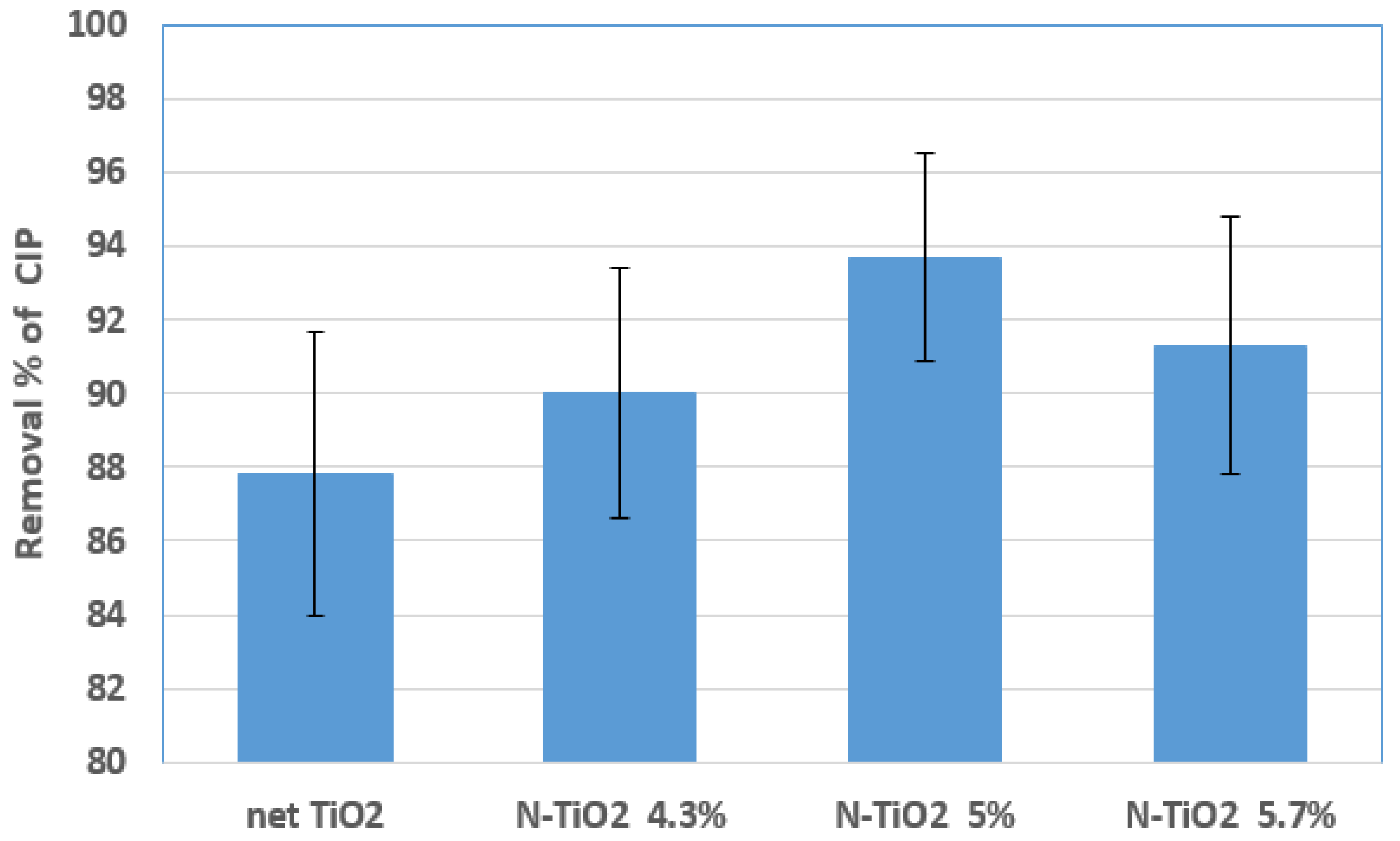
2.2.2. Photocatalytic Degradation with the Suspension of N-Doped TiO2
2.2.3. Photocatalytic Degradation with the N-Doped TiO2 after the Coating Process
2.3. Effect of the Operating Parameters on the Removal Efficiency
2.3.1. Effect of Initial Ciprofloxacin Concentration
2.3.2. Effect of Feed Flow Rate
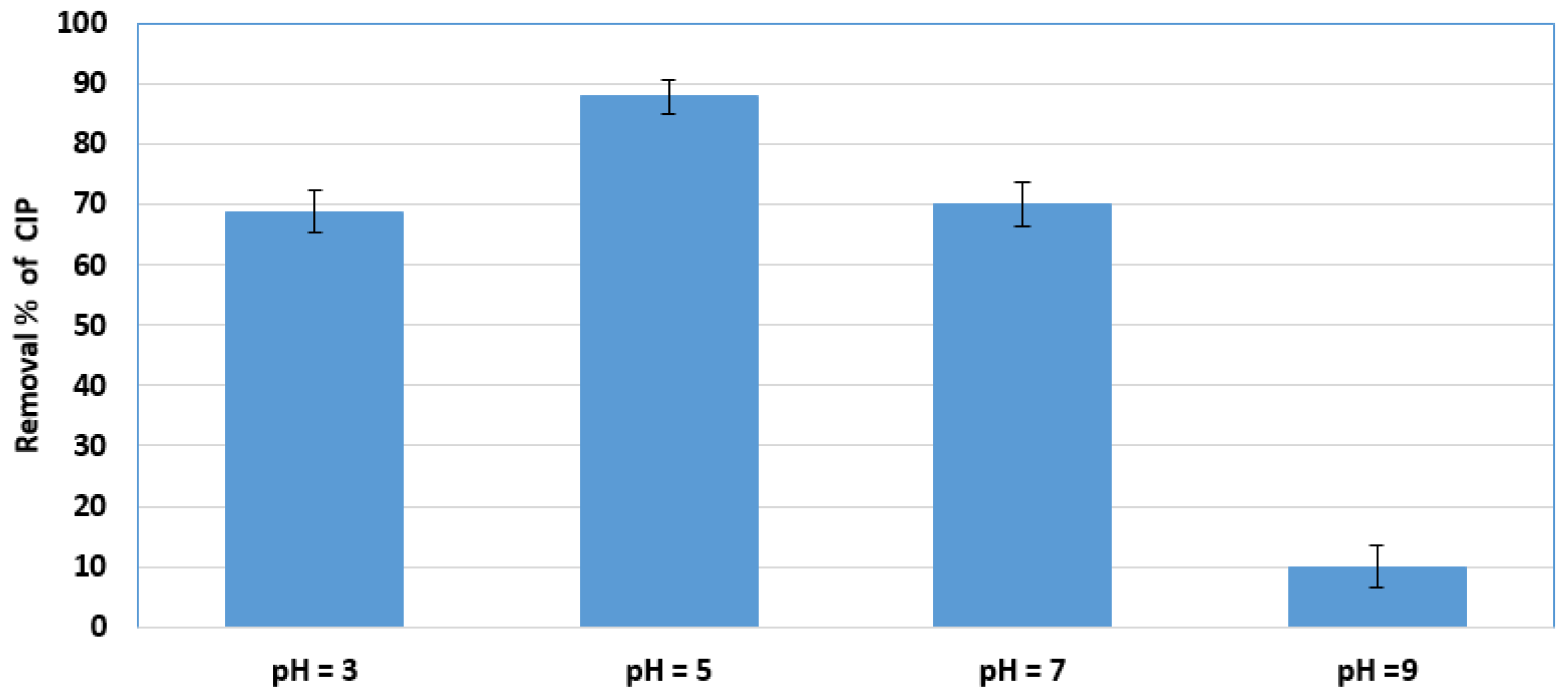
2.3.3. Effect of pH
- Adsorption of CIP on the TiO2 surface;
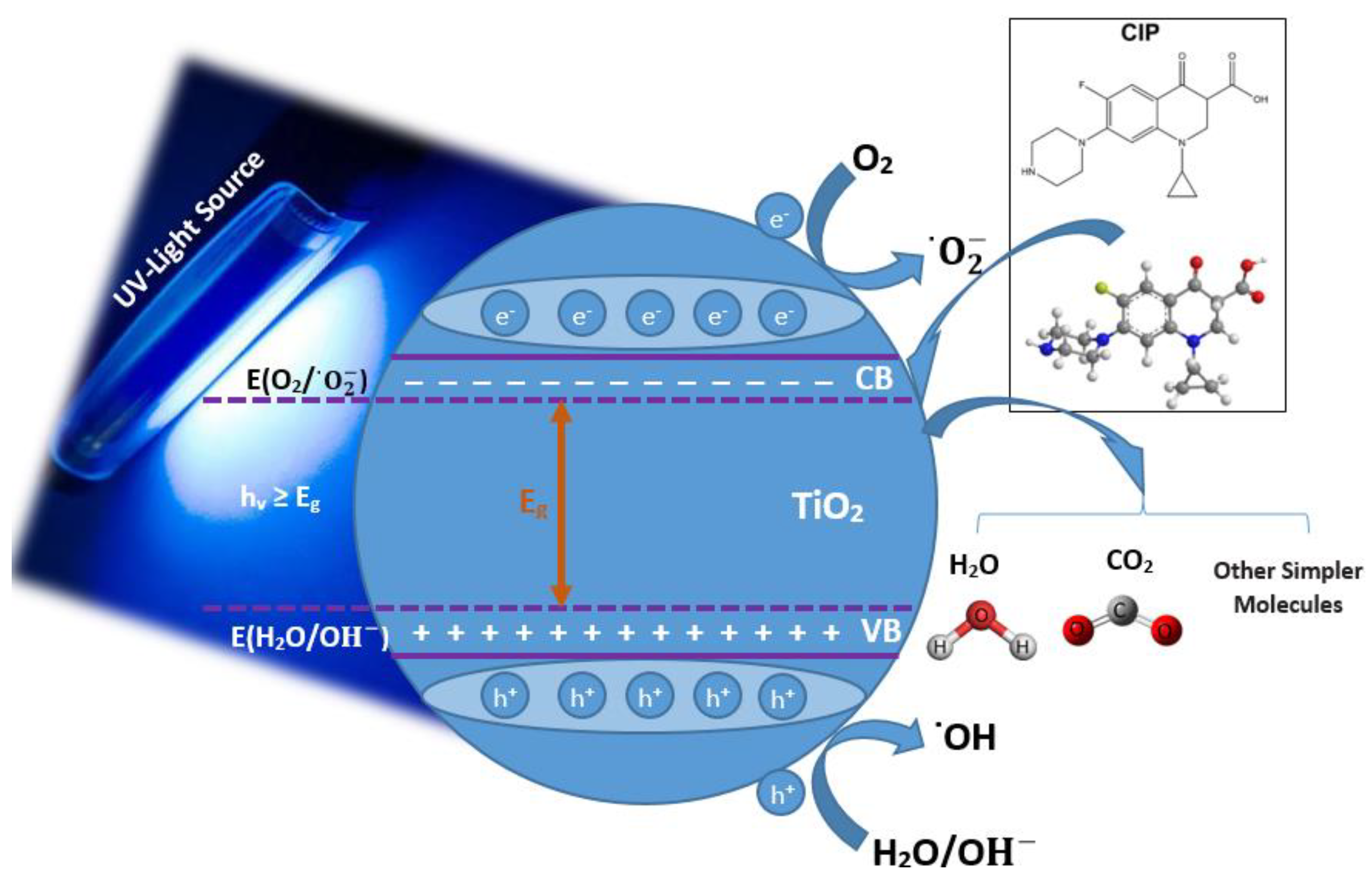
- The photocatalytic degradation of the adsorbed CIP via oxidation–reduction reactions with photogenerated electrons (e−), holes (h+), and reactive species (depicted in Figure 11);
- Desorption of degradation products.
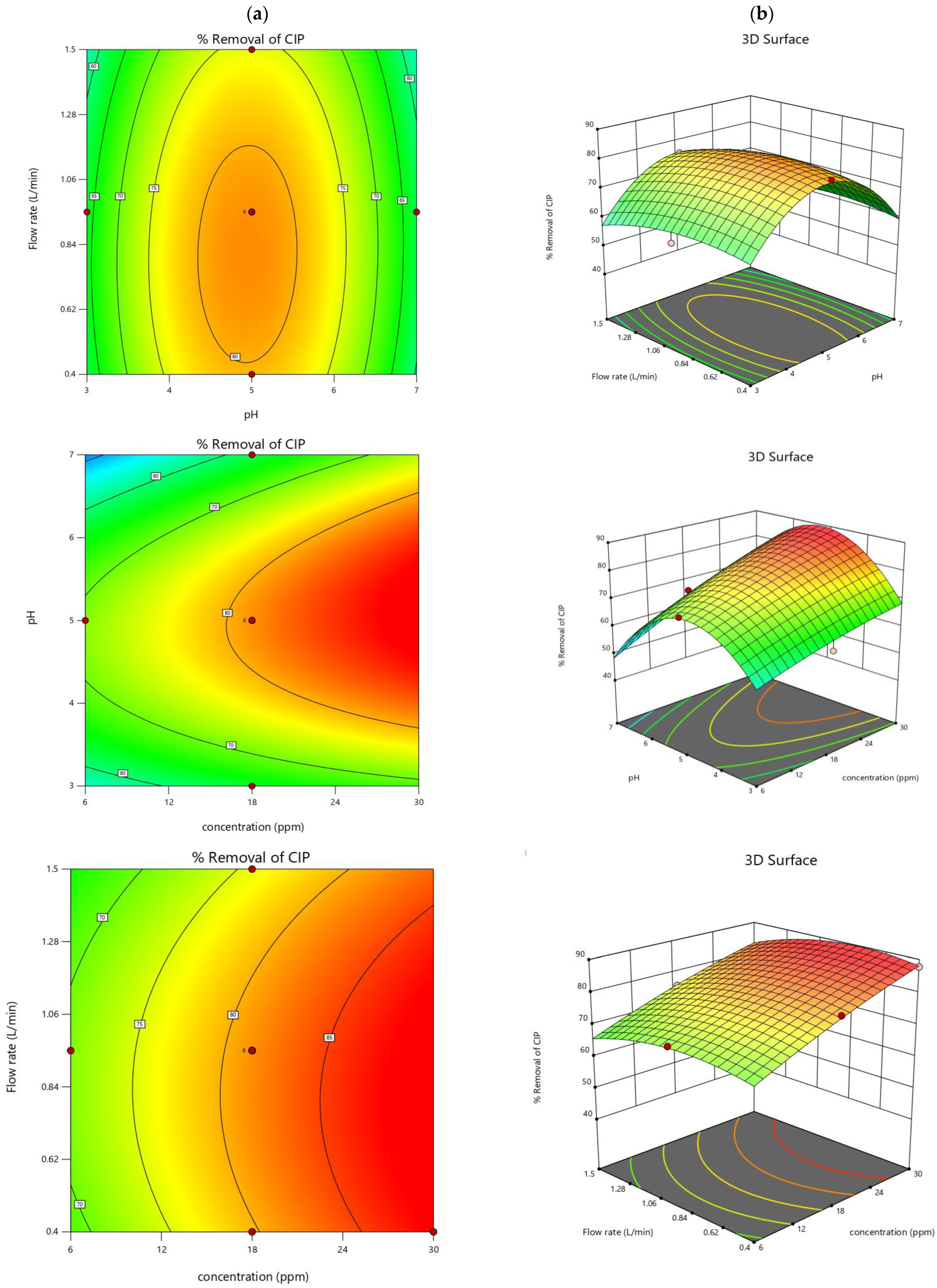
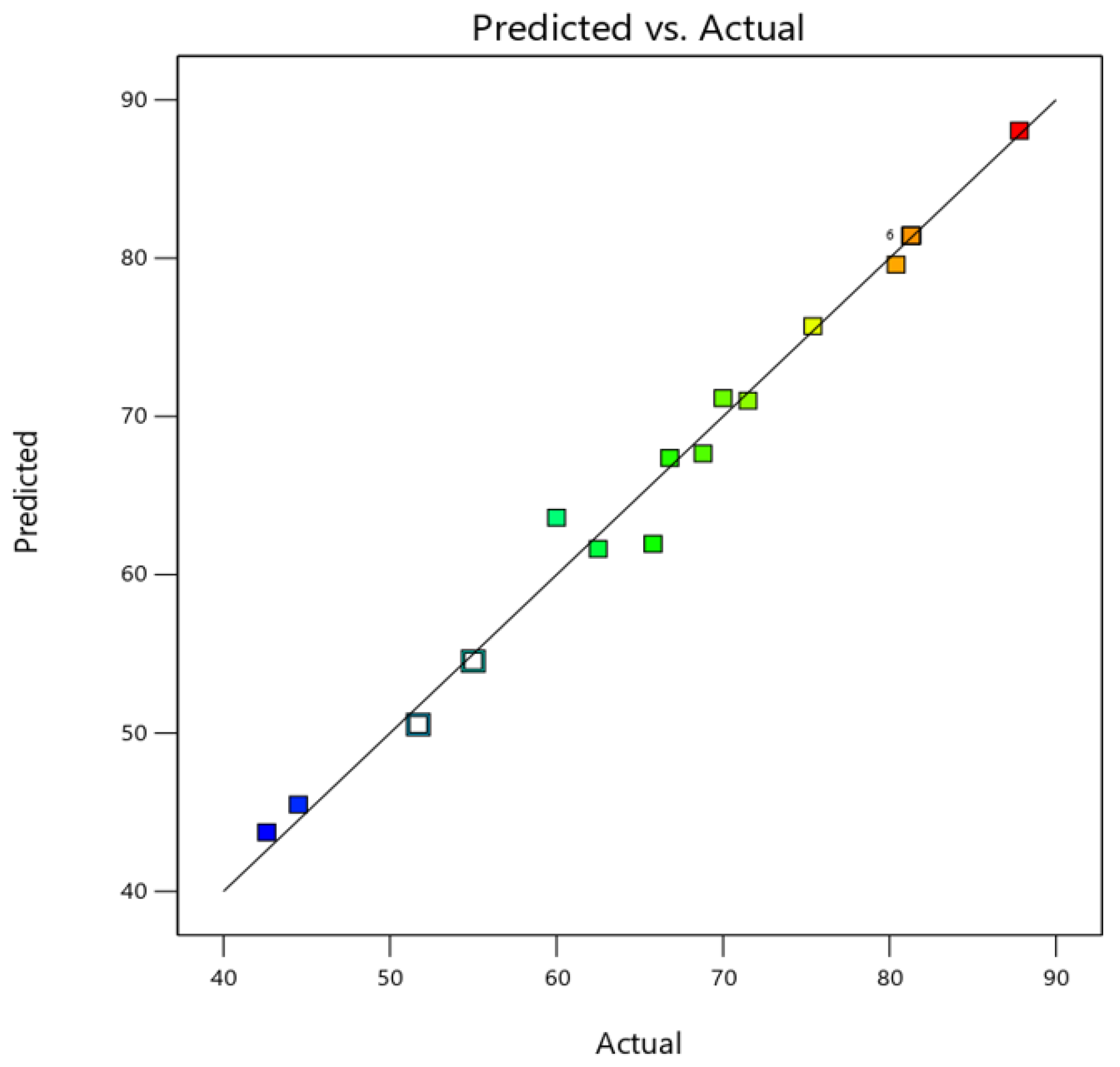
2.4. Design-Expert Software Results
2.4.1. Graphical Interpretation by 2D and 3D Response Surfaces
2.4.2. Predicted Model
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of N-Doped TiO2
3.2.2. Coating Process Using the Nitrogen-Doped Catalyst
3.2.3. Analysis Methods
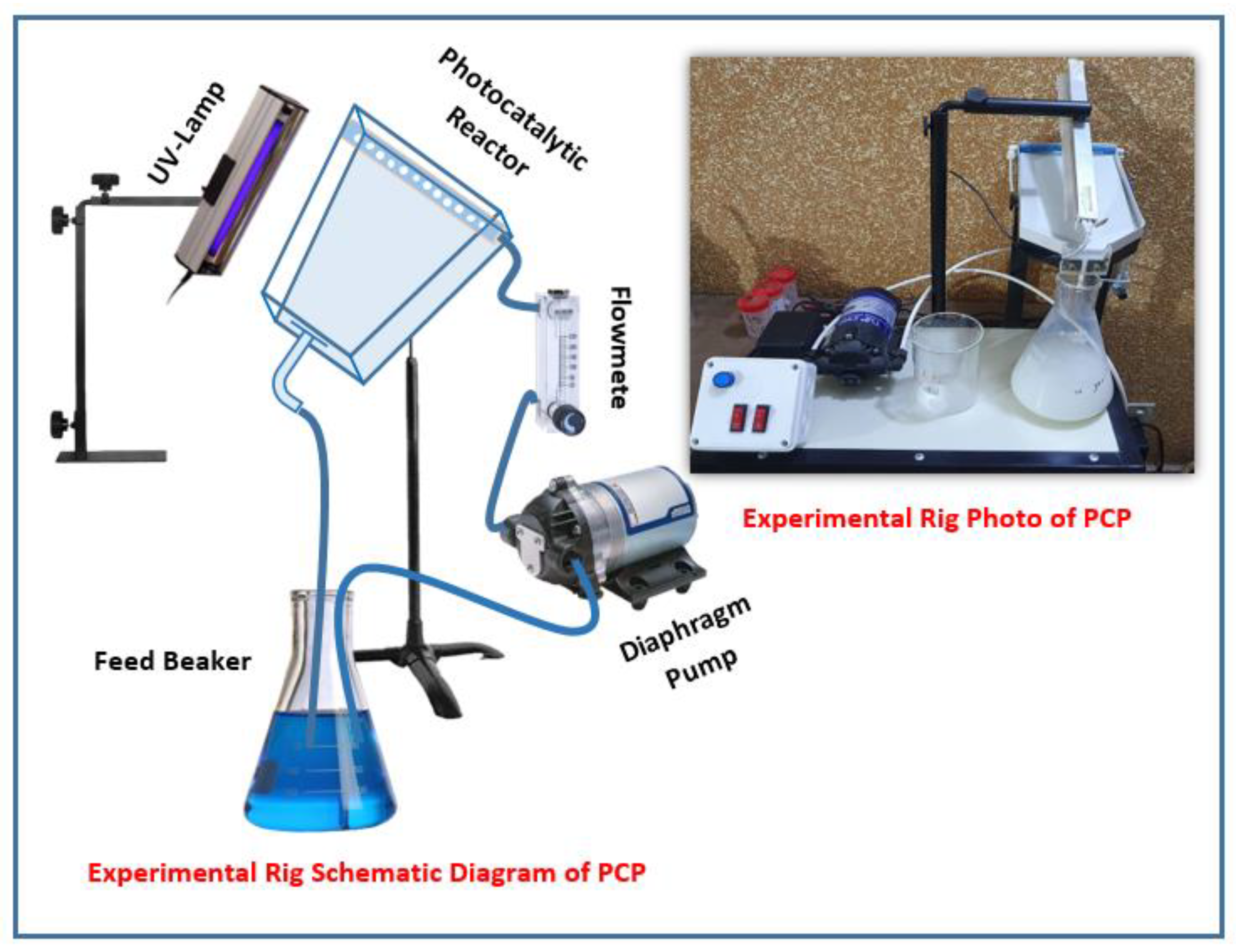
3.2.4. Experimental Setup
3.2.5. Experimental Design and Optimization Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton’s Reaction Chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef] [PubMed]
- Lopez, R.; Menendez, M.I.; Diaz, N.; Suarez, D.; Campomanes, P.; Ardura, D.; Sordo, T.L. Theoretical studies on the ring opening of β-lactams: Processes in solution and in enzymatic media. Curr. Org. Chem. 2006, 10, 805–821. [Google Scholar] [CrossRef]
- Nikolaous, A.; Meric, S.; Fatta, D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef]
- Jayasiri, H.B.; Purushothaman, C.S.; Vennila, A. Pharmaceutically active compounds (PhACs): A threat for aquatic environment. J. Mar. Sci. Res. Dev. 2013, 4, e122. [Google Scholar] [CrossRef]
- Alonso, J.J.S.; El Kori, N.; Melián-Martel, N.; Del Río-Gamero, B. Removal of ciprofoxacin from seawater by reverse osmosis. J. Environ. Manage. 2018, 217, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; McDonald, T.J.; Kim, H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef]
- Peng, X.; Hu, F.; Zhang, T.; Qiu, F.; Dai, H. Amine-functionalized magnetic bamboo-based activated carbon adsorptive removal of ciprofoxacin and norfoxacin: A batch and fxed-bed column study. Bioresour. Technol. 2018, 249, 924–934. [Google Scholar] [CrossRef]
- Mekhamer, W.; Al-Tamimi, S. Removal of ciprofloxacin from simulated wastewater by pomegranate peels. Environ. Sci. Pollut. Res. 2018, 26, 2297–2304. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, J.; Liu, M.; Li, Y.; Ruan, J.; Li, Q.; Tian, J.; Zhu, X.; Zhang, X.; Wei, Y. Synthesis of polyacrylamide immobilized molybdenum disulfide (MoS2@PDA@PAM) composites via mussel-inspired chemistry and surface-initiated atom transfer radical polymerization for removal of copper (II) ions. J. Taiwan Inst. Chem. Eng. 2018, 86, 174–184. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, J.; Liu, M.; Chen, J.; Zhu, X.; Wu, T.; Tian, J.; Wen, Y.; Zhang, X.; Wei, Y. Preparation of polyethylene polyamine@tannic acid encapsulated MgAl-layered double hydroxide for the efficient removal of copper (II) ions from aqueous solution. J. Taiwan Inst. Chem. Eng. 2018, 82, 92–101. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, M.; Zhao, J.; Chen, J.; Zeng, G.; Huang, H.; Tian, J.; Wen, Y.; Zhang, X.; Wei, Y. Facile preparation of polyethylenimine-tannins coated SiO2 hybrid materials for Cu2+ removal. Appl. Surf. Sci. 2018, 427, 535–544. [Google Scholar] [CrossRef]
- Shah, L.A.; Malik, T.; Siddiq, M.; Haleem, A.; Sayed, M.; Naeem, A. TiO2 nanotubes doped poly(vinylidene fluoride) polymer membranes (PVDF/TNT) for efficient photocatalytic degradation of brilliant green dye. J. Environ. Chem. Eng. 2019, 7, 103291. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, D.T.C.; Le, H.T.; Tu, T.T.; Le, N.D.; Lim, K.T.; Bach, L.G.; Nguyen, T.D. MIL-53 (Fe)-directed synthesis of hierarchically mesoporous carbon and its utilization for ciprofloxacin antibiotic remediation. J. Environ. Chem. Eng. 2019, 7, 102881. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Bui, X.-T.; Luu, V.-P.; Nguyen, P.-D.; Guo, W.; Ngo, H.-H. Removal of antibiotics in sponge membrane bioreactors treating hospital wastewater: Comparison between hollow fiber and flat sheet membrane systems. Bioresour. Technol. 2017, 240, 42–49. [Google Scholar] [CrossRef]
- Wajahat, R.; Yasar, A.; Khan, A.M.; Tabinda, A.B.; Bhatti, S.G. Ozonation and Photo-Driven Oxidation of Ciprofloxacin in Pharmaceutical Wastewater: Degradation Kinetics and Energy Requirements. Pol. J. Environ. Stud. 2019, 28, 1933–1938. [Google Scholar] [CrossRef]
- Tasca, A.L.; Clematis, D.; Stefanelli, E.; Panizza, M.; Puccini, M. Ciprofloxacin removal: BDD anode coupled with solid polymer electrolyte and ultrasound irradiation. J. Water Process. Eng. 2020, 33, 101074. [Google Scholar] [CrossRef]
- Mohd Adnan, M.A.; Julkapli, N.M.; Amir, M.N.I.; Maamor, A. Effect on different TiO2 photocatalyst supports on photodecolorization of synthetic dyes: A review. Int. J. Environ. Sci. Technol. 2018, 16, 547–566. [Google Scholar] [CrossRef]
- Rehman, S.; Ullah, R.; Butt, A.; Gohar, N. Strategies of making TiO2 and ZnO visible light active. J. Hazard. Mater. 2009, 170, 560–569. [Google Scholar] [CrossRef]
- Padervand, M.; Ghasemi, S.; Hajiahmadi, S.; Rhimi, B.; Nejad, Z.G.; Karima, S.; Shahsavari, Z.; Wang, C. Multifunctional Ag/AgCl/ZnTiO3 structures as highly efficient photocatalysts for the removal of nitrophenols, CO2 photoreduction, biomedical waste treatment, and bacteria inactivation. Appl. Catal. A Gen. 2022, 643, 118794. [Google Scholar] [CrossRef]
- Wang, H.; Lewis, J.P. Second-generation photocatalytic materials: Anion-doped TiO2. J. Phys. Condens. Matter 2005, 18, 421–434. [Google Scholar] [CrossRef]
- Padervand, M.; Rhimi, B.; Wang, C. One-pot synthesis of novel ternary Fe3N/Fe2O3/C3N4 photocatalyst for efficient removal of rhodamine B and CO2 reduction. J. Alloy. Compd. 2021, 852, 156955. [Google Scholar] [CrossRef]
- Tan, Y.N.; Wong, C.L.; Mohamed, A.R. An Overview on the Photocatalytic Activity of Nano-Doped-TiO2 in the Degradation of Organic Pollutants. ISRN Mater. Sci. 2011, 2011, 261219. [Google Scholar] [CrossRef] [Green Version]
- Bibi, S.; Ahmad, A.; Anjum, M.A.R.; Haleem, A.; Siddiq, M.; Shah, S.S.; Al Kahtani, A. Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105580. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Nitrogen and sulfur co-doped TiO2 nanosheets with exposed {001} facets: Synthesis, characterization and visible-light photocatalytic activity. Phys. Chem. Chem. Phys. 2011, 13, 4853–4861. [Google Scholar] [CrossRef]
- Mozia, S.; Darowna, D.; Przepiórski, J.; Morawski, A.W. Evaluation of Performance of Hybrid Photolysis-DCMD and Photocatalysis-DCMD Systems Utilizing UV-C Radiation for Removal of Diclofenac Sodium Salt From Water. Pol. J. Chem. Technol. 2013, 15, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Gad-Allah, T.A.; Ali, M.E.; Badawy, M.I. Photocatalytic oxidation of ciprofloxacin under simulated sunlight. J. Hazard. Mater. 2011, 186, 751–755. [Google Scholar] [CrossRef]
- Durán-Álvarez, J.C.; Avella, E.; Ramírez-Zamora, R.M.; Zanella, R. Photocatalytic degradation of ciprofloxacin using mono-(Au, Ag and Cu) and bi-(Au–Ag and Au–Cu) metallic nanoparticles supported on TiO2 under UV-C and simulated sunlight. Catal. Today 2016, 266, 175–187. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, S.; Wang, Y.; Sun, X.; Gao, Y.; Gao, B. Enhanced degradation of ciprofloxacin by graphitized mesoporous carbon (GMC)-TiO2 nanocomposite: Strong synergy of adsorption-photocatalysis and antibiotics degradation mechanism. J. Colloid Interface Sci. 2018, 527, 202–213. [Google Scholar] [CrossRef]
- Malakootian, M.; Nasiri, A.; Amiri Gharaghani, M. Photocatalytic degradation of ciprofloxacin antibiotic by TiO2 nanoparticles immobilized on a glass plate. Chem. Eng. Commun. 2019, 207, 56–72. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Nguyen, H.T.; Pham, T.D.; Tran, T.D.; Chu, H.T.; Dang, H.T.; Nguyen, V.-H.; Nguyen, K.M.; Pham, T.T.; Van der Bruggen, B. UV–Visible Light Driven Photocatalytic Degradation of Ciprofloxacin by N,S Co-doped TiO2: The Effect of Operational Parameters. Top. Catal. 2020, 63, 985–995. [Google Scholar] [CrossRef]
- Manasa, M.; Chandewar, P.R.; Mahalingam, H. Photocatalytic degradation of ciprofloxacin & norfloxacin and disinfection studies under solar light using boron & cerium doped TiO2 catalysts synthesized by green EDTA-citrate method. Catal. Today 2021, 375, 522–536. [Google Scholar] [CrossRef]
- Wafi, A.; Szabó-Bárdos, E.; Horváth, O.; Makó, É.; Jakab, M.; Zsirka, B. Coumarin-based quantification of hydroxyl radicals and other reactive species generated on excited nitrogen-doped TiO2. J. Photochem. Photobiol. A Chem. 2021, 404, 112913. [Google Scholar] [CrossRef]
- Mozia, S.; Tomaszewska, M.; Morawski, A.W. Photodegradation of azo dye Acid Red 18 in a quartz labyrinth flow reactor with immobilized TiO2 bed. Dye. Pigment. 2007, 75, 60–66. [Google Scholar] [CrossRef]
- Kamalakkannan, J.; Chandraboss, V.L.; Prabha, S.; Senthilvelan, S. Activated Carbon Loaded N, S Co-Doped TiO2 Nanomaterial and its Dye Wastewater Treatment. Int. Lett. Chem. Phys. Astron. 2015, 47, 147–164. [Google Scholar] [CrossRef]
- Noophum, B.; Sikong, L.; Kooptanond, K. Photocatalytic properties of nitrogen-sulfur co-doped TiO2 films coated on glass fiber. Adv. Mater. Res. 2013, 781–784, 2237–2240. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Xiong, W.; Zhang, Z. Photocatalytic reduction of nitroarenes to azo compounds over N-doped TiO2: Relationship between catalysts and chemical reactivity. Res. Chem. Intermed. 2013, 41, 3981–3997. [Google Scholar] [CrossRef]
- Brindha, A.; Sivakumar, T. Visible active N, S co-doped TiO2/graphene photocatalysts for the degradation of hazardous dyes. J. Photochem. Photobiol. A Chem. 2017, 340, 146–156. [Google Scholar] [CrossRef]
- Mozia, S.; Morawski, A.W. The performance of a hybrid photocatalysis–MD system for the treatment of tap water contaminated with ibuprofen. Catal. Today 2012, 193, 213–220. [Google Scholar] [CrossRef]
- Soares, S.F.; Nogueira, J.; Trindade, T.; Daniel-da-Silva, A.L. Towards efficient ciprofloxacin adsorption using magnetic hybrid nanoparticles prepared with κ-, ι-, and λ-carrageenan. J. Nanostructure Chem. 2022. [Google Scholar] [CrossRef]
- Abid, M.F.; Hamza, N.H. Removal of Methyl Violet Dye from Synthetic Wastewater Using a Hybrid Detoxification Process. Eng. Technol. J. 2014, 32, 1544–1561. [Google Scholar]
- Plakas, K.V.; Sarasidis, V.C.; Patsios, S.I.; Lambropoulou, D.A.; Karabelas, A.J. Novel pilot scale continuous photocatalytic membrane reactor for removal of organic micropollutants from water. Chem. Eng. J. 2016, 304, 335–343. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Giraldo-Aguirre, A.L.; Silva-Agredo, J.; Flórez-Acosta, O.A.; Torres-Palma, R.A. Removal of antibiotic cloxacillin by means of electrochemical oxidation, TiO2 photocatalysis, and photo-Fenton processes: Analysis of degradation pathways and effect of the water matrix on the elimination of antimicrobial activity. Environ. Sci. Pollut. Res. 2017, 24, 6339–6352. [Google Scholar] [CrossRef] [PubMed]
- Nakarmi, K.J.; Daneshvar, E.; Eshaq, G.; Puro, L.; Maiti, A.; Nidheesh, P.; Wang, H.; Bhatnagar, A. Synthesis of biochar from iron-free and iron-containing microalgal biomass for the removal of pharmaceuticals from water. Environ. Res. 2022, 214, 114041. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.-M.; Zhang, Y.-Q.; Zheng, Y.-F.; Xu, C.-S.; Liu, G.-M. Enhanced visible light photocatalytic activity of N, F-codoped TiO2 powders with high thermal stability. Environ. Technol. 2018, 40, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yang, Q.; Yao, F.; Wang, S.; Sun, J.; An, H.; Yi, K.; Wang, Y.; Zhou, Y.; Wang, L.; et al. Visible-light photocatalytic degradation of multiple antibiotics by AgI nanoparticle-sensitized Bi5O7I microspheres: Enhanced interfacial charge transfer based on Z-scheme heterojunctions. J. Catal. 2017, 352, 160–170. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, T.; Chen, B.; Malkoske, T.; Yu, S.; Tang, Y. Degradation of Tetracycline with BiFeO3 Prepared by a Simple Hydrothermal Method. Materials 2015, 8, 6360–6378. [Google Scholar] [CrossRef]
- Jiang, W.-T.; Chang, P.-H.; Wang, Y.-S.; Tsai, Y.; Jean, J.-S.; Li, Z.; Krukowski, K. Removal of ciprofloxacin from water by birnessite. J. Hazard. Mater. 2013, 250–251, 362–369. [Google Scholar] [CrossRef]
- Yu, X.; Kou, S.; Zhang, J.; Tang, X.; Yang, Q.; Yao, B. Preparation and characterization of Cu2O nano-particles and their photocatalytic degradation of fluroxypyr. Environ. Technol. 2018, 39, 2967–2976. [Google Scholar] [CrossRef]
- Zhou, F.; Yan, C.; Liang, T.; Sun, Q.; Wang, H. Photocatalytic degradation of orange G using sepiolite-TiO2 nanocomposites: Optimization of physicochemical parameters and kinetics studies. Chem. Eng. Sci. 2018, 183, 231–239. [Google Scholar] [CrossRef]






| Conc. of CIP (ppm) | Conc. of Catalyst (mg/L) | Catalyst Type-Form | pH | λmax (nm) | Time (min) | R (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 50 | 1000 | Net-TiO2-Sus. | 5.8 | Sunlight | 120 | 95 | [28] |
| 30 | 500 | Au-TiO2-Sus. | 6.1 | 254 | 180 | 63 | [29] |
| 15 | 350 | GMC-TiO2-Sus. | 5.8 | 254 | 90 | 94 | [30] |
| 3 | 1000 | Net-TiO2-Cot. | 5 | 276 | 105 | 92.8 | [31] |
| 30 | 2000 | S,N-TiO2-Sus. | 5.5 | 276 | 180 | 78.7 | [32] |
| 40 | 500 | B-TiO2-Sus. Ce-TiO2-Sus. | 5.5–6 | 276 | 180 | 90–93 | [33] |
| 30 | 1000 | N-TiO2-Cot. | 5 | 257 | 120 | 94.5 | present work |
| Std | Run | Con. (ppm) | pH | Flowrate (L/min) | R (%) |
|---|---|---|---|---|---|
| 17 | 1 | 18 | 5 | 0.95 | 81.3 |
| 20 | 2 | 18 | 5 | 0.95 | 81.3 |
| 6 | 3 | 30 | 3 | 1.5 | 62.5 |
| 13 | 4 | 18 | 5 | 0.4 | 80.4 |
| 15 | 5 | 18 | 5 | 0.95 | 81.3 |
| 3 | 6 | 6 | 7 | 0.4 | 44.5 |
| 5 | 7 | 6 | 3 | 1.5 | 51.7 |
| 1 | 8 | 6 | 3 | 0.4 | 55.0 |
| 10 | 9 | 30 | 5 | 0.4 | 87.8 |
| 9 | 10 | 6 | 5 | 0.95 | 71.5 |
| 2 | 11 | 30 | 3 | 0.4 | 68.8 |
| 18 | 12 | 18 | 5 | 0.95 | 81.3 |
| 19 | 13 | 18 | 5 | 0.95 | 81.3 |
| 15 | 14 | 18 | 5 | 0.95 | 81.3 |
| 14 | 15 | 18 | 5 | 1.5 | 75.4 |
| 8 | 16 | 30 | 7 | 1.5 | 66.8 |
| 11 | 17 | 18 | 3 | 0.95 | 60.0 |
| 7 | 18 | 6 | 7 | 1.5 | 42.6 |
| 12 | 19 | 18 | 7 | 0.95 | 65.8 |
| 4 | 20 | 30 | 7 | 0.4 | 70.0 |
| Source | SS | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 3328.09 | 9 | 369.79 | 101.29 | <0.0001 |
| A ≡ concentration | 796.65 | 1 | 796.65 | 218.22 | <0.0001 |
| B ≡ pH | 6.89 | 1 | 6.89 | 1.89 | 0.1995 |
| C ≡ flow rate | 39.90 | 1 | 39.90 | 10.93 | 0.0079 |
| AB | 78.75 | 1 | 78.75 | 21.57 | 0.0009 |
| AC | 2.19 | 1 | 2.19 | 0.5989 | 0.4569 |
| BC | 2.53 | 1 | 2.53 | 0.6934 | 0.4245 |
| A2 | 3.12 | 1 | 3.12 | 0.8536 | 0.3773 |
| B2 | 1047.05 | 1 | 1047.05 | 286.80 | <0.0001 |
| C2 | 33.47 | 1 | 33.47 | 9.17 | 0.0127 |
| Operating Conditions | Low | High |
|---|---|---|
| Initial concentration (ppm) | 6 | 30 |
| pH | 3 | 7 |
| Flow rate (L/min) | 0.4 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulrahman, S.A.; Shnain, Z.Y.; Ibrahim, S.S.; Majdi, H.S. Photocatalytic Degradation of Ciprofloxacin by UV Light Using N-Doped TiO2 in Suspension and Coated Forms. Catalysts 2022, 12, 1663. https://doi.org/10.3390/catal12121663
Abdulrahman SA, Shnain ZY, Ibrahim SS, Majdi HS. Photocatalytic Degradation of Ciprofloxacin by UV Light Using N-Doped TiO2 in Suspension and Coated Forms. Catalysts. 2022; 12(12):1663. https://doi.org/10.3390/catal12121663
Chicago/Turabian StyleAbdulrahman, Sarah A., Zainab Y. Shnain, Salah S. Ibrahim, and Hasan Sh. Majdi. 2022. "Photocatalytic Degradation of Ciprofloxacin by UV Light Using N-Doped TiO2 in Suspension and Coated Forms" Catalysts 12, no. 12: 1663. https://doi.org/10.3390/catal12121663
APA StyleAbdulrahman, S. A., Shnain, Z. Y., Ibrahim, S. S., & Majdi, H. S. (2022). Photocatalytic Degradation of Ciprofloxacin by UV Light Using N-Doped TiO2 in Suspension and Coated Forms. Catalysts, 12(12), 1663. https://doi.org/10.3390/catal12121663









