Synthesis, Characterization and Photocatalytic Activity of Spherulite-like r-TiO2 in Hydrogen Evolution Reaction and Methyl Violet Photodegradation
Abstract
:1. Introduction
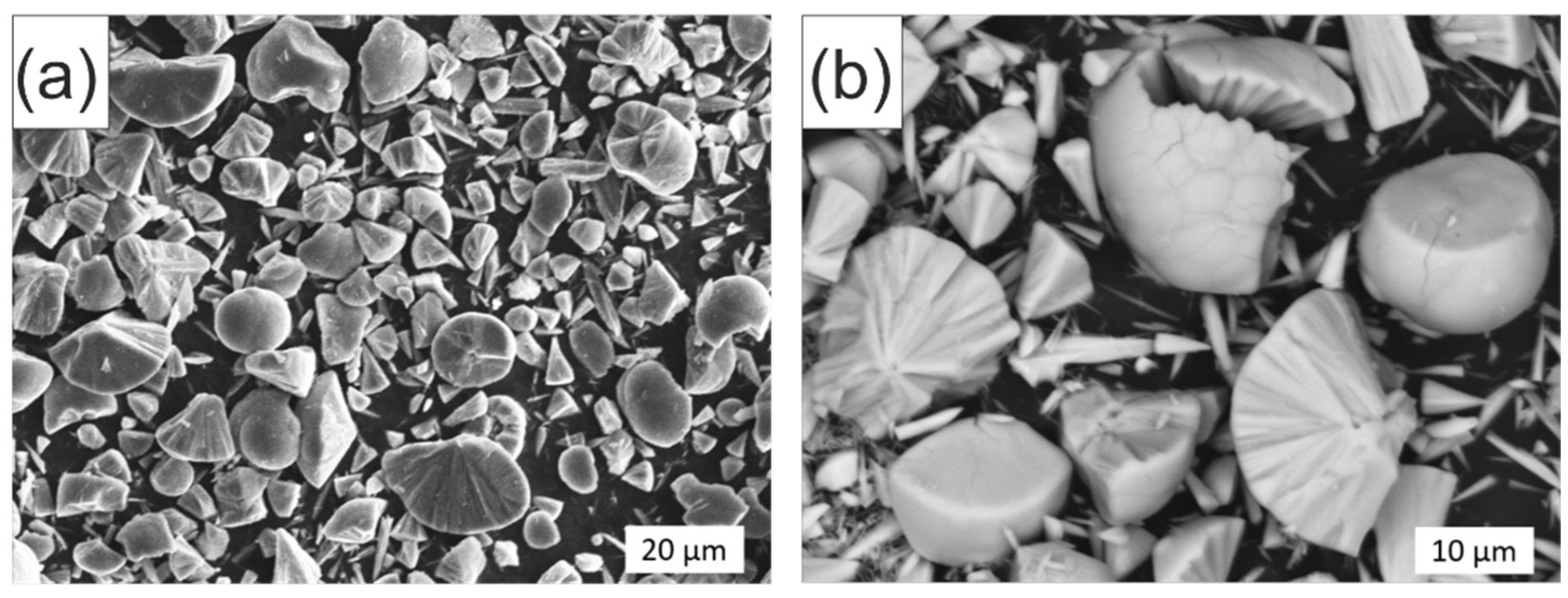
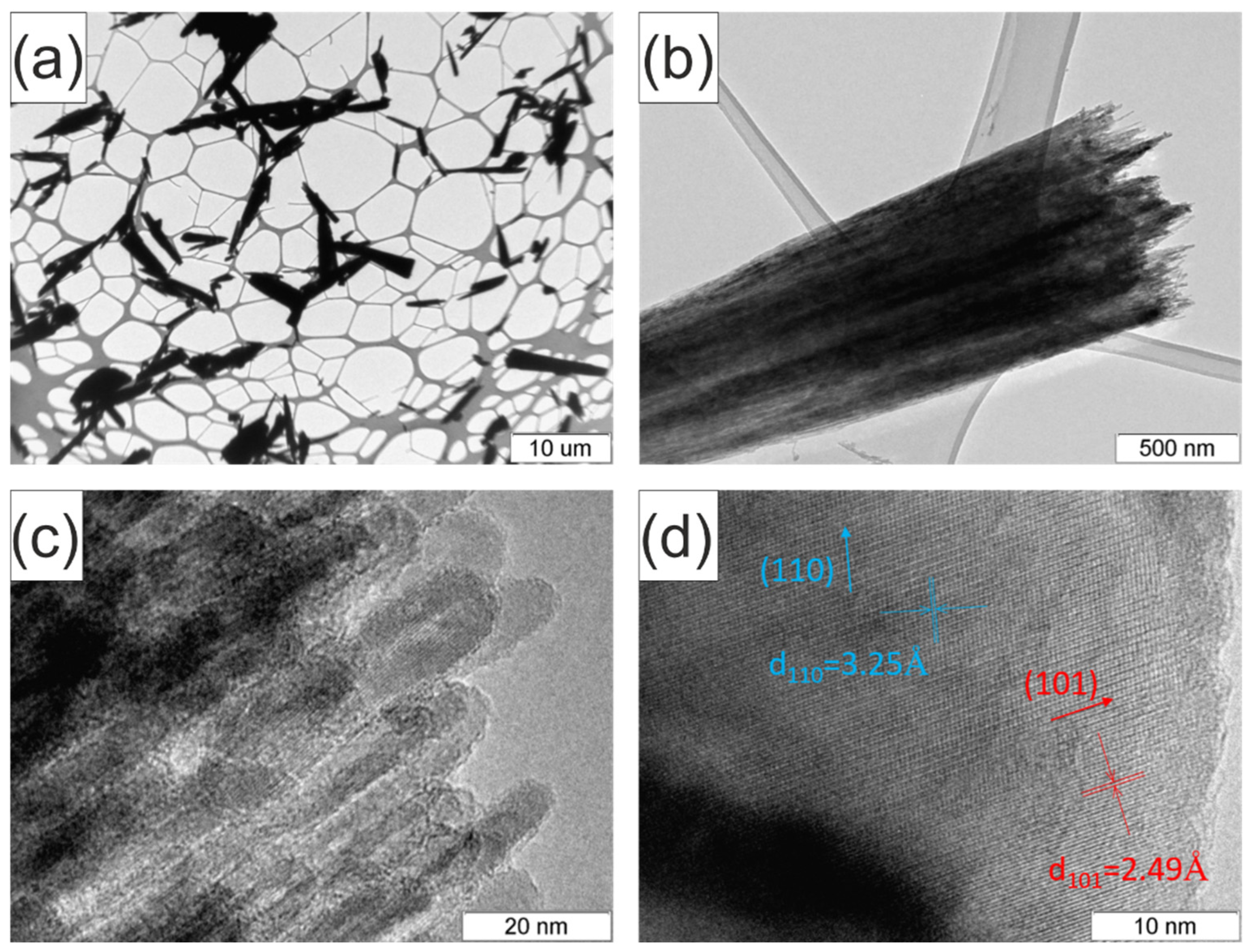
2. Results and Discussion
C2H5OH → CH3CHO + H2↑
3. Materials and Methods
3.1. Synthesis Procedure
3.2. Physico-Chemical Characterization
3.3. Photocatalytic Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Cui, H.; Humayun, M.; Qu, Y.; Fan, N.; Sun, X.; Jing, L. Exceptional Performance of Photoelectrochemical Water Oxidation of Single-Crystal Rutile TiO2 Nanorods Dependent on the Hole Trapping of Modified Chloride. Sci. Rep. 2016, 6, 21430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification Strategies of TiO2 for Potential Applications in Photocatalysis: A Critical Review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the Anatase to Rutile Phase Transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Selishchev, D.; Stepanov, G.; Sergeeva, M.; Solovyeva, M.; Zhuravlev, E.; Komissarov, A.; Richter, V.; Kozlov, D. Inactivation and Degradation of Influenza a Virus on the Surface of Photoactive Self-Cleaning Cotton Fabric Functionalized with Nanocrystalline TiO2. Catalysts 2022, 12, 1298. [Google Scholar] [CrossRef]
- Xu, M.; Gao, Y.; Moreno, E.M.; Kunst, M.; Muhler, M.; Wang, Y.; Idriss, H.; Wöll, C. Photocatalytic Activity of Bulk TiO2 Anatase and Rutile Single Crystals Using Infrared Absorption Spectroscopy. Phys. Rev. Lett. 2011, 106, 138302. [Google Scholar] [CrossRef] [Green Version]
- Rempel, A.A.; Valeeva, A.A. Nanostructured Titanium Dioxide for Medicinal Chemistry. Russ. Chem. Bull. 2019, 68, 2163–2171. [Google Scholar] [CrossRef]
- Rempel, A.A.; Kuznetsova, Y.V.; Dorosheva, I.B.; Valeeva, A.A.; Weinstein, I.A.; Kozlova, E.A.; Saraev, A.A.; Selishchev, D.S. High Photocatalytic Activity Under Visible Light of Sandwich Structures Based on Anodic TiO2/CdS Nanoparticles/Sol–Gel TiO2. Top. Catal. 2020, 63, 130–138. [Google Scholar] [CrossRef]
- Rempel, A.A.; Valeeva, A.A.; Vokhmintsev, A.S.; Weinstein, I.A. Titanium Dioxide Nanotubes: Synthesis, Structure, Properties and Applications. Russ. Chem. Rev. 2021, 90, 1397–1414. [Google Scholar] [CrossRef]
- Swamy, V.; Gale, J.D.; Dubrovinsky, L.S. Atomistic Simulation of the Crystal Structures and Bulk Moduli of TiO2 Polymorphs. J. Phys. Chem. Solids 2001, 62, 887–895. [Google Scholar] [CrossRef]
- Gurenko, V.E.; Popkov, V.I. Hydrothermal-Assisted Synthesis of Sponge-like Brookite for Pseudo-Fenton Decomposition of Crystal Violet under UV–Light. Nano-Struct. Nano-Objects 2021, 28, 100801. [Google Scholar] [CrossRef]
- Bachina; Almjasheva, O.V.; Popkov, V.I.; Nevedomskiy, V.N.; Gusarov, V.V. Heat-Stimulated Crystallization and Phase Transformation of Titania Nanoparticles. J. Cryst. Growth 2021, 576, 126371. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, N.K.; Singla, M.L. Size Dependent Reflective Properties of TiO2 Nanoparticles and Reflectors Made Thereof. Dig. J. Nanomater. Biostruct. 2012, 7, 607–619. [Google Scholar]
- Huber, B.; Brodyanski, A.; Scheib, M.; Orendorz, A.; Ziegler, C.; Gnaser, H. Nanocrystalline Anatase TiO2 Thin Films: Preparation and Crystallite Size-Dependent Properties. Thin Solid Films 2005, 472, 114–124. [Google Scholar] [CrossRef]
- Vasilevskaia, A.K.; Popkov, V.I.; Valeeva, A.A.; Rempel’, A.A. Formation of Nonstoichiometric Titanium Oxides Nanoparticles TinO2n–1 upon Heat-Treatments of Titanium Hydroxide and Anatase Nanoparticles in a Hydrogen Flow. Russ. J. Appl. Chem. 2016, 89, 1211–1220. [Google Scholar] [CrossRef]
- Dorosheva, I.B.; Valeeva, A.A.; Rempel, A.A.; Trestsova, M.A.; Utepova, I.A.; Chupakhin, O.N. Synthesis and Physicochemical Properties of Nanostructured TiO2 with Enhanced Photocatalytic Activity. Inorg. Mater. 2021, 57, 503–510. [Google Scholar] [CrossRef]
- Valeeva, A.A.; Dorosheva, I.B.; Kozlova, E.A.; Sushnikova, A.A.; Kurenkova, A.Y.; Saraev, A.; Schroettner, H.; Rempel, A. Solar Photocatalysts Based on Titanium Dioxide Nanotubes for Hydrogen Evolution from Aqueous Solutions of Ethanol. Int. J. Hydrogen Energy 2021, 46, 16917–16924. [Google Scholar] [CrossRef]
- Barnard, A.S.; Zapol, P. Predicting the Energetics, Phase Stability, and Morphology Evolution of Faceted and Spherical Anatase Nanocrystals. J. Phys. Chem. B 2004, 108, 18435–18440. [Google Scholar] [CrossRef]
- Li, Y.; Lee, N.H.; Lee, E.G.; Song, J.S.; Kim, S.J. The Characterization and Photocatalytic Properties of Mesoporous Rutile TiO2 Powder Synthesized through Self-Assembly of Nano Crystals. Chem. Phys. Lett. 2004, 389, 124–128. [Google Scholar] [CrossRef]
- Popkov, V.I.; Bachina, A.K.; Valeeva, A.A.; Lobinsky, A.A.; Gerasimov, E.Y.; Rempel, A.A. Synthesis, Morphology and Electrochemical Properties of Spherulite Titania Nanocrystals. Ceram. Int. 2020, 46, 24483–24487. [Google Scholar] [CrossRef]
- Zhang, Y.; Harris, C.X.; Wallenmeyer, P.; Murowchick, J.; Chen, X. Asymmetric Lattice Vibrational Characteristics of Rutile TiO2 as Revealed by Laser Power Dependent Raman Spectroscopy. J. Phys. Chem. C 2013, 117, 24015–24022. [Google Scholar] [CrossRef]
- Ocaña, M.; Fornés, V.; Ramos, J.V.G.; Serna, C.J. Factors Affecting the Infrared and Raman Spectra of Rutile Powders. J. Solid State Chem. 1988, 75, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Swamy, V. Size-Dependent Modifications of the First-Order Raman Spectra of Nanostructured Rutile TiO2. Phys. Rev. B-Condens. Matter Mater. Phys. 2008, 77, 15–18. [Google Scholar] [CrossRef]
- Swamy, V.; Muddle, B.C.; Dai, Q. Size-Dependent Modifications of the Raman Spectrum of Rutile TiO2. Appl. Phys. Lett. 2006, 89, 163118. [Google Scholar] [CrossRef]
- Mazza, T.; Barborini, E.; Piseri, P.; Milani, P.; Cattaneo, D.; Li Bassi, A.; Bottani, C.E.; Ducati, C. Raman Spectroscopy Characterization of TiO2 Rutile Nanocrystals. Phys. Rev. B-Condens. Matter Mater. Phys. 2007, 75, 045416. [Google Scholar] [CrossRef]
- Moulder, J.F.; Strickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1995; ISBN 0962702625. [Google Scholar]
- Stefanov, P.; Shipochka, M.; Stefchev, P.; Raicheva, Z.; Lazarova, V.; Spassov, L. XPS Characterization of TiO2 Layers Deposited on Quartz Plates. J. Phys. Conf. Ser. 2008, 100, 012039. [Google Scholar] [CrossRef] [Green Version]
- Kubelka, P.; Munk, F. Ein Beitrag Zur Optik Der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Harb, M.; Jeantelot, G.; Basset, J.M. Insights into the Most Suitable TiO2 Surfaces for Photocatalytic O2 and H2 Evolution Reactions from DFT Calculations. J. Phys. Chem. C 2019, 123, 28210–28218. [Google Scholar] [CrossRef]
- Pan, J.; Liu, G.; Lu, G.Q.; Cheng, H.M. On the True Photoreactivity Order of {001}, {010}, and {101} Facets of Anatase TiO2 Crystals. Angew. Chem.-Int. Ed. 2011, 50, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Ruso, J.M.; Verdinelli, V.; Hassan, N.; Pieroni, O.; Messina, P.V. Enhancing CaP Biomimetic Growth on TiO2 Cuboids Nanoparticles via Highly Reactive Facets. Langmuir 2013, 29, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
- Batzill, M. Fundamental Aspects of Surface Engineering of Transition Metal Oxide Photocatalysts. Energy Environ. Sci. 2011, 4, 3275–3286. [Google Scholar] [CrossRef]
- Viswanatha, R.; Sapra, S.; Satpati, B.; Satyam, P.V.; Dev, B.N.; Sarma, D.D. Understanding the Quantum Size Effects in ZnO Nanocrystals. J. Mater. Chem. 2004, 14, 661–668. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chang, Y.S.; Teoh, L.G.; Huang, Y.L.; Shen, Y.C. The Effects of the Nanostructure of Mesoporous TiO2 on Optical Band Gap Energy. J. Sol-Gel Sci. Technol. 2010, 56, 33–38. [Google Scholar] [CrossRef]
- Singh, M.; Goyal, M.; Devlal, K. Size and Shape Effects on the Band Gap of Semiconductor Compound Nanomaterials. J. Taibah Univ. Sci. 2018, 12, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Fang, P.; Gao, Y.; Liu, Z.; Liu, Y.; Dai, Y. Effective Removal of High-Chroma Crystal Violet over TiO2-Based Nanosheet by Adsorption-Photocatalytic Degradation. Chem. Eng. J. 2012, 204–205, 107–113. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, Z.; Chen, X.; Yang, X. Effective Synthesis of Nanoscale Anatase TiO2 Single Crystals Using Activated Carbon Template to Enhance the Photodegradation of Crystal Violet. Appl. Organomet. Chem. 2019, 33, e4664. [Google Scholar] [CrossRef]
- Devi, L.G.; Nithya, P.M.; Abraham, C.; Kavitha, R. Influence of Surface Metallic Silver Deposit and Surface Fluorination on the Photocatalytic Activity of Rutile TiO2 for the Degradation of Crystal Violet a Cationic Dye under UV Light Irradiation. Mater. Today Commun. 2017, 10, 1–13. [Google Scholar] [CrossRef]
- Markovskaya, D.V.; Kozlova, E.A. Formal Kinetic Description of Photocatalytic Hydrogen Evolution from Ethanol Aqueous Solutions in the Presence of Sodium Hydroxide. Kinet. Catal. 2018, 59, 727–734. [Google Scholar] [CrossRef]
- Sun, B.; Vorontsov, A.V.; Smirniotis, P.G. Role of Platinum Deposited on TiO2 in Phenol Photocatalytic Oxidation. Langmuir 2003, 19, 3151–3156. [Google Scholar] [CrossRef]
- Moiseev, I.I. Green Chemistry: Development Trajectory. Russ. Chem. Rev. 2013, 82, 616–623. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Poudyal, R.S.; Tiwari, I.; Voloshin, R.A.; Zharmukhamedov, S.K.; Nam, H.G.; Zayadan, B.K.; Bruce, B.D.; Hou, H.J.M.; Allakhverdiev, S.I. Biofuel Production: Challenges and Opportunities. Int. J. Hydrogen Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Markovskaya, D.V.; Cherepanova, S.V.; Saraev, A.A.; Gerasimov, E.Y.; Perevalov, T.V.; Kaichev, V.V.; Parmon, V.N. Novel Photocatalysts Based on Cd1−xZnxS/Zn(OH)2 for the Hydrogen Evolution from Water Solutions of Ethanol. Int. J. Hydrogen Energy 2014, 39, 18758–18769. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Cherepanova, S.V.; Markovskaya, D.V.; Saraev, A.A.; Gerasimov, E.Y.; Parmon, V.N. Novel Photocatalysts Pt/Cd1-xZnxS/ZnO/Zn(OH)2: Activation during Hydrogen Evolution from Aqueous Solutions of Ethanol under Visible Light. Appl. Catal. B Environ. 2016, 183, 197–205. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Kurenkova, A.Y.; Gerasimov, E.Y.; Gromov, N.V.; Medvedeva, T.B.; Saraev, A.A.; Kaichev, V.V. Comparative Study of Photoreforming of Glycerol on Pt/TiO2 and CuOx/TiO2 Photocatalysts under UV Light. Mater. Lett. 2021, 283, 128901. [Google Scholar] [CrossRef]
- Le Bail, A. Whole Powder Pattern Decomposition Methods and Applications: A Retrospection. Powder Diffr. 2005, 20, 316–326. [Google Scholar] [CrossRef] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Kurenkova, A.Y.; Kremneva, A.M.; Saraev, A.A.; Murzin, V.; Kozlova, E.A.; Kaichev, V.V. Influence of Thermal Activation of Titania on Photoreactivity of Pt/TiO2 in Hydrogen Production. Catal. Lett. 2021, 151, 748–754. [Google Scholar] [CrossRef]





| Sample | Phase Composition | Ss, m2/g | Morphology/ Crystallite Size, nm | Synthesis Procedure/ Precursor | k, min−1 | Reference |
|---|---|---|---|---|---|---|
| r-TiO2 | Rutile | 102.6 | Spherulite agglomerates of rod-like/5 × 15 | HTT */ titanyl citrate | 0.0238 | this work |
| TiO2 | Brookite | 93.4 | Sponge-like/ 30 | HTT */ titanium (IV) peroxocomlex | 0.0234 | [12] |
| TNS | Anatase, rutile | 207 | Sheet-like | HTT *, Degussa P25 | 0.01812 | [38] |
| TiO2 | Anatase | ~36 | 12.3 | Sol–gel, titanium(IV) butoxide | 0.00994 | [39] |
| TiO2 | Anatase | 106.1 | 8.7 | Sol–gel, titanium(IV) butoxide, activated carbone | 0.03914 | [7] |
| TiO2 | Rutile | - | Agglomerated flakes/53 | Sol–gel/ TiCl4 | 0.01156 | [40] |
| FRT | Rutile | - | Agglomerated flakes/39 | Impregnation TiO2 with HF | 0.02541 | [40] |
| SRT | Rutile, silver | - | Agglomerated flakes/48 | Photoreduction of AgNO3 on TiO2 | 0.02827 | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachina, A.K.; Popkov, V.I.; Seroglazova, A.S.; Enikeeva, M.O.; Kurenkova, A.Y.; Kozlova, E.A.; Gerasimov, E.Y.; Valeeva, A.A.; Rempel, A.A. Synthesis, Characterization and Photocatalytic Activity of Spherulite-like r-TiO2 in Hydrogen Evolution Reaction and Methyl Violet Photodegradation. Catalysts 2022, 12, 1546. https://doi.org/10.3390/catal12121546
Bachina AK, Popkov VI, Seroglazova AS, Enikeeva MO, Kurenkova AY, Kozlova EA, Gerasimov EY, Valeeva AA, Rempel AA. Synthesis, Characterization and Photocatalytic Activity of Spherulite-like r-TiO2 in Hydrogen Evolution Reaction and Methyl Violet Photodegradation. Catalysts. 2022; 12(12):1546. https://doi.org/10.3390/catal12121546
Chicago/Turabian StyleBachina, Anastasia K., Vadim I. Popkov, Anna S. Seroglazova, Maria O. Enikeeva, Anna Yu. Kurenkova, Ekaterina A. Kozlova, Evgeny Y. Gerasimov, Albina A. Valeeva, and Andrey A. Rempel. 2022. "Synthesis, Characterization and Photocatalytic Activity of Spherulite-like r-TiO2 in Hydrogen Evolution Reaction and Methyl Violet Photodegradation" Catalysts 12, no. 12: 1546. https://doi.org/10.3390/catal12121546







