Chemo-Enzymatic Synthesis of Enantiopure β-Antagonist (S)-Betaxolol
Abstract
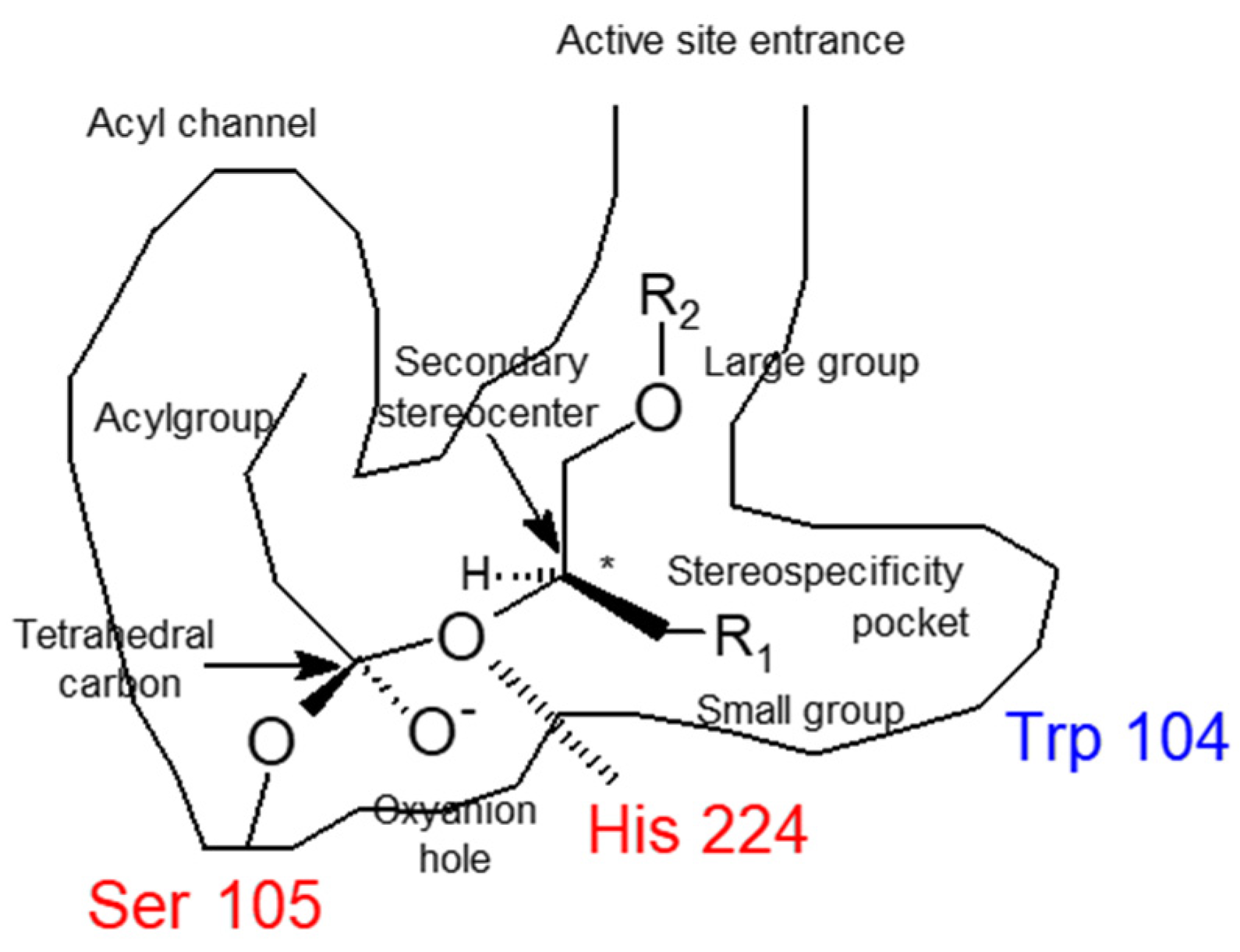
1. Introduction
2. Results and Discussion
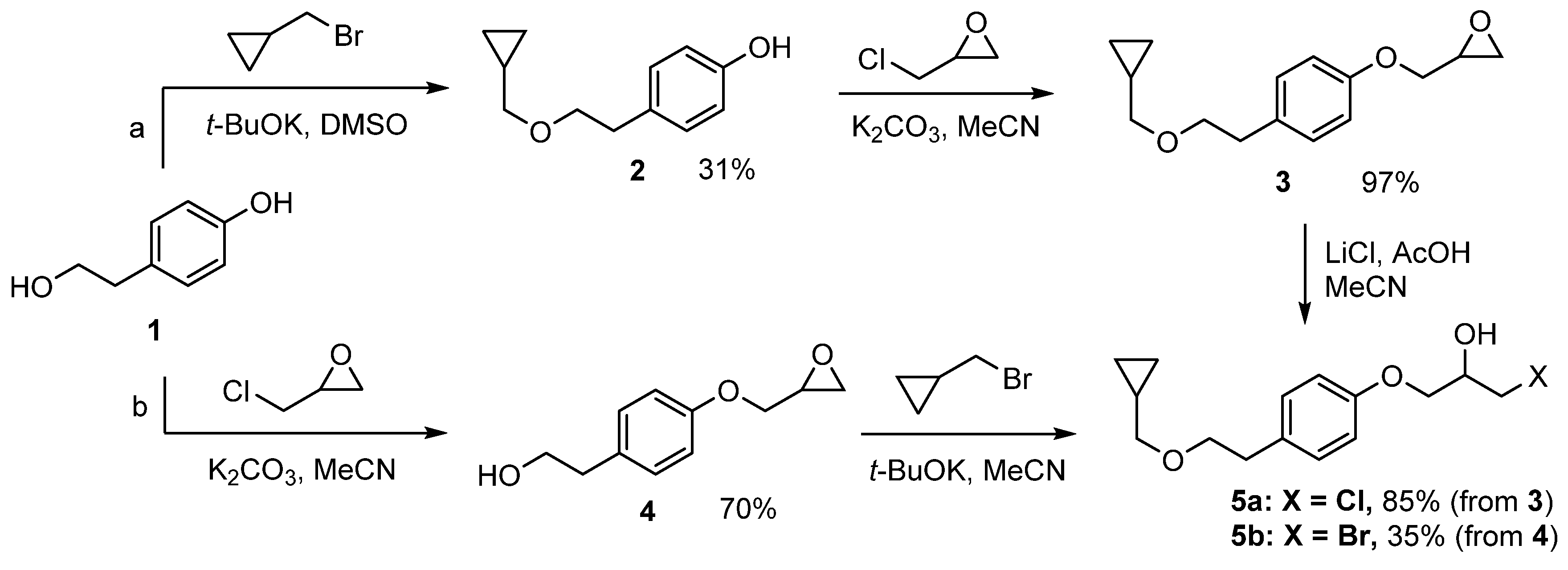
2.1. Synthesis of 1-chloro-3-(4-(2-(cyclopropylmethoxy)ethyl)phenoxy)propan-2-ol (5a) and 1-bromo-3-(4-(2-(cyclopropylmethoxy)ethyl)phenoxy)propan-2-ol (5b)
2.1.1. Synthesis of 1-chloro-3-(4-(2-(cyclopropylmethoxy)ethyl)phenoxy)propan-2-ol (5a)
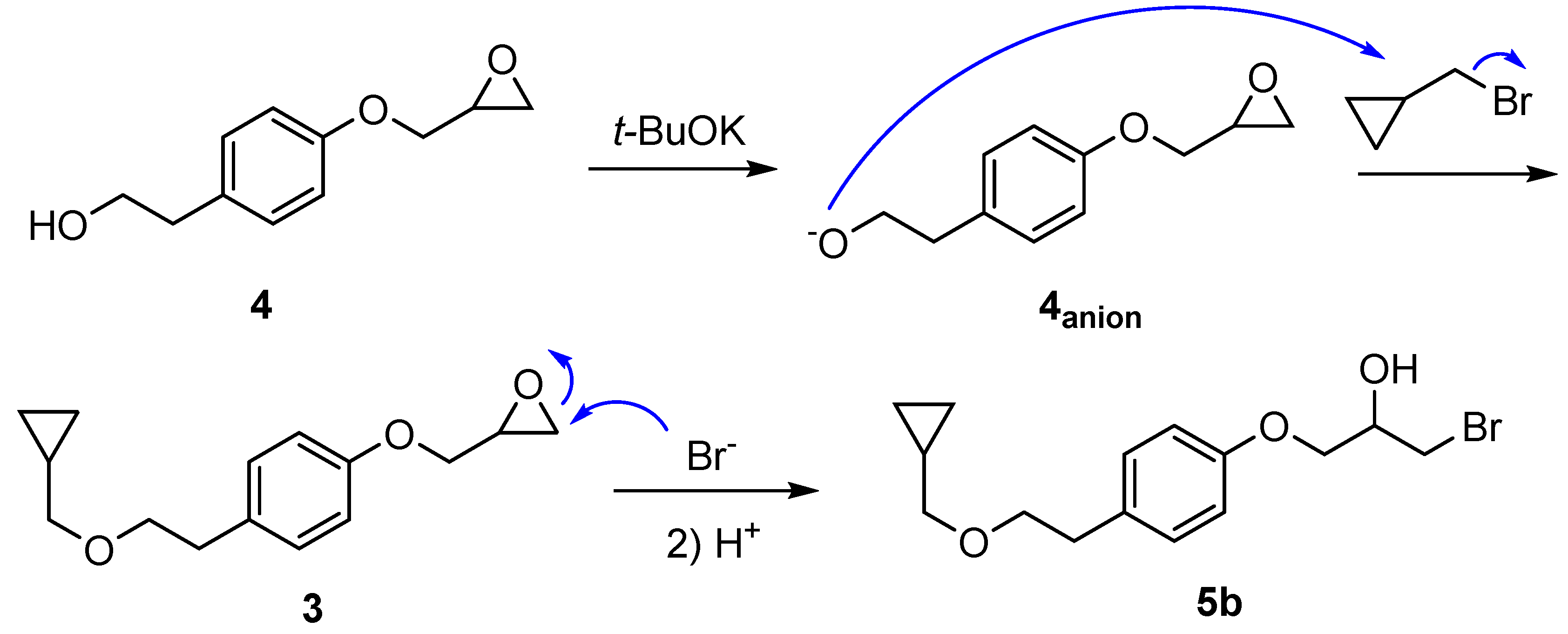
2.1.2. Synthesis of 1-bromo-3-(4-(2-(cyclopropylmethoxy)ethyl)phenoxy)propan-2-ol (5b)
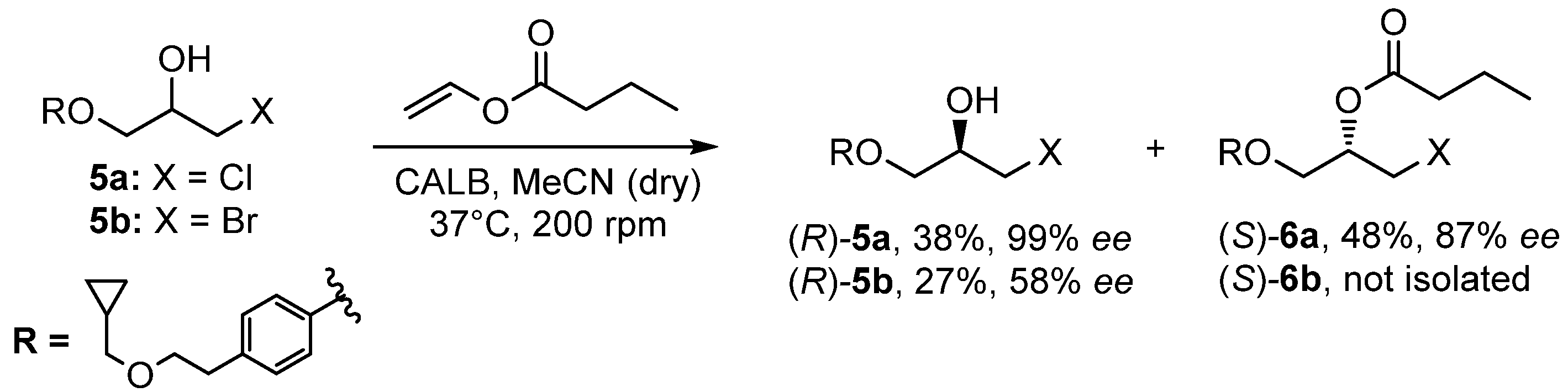
2.2. CALB Catalyzed Kinetic Resolution of 1-chloro-3-(4-(2-(cyclopropylmethoxy)ethyl)phenoxy)propan-2-ol (5a) and 1-bromo-3-(4-(2-(cyclopropylmethoxy)ethyl)phenoxy)propan-2-ol (5b)
2.3. Synthesis of (S)-betaxolol ((S)-7)
3. Materials and Methods
3.1. Chemicals and Solvents
3.2. TLC-Analyses and Column Chromatography
3.3. Enzyme Preparations
3.4. NMR Analyses
3.5. Optical Rotation
3.6. Chiral HPLC Analyses
3.7. Liquid Chromatography-Mass Spectroscopy (LC-MS)
3.8. Mass Spectrometry Analysis
3.9. Synthesis Protocols
3.9.1. 4-(2-(Cyclopropylmethoxy)ethyl)phenol (2)
3.9.2. 2-((4-(2-(Cyclopropylmethoxy)ethyl)phenoxy)methyl)oxirane (3)
3.9.3. 2-(4-(Oxiran-2-ylmethoxy)phenyl)ethan-1-ol (4)
3.9.4. 1-Chloro-3-(4-(2-(cyclopropylmethoxy)ethyl)phenoxy)propan-2-ol (5a)
3.9.5. 1-Bromo-3-(4-(2-(cyclopropylmethoxy)ethyl)phenoxy)propan-2-ol (5b) Correct Procedure
3.9.6. Synthesis of 1-chloro-3-(4-(2-(cyclopropylmethoxy)ethyl)phenoxy)propan-2-yl butanoate (6a)
3.9.7. Synthesis of Racemic Betaxolol (7)
3.9.8. CALB Catalyzed Kinetic Resolution of 1-chloro-3-(4-(2-(cyclopropylme-thoxy)ethyl)phenoxy)propan-2-ol (5a)
3.9.9. CALB catalyzed Kinetic Resolution of 1-bromo-3-(4-(2-(cyclopropylme-thoxy)ethyl)phenoxy)propan-2-ol (5b)
3.9.10. Synthesis of (S)-betaxolol ((S)-7) from (R)-5a
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-wadei, M.J.; Bakheit, A.H.; Abdel-Aziz, A.A.; Wani, T.A. Chapter Three-Betaxolol: A Comprehensive Profile in Profiles of Drug Substances, Excipients and Related Methodology; Al-Majed, A., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 91–136. ISBN 18715125. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Agustian, J.; Kamaruddin, A.; Bhatia, S. Single enantiomeric b-blockers. The existing technologies. Process Biochem. 2010, 45, 1587–1604. [Google Scholar] [CrossRef]
- FDA’s policy statement for the development of new stereoisomeric drugs. Chirality 1992, 4, 338–340. [CrossRef]
- Calcaterra, A.; D’Acquarica, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef]
- Manoury, P.M.; Binet, J.L.; Rousseau, J.; Lefevre-Borg, F.M.; Cavero, I.G. Synthesis of a series of compounds related to betaxolol, a new b1-adrenoceptor antagonist with a pharmacological and pharmacokinetic profile optimized for the treatment of chronic cardiovascular diseases. J. Med. Chem. 1987, 30, 1003–1011. [Google Scholar] [CrossRef]
- Joshi, R.A.; Garud, D.R.; Muthukrishnan, M.; Joshi, R.R.; Gurjar, M.K. A convenient synthesis of the enantiomerically pure b-blocker (S)-betaxolol using hydrolytic kinetic resolution. Tetrahedron: Asymmetry 2005, 16, 3802–3806. [Google Scholar] [CrossRef]
- Wang, X.W.; Bhatia, A.V.; Chamberlin, S.A.; Luping, L. Selective alkylation of an alcohol substituted phenol compound. U.S. Patent 5731463, 24 March 1998. [Google Scholar]
- Datta, G.K.; von Schenck, H.; Hallberg, A.; Larhed, M. Selective Terminal Heck Arylation of Vinyl Ethers with Aryl Chlorides, A Combined Experimental Computational Approach Including Synthesis of Betaxolol. J. Org. Chem. 2006, 71, 3896–3903. [Google Scholar] [CrossRef]
- Muthukrishnan, M.; Garud, D.R.; Joshi, R.R.; Joshi, R.A. Concise synthesis of b-blockers (S)-metoprolol and (S)-betaxolol using hydrolytic kinetic resolution. Tetrahedron 2007, 63, 1872–1876. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Liu, H.-M.; Wang, X.-J.; Wang, P.; Zheng, J.-X. Application of kinetic resolution using HCS as chiral auxiliary, Novel synthesis of b-blockers (S)-betaxolol and (S)-metoprolol. Chirality 2009, 21, 745–750. [Google Scholar] [CrossRef]
- Liu, H.-M.; Liu, F.-W.; Song, X.-P.; Zhang, J.-Y.; Yan, L. A novel free C-12 higher carbon sugar: Asymmetric synthesis and reactivity with nucleophiles. Tetrahedron: Asymmetry 2006, 17, 3230–3236. [Google Scholar] [CrossRef]
- Di Bono, G.; Scilimati, A. A Chemoenzymatic Route to Both Enantiomers of Betaxolol. Synthesis 1995, 6, 699–702. [Google Scholar] [CrossRef]
- Li, Y.-H.; Huang, L.-H.; Liu, H.-M. Chemoenzymatic Route to S-Betaxolol. Synth. Commun. 2011, 41, 2468–2474. [Google Scholar] [CrossRef]
- Lund, I.T.; Bøckmann, P.L.; Jacobsen, E.E. Highly enantioselective CALB catalyzed kinetic resolution of building blocks for b-blocker atenolol. Tetrahedron 2016, 72, 7288–7292. [Google Scholar] [CrossRef]
- Gundersen, M.A.; Austli, G.B.; Løvland, S.S.; Hansen, M.B.; Rødseth, M.; Jacobsen, E.E. Lipase Catalyzed Synthesis of Enantiopure Precursors and Derivatives for β-Blockers Practolol, Pindolol and Carteolol. Catalysts 2021, 11, 503. [Google Scholar] [CrossRef]
- Troøyen, S.H.; Bocquin, L.; Tennfjord, A.L.; Klungseth, K.; Jacobsen, E.E. Green Chemo-Enzymatic Protocols for the Synthesis of Enantiopure b-Blockers (S)-Esmolol and (S)-Penbutolol. Catalysts 2022, 12, 980. [Google Scholar] [CrossRef]
- Uppenberg, J.; Öhrner, N.; Norin, M.; Hult, K.; Kleywegt, G.J.; Patkar, S.; Waagen, V.; Anthonsen, T.; Jones, T.A. Crystallographic and molecular modeling studies of lipase B from Candida antarctica reveal a stereospecificity pocket for secondary alcohols. Biochemistry 1995, 34, 16838–16851. [Google Scholar] [CrossRef]
- Jacobsen, E.E.; Hoff, B.H.; Anthonsen, T. Enantiopure derivatives of 1,2-alkanediols. Substrate requirements for lipase B fromCandida antarctica. Chirality 2000, 12, 654–659. [Google Scholar] [CrossRef]
- Jacobsen, E.E.; Anthonsen, T. Water content influences the selectivity of CALB-catalyzed kinetic resolution of phenoxymethyl-substituted secondary alcohols. Can. J. Chem. 2002, 80, 577–581. [Google Scholar] [CrossRef]
- Brittain, W.D.G.; Cobb, S.L. Tetrafluuoropyridyl (TFP): A general phenol protecting group readily cleaved under mild conditions. Org. Biomol. Chem. 2019, 17, 2110–2115. [Google Scholar] [CrossRef]
- Banoth, L.; Banerjee, U.C. New chemical and chemo-enzymatic synthesis of (RS)-, (R)-, and (S)-esmolol. Arab. J. Chem. 2017, 10, S3603–S3613. [Google Scholar] [CrossRef]
- Anthonsen, H.W.; Hoff, B.H.; Anthonsen, T. Calculation of enantiomer ratio and equilibrium constants in biocatalytic ping-pong bi-bi resolutions. Tetrahedron Asymmetry 1996, 7, 2633–2638. [Google Scholar] [CrossRef]




| Enantiomer | E-Value | Specific Rotation, ee % | Literature Value |
|---|---|---|---|
| (R)-5a | 57 | (c 1.04, CHCl3), 99% ee | (c 1.0, CHCl3), 91% ee [14] |
| (R)-5b | - | ||
| (S)-6a | 57 | (c 0.97, CHCl3), 87 % ee | |
| (S)-7 | - | (c 0.97, CHCl3), 99% ee | (c 1.0, CHCl3), 99% ee [8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troøyen, S.H.; Jacobsen, E.E. Chemo-Enzymatic Synthesis of Enantiopure β-Antagonist (S)-Betaxolol. Catalysts 2022, 12, 1645. https://doi.org/10.3390/catal12121645
Troøyen SH, Jacobsen EE. Chemo-Enzymatic Synthesis of Enantiopure β-Antagonist (S)-Betaxolol. Catalysts. 2022; 12(12):1645. https://doi.org/10.3390/catal12121645
Chicago/Turabian StyleTroøyen, Susanne Hansen, and Elisabeth Egholm Jacobsen. 2022. "Chemo-Enzymatic Synthesis of Enantiopure β-Antagonist (S)-Betaxolol" Catalysts 12, no. 12: 1645. https://doi.org/10.3390/catal12121645
APA StyleTroøyen, S. H., & Jacobsen, E. E. (2022). Chemo-Enzymatic Synthesis of Enantiopure β-Antagonist (S)-Betaxolol. Catalysts, 12(12), 1645. https://doi.org/10.3390/catal12121645








