Checking the Efficiency of a Magnetic Graphene Oxide–Titania Material for Catalytic and Photocatalytic Ozonation Reactions in Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Activity Tests
2.3. pH Stability Range and FeGOTi Reusage
2.4. Catalysts’ Efficiency Comparison
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Photocatalytic Test
3.4. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Issaka, E.; AMU-Darko, J.N.O.; Yakubu, S.; Fapohunda, F.O.; Ali, N.; Bilal, M. Advanced catalytic ozonation for degradation of pharmaceutical pollutants―A review. Chemosphere 2022, 289, 133208. [Google Scholar] [CrossRef] [PubMed]
- Mecha, A.C.; Chollom, M.N. Photocatalytic ozonation of wastewater: A review. Environ. Chem. Lett. 2020, 18, 1491–1507. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rey, A.; Gimeno, O. The role of catalytic ozonation processes on the elimination of dbps and their precursors in drinking water treatment. Catalysts 2021, 11, 521. [Google Scholar] [CrossRef]
- Gomes, J.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Application of ozonation for pharmaceuticals and personal care products removal from water. Sci. Total Environ. 2017, 586, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Gunten, U. Von Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.W. Advanced Oxidation Processes: Test of a Kinetic Model for the Oxidation of Organic Compounds with Ozone and Hydrogen Peroxide in a Semibatch Reactor. Ind. Eng. Chem. Res. 1989, 28, 1580–1587. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.-W.; Chapin, D.H. The Chemistry of Water Treatment Processes Involving Ozone, Hydrogen Peroxide and Ultraviolet Radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Orge, C.A.; Sousa, J.P.S.; Gonçalves, F.; Freire, C.; Órfão, J.J.M.; Pereira, M.F.R. Development of novel mesoporous carbon materials for the catalytic ozonation of organic pollutants. Catal. Lett. 2009, 132, 1–9. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Yang, H.; Wen, L.; Yi, Z.; Zhou, Z.; Dai, B.; Zhang, J.; Wu, X.; Wu, P. Multi-mode surface plasmon resonance absorber based on dart-type single-layer graphene. RSC Adv. 2022, 12, 7821–7829. [Google Scholar] [CrossRef]
- Shangguan, Q.; Chen, Z.; Yang, H.; Cheng, S.; Yang, W.; Yi, Z.; Wu, X.; Wang, S.; Yi, Y.; Wu, P. Design of Ultra-Narrow Band Graphene Refractive Index Sensor. Sensors 2022, 22, 6483. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, F.J.; Álvarez, P.M.; Gimeno, O. Graphene-based catalysts for ozone processes to decontaminate water. Molecules 2019, 24, 3438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, E.M.; Rey, A.; Mena, E.; Beltrán, F.J. Application of solar photocatalytic ozonation in water treatment using supported TiO2. Appl. Catal. B Environ. 2019, 254, 237–245. [Google Scholar] [CrossRef]
- Rosal, R.; Gonzalo, M.S.; Rodríguez, A.; García-Calvo, E. Catalytic ozonation of fenofibric acid over alumina-supported manganese oxide. J. Hazard. Mater. 2010, 183, 271–278. [Google Scholar] [CrossRef]
- Chávez, A.M.; Solís, R.R.; Beltrán, F.J. Magnetic graphene TiO2-based photocatalyst for the removal of pollutants of emerging concern in water by simulated sunlight aided photocatalytic ozonation. Appl. Catal. B Environ. 2020, 262, 118275. [Google Scholar] [CrossRef]
- Chang, Y.N.; Ou, X.M.; Zeng, G.M.; Gong, J.L.; Deng, C.H.; Jiang, Y.; Liang, J.; Yuan, G.Q.; Liu, H.Y.; He, X. Synthesis of magnetic graphene oxide-TiO 2 and their antibacterial properties under solar irradiation. Appl. Surf. Sci. 2015, 343, 1–10. [Google Scholar] [CrossRef]
- Nada, A.A.; Tantawy, H.R.; Elsayed, M.A.; Bechelany, M.; Elmowafy, M.E. Elaboration of nano titania-magnetic reduced graphene oxide for degradation of tartrazine dye in aqueous solution. Solid State Sci. 2018, 78, 116–125. [Google Scholar] [CrossRef]
- Guskos, N.; Zolnierkiewicz, G.; Guskos, A.; Aidinis, K.; Wanag, A.; Kusiak-Nejman, E.; Narkiewicz, U.; Morawski, A.W. Magnetic moment centers in titanium dioxide photocatalysts loaded on reduced graphene oxide flakes. Rev. Adv. Mater. Sci. 2021, 60, 57–63. [Google Scholar] [CrossRef]
- Pedrosa, M.; Pastrana-Martínez, L.M.; Pereira, M.F.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T. N/S-doped graphene derivatives and TiO2 for catalytic ozonation and photocatalysis of water pollutants. Chem. Eng. J. 2018, 348, 888–897. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Figueiredo, J.L.; Faria, J.L.; Falaras, P.; Silva, A.M.T. Advanced nanostructured photocatalysts based on reduced graphene oxide-TiO2 composites for degradation of diphenhydramine pharmaceutical and methyl orange dye. Appl. Catal. B Environ. 2012, 123–124, 241–256. [Google Scholar] [CrossRef]
- Hass, U.; Duennbier, U.; Massmann, G. Occurrence and distribution of psychoactive compounds and their metabolites in the urban water cycle of Berlin (Germany). Water Res. 2012, 46, 6013–6022. [Google Scholar] [CrossRef]
- Real, F.J.; Javier Benitez, F.; Acero, J.L.; Sagasti, J.J.P.; Casas, F. Kinetics of the chemical oxidation of the pharmaceuticals primidone, ketoprofen, and diatrizoate in ultrapure and natural waters. Ind. Eng. Chem. Res. 2009, 48, 3380–3388. [Google Scholar] [CrossRef]
- Chen, P.; Liu, F.; Ding, H.; Chen, S.; Chen, L.; Li, Y.-J.; Au, C.-T.; Yin, S.-F. Porous double-shell CdS@C3N4 octahedron derived by in situ supramolecular self-assembly for enhanced photocatalytic activity. Appl. Catal. B Environ. 2019, 252, 33–40. [Google Scholar] [CrossRef]
- Cao, W.; Lin, L.; Qi, H.; He, Q.; Wu, Z.; Wang, A.; Luo, W.; Zhang, T. In-situ synthesis of single-atom Ir by utilizing metal-organic frameworks: An acid-resistant catalyst for hydrogenation of levulinic acid to γ-valerolactone. J. Catal. 2019, 373, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.; Sharma, N.; Singh, U.; Arif, M.; Singh, A. Higher oxidation level in graphene oxide. Optik 2017, 143, 115–124. [Google Scholar] [CrossRef]
- Quiñones, D.H.; Rey, A.; Álvarez, P.M.; Beltrán, F.J.; Plucinski, P.K. Enhanced activity and reusability of TiO2 loaded magnetic activated carbon for solar photocatalytic ozonation. Appl. Catal. B Environ. 2014, 144, 96–106. [Google Scholar] [CrossRef]
- Nuñez, J.M.; Hettler, S.; Lima Jr, E.; Goya, G.F.; Arenal, R.; Zysler, R.D.; Aguirre, M.H.; Winkler, E.L. Onion-like Fe3O4/MgO/CoFe2O4 magnetic nanoparticles: New ways to control magnetic coupling between soft and hard magnetic phases. J. Mater. Chem. C 2022, 10, 15339–15352. [Google Scholar] [CrossRef]
- Majidnia, Z.; Idris, A. Combination of maghemite and titanium oxide nanoparticles in polyvinyl alcohol-alginate encapsulated beads for cadmium ions removal. Korean J. Chem. Eng. 2015, 32, 1094–1100. [Google Scholar] [CrossRef]
- Shokrollahi, H. A review of the magnetic properties, synthesis methods and applications of maghemite. J. Magn. Magn. Mater. 2017, 426, 74–81. [Google Scholar] [CrossRef]
- Mei, X.; Meng, X.; Wu, F. Hydrothermal method for the production of reduced graphene oxide. Phys. E Low-Dimens. Syst. Nanostruct. 2015, 68, 81–86. [Google Scholar] [CrossRef]
- Dai, X.; Lu, G.; Hu, Y.; Xie, X.; Wang, X.; Sun, J. Reversible redox behavior of Fe2O3/TiO2 composites in the gaseous photodegradation process. Ceram. Int. 2019, 45, 13187–13192. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J. Environ. Manag. 2020, 258, 110050. [Google Scholar] [CrossRef] [PubMed]
- Figueredo, M.A.; Rodríguez, E.M.; Checa, M.; Beltran, F.J. Ozone-Based Advanced Oxidation Processes for Primidone Removal in Water using Simulated Solar Radiation and TiO2 or WO3 as Photocatalyst. Molecules 2019, 24, 1728. [Google Scholar] [CrossRef] [Green Version]
- Checa, M.; Figueredo, M.; Aguinaco, A.; Beltrán, F.J. Graphene oxide/titania photocatalytic ozonation of primidone in a visible LED photoreactor. J. Hazard. Mater. 2019, 369, 70–78. [Google Scholar] [CrossRef]
- Abbas, N.; Shao, G.N.; Haider, M.S.; Imran, S.M.; Park, S.S.; Kim, H.T. Sol–gel synthesis of TiO2-Fe2O3 systems: Effects of Fe2O3 content and their photocatalytic properties. J. Ind. Eng. Chem. 2016, 39, 112–120. [Google Scholar] [CrossRef]
- Kurien, U.; Hu, Z.; Lee, H.; Dastoor, A.P.; Ariya, P.A. Radiation enhanced uptake of Hg0(g) on iron (oxyhydr)oxide nanoparticles. RSC Adv. 2017, 7, 45010–45021. [Google Scholar] [CrossRef] [Green Version]
- Mishra, M.; Chun, D.M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Environmental photochemistry: Is iron oxide (hematite) an active photocatalyst? A comparative study: α-Fe2O3, ZnO, TiO2. J. Photochem. Photobiol. A Chem. 1989, 48, 161–169. [Google Scholar] [CrossRef]
- Palanisamy, B.; Babu, C.M.; Sundaravel, B.; Anandan, S.; Murugesan, V. Sol-gel synthesis of mesoporous mixed Fe2O3/TiO2 photocatalyst: Application for degradation of 4-chlorophenol. J. Hazard. Mater. 2013, 252–253, 233–242. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rivas, J.; Álvarez, P.; Montero-de-Espinosa, R. Kinetics of heterogeneous catalytic ozone decomposition in water on an activated carbon. Ozone Sci. Eng. 2002, 24, 227–237. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, X.; Chen, L.; Wang, X.; Zhang, N.; Liu, Y.; Fang, Y. Oxygen vacancies enabled enhancement of catalytic property of Al reduced anatase TiO2 in the decomposition of high concentration ozone. J. Solid State Chem. 2017, 250, 121–127. [Google Scholar] [CrossRef]
- Yan, L.; Bing, J.; Wu, H. The behavior of ozone on different iron oxides surface sites in water. Sci. Rep. 2019, 9, 14752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro Da Silva, M.F.; Soeira, L.S.; Daghastanli, K.R.P.; Martins, T.S.; Cuccovia, I.M.; Freire, R.S.; Isolani, P.C. CeO2-catalyzed ozonation of phenol: The role of cerium citrate as precursor of CeO2. J. Therm. Anal. Calorim. 2010, 102, 907–913. [Google Scholar] [CrossRef]
- Bing, J.; Hu, C.; Zhang, L. Enhanced mineralization of pharmaceuticals by surface oxidation over mesoporous γ-Ti-Al2O3 suspension with ozone. Appl. Catal. B Environ. 2017, 202, 118–126. [Google Scholar] [CrossRef]
- Kongsuebchart, W.; Praserthdam, P.; Panpranot, J.; Sirisuk, A.; Supphasrirongjaroen, P.; Satayaprasert, C. Effect of crystallite size on the surface defect of nano-TiO2 prepared via solvothermal synthesis. J. Cryst. Growth 2006, 297, 234–238. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Ji, J.; Li, K.; Cao, J.; Feng, Q.; Huang, H. Effective regulation of surface bridging hydroxyls on TiO2 for superior photocatalytic activity via ozone treatment. Appl. Catal. B Environ. 2022, 304, 120952. [Google Scholar] [CrossRef]
- Solís, R.R.; Rivas, F.J.; Gimeno, O.; Pérez-Bote, J.L. Photocatalytic ozonation of clopyralid, picloram and triclopyr. Kinetics, toxicity and influence of operational parameters. J. Chem. Technol. Biotechnol. 2016, 91, 51–58. [Google Scholar] [CrossRef]
- Li, J.; Guan, W.; Yan, X.; Wu, Z.; Shi, W. Photocatalytic Ozonation of 2,4-Dichlorophenoxyacetic Acid using LaFeO3 Photocatalyst Under Visible Light Irradiation. Catal. Letters 2018, 148, 23–29. [Google Scholar] [CrossRef]
- Kumar, P.; Joshi, C.; Barras, A.; Sieber, B.; Addad, A.; Boussekey, L.; Szunerits, S.; Boukherroub, R.; Jain, S.L. Core–shell structured reduced graphene oxide wrapped magnetically separable rGO@CuZnO@Fe3O4 microspheres as superior photocatalyst for CO2 reduction under visible light. Appl. Catal. B Environ. 2017, 205, 654–665. [Google Scholar] [CrossRef]
- Jo, W.K.; Kumar, S.; Isaacs, M.A.; Lee, A.F.; Karthikeyan, S. Cobalt promoted TiO2/GO for the photocatalytic degradation of oxytetracycline and Congo Red. Appl. Catal. B Environ. 2017, 201, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Che, H.; Che, G.; Jiang, E.; Liu, C.; Dong, H.; Li, C. A novel Z-Scheme CdS/Bi3O4Cl heterostructure for photocatalytic degradation of antibiotics: Mineralization activity, degradation pathways and mechanism insight. J. Taiwan Inst. Chem. Eng. 2018, 91, 224–234. [Google Scholar] [CrossRef]
- Kaus, N.H.M.; Rithwan, A.F.; Adnan, R.; Ibrahim, M.L.; Thongmee, S.; Yusoff, S.F.M. Effective strategies, mechanisms, and photocatalytic efficiency of semiconductor nanomaterials incorporating rgo for environmental contaminant degradation. Catalysts 2021, 11, 302. [Google Scholar] [CrossRef]
- Ahn, Y.; Oh, H.; Yoon, Y.; Park, W.K.; Yang, W.S.; Kang, J.W. Effect of graphene oxidation degree on the catalytic activity of graphene for ozone catalysis. J. Environ. Chem. Eng. 2017, 5, 3882–3894. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, M.; Wang, Z.; Ji, H.; Zheng, B.; Xiao, D.; Wu, L.; Guo, Y. The role of ozone in the ozonation process of graphene oxide: Oxidation or decomposition? RSC Adv. 2014, 4, 58325–58328. [Google Scholar] [CrossRef]
- Decoret, C.; Royer, J.; Legube, B.; Dore, M. Experimental and theoretical studies of the mechanism of the initial attack of ozone on some aromatics in aqueous medium. Environ. Technol. Lett. 1984, 5, 207–218. [Google Scholar] [CrossRef]
- Staehelin, J.; Hoigne, J. Decomposition of ozone in water: Rate of initiation by hydroxide ions and hydrogen peroxide. Environ. Sci. Technol. 1982, 16, 676–681. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Checa, M.; Rivas, J.; García-Araya, J.F. Modeling the Mineralization Kinetics of Visible Led Graphene Oxide/Titania Photocatalytic Ozonation of an Urban Wastewater Containing Pharmaceutical Compounds. Catalysts 2020, 10, 1256. [Google Scholar] [CrossRef]
- Kiwi, J.; Lopez, A.; Nadtochenko, V. Mechanism and kinetics of the OH-radical intervention during Fenton oxidation in the presence of a significant amount of radical scavenger (Cl-). Environ. Sci. Technol. 2000, 34, 2162–2168. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation—A review. Appl. Catal. B Environ. 2015, 176–177, 249–265. [Google Scholar] [CrossRef]
- Matafonova, G.; Batoev, V. Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: A review. Water Res. 2018, 132, 177–189. [Google Scholar] [CrossRef]
- Rodríguez, E.M.; Márquez, G.; Tena, M.; Álvarez, P.M.; Beltrán, F.J. Determination of main species involved in the first steps of TiO2 photocatalytic degradation of organics with the use of scavengers: The case of ofloxacin. Appl. Catal. B Environ. 2014, 178, 44–53. [Google Scholar] [CrossRef]
- Wu, D.; Li, X.; Tang, Y.; Lu, P.; Chen, W.; Xu, X.; Li, L. Mechanism insight of PFOA degradation by ZnO assisted-photocatalytic ozonation: Efficiency and intermediates. Chemosphere 2017, 180, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Buffle, M.O.; Schumacher, J.; Meylan, S.; Jekel, M.; Von Gunten, U. Ozonation and advanced oxidation of wastewater: Effect of O3 dose, pH, DOM and HO.-scavengers on ozone decomposition and HO. generation. Ozone Sci. Eng. 2006, 28, 247–259. [Google Scholar] [CrossRef]
- Xu, P.; Janex, M.L.; Savoye, P.; Cockx, A.; Lazarova, V. Wastewater disinfection by ozone: Main parameters for process design. Water Res. 2002, 36, 1043–1055. [Google Scholar] [CrossRef]
- Elovitz, M.S.; Von Gunten, U. Hydroxyl radical/ozone ratios during ozonation processes. I. The R(ct) concept. Ozone Sci. Eng. 1999, 21, 239–260. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Aguinaco, A.; García-Araya, J.F. Kinetic modelling of TOC removal in the photocatalytic ozonation of diclofenac aqueous solutions. Appl. Catal. B Environ. 2010, 100, 289–298. [Google Scholar] [CrossRef]
- Justo, A.; González, O.; Aceña, J.; Pérez, S.; Barceló, D.; Sans, C.; Esplugas, S. Pharmaceuticals and organic pollution mitigation in reclamation osmosis brines by UV/H2O2 and ozone. J. Hazard. Mater. 2013, 263, 268–274. [Google Scholar] [CrossRef]
- Kwon, M.; Kye, H.; Jung, Y.; Yoon, Y.; Kang, J.W. Performance characterization and kinetic modeling of ozonation using a new method: ROH,O3 concept. Water Res. 2017, 122, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Hermes, N.; Jewell, K.S.; Schulz, M.; Müller, J.; Hübner, U.; Wick, A.; Drewes, J.E.; Ternes, T.A. Elucidation of removal processes in sequential biofiltration (SBF) and soil aquifer treatment (SAT) by analysis of a broad range of trace organic chemicals (TOrCs) and their transformation products (TPs). Water Res. 2019, 163, 114857. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Ding, X.; Li, M.; Wang, L.; Wang, Y. Degradation efficiency and mechanism of primidone by UV/chorine process. Chinese J. Environ. Eng. 2021, 15, 3524–3535. [Google Scholar] [CrossRef]
- Solís, R.R.; Rivas, F.J.; Chávez, A.M.; Dionysiou, D.D. Peroxymonosulfate/solar radiation process for the removal of aqueous microcontaminants. Kinetic modeling, influence of variables and matrix constituents. J. Hazard. Mater. 2020, 400, 123118. [Google Scholar] [CrossRef] [PubMed]
- Rosario-Ortiz, F.L.; Wert, E.C.; Snyder, S.A. Evaluation of UV/H2O2 treatment for the oxidation of pharmaceuticals in wastewater. Water Res. 2010, 44, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Figueredo, M.; Rodríguez, E.M.; Rivas, J.; Beltrán, F.J. Kinetic model basis of ozone/light-based advanced oxidation processes: A pseudoempirical approach. Environ. Sci. Water Res. Technol. 2020, 6, 1176–1185. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Checa, M. Comparison of graphene oxide titania catalysts for their use in photocatalytic ozonation of water contaminants: Application to oxalic acid removal. Chem. Eng. J. 2020, 385, 123922. [Google Scholar] [CrossRef]
- Lin, Y.; Geng, Z.; Cai, H.; Ma, L.; Chen, J.; Zeng, J.; Pan, N.; Wang, X. Ternary graphene-TiO2-Fe3O4 nanocomposite as a recollectable photocatalyst with enhanced durability. Eur. J. Inorg. Chem. 2012, 2012, 4439–4444. [Google Scholar] [CrossRef]
- Bader, H.; Hoigné, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–456. [Google Scholar] [CrossRef]
- Masschelein, W.; Denis, M.; Ledent, R. Spectrophotometric determination of residual hydrogen peroxide. Water Sew. Work. 1977, 124, 69–72. [Google Scholar]
- Wu, W.; Jiang, C.; Roy, V.A.L. Recent Progress in Magnetic Iron Oxide-Semiconductor Composite Nanomaterials as Promising Photocatalysts. Nanoscale 2015, 7, 38–58. [Google Scholar] [CrossRef]
 ) and maghemite/magnetite (
) and maghemite/magnetite ( ). (b) Magnetic moment, Ms, versus applied magnetic field, H, for calcined magnetite (black) and FeGOTi catalyst (red).
). (b) Magnetic moment, Ms, versus applied magnetic field, H, for calcined magnetite (black) and FeGOTi catalyst (red).
 ) and maghemite/magnetite (
) and maghemite/magnetite ( ). (b) Magnetic moment, Ms, versus applied magnetic field, H, for calcined magnetite (black) and FeGOTi catalyst (red).
). (b) Magnetic moment, Ms, versus applied magnetic field, H, for calcined magnetite (black) and FeGOTi catalyst (red).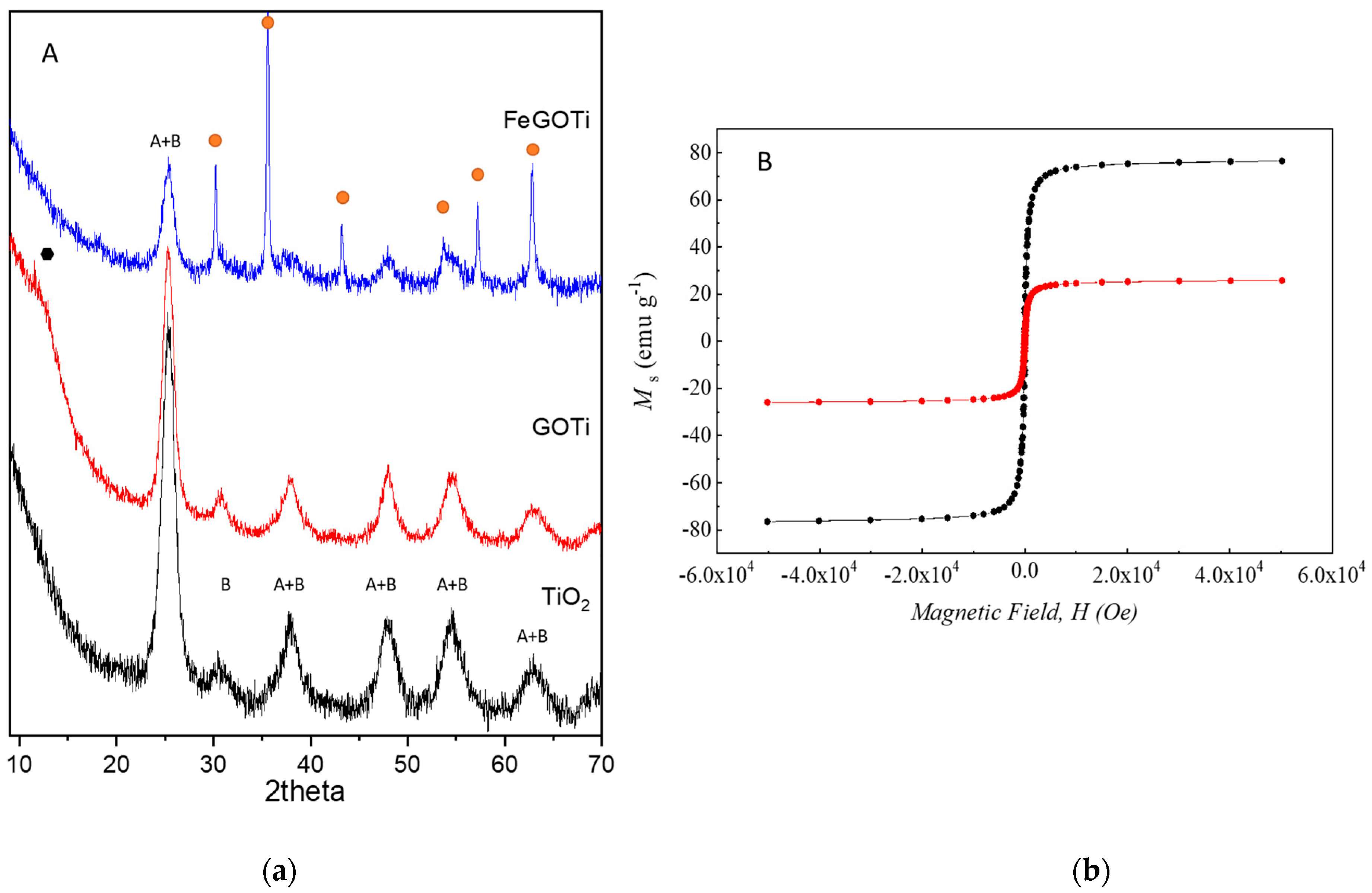
 ) and PhCat (◆). Conditions: gas flow rate, 35 L h−1; inlet ozone gas concentration, 10 mg L−1; primidone initial concentration, C0 = 10 mg L−1; catalyst dosage, 0.25 g L−1; agitation speed, 700 rpm.
) and PhCat (◆). Conditions: gas flow rate, 35 L h−1; inlet ozone gas concentration, 10 mg L−1; primidone initial concentration, C0 = 10 mg L−1; catalyst dosage, 0.25 g L−1; agitation speed, 700 rpm.
 ) and PhCat (◆). Conditions: gas flow rate, 35 L h−1; inlet ozone gas concentration, 10 mg L−1; primidone initial concentration, C0 = 10 mg L−1; catalyst dosage, 0.25 g L−1; agitation speed, 700 rpm.
) and PhCat (◆). Conditions: gas flow rate, 35 L h−1; inlet ozone gas concentration, 10 mg L−1; primidone initial concentration, C0 = 10 mg L−1; catalyst dosage, 0.25 g L−1; agitation speed, 700 rpm.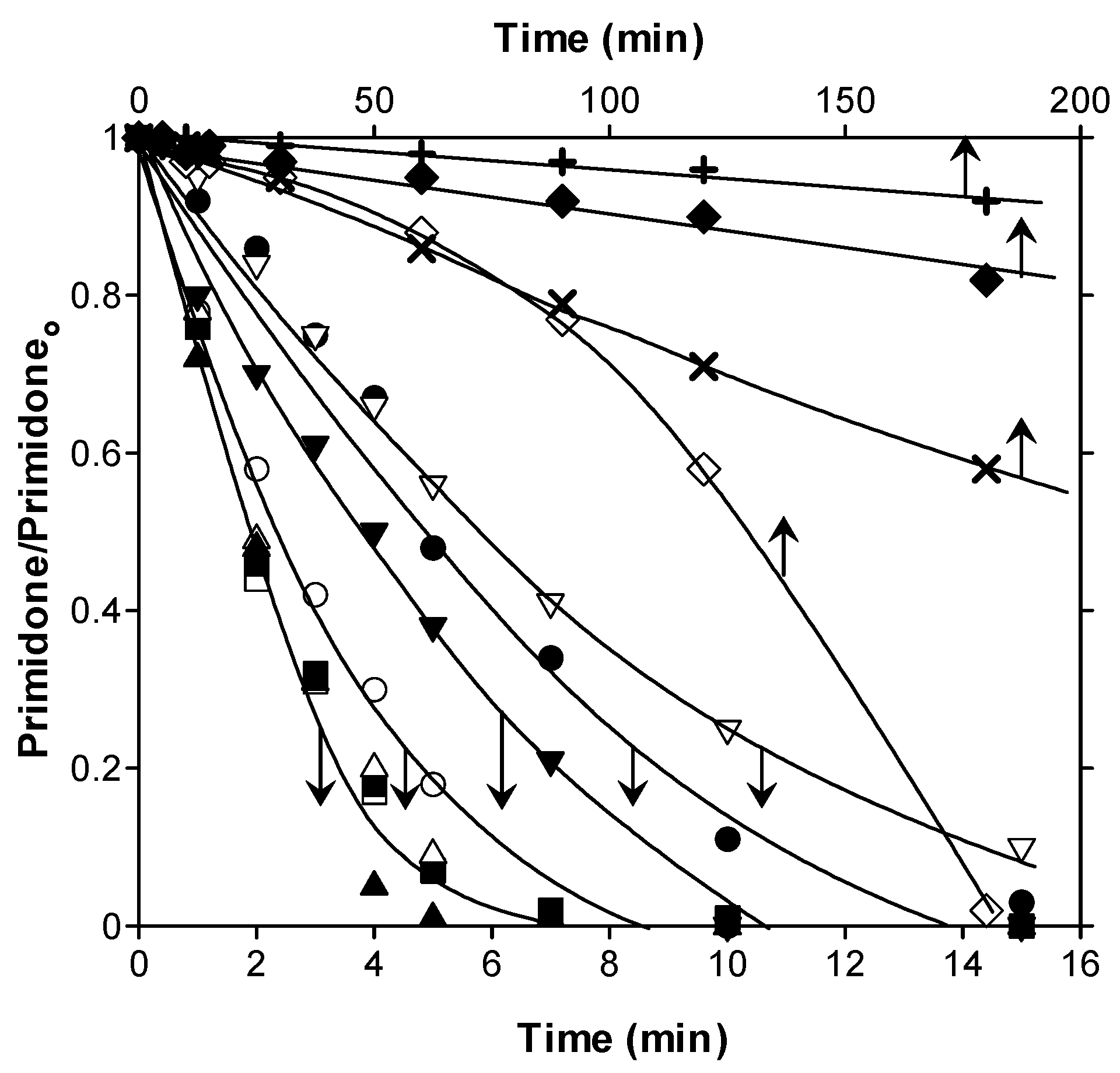
 ) and PhCatOz (○). Conditions: gas flow rate, 35 L h−1; inlet ozone gas concentration, 10 mg L−1; initial TOC = 6.6 mg L−1; catalyst dosage, 0.25 g L−1; agitation speed, 700 rpm.
) and PhCatOz (○). Conditions: gas flow rate, 35 L h−1; inlet ozone gas concentration, 10 mg L−1; initial TOC = 6.6 mg L−1; catalyst dosage, 0.25 g L−1; agitation speed, 700 rpm.
 ) and PhCatOz (○). Conditions: gas flow rate, 35 L h−1; inlet ozone gas concentration, 10 mg L−1; initial TOC = 6.6 mg L−1; catalyst dosage, 0.25 g L−1; agitation speed, 700 rpm.
) and PhCatOz (○). Conditions: gas flow rate, 35 L h−1; inlet ozone gas concentration, 10 mg L−1; initial TOC = 6.6 mg L−1; catalyst dosage, 0.25 g L−1; agitation speed, 700 rpm.

 ), GOTi (
), GOTi ( ) and FeGOTi (
) and FeGOTi ( ). Conditions: gas flow rate, 35 L h−1; inlet ozone gas concentration, 10 mg L−1; primidone, C0 = 10 mg L−1; TOC0 = 6.6. mgL−1; catalyst dosage, 0.25 g L−1; agitation speed, 700 rpm.
). Conditions: gas flow rate, 35 L h−1; inlet ozone gas concentration, 10 mg L−1; primidone, C0 = 10 mg L−1; TOC0 = 6.6. mgL−1; catalyst dosage, 0.25 g L−1; agitation speed, 700 rpm.
 ), GOTi (
), GOTi ( ) and FeGOTi (
) and FeGOTi ( ). Conditions: gas flow rate, 35 L h−1; inlet ozone gas concentration, 10 mg L−1; primidone, C0 = 10 mg L−1; TOC0 = 6.6. mgL−1; catalyst dosage, 0.25 g L−1; agitation speed, 700 rpm.
). Conditions: gas flow rate, 35 L h−1; inlet ozone gas concentration, 10 mg L−1; primidone, C0 = 10 mg L−1; TOC0 = 6.6. mgL−1; catalyst dosage, 0.25 g L−1; agitation speed, 700 rpm.
| Catalyst | Band Gap (eV) | SB.E.T. m2 g−1 | TGA (% C) | XRD (nm) * | Crystallinity Degree | Raman (ID/IG) |
|---|---|---|---|---|---|---|
| TiO2 | 3.02 | 195.0 | - | 6.9 (A) | 69.7 | - |
| GOTi | 2.86 | 184.2 | 1.24 | 7.3 (A) | 70.0 | 1.31 |
| FeGOTi | 1.83 | 144.3 | 0.49 | 38.6 (M); 8.7 (A) | 84.0 | 1.29 |
| PhCat | PhCatOz | SF | |||
|---|---|---|---|---|---|
| kap (min−1) | R2 | kap (min−1) | R2 | ||
| No catalyst | 0 a | - | 0.0079 b | 0.98 | - |
| FeGOTi | 0 | - | 0.0095 | 0.97 | 1.20 |
| GOTi | 0.0016 | 0.95 | 0.0405 | 0.90 | 4.23 |
| TiO2 | 0.0007 | 0.95 | 0.0176 | 0.98 | 2.03 |
| pH | % TOC Removal | % Fe Leached |
|---|---|---|
| 3.8 | 99.2 | 2.64 |
| 4.0 | 74.6 | 0.12 |
| 6.0 | 77.5 | 0.16 |
| 8.0 | 68.0 | 1.68 |
| PhCatOz | CatOz | |||
|---|---|---|---|---|
| Catalyst | RCT | RO3HO | RCT | RO3HO |
| FeGOTi | 3.28 × 10−4 | 5.52 × 10−9 | 1.04 × 10−4 | 2.25 × 10−9 |
| GOTi | 1.26 × 10−3 | 5.95 × 10−8 | 6.80 × 10−5 | 1.38 × 10−9 |
| TiO2 | 1.49 × 10−4 | 2.72 × 10−9 | 5.27 × 10−5 | 1.02 × 10−9 |
| Removal, % (Time, h) of: | |||||
|---|---|---|---|---|---|
| Process | Radiation Source | Catalyst (Dosage) | Primidone | TOC | Reference |
| UV/Cl2 | Low Pressure Hg lamp | - | 80–90 (0.17) | 5 (1 h) | [71] |
| UV/Cl2 | Low Pressure Hg lamp | FeCl3 (50 μmol/L) | 100 (0.085) | 6.8 (1 h) | [71] |
| Peroxymonosulfate | Solar radiation | PMS (5 mM) | 95–100 (2) | [72] | |
| UV/H2O2 | Two G15T8 germicidal lamps | H2O2 (20 mg/L) | 10–89 (0.33) | - | [73] |
| O3/UV Semicontinuous | UV-LED | TiO2 (0.5 g/L) | 100 (0.25) | 95 (1 h) | [74] |
| O3/Vis | 425 nm Vis-LED | TiO2 (0.25 g/L) | 100 (0.17) | 90 (2 h) | [35] |
| O3/Vis | 425 nm Vis-LED | GO/TiO2 (0.25 g/L) | 100 (0.17) | 80 (2 h) | [35] |
| O3/UV-Vis | Solar radiation | TiO2 (0.25 g/L) | 100 (0.1) | 95 (3 h) | This work |
| O3/UV-Vis | Solar radiation | GO/TiO2 (0.25 g/L) | 100 (0.1) | 100 (2 h) | This work |
| O3/UV-Vis | Solar radiation | FeGOTi (0.25 g/L) | 100 (0.25) | 90 (3 h) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Checa, M.; Montes, V.; Rivas, J.; Beltrán, F.J. Checking the Efficiency of a Magnetic Graphene Oxide–Titania Material for Catalytic and Photocatalytic Ozonation Reactions in Water. Catalysts 2022, 12, 1587. https://doi.org/10.3390/catal12121587
Checa M, Montes V, Rivas J, Beltrán FJ. Checking the Efficiency of a Magnetic Graphene Oxide–Titania Material for Catalytic and Photocatalytic Ozonation Reactions in Water. Catalysts. 2022; 12(12):1587. https://doi.org/10.3390/catal12121587
Chicago/Turabian StyleCheca, Manuel, Vicente Montes, Javier Rivas, and Fernando J. Beltrán. 2022. "Checking the Efficiency of a Magnetic Graphene Oxide–Titania Material for Catalytic and Photocatalytic Ozonation Reactions in Water" Catalysts 12, no. 12: 1587. https://doi.org/10.3390/catal12121587
APA StyleCheca, M., Montes, V., Rivas, J., & Beltrán, F. J. (2022). Checking the Efficiency of a Magnetic Graphene Oxide–Titania Material for Catalytic and Photocatalytic Ozonation Reactions in Water. Catalysts, 12(12), 1587. https://doi.org/10.3390/catal12121587