Heterogeneous Catalysis as an Efficient Tool for Selective Hydrogenation of Oximes to Amines and Hydroxylamines
Abstract
:1. Introduction
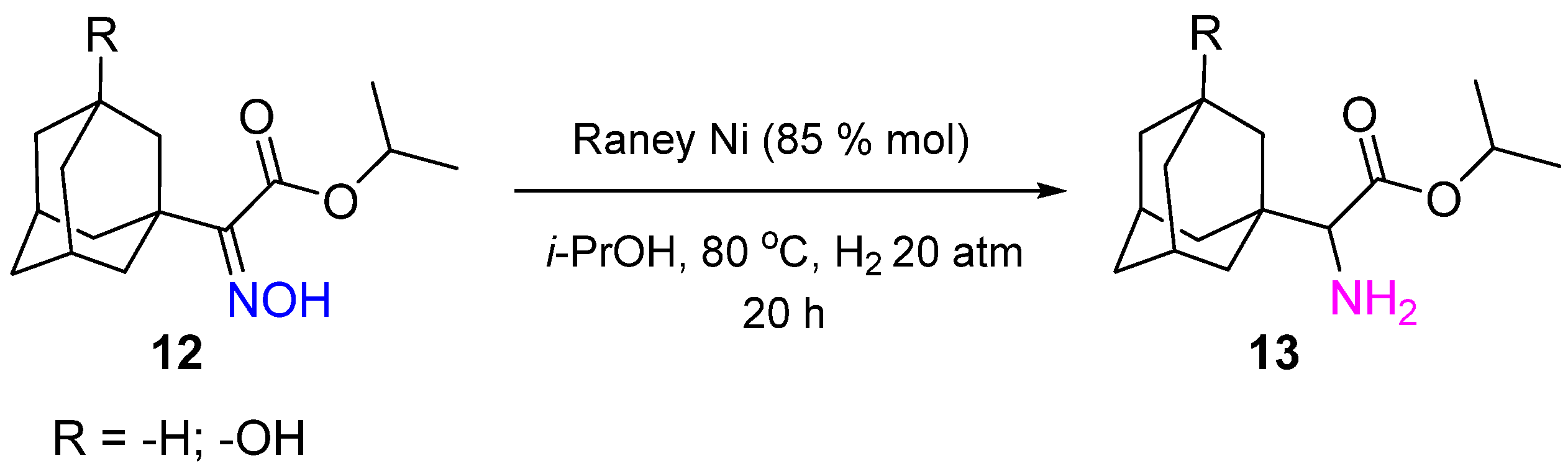
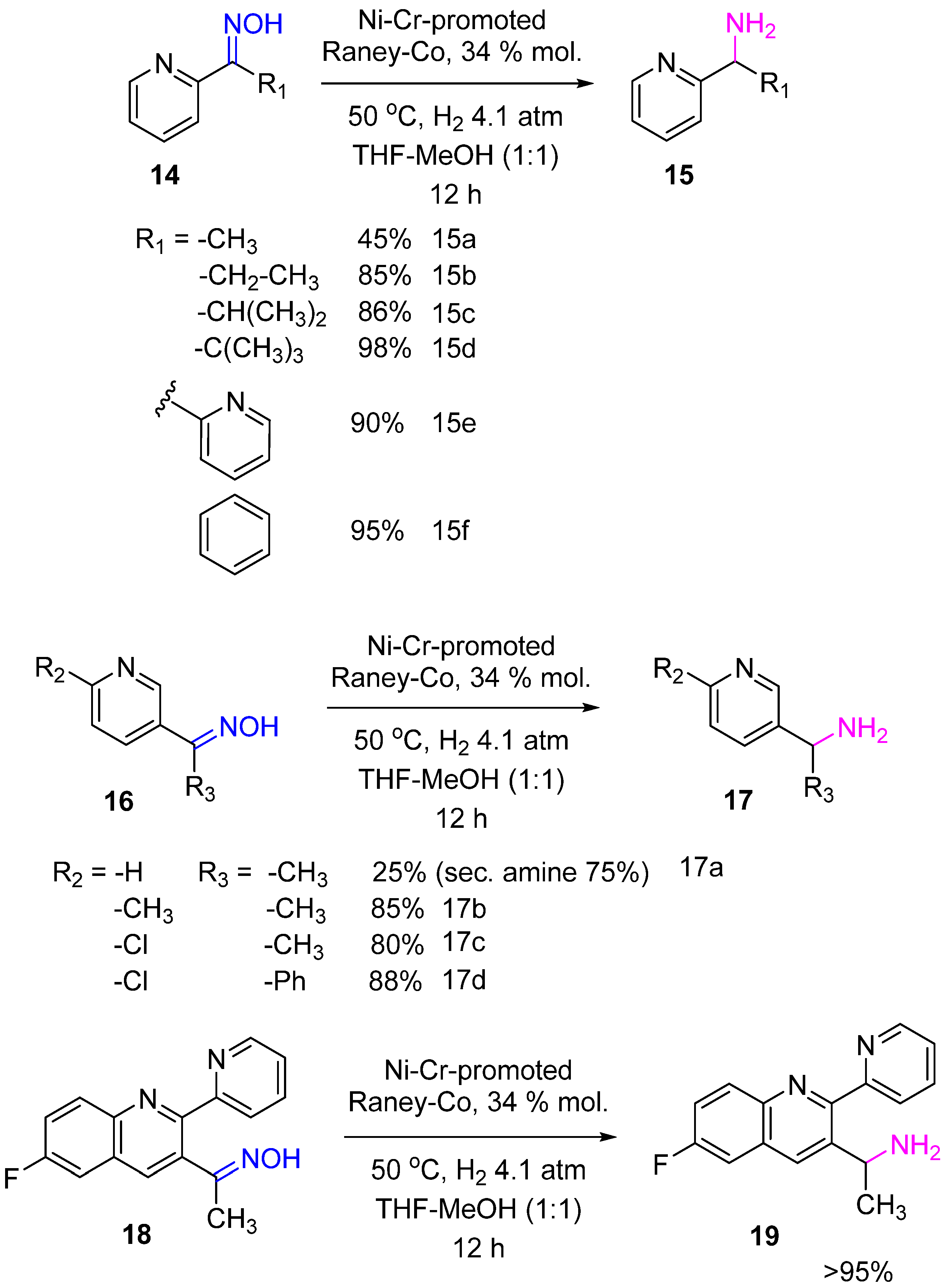
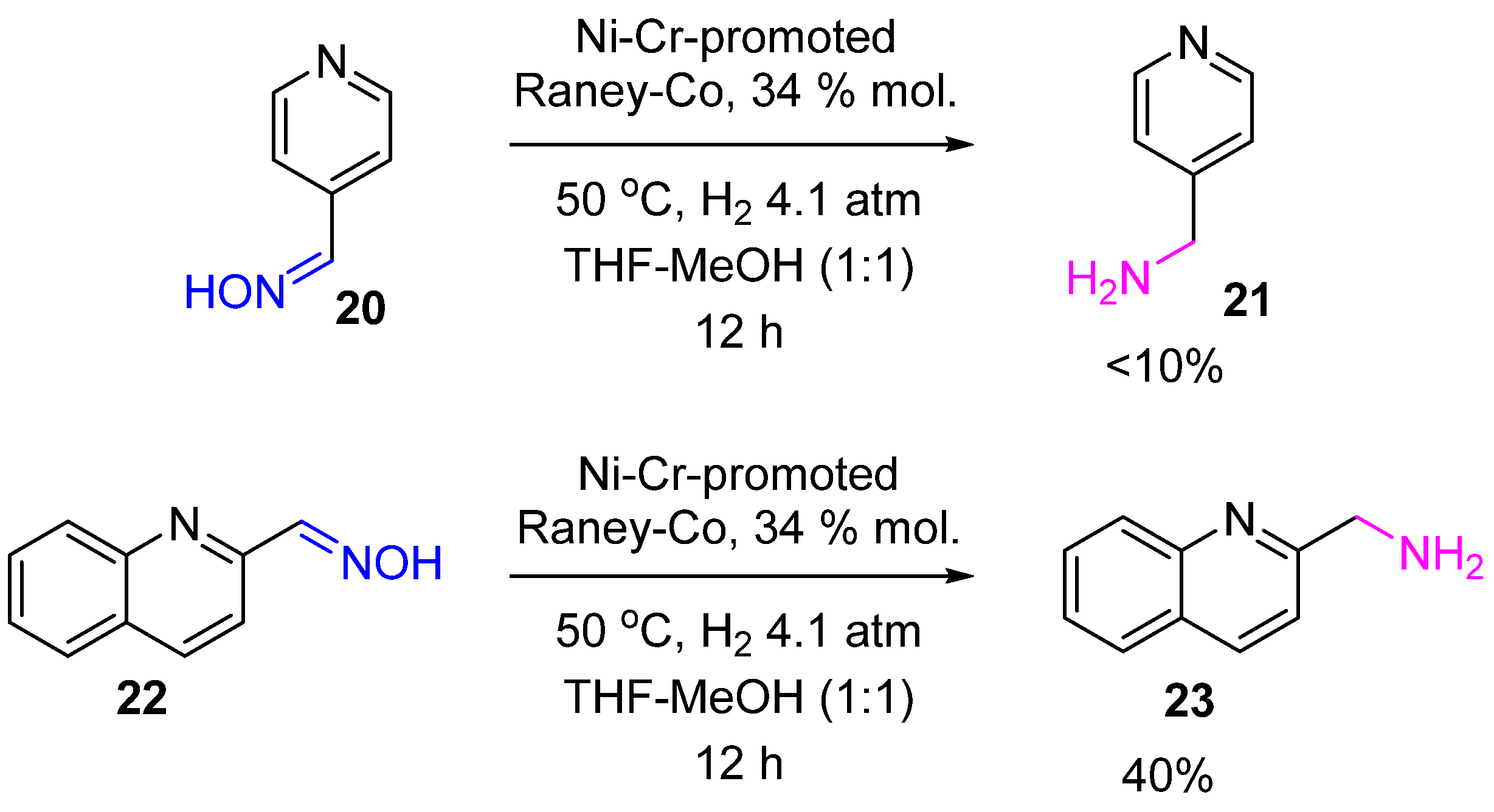
2. Hydrogenation of Ketoximes to Amines
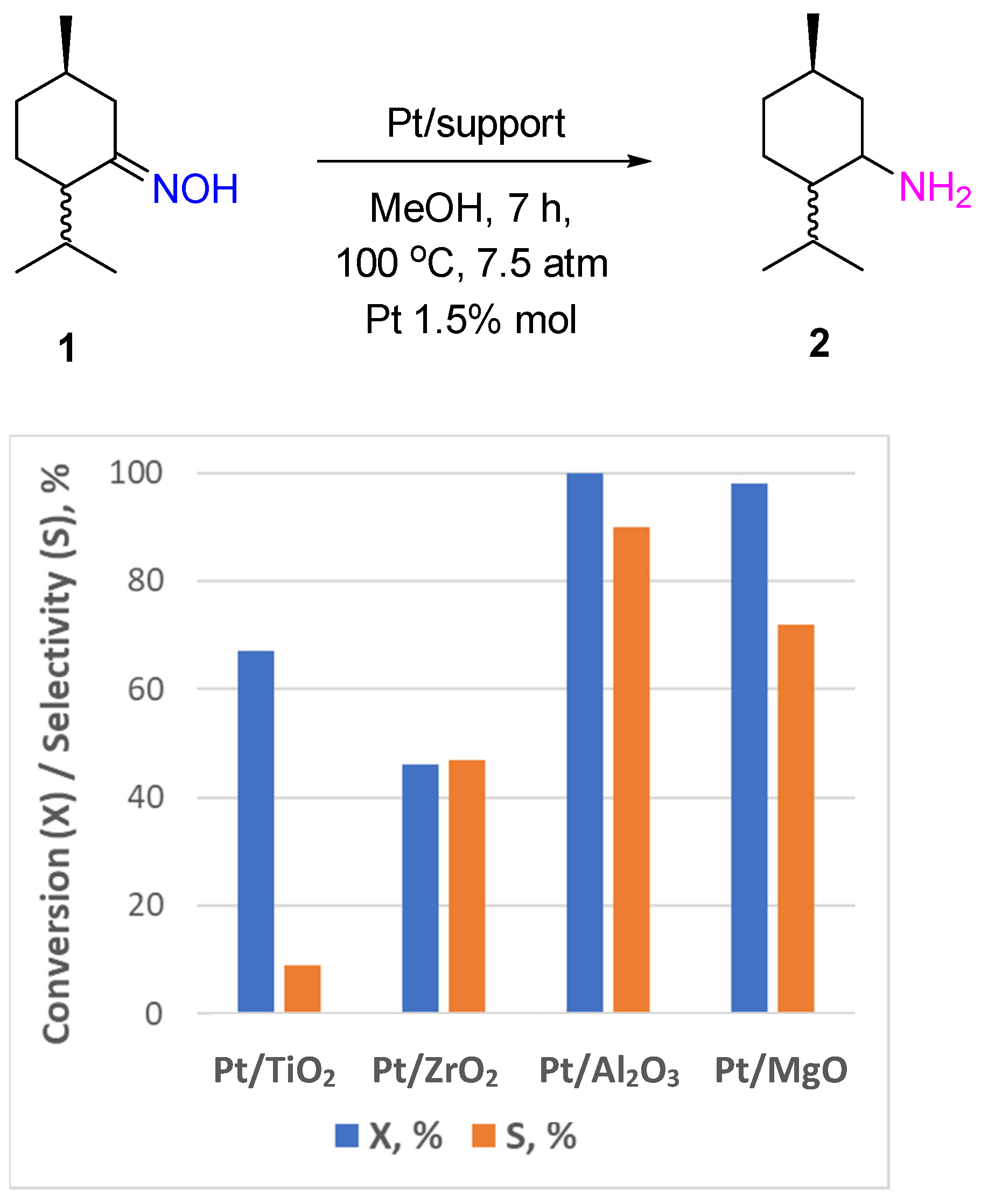
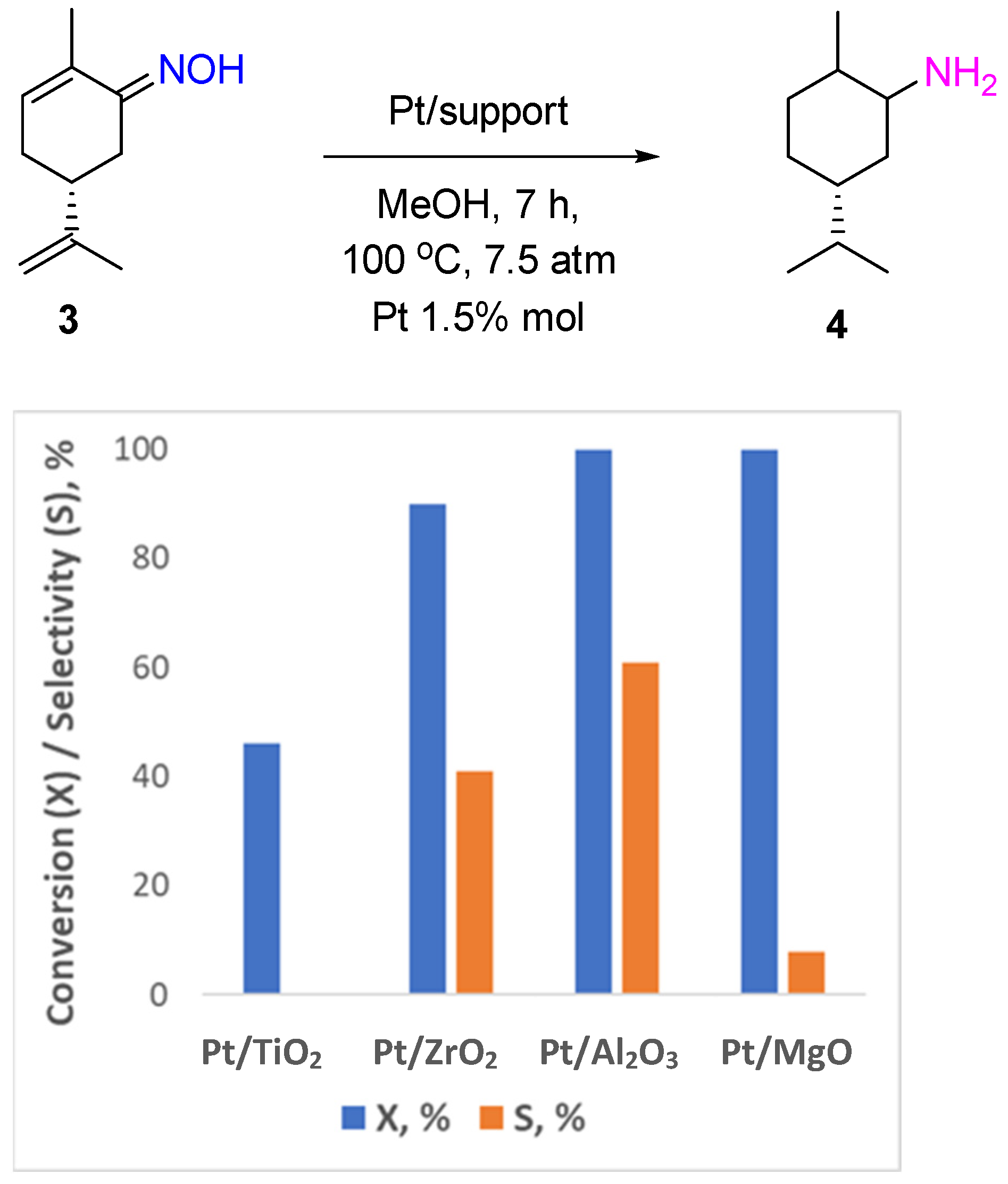
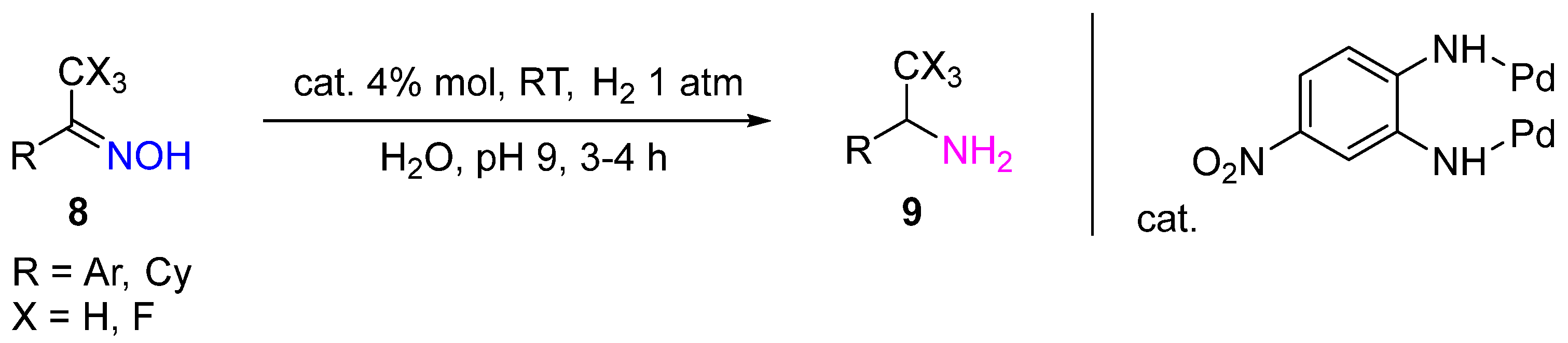
2.1. Noble Metal Catalysts
2.2. Non-Noble-Metal Catalysts
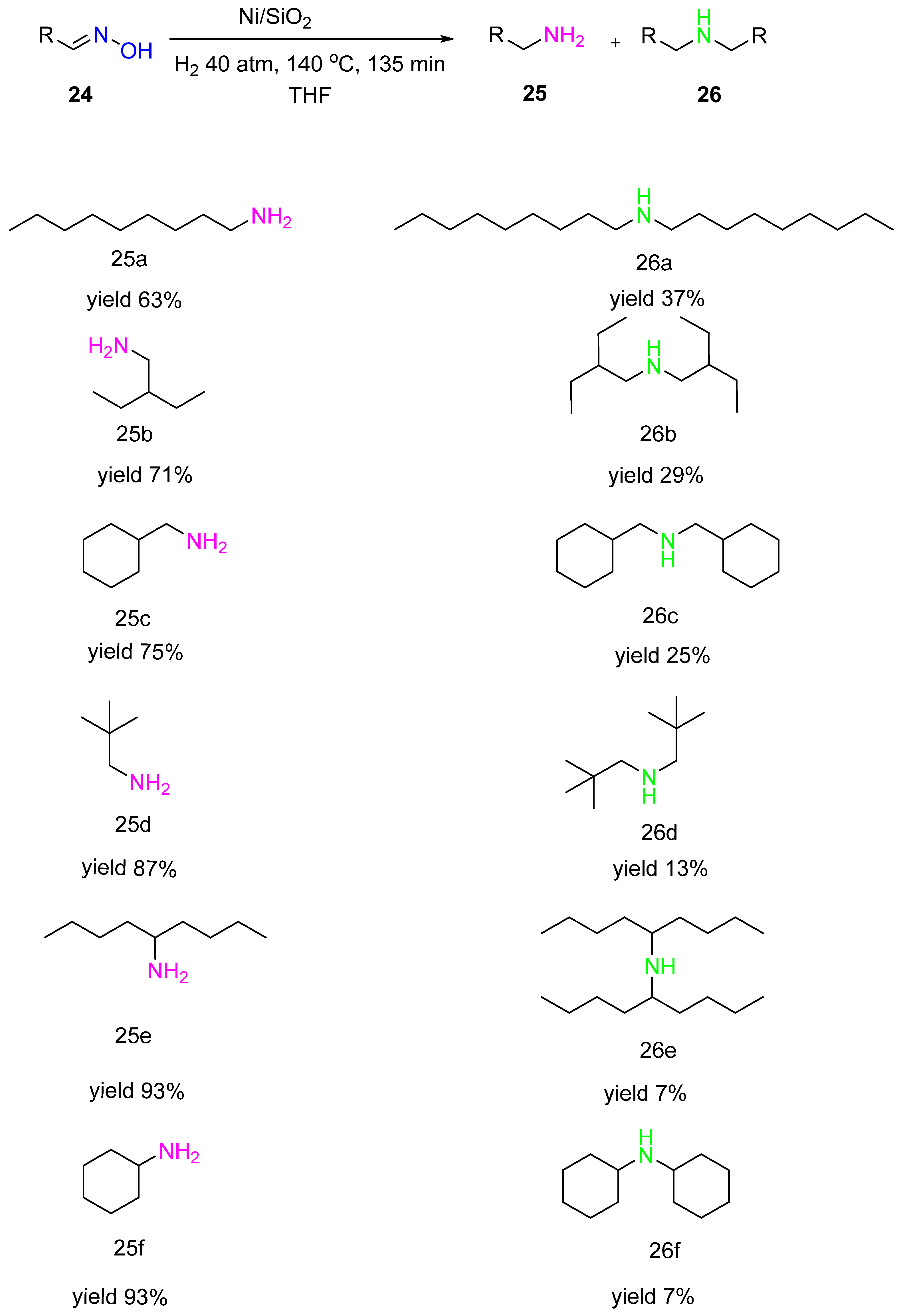
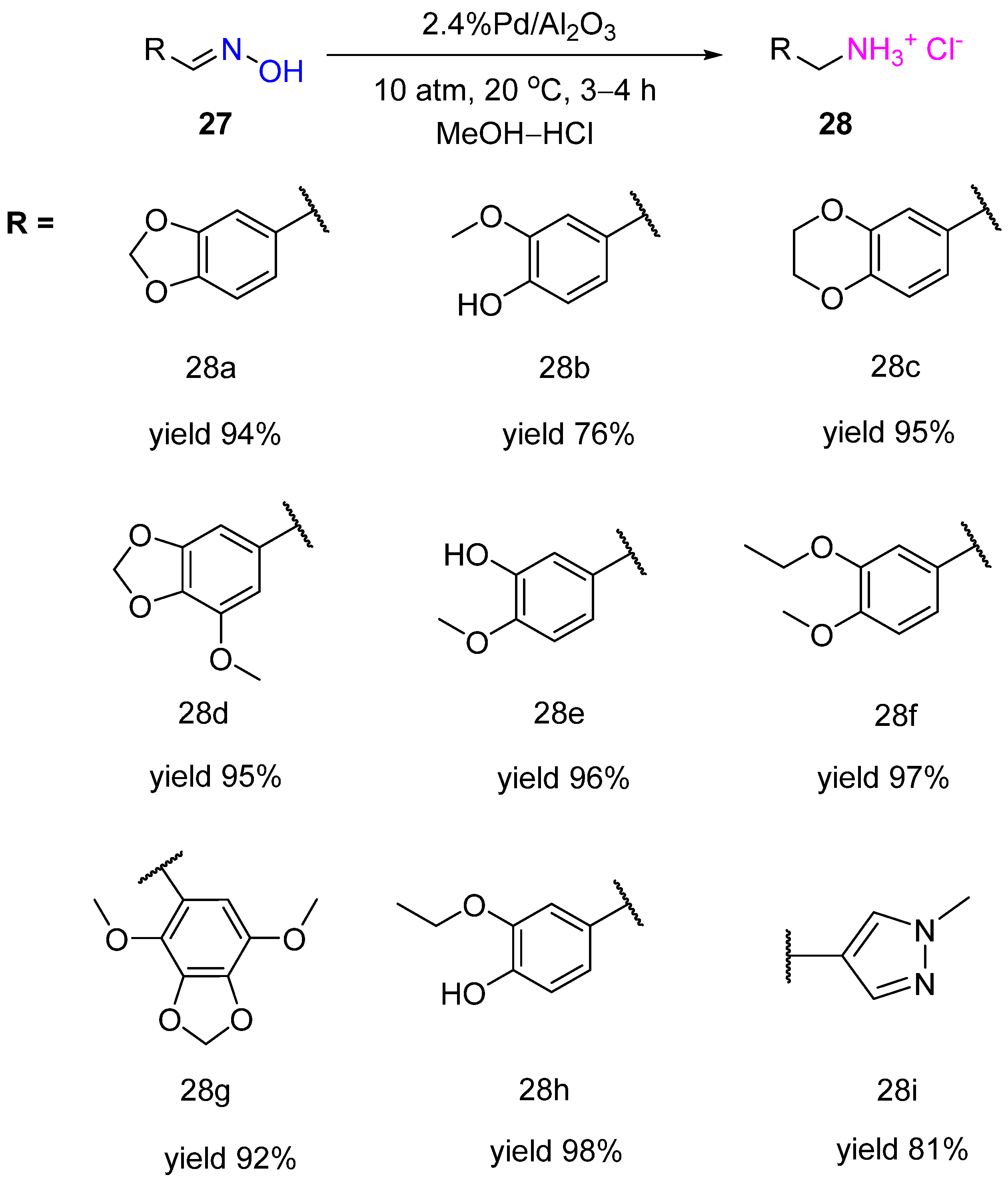
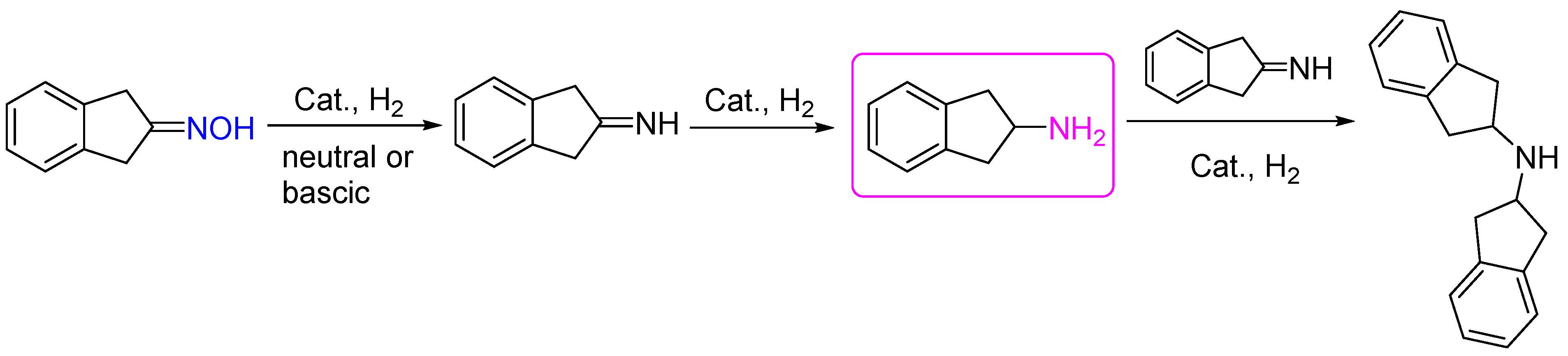
3. Hydrogenation of Aldoximes to Amines
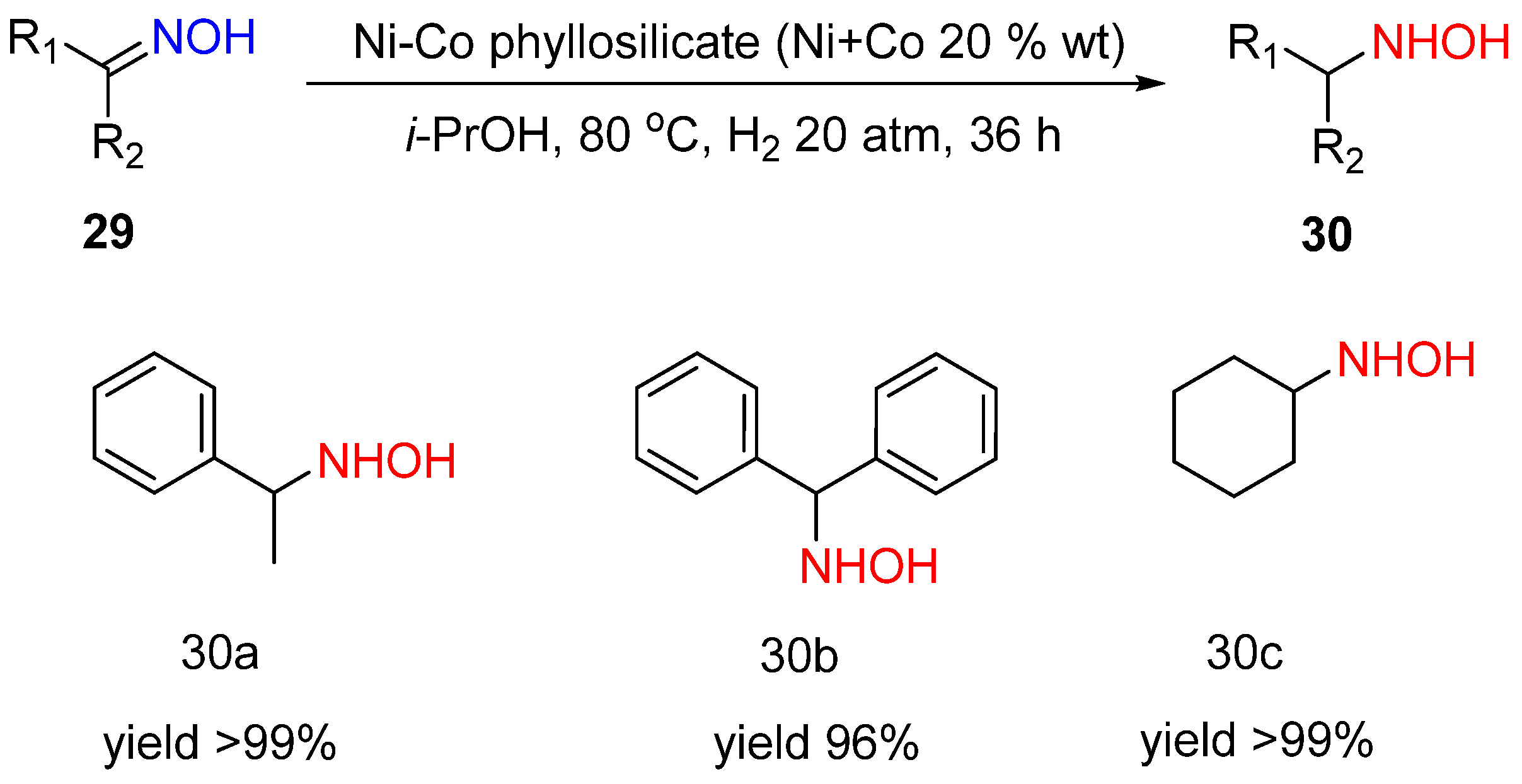
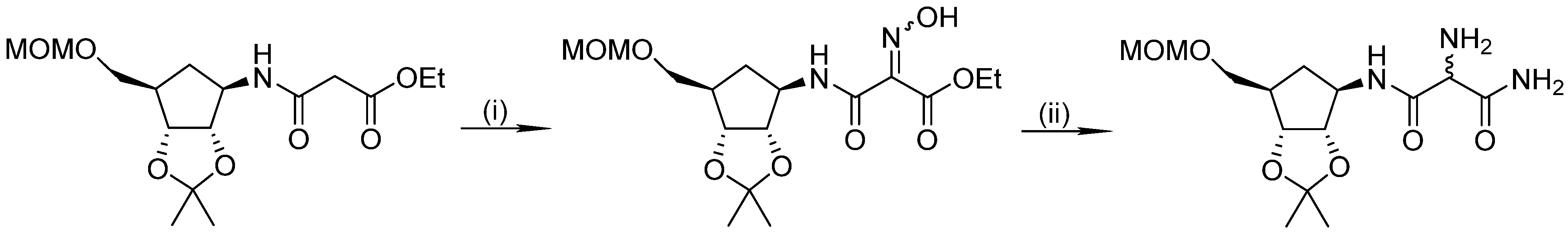
4. Hydrogenation of Oximes to Hydroxylamines
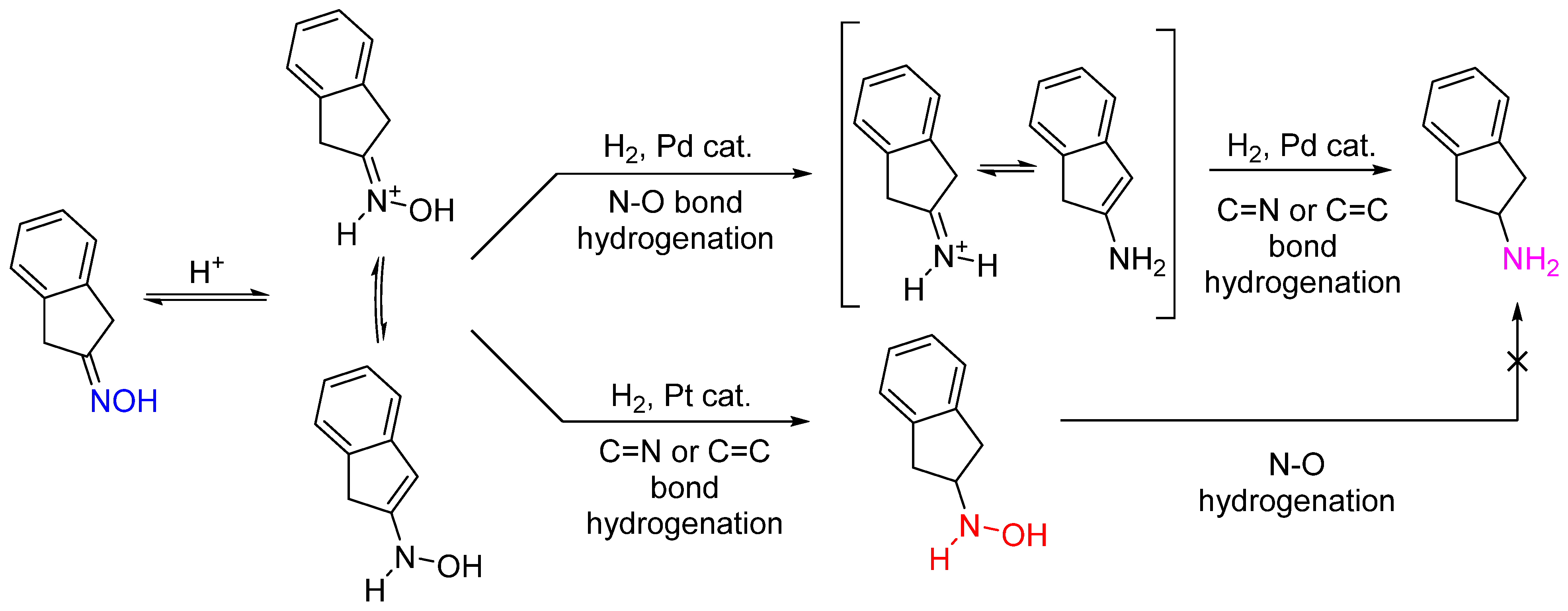
5. General Mechanisms of Hydrogenation of Oximes
5.1. Hydrogenation of Oximes to Amines and Hydroxylamines
5.2. Hydrogenation of Aldoximes
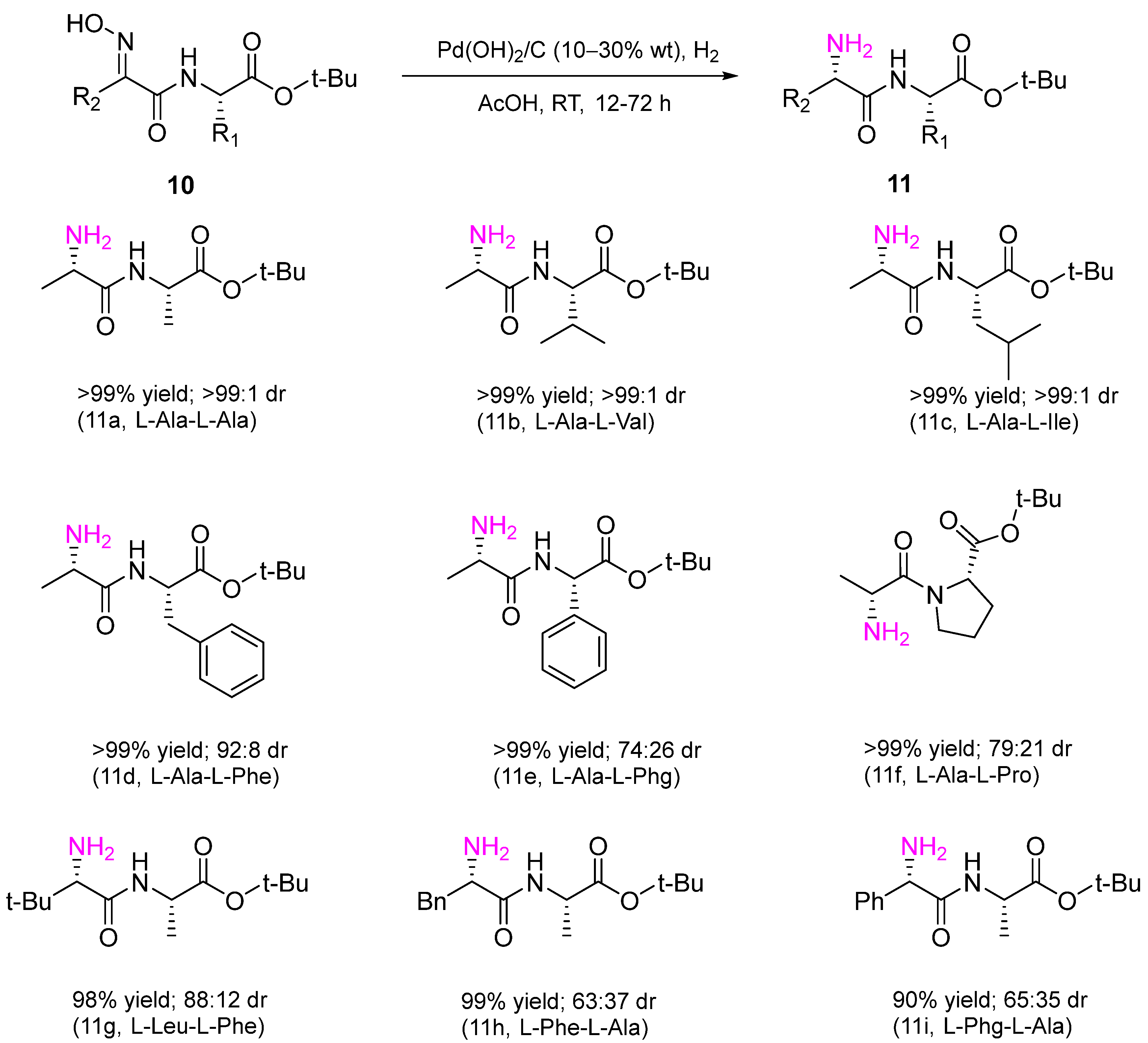
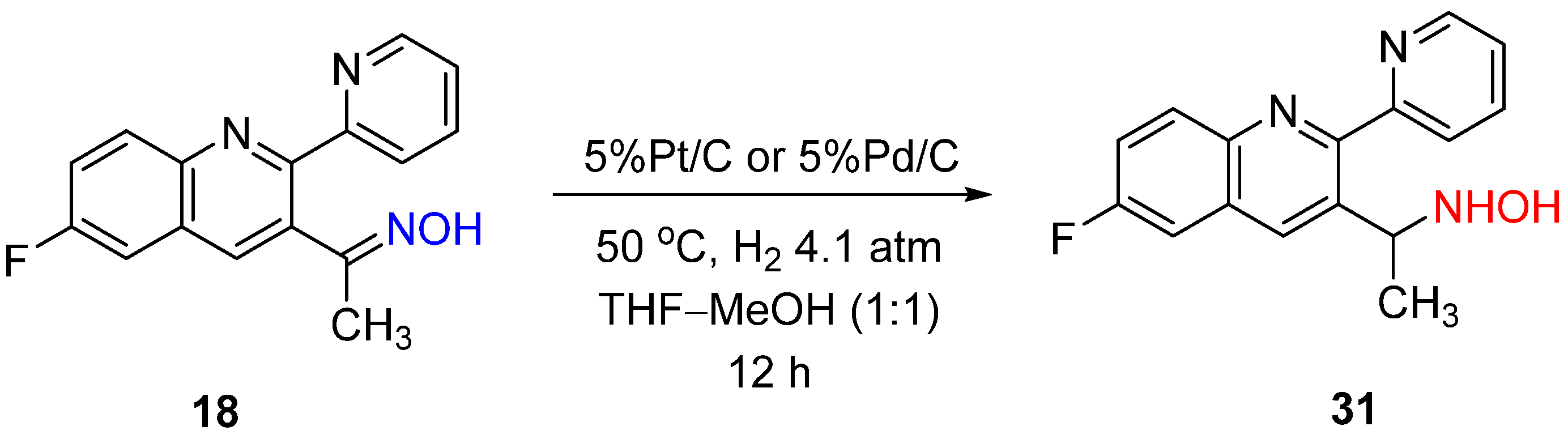
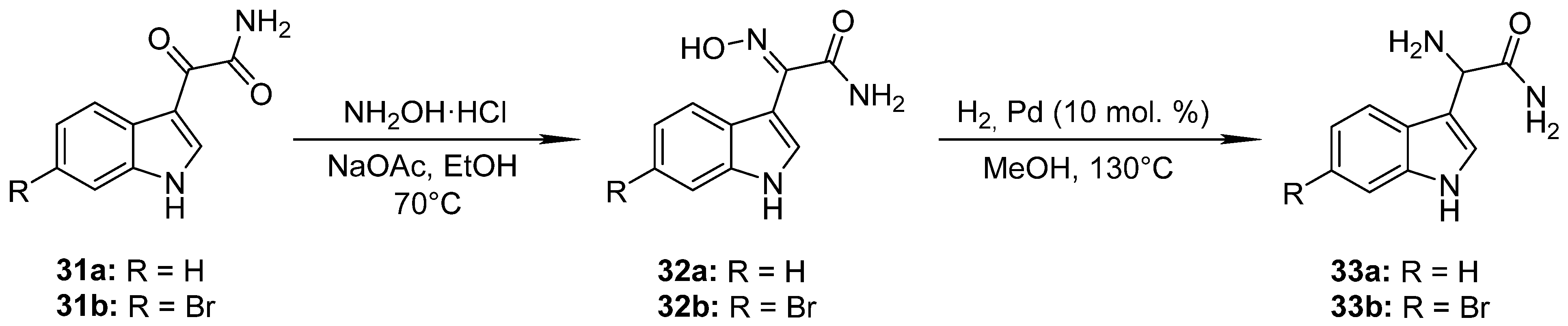
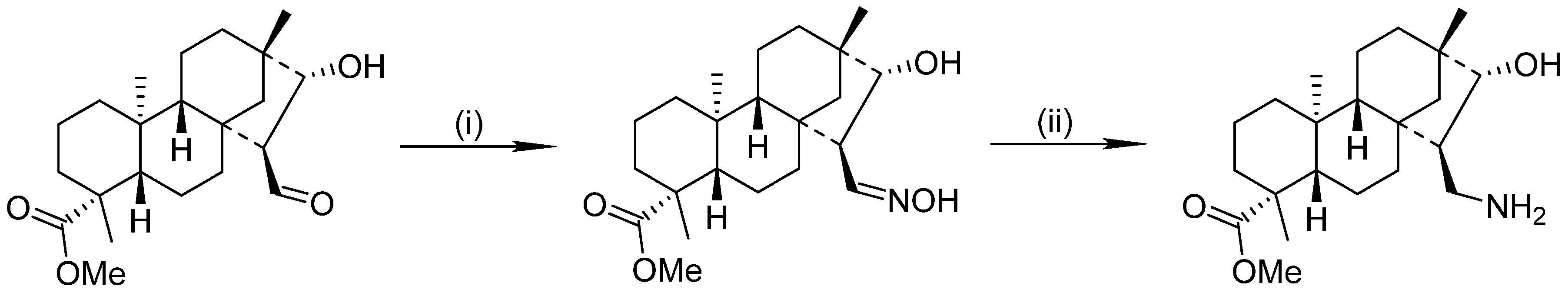
6. Hydrogenation of Oximes in Pharmaceutical Synthesis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Vries, J.G. Catalytic Reduction in Organic Synthesis, 2nd ed.; Georg Thieme: Stuttgart, Germany, 2018; p. b-005-145235. ISBN 978-3-13-240626-1. [Google Scholar]
- Terent’ev, A.O.; Krylov, I.B.; Timofeev, V.P.; Starikova, Z.A.; Merkulova, V.M.; Ilovaisky, A.I.; Nikishin, G.I. Oxidative CO Cross-Coupling of 1,3-Dicarbonyl Compounds and Their Heteroanalogues with N-Substituted Hydroxamic Acids and N-Hydroxyimides. Adv. Synth. Catal. 2013, 355, 2375–2390. [Google Scholar] [CrossRef]
- Mas-Roselló, J.; Cramer, N. Catalytic Reduction of Oximes to Hydroxylamines: Current Methods, Challenges and Opportunities. Chem.–A Eur. J. 2022, 28, e202103683. [Google Scholar] [CrossRef]
- Vavon, G.; Krajcinovic, N. Catalytic Hydrogenation of Oximes and Their Transformation into β-Hydroxylamines. Bull. Soc. Chim. Fr. 1928, 43, 231–237. [Google Scholar]
- Kozlov, N.G. Advances in the Field of the Synthesis of Amino Derivatives of Terpenoids. Chem. Nat. Compd. 1982, 18, 131–143. [Google Scholar] [CrossRef]
- Demidova, Y.S.; Mozhaitsev, E.S.; Munkuev, A.A.; Suslov, E.V.; Saraev, A.A.; Volcho, K.P.; Salakhutdinov, N.F.; Simakova, I.L.; Murzin, D.Y. Monoterpenoid Oximes Hydrogenation over Platinum Catalysts. Top. Catal. 2020, 63, 187–195. [Google Scholar] [CrossRef]
- Rosen, W.E.; Green, M.J. The Reduction of 2-Indanone Oxime to 2-Aminoindane. Methods and Mechanisms. J. Org. Chem. 1963, 28, 2797–2804. [Google Scholar] [CrossRef]
- Liu, Y.; Quan, Z.; He, S.; Zhao, Z.; Wang, J.; Wang, B. Heterogeneous Palladium-Based Catalyst Promoted Reduction of Oximes to Amines: Using H2 at 1 Atm in H2O under Mild Conditions. React. Chem. Eng. 2019, 4, 1145–1152. [Google Scholar] [CrossRef]
- Muramatsu, W.; Tsuji, H.; Yamamoto, H. Catalytic Peptide Synthesis: Amidation of N-Hydroxyimino Esters. ACS Catal. 2018, 8, 2181–2187. [Google Scholar] [CrossRef]
- Borszeky, K.; Mallat, T.; Aeschiman, R.; Schweizer, W.B.; Baiker, A. Enantioselective Hydrogenation of Pyruvic Acid Oxime to Alanine on Pd/Alumina. J. Catal. 1996, 161, 451–458. [Google Scholar] [CrossRef]
- Nakamura, Y. Asymmetrische Synteese. III. Versuch Zur Darstellung Eines Optisch-Aktiven Amins Durch Die Reduktion Des Ketoxims Beim Vorhandensein von Optisch-Aktiver Säure. Bull. Chem. Soc. Jpn. 1941, 16, 367–370. [Google Scholar] [CrossRef]
- Blaser, H.-U.; Müller, M. Enantioselective Catalysis by Chiral Solids: Approaches and Results. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1991; Volume 59, pp. 73–92. ISBN 978-0-444-88514-2. [Google Scholar]
- Tan, F.; Zheng, P.; Zou, Y.-Q. Highly Selective Asymmetric Hydrogenation of Oximes to Hydroxylamine Derivatives. Chem 2020, 6, 1517–1519. [Google Scholar] [CrossRef]
- Mas-Roselló, J.; Smejkal, T.; Cramer, N. Iridium-Catalyzed Acid-Assisted Asymmetric Hydrogenation of Oximes to Hydroxylamines. Science 2020, 368, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, A.N.; Martynova, N.A.; Sibiryakova, A.E.; Klimochkin, Y.N. Synthesis of α-Imino Derivatives of 1-Adamantylacetic and (3-Hydroxy-1-Adamantyl)Acetic Acids. Russ. J. Gen. Chem. 2015, 85, 2024–2029. [Google Scholar] [CrossRef]
- Baucom, K.; Guram, A.; Borths, C. Effective Conversion of Heteroaromatic Ketones into Primary Amines via Hydrogenation of Intermediate Ketoximes. Synlett 2014, 26, 201–204. [Google Scholar] [CrossRef]
- Gebauer-Henke, E.; Leitner, W.; Prokofieva, A.; Vogt, H.; Müller, T.E. Controlling Selectivity in the Reaction Network of Aldoxime Hydrogenation to Primary Amines. Catal. Sci. Technol. 2012, 2, 2539–2548. [Google Scholar] [CrossRef]
- Ignatov, A.V.; Varakutin, A.E.; Solov’eva, I.N.; Karmanova, I.B.; Kozlov, I.A.; Semenova, M.N.; Semenov, V.V. Efficient Hydrogenation of Benzaldoximes and Schiff Bases on Ceramic High-Porosity Palladium Catalysts. Russ. Chem. Bull. 2018, 67, 1394–1400. [Google Scholar] [CrossRef]
- Ciotonea, C.; Hammi, N.; Dhainaut, J.; Marinova, M.; Ungureanu, A.; El Kadib, A.; Michon, C.; Royer, S. Phyllosilicate-derived Nickel-cobalt Bimetallic Nanoparticles for the Catalytic Hydrogenation of Imines, Oximes and N-heteroarenes. ChemCatChem 2020, 12, 4652–4663. [Google Scholar] [CrossRef]
- Mas-Roselló, J.; Cope, C.J.; Tan, E.; Pinson, B.; Robinson, A.; Smejkal, T.; Cramer, N. Iridium-Catalyzed Acid-Assisted Hydrogenation of Oximes to Hydroxylamines. Angew. Chem. Int. Ed. 2021, 60, 15524–15532. [Google Scholar] [CrossRef]
- Srinivasan, A.; Banerjee, S.; Pachore, S.; Kumar, U.S. Three-Component Coupling–Oxidative Amidation–Heterocycloannulation: Synthesis of the Indole Alkaloids Hamacanthin A and Trans-2,5-Bis(3′-Indolyl)Piperazine. Synlett 2017, 28, 1057–1064. [Google Scholar] [CrossRef] [Green Version]
- Garg, N.K.; Sarpong, R.; Stoltz, B.M. The First Total Synthesis of Dragmacidin D. J. Am. Chem. Soc. 2002, 124, 13179–13184. [Google Scholar] [CrossRef] [Green Version]
- Haadsma-Svensson, S.R.; Cleek, K.A.; Dinh, D.M.; Duncan, J.N.; Haber, C.L.; Huff, R.M.; Lajiness, M.E.; Nichols, N.F.; Smith, M.W.; Svensson, K.A.; et al. Dopamine D3 Receptor Antagonists. 1. Synthesis and Structure−Activity Relationships of 5,6-Dimethoxy-N-Alkyl- and N-Alkylaryl-Substituted 2-Aminoindans. J. Med. Chem. 2001, 44, 4716–4732. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.M.; Chen, X.; Cryan, E.; Hlasta, D.J.; Rybczynski, P.J.; Strauss, K.; Tang, Y.; Xu, J.Z.; Yang, M.; Zhou, L.; et al. Design and Synthesis of Indane-Ureido-Thioisobutyric Acids: A Novel Class of PPARα Agonists. Bioorganic Med. Chem. Lett. 2007, 17, 6773–6778. [Google Scholar] [CrossRef] [PubMed]
- Ozsvár, D.; Nagy, V.; Zupkó, I.; Szakonyi, Z. Synthesis and Biological Application of Isosteviol-Based 1,3-Aminoalcohols. Int. J. Mol. Sci. 2021, 22, 11232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Ma, Y.; Pan, Q.; Bai, Z.-G.; Qi, H.; Zhang, Q.-Z. Synthesis of (5,6-Dihydro-4H-Pyrrolo[1,2-b]Pyrazol-3-Yl)-Methanamine. Heterocycles 2017, 94, 1923. [Google Scholar] [CrossRef]
- Sanz-Cervera, J.F.; Blasco, R.; Piera, J.; Cynamon, M.; Ibáñez, I.; Murguía, M.; Fustero, S. Solution versus Fluorous versus Solid-Phase Synthesis of 2,5-Disubstituted 1,3-Azoles. Preliminary Antibacterial Activity Studies. J. Org. Chem. 2009, 74, 8988–8996. [Google Scholar] [CrossRef]
- Laufer, S.A.; Liedtke, A.J. A Concise and Optimized Four-Step Approach toward 2-(Aryl-)Alkylsulfanyl-, 4(5)-Aryl-, 5(4)-Heteroaryl-Substituted Imidazoles Using Alkyl- or Arylalkyl Thiocyanates. Tetrahedron Lett. 2006, 47, 7199–7203. [Google Scholar] [CrossRef]
- Nair, V.; Zhang, F. Synthesis of a Novel Carbocyclic Analog of Bredinin. Molecules 2013, 18, 11576–11585. [Google Scholar] [CrossRef]


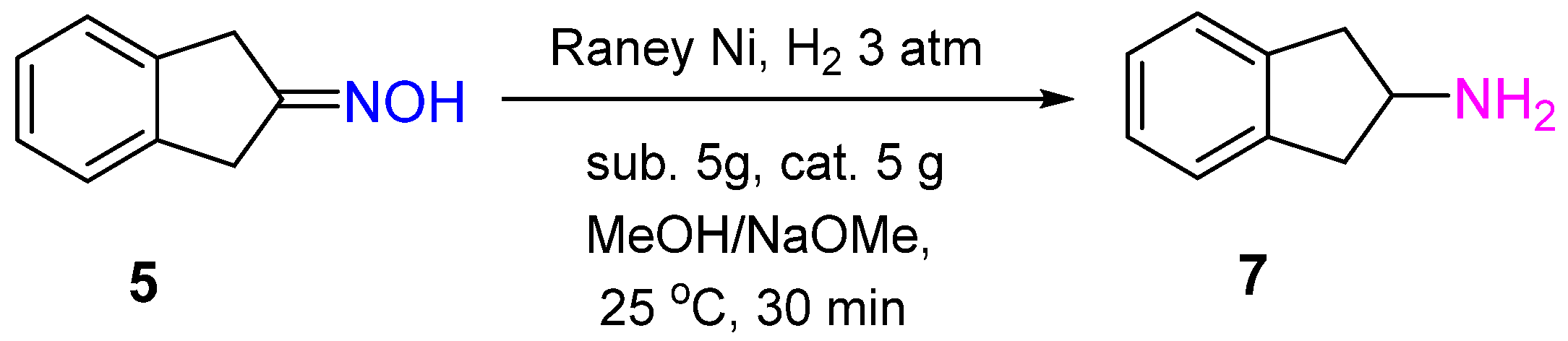


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redina, E.A.; Ivanova, I.I.; Arkhipova, N.Y.; Kustov, L.M. Heterogeneous Catalysis as an Efficient Tool for Selective Hydrogenation of Oximes to Amines and Hydroxylamines. Catalysts 2022, 12, 1614. https://doi.org/10.3390/catal12121614
Redina EA, Ivanova II, Arkhipova NY, Kustov LM. Heterogeneous Catalysis as an Efficient Tool for Selective Hydrogenation of Oximes to Amines and Hydroxylamines. Catalysts. 2022; 12(12):1614. https://doi.org/10.3390/catal12121614
Chicago/Turabian StyleRedina, Elena A., Inna I. Ivanova, Natalia Y. Arkhipova, and Leonid M. Kustov. 2022. "Heterogeneous Catalysis as an Efficient Tool for Selective Hydrogenation of Oximes to Amines and Hydroxylamines" Catalysts 12, no. 12: 1614. https://doi.org/10.3390/catal12121614
APA StyleRedina, E. A., Ivanova, I. I., Arkhipova, N. Y., & Kustov, L. M. (2022). Heterogeneous Catalysis as an Efficient Tool for Selective Hydrogenation of Oximes to Amines and Hydroxylamines. Catalysts, 12(12), 1614. https://doi.org/10.3390/catal12121614






