Improving the Hydrothermal Stability of ZSM-5 Zeolites in 1-Octene Aromatization by Sequential Alkali Treatment and Phosphorus Modification
Abstract
:1. Introduction
2. Results and Discussion
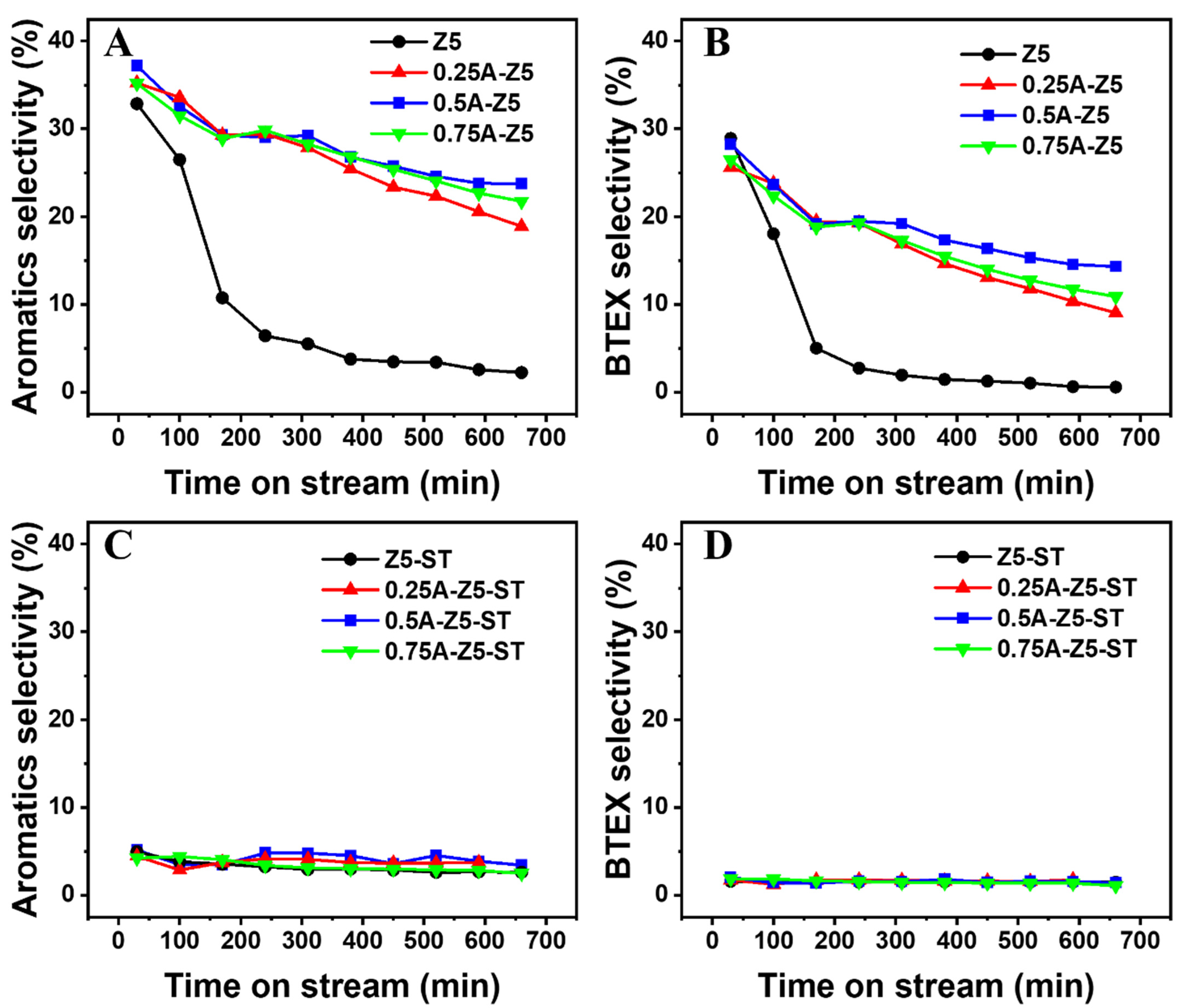
2.1. Evaluation of the Z5 and xA-Z5 Catalysts for 1-Octene Aromatization
2.2. Structural Foundation for the Improved Catalytic Stability of the Alkali-Treated ZSM-5 Samples
2.2.1. Crystal Structure
2.2.2. Textural Properties
2.2.3. Acidic Properties
2.3. Evaluation of the ZSM-5 Catalysts with P Modification in 1-Octene Aromatization
2.4. Structural Characterizations of the ZSM-5 Catalysts with P Modifications
2.4.1. Crystal Structure
2.4.2. Textural Properties
2.4.3. Acidic Properties
| Chemical Shift/ppm | Description | P-0.25A-Z5 | P-0.5A-Z5 | P-0.75A-Z5 |
|---|---|---|---|---|
| 0 | Monomeric phosphates [39] | 2.7% | 0.0% | 5.3% |
| −4–−6 | Terminal phosphates [20,33] | 12.1% | 15.3% | 3.8% |
| −11 | Middle group phosphates [17] | 5.2% | 17.0% | 11.9% |
| −15–−17 | Physical interaction with framework aluminum, nonbonded [34] | 22.6% | 6.1% | 32.3% |
| −26 | Interaction between phosphorus and framework aluminum (similar to AlPO4 structure) [40,41] | 20.9% | 32.5% | 18.6% |
| −32–−38 | Extra-frameworkaluminum phosphate [42] | 12.6% | 16.5% | 6.1% |
| −42–−56 | Condensed polyphosphates [43] | 23.9% | 12.6% | 22.0% |
3. Materials and Methods
3.1. Alkaline Treatment of ZSM-5 Zeolite
3.2. Phosphorus Modification
3.3. Hydrothermal Treatment
3.4. Evaluation of the Catalytic Performance
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, X.; Wang, G.; Bai, X.; Wu, W.; Xiao, L.; Fang, Y.; Zhang, J. Synthesis of nanosized HZSM-5 zeolites isomorphously substituted by gallium and their catalytic performance in the aromatization. Chem. Eng. J. 2016, 293, 365–375. [Google Scholar] [CrossRef]
- Pan, T.; Ge, S.; Yu, M.; Ju, Y.; Zhang, R.; Wu, P.; Zhou, K.; Wu, Z. Synthesis and consequence of Zn modified ZSM-5 zeolite supported Ni catalyst for catalytic aromatization of olefin/paraffin. Fuel 2021, 311, 122629. [Google Scholar] [CrossRef]
- Song, S.; Li, T.; Ju, Y.; Li, Y.; Lv, Z.; Zheng, P.; Duan, A.; Wu, P.; Wang, X. Lanthanum/Gallium-modified Zn/ZSM-5 zeolite for efficient isomerization/aromatization of FCC light gasoline. Ind. Eng. Chem. Res. 2022, 61, 9667–9677. [Google Scholar] [CrossRef]
- Gao, D.; Zhi, Y.; Cao, L.; Zhao, L.; Gao, J.; Xu, C. Optimizing the acid properties of the HZSM-5 catalyst for increasing the p-Xylene yield in 1-Hexene aromatization. Ind. Eng. Chem. Res. 2022, 61, 3539–3549. [Google Scholar] [CrossRef]
- Nash, R.J.; Dry, M.E.; O’Connor, C.T. Aromatization of 1-hexene and 1-octene by gallium/H-ZSM-5 catalysts. Appl. Catal. A 1996, 134, 285–297. [Google Scholar] [CrossRef]
- Wang, H.; Hou, Y.; Sun, W.; Hu, Q.; Xiong, H.; Wang, T.; Yan, B.; Qian, W. Insight into the effects of water on the ethene to aromatics reaction with HZSM-5. ACS Catal. 2020, 10, 5288–5298. [Google Scholar] [CrossRef]
- Wannapakdee, W.; Suttipat, D.; Dugkhuntod, P.; Yutthalekha, T.; Thivasasith, A.; Kidkhunthod, P.; Nokbin, S.; Pengpanich, S.; Limtrakul, J.; Wattanakit, C. Aromatization of C5 hydrocarbons over Ga-modified hierarchical HZSM-5 nanosheets. Fuel 2019, 236, 1243–1253. [Google Scholar] [CrossRef]
- Zhang, C.; Kwak, G.; Lee, Y.-J.; Jun, K.-W.; Gao, R.; Park, H.-G.; Kim, S.; Min, J.-E.; Kang, S.C.; Guan, G. Light hydrocarbons to BTEX aromatics over Zn-modified hierarchical ZSM-5 combined with enhanced catalytic activity and stability. Microporous Mesoporous Mater. 2019, 284, 316–326. [Google Scholar] [CrossRef]
- Ma, Q.; Fu, T.; Li, H.; Cui, L.; Li, Z. Insight into the selection of the post-treatment strategy for ZSM-5 zeolites for the improvement of catalytic stability in the conversion of methanol to hydrocarbons. Ind. Eng. Chem. Res. 2020, 59, 11125–11138. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, X.; Gao, D.; Ma, X.; Li, S.; Wang, Y.; Song, G.; Duan, A.; Chen, G. Hierarchically porous ZSM-5/SBA-15 zeolite: Tuning pore structure and acidity for enhanced hydro-upgrading of FCC gasoline. Ind. Eng. Chem. Res. 2018, 57, 14031–14043. [Google Scholar] [CrossRef]
- Yan, P.; Wang, H.; Liao, Y.; Sun, P.; Wang, C. Introducing mesopore and regulating Al distribution for improving catalytic performances of ZSM-5 in furfuryl alcohol to levulinic acid. Fuel 2022, 329, 125213. [Google Scholar] [CrossRef]
- Seemala, B.; Wyman, C.E. Relationship between ZSM-5 pore modifications and gallium proximity and liquid hydrocarbon number distribution from ethanol oligomerization. Catal. Sci. Technol. 2022, 12, 4903–4916. [Google Scholar] [CrossRef]
- Jin, R.; Ma, K.; Xu, S.; Wei, Y.; Song, L.; Li, Z.; Zhang, P.; Wang, Y.; Wang, J.; Zhang, Z.; et al. Effect of acid distribution and pore structure of ZSM-5 on catalytic performance. React. Chem. Eng. 2022, 7, 2152–2162. [Google Scholar] [CrossRef]
- Védrine, J.C.; Auroux, A.; Dejaifve, P.; Ducarme, V.; Hoser, H.; Zhou, S. Catalytic and physical properties of phosphorus-modified ZSM-5 zeolite. J. Catal. 1982, 73, 147–160. [Google Scholar] [CrossRef]
- Caro, J. NMR and IR studies of zeolite H-ZSM-5 modified with orthophosphoric acid. J. Catal. 1990, 124, 367–375. [Google Scholar] [CrossRef]
- Huangfu, J.; Mao, D.; Zhai, X.; Guo, Q. Remarkably enhanced stability of HZSM-5 zeolite co-modified with alkaline and phosphorous for the selective conversion of bio-ethanol to propylene. Appl. Catal. A 2016, 520, 99–104. [Google Scholar] [CrossRef]
- Ding, J.; Wang, M.; Peng, L.; Xue, N.; Wang, Y.; He, M.-Y. Combined desilication and phosphorus modification for high-silica ZSM-5 zeolite with related study of hydrocarbon cracking performance. Appl. Catal. A 2015, 503, 147–155. [Google Scholar] [CrossRef]
- Sang, Y.; Li, H. Effect of phosphorus and mesopore modification on the HZSM-5 zeolites for n-decane cracking. J. Solid State Chem. 2019, 271, 326–333. [Google Scholar] [CrossRef]
- Gao, X.; Tang, Z.; Ji, D.; Zhang, H. Modification of ZSM-5 zeolite for maximizing propylene in fluid catalytic cracking reaction. Catal. Commun. 2009, 10, 1787–1790. [Google Scholar] [CrossRef]
- Gao, X.; Tang, Z.; Zhang, H.; Liu, C.; Zhang, Z.; Lu, G.; Ji, D. High performance phosphorus-modified ZSM-5 zeolite for butene catalytic cracking. Korean J. Chem. Eng. 2010, 27, 812–815. [Google Scholar] [CrossRef]
- Li, Y.; Shen, B.; Hao, K.; Tao, Z.; Guo, Y.; Shen, B.; Yin, S.; Lu, Y.; Wang, X.; Fan, L.; et al. The invention relates to a catalyst for producing high octane component gasoline and a preparation method and application thereof. CN Patent 112371167 B, 30 September 2022. [Google Scholar]
- Hou, R.; Hao, K.; Yang, D.; Tao, Z.; Jiang, D.; Fu, L.; Wang, X.; Xu, Z.; Yang, Y.; Li, Y. The invention relates to a method and device for generating high octane gasoline from Fischer-Tropsch synthetic oil. CN Patent 112480961 B, 8 April 2022. [Google Scholar]
- Miyamoto, M.; Kamada, J.; Oumi, Y.; Uemiya, S. Effect of silicalite-1 coating on product selectivity over MFI type galloaluminosilicate in aromatization of light alkenes. Adv. Porous Mater. 2016, 4, 102–109. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Panjala, D.; Banerjee, S. Aromatization of propene and n-butene over H-galloaluminosilicate (ZSM-5 type) zeolite. Appl. Catal. A 2002, 231, 243–251. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Guo, Z.; Zhu, X.; Liu, S.; Niu, X.; Xu, L. Effect of variations in acid properties of HZSM-5 on the coking behavior and reaction stability in butene aromatization. Appl. Catal. A 2005, 292, 162–170. [Google Scholar] [CrossRef]
- Song, Y.Q.; Zhu, X.X.; Song, Y.; Wang, Q.X.; Xu, L.Y. An effective method to enhance the stability on-stream of butene aromatization: Post-treatment of ZSM-5 by alkali solution of sodium hydroxide. Appl. Catal. A 2006, 302, 69–77. [Google Scholar] [CrossRef]
- Solymosi, F.; Szechenyi, A. Aromatization of n-butane and 1-butene over supported Mo2C catalyst. J. Catal. 2004, 223, 221–231. [Google Scholar] [CrossRef]
- Vorob’ev, B.L.; Koshelev, Y.N.; Trishin, P.Y.; Khvorova, E.P. Coking and deactivation of a zeolite-containing catalyst during aromatization of C-3-C-4 olefins. Russ. J. Appl. Chem. 1997, 70, 1434–1436. [Google Scholar]
- Zhang, L.; Zhang, H.; Chen, Z.; Ning, Q.; Liu, S.; Ren, J.; Wen, X.; Li, Y.-W. Insight into the impact of Al distribution on the catalytic performance of 1-octene aromatization over ZSM-5 zeolite. Catal. Sci. Technol. 2019, 9, 7034–7044. [Google Scholar] [CrossRef]
- Long, H.; Wang, X.; Sun, W.; Xiong, G.; Wang, K. Effect of acidity on n-octene reaction over potassium modified nanoscale HZSM-5. Fuel 2008, 87, 3660–3663. [Google Scholar] [CrossRef]
- Long, H.; Wang, X.; Sun, W. Study of n-octene aromatization over nanoscale HZSM-5 zeolite. Microporous Mesoporous Mater. 2009, 119, 18–22. [Google Scholar] [CrossRef]
- Lin, W.; Song, Y.; Han, L.; Yang, X.; Liu, J.; Peng, B. Dehydrogenative aromatization of 1-octene over multifunctional Ni/ZSM-5-P-Fe catalyst. Fuel 2021, 299, 120890. [Google Scholar] [CrossRef]
- Chen, L.-H.; Sun, M.-H.; Wang, Z.; Yang, W.; Xie, Z.; Su, B.-L. Hierarchically structured zeolites: From design to application. Chem. Rev. 2020, 120, 11194–11294. [Google Scholar] [CrossRef] [PubMed]
- van der Bij, H.E.; Meirer, F.; Kalirai, S.; Wang, J.; Weckhuysen, B.M. Hexane cracking over steamed phosphated zeolite H-ZSM-5: Promotional effect on catalyst performance and stability. Chem. Eur. J. 2014, 20, 16922–16932. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Yokoi, T.; Imai, H.; Namba, S.; Kondo, J.N.; Tatsumi, T. Effect of desilication of H-ZSM-5 by alkali treatment on catalytic performance in hexane cracking. Appl. Catal. A 2012, 449, 188–197. [Google Scholar] [CrossRef]
- Gohlich, M.; Reschetilowski, W.; Paasch, S. Spectroscopic study of phosphorus modified H-ZSM-5. Microporous Mesoporous Mater. 2011, 142, 178–183. [Google Scholar] [CrossRef]
- Han, L.; Ouyang, Y.; Xing, E.; Luo, Y.; Da, Z. Enhancing hydrothermal stability of framework Al in ZSM-5: From the view on the transformation between P and Al species by solid-state NMR spectroscopy. Chin. J. Chem. Eng. 2020, 28, 3052–3060. [Google Scholar] [CrossRef]
- Wang, C.; Ouyang, Y.; Xing, E.; Luo, Y.; Shu, X. Pressured hydrothermal activation on phosphorus to stabilize framework Al for better ZSM-5-based cracking catalysts. Microporous Mesoporous Mater. 2021, 323, 111205. [Google Scholar] [CrossRef]
- Blasco, T.; Corma, A.; Martinez-Triguero, J. Hydrothermal stabilization of ZSM-5 catalytic-cracking additives by phosphorus addition. J. Catal. 2006, 237, 267–277. [Google Scholar] [CrossRef]
- Peeters, M.P.J.; de Haan, J.W.; van de Ven, L.J.M.; van Hooff, J.H.C. Hydration of AlPO4-11 studied with x-ray powder diffraction and aluminum-27 and phosphorus-31 NMR. J. Phys. Chem. 2002, 97, 5363–5369. [Google Scholar] [CrossRef] [Green Version]
- Van Der Bij, H.E.; Aramburo, L.R.; Arstad, B.; Dynes, J.J.; Wang, J.; Weckhuysen, B.M. Phosphatation of zeolite H-ZSM-5: A combined microscopy and spectroscopy study. Chemphyschem 2014, 15, 283–292. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, L.-L.; Li, G.-D.; Shang, Y.-S.; Zhao, X.-M.; Ma, T.; Zhang, L.-M.; Zhai, Y.-L.; Gong, Y.-J.; Xu, J.; et al. ZSM-5 extrudates modified with phosphorus as a super effective MTP catalyst: Impact of the acidity on binder. Fuel Process. Technol. 2017, 168, 105–115. [Google Scholar] [CrossRef]
- Damodaran, K.; Wiench, J.W.; de Menezes, S.M.C.; Lam, Y.L.; Trebosc, J.; Amoureux, J.P.; Pruski, M. Modification of H-ZSM-5 zeolites with phosphorus. 2. Interaction between phosphorus and aluminum studied by solid-state NMR spectroscopy. Microporous Mesoporous Mater. 2006, 95, 296–305. [Google Scholar] [CrossRef]






| Sample | BET Surface Area [m2/g] | t-Plot External Surface Area [m2/g] | t-Plot Micropore Area [m2/g] | t-Plot Micropore Volume [cm3/g] | Mesopore Volume [cm3/g] | Micropore Retention Rate [%] |
|---|---|---|---|---|---|---|
| Z5 | 374 | 32 | 342 | 0.166 | 0.079 | 100.0 |
| 0.25A-Z5 | 364 | 60 | 304 | 0.149 | 0.130 | 89.7 |
| 0.5A-Z5 | 380 | 105 | 275 | 0.135 | 0.248 | 81.3 |
| 0.75A-Z5 | 371 | 116 | 255 | 0.120 | 0.364 | 72.3 |
| Z5-ST | 285 | 125 | 160 | 0.082 | 0.154 | 49.4 |
| 0.25A-Z5-ST | 294 | 113 | 181 | 0.091 | 0.195 | 54.8 |
| 0.5A-Z5-ST | 302 | 105 | 197 | 0.097 | 0.273 | 58.4 |
| 0.75A-Z5-ST | 272 | 111 | 161 | 0.079 | 0.347 | 47.6 |
| Sample | 200 °C (μmol Py/g) | 350 °C (μmol Py/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| BAS | LAS | Total | L/B | BAS | LAS | Total | L/B | |
| Z5 | 467.8 | 25.6 | 493.4 | 0.05 | 378.0 | 19.7 | 397.7 | 0.05 |
| 0.25A-Z5 | 285.2 | 51.4 | 336.6 | 0.18 | 213.8 | 45.1 | 258.9 | 0.21 |
| 0.5A-Z5 | 324.0 | 85.2 | 409.2 | 0.26 | 218.2 | 75.6 | 293.8 | 0.35 |
| 0.75A-Z5 | 308.0 | 103.5 | 411.5 | 0.34 | 205.4 | 102.3 | 307.7 | 0.50 |
| Z5-ST | 16.3 | 4.9 | 21.2 | 0.30 | 7.3 | 2.2 | 9.5 | 0.30 |
| 0.25A-Z5-ST | 19.5 | 9.5 | 29.0 | 0.49 | 2.7 | 6.7 | 9.4 | 2.48 |
| 0.5A-Z5-ST | 23.1 | 24.1 | 47.2 | 1.04 | 6.0 | 15.8 | 21.8 | 2.63 |
| 0.75A-Z5-ST | 25.8 | 28.3 | 54.1 | 1.10 | 5.9 | 23.2 | 29.1 | 3.93 |
| Sample | BET Surface Area [m2/g] | t-Plot Micropore Area [m2/g] | t-Plot External Surface Area [m2/g] | t-Plot Micropore Volume [cm3/g] | Mesopore Volume [cm3/g] | Micropore Retention Rate [%] |
|---|---|---|---|---|---|---|
| P-Z5 | 283 | 259 | 24 | 0.128 | 0.079 | 77.1 |
| P-0.25A-Z5 | 269 | 233 | 36 | 0.115 | 0.112 | 69.3 |
| P-0.5A-Z5 | 285 | 232 | 53 | 0.114 | 0.197 | 68.7 |
| P-0.75A-Z5 | 239 | 171 | 68 | 0.085 | 0.251 | 51.2 |
| P-Z5-ST | 296 | 280 | 16 | 0.138 | 0.094 | 83.1 |
| P-0.25A-Z5-ST | 303 | 276 | 27 | 0.135 | 0.126 | 81.3 |
| P-0.5A-Z5-ST | 293 | 244 | 49 | 0.122 | 0.209 | 73.5 |
| P-0.75A-Z5-ST | 242 | 184 | 58 | 0.090 | 0.255 | 54.2 |
| Sample | 200 °C (μmol Py/g) | 350 °C (μmol Py/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| BAS | LAS | Total | L/B | BAS | LAS | Total | L/B | |
| P-Z5 | 167.7 | 12.4 | 180.1 | 0.07 | 121.6 | 10.1 | 131.7 | 0.08 |
| P-0.25A-Z5 | 134.1 | 13.3 | 147.4 | 0.10 | 91.7 | 11.2 | 102.9 | 0.12 |
| P-0.5A-Z5 | 121.5 | 16.3 | 137.8 | 0.13 | 97.9 | 14.9 | 112.8 | 0.15 |
| P-0.75A-Z5 | 89.2 | 10.5 | 99.7 | 0.12 | 64.4 | 10.2 | 74.6 | 0.16 |
| P-Z5-ST | 35.7 | 8.7 | 44.4 | 0.24 | 16.9 | 3.6 | 20.5 | 0.21 |
| P-0.25A-Z5-ST | 52.0 | 11.8 | 63.8 | 0.23 | 32.0 | 11.5 | 43.5 | 0.36 |
| P-0.5A-Z5-ST | 53.0 | 16.5 | 69.5 | 0.31 | 29.9 | 12.9 | 42.8 | 0.43 |
| P-0.75A-Z5-ST | 53.2 | 6.3 | 59.5 | 0.12 | 18.0 | 4.4 | 22.4 | 0.24 |
| Catalyst | Si/Al a | Si/Al b | P/% a | Bulk P/Al a | Surface P/Al b |
|---|---|---|---|---|---|
| P-Z5 | 15 | 15 | 1.3 | 0.4 | 0.8 |
| P-0.25A-Z5 | 14 | 13 | 1.7 | 0.5 | 1.0 |
| P-0.5A-Z5 | 11 | 10 | 2.2 | 0.6 | 0.8 |
| P-0.75A-Z5 | 10 | 9 | 5.8 | 1.5 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Xing, M.; Han, Y.; Hao, K.; Zhang, L.; Wang, F.; Zheng, W.; Tao, Z.; Wen, X.; Yang, Y.; et al. Improving the Hydrothermal Stability of ZSM-5 Zeolites in 1-Octene Aromatization by Sequential Alkali Treatment and Phosphorus Modification. Catalysts 2022, 12, 1629. https://doi.org/10.3390/catal12121629
Cao J, Xing M, Han Y, Hao K, Zhang L, Wang F, Zheng W, Tao Z, Wen X, Yang Y, et al. Improving the Hydrothermal Stability of ZSM-5 Zeolites in 1-Octene Aromatization by Sequential Alkali Treatment and Phosphorus Modification. Catalysts. 2022; 12(12):1629. https://doi.org/10.3390/catal12121629
Chicago/Turabian StyleCao, Jian, Mengjiao Xing, Yuanlong Han, Kun Hao, Ling Zhang, Fei Wang, Wentao Zheng, Zhichao Tao, Xiaodong Wen, Yong Yang, and et al. 2022. "Improving the Hydrothermal Stability of ZSM-5 Zeolites in 1-Octene Aromatization by Sequential Alkali Treatment and Phosphorus Modification" Catalysts 12, no. 12: 1629. https://doi.org/10.3390/catal12121629
APA StyleCao, J., Xing, M., Han, Y., Hao, K., Zhang, L., Wang, F., Zheng, W., Tao, Z., Wen, X., Yang, Y., & Li, Y. (2022). Improving the Hydrothermal Stability of ZSM-5 Zeolites in 1-Octene Aromatization by Sequential Alkali Treatment and Phosphorus Modification. Catalysts, 12(12), 1629. https://doi.org/10.3390/catal12121629






