Egg-Shell-Type MgAl2O4 Pellet Catalyst for Steam Methane Reforming Reaction Activity: Effect of Pellet Preparation Temperature
Abstract
1. Introduction
2. Results and Discussion
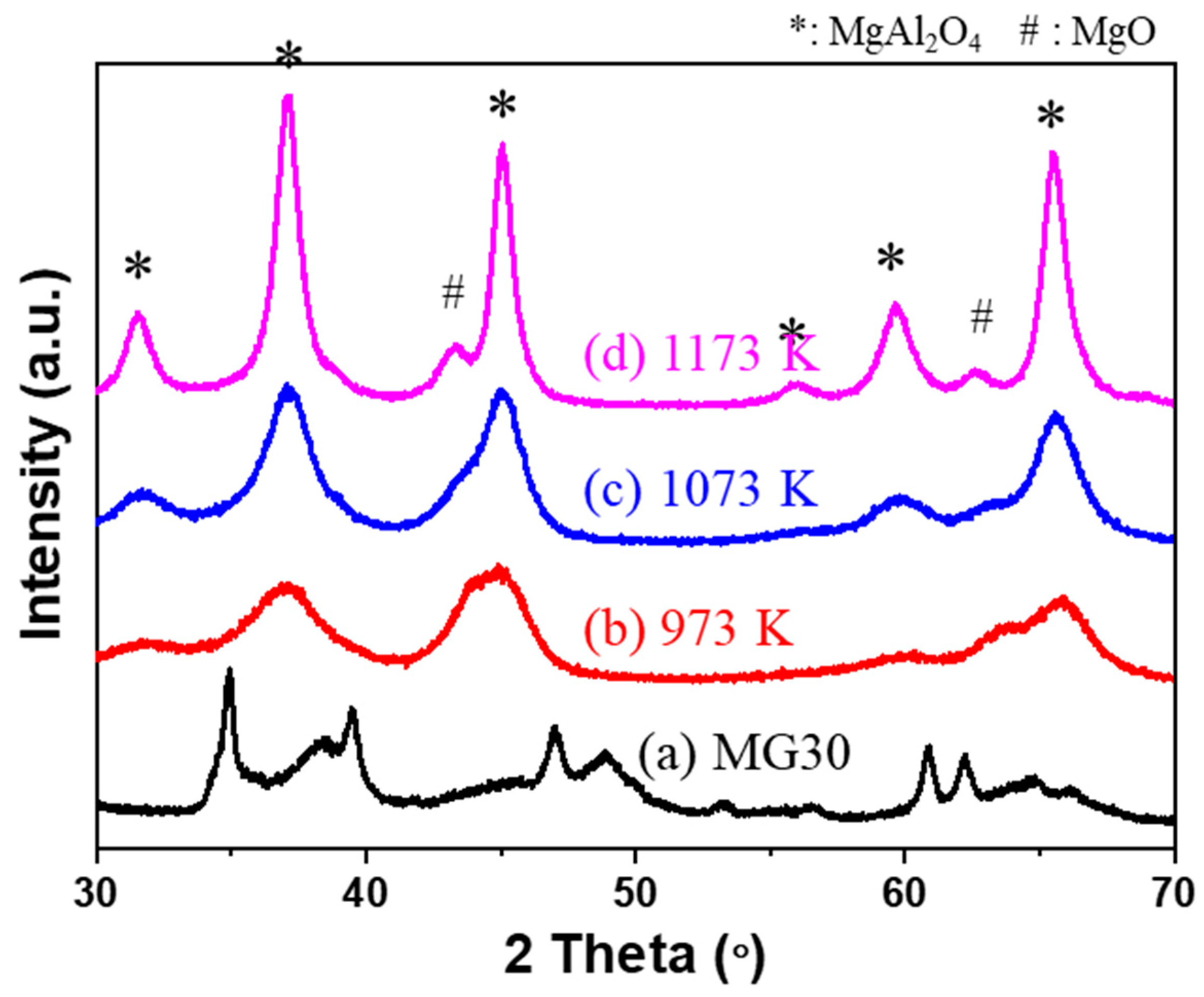
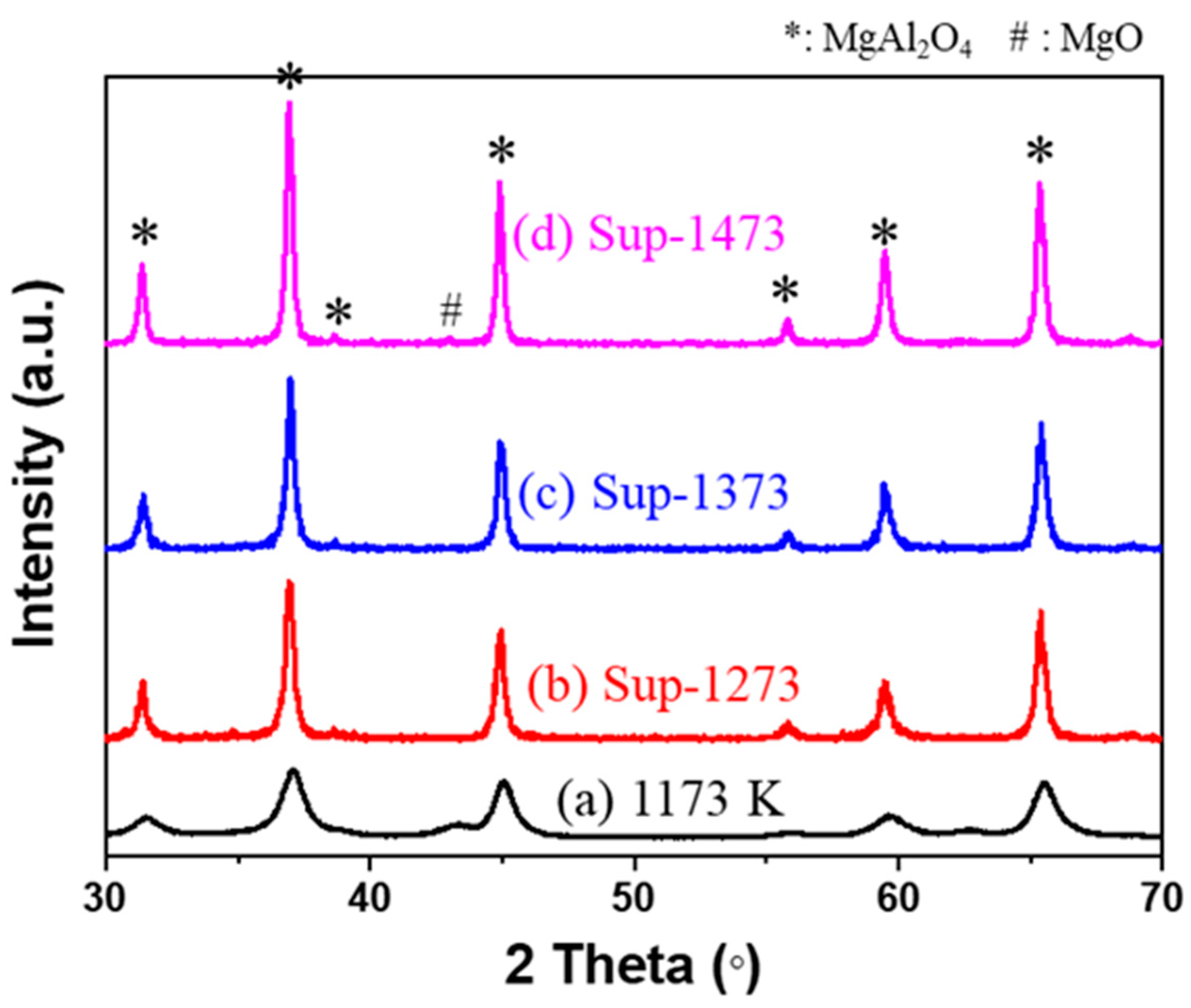
2.1. Analysis of Supports
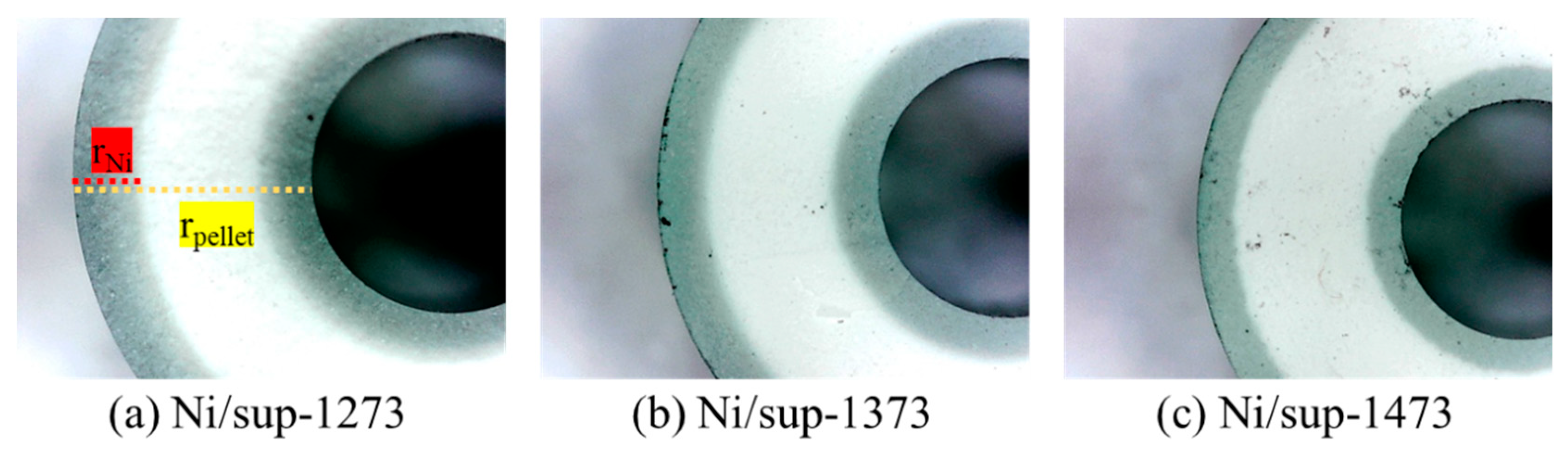
2.2. Egg-Shell-Type 3.5 wt% Ni/MgAl2O4Pellet Catalysts
2.3. Catalytic Activity
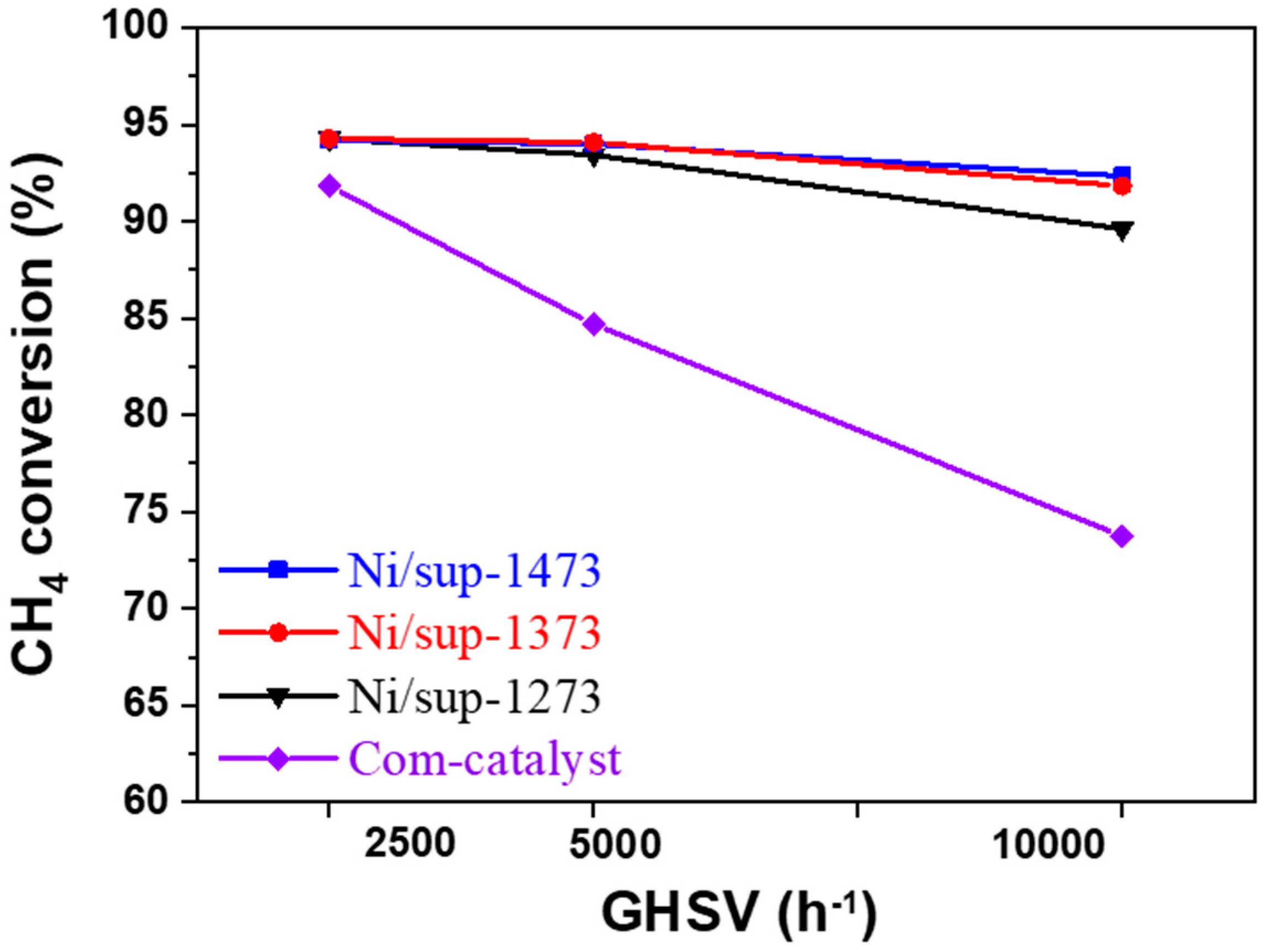
2.3.1. Evaluation of the SMR Reaction According to Space Velocity
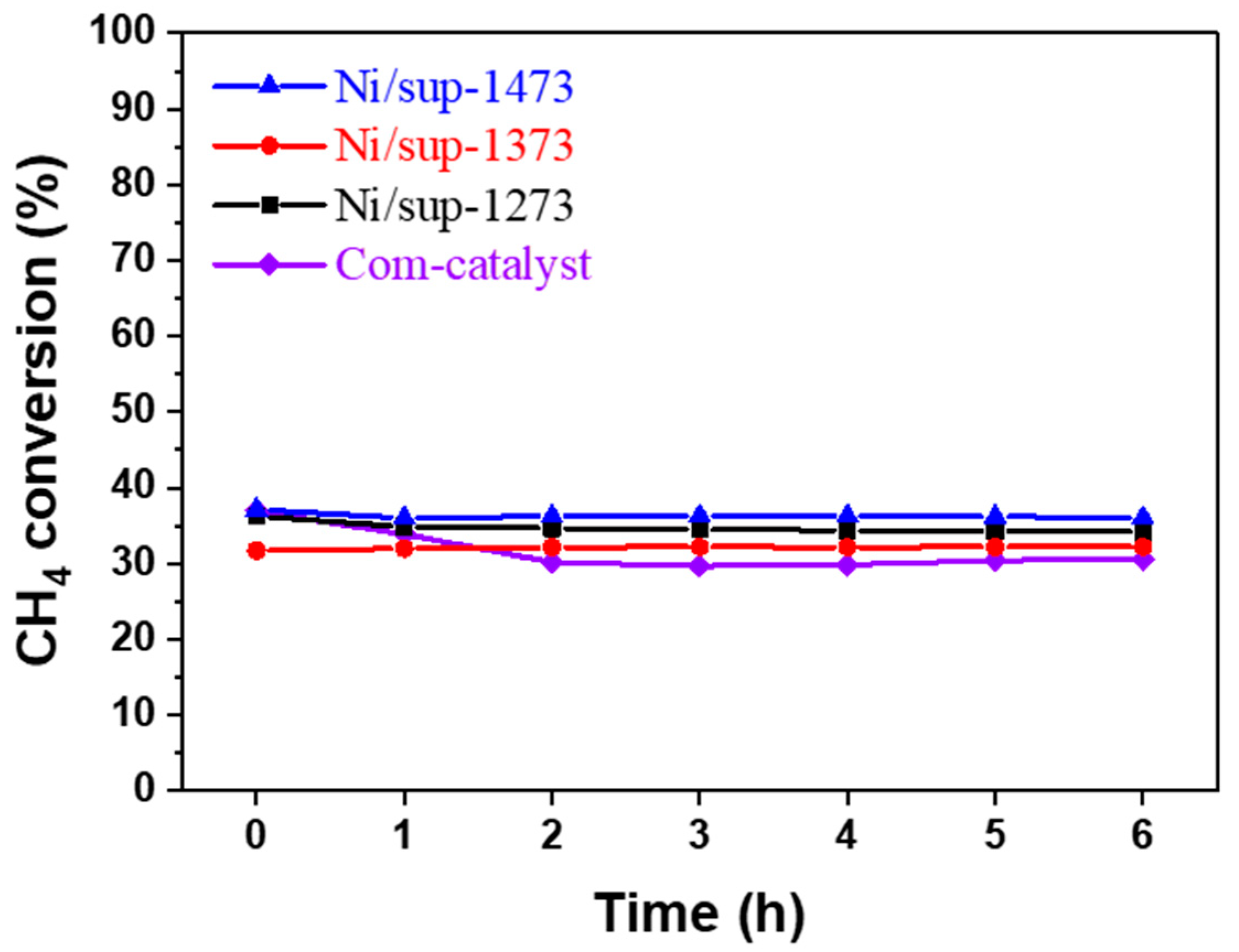
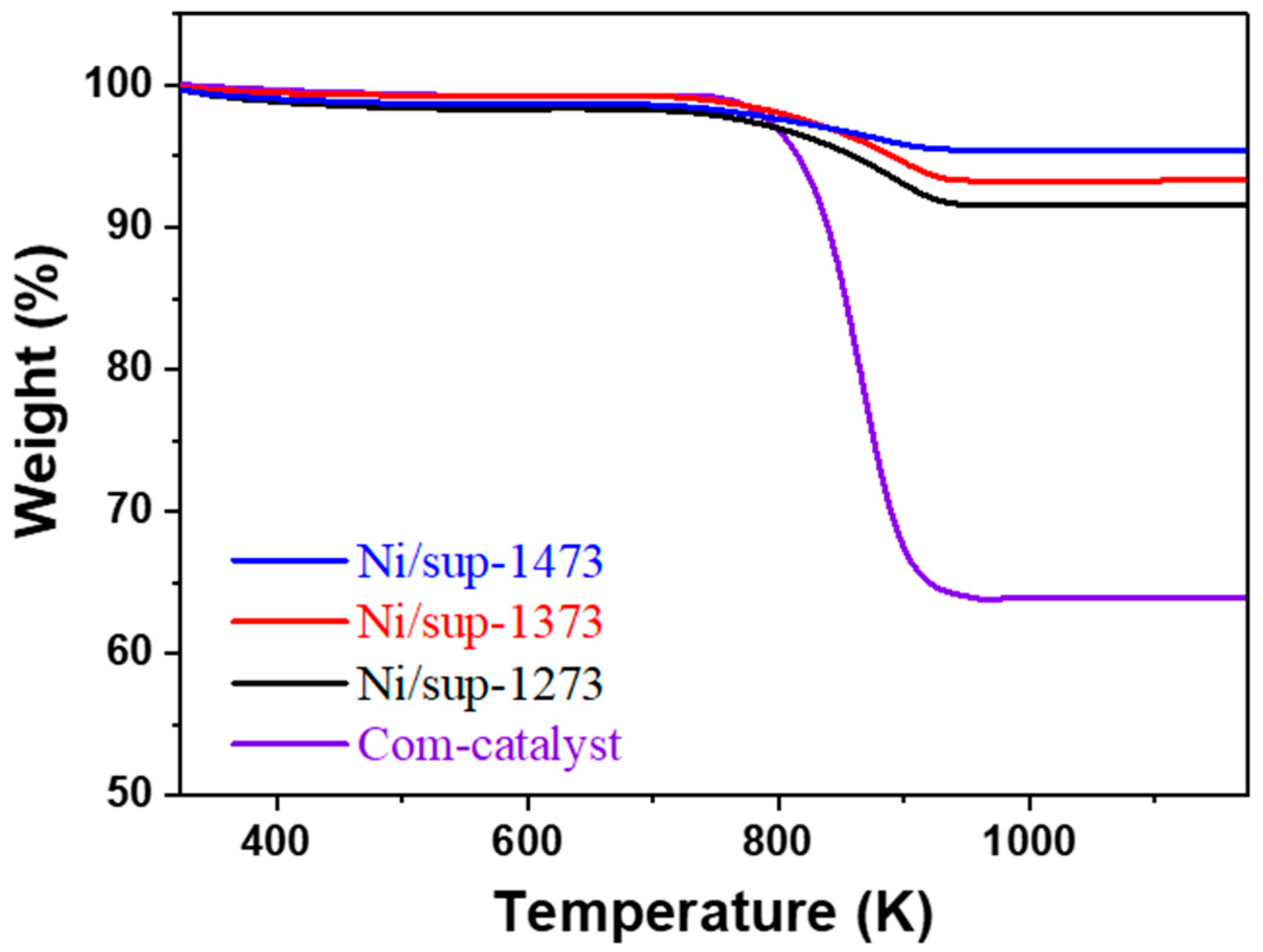
2.3.2. Evaluation of Durability and Resistance to Carbon Deposition
3. Conclusions
4. Materials and Methods
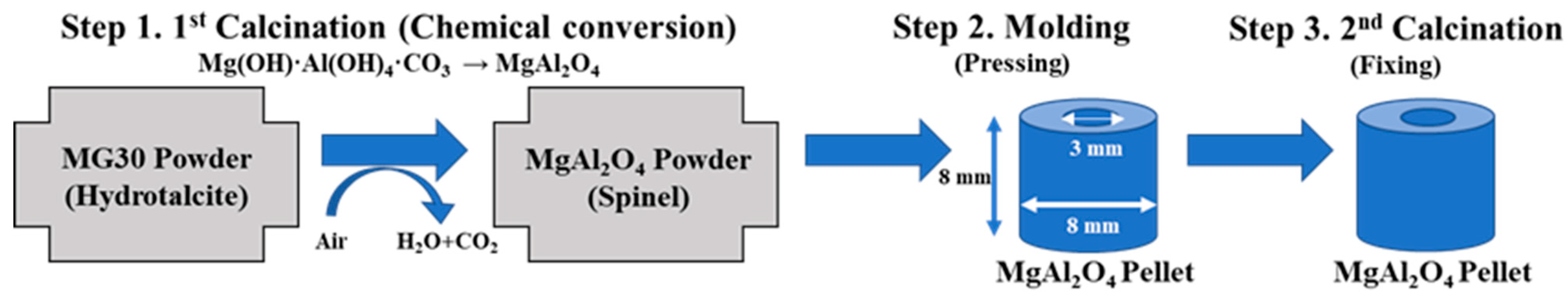
4.1. Manufacturing Pellet Type Supports
4.2. Preparation of Egg-Shell-Type Catalysts
4.3. Conditions for SMR Reaction
4.4. Characterization
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Keeling, C.D.; Piper, S.C.; Bacastow, R.B.; Wahlen, M.; Whorf, T.P.; Heimann, M.; Meijer, H.A. Atmospheric CO2 and 13CO2 exchange with the terrestrial biosphere and oceans from 1978 to 2000: Observations and carbon cycle implications. In A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems; Springer: New York, NY, USA, 2005; pp. 83–113. [Google Scholar]
- Shaner, M.R.; Atwater, H.A.; Lewis, N.S.; McFarland, E.W. A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ. Sci. 2016, 9, 2354–2371. [Google Scholar] [CrossRef]
- Kapner, R.S.; Lannus, A. Thermodynamic analysis of energy efficiency in catalytic reforming. Energy 1980, 5, 915–924. [Google Scholar] [CrossRef]
- Suelves, I.; Lázaro, M.; Moliner, R.; Corbella, B.; Palacios, J. Hydrogen production by thermo catalytic decomposition of methane on Ni-based catalysts: Influence of operating conditions on catalyst deactivation and carbon characteristics. Int. J. Hydrogen Energy 2005, 30, 1555–1567. [Google Scholar] [CrossRef]
- Forzatti, P.; Lietti, L. Catalyst deactivation. Catal. Today 1999, 52, 165–181. [Google Scholar] [CrossRef]
- Roh, H.-S.; Jun, K.-W.; Dong, W.-S.; Chang, J.-S.; Park, S.-E.; Joe, Y.-I. Highly active and stable Ni/Ce–ZrO2 catalyst for H2 production from methane. J. Mol. Catal. A Chem. 2002, 181, 137–142. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Tan, K.F.; Borgna, A.; Saeys, M. Effect of boron on the stability of Ni catalysts during steam methane reforming. J. Catal. 2009, 261, 158–165. [Google Scholar] [CrossRef]
- Nieva, M.A.; Villaverde, M.M.; Monzón, A.; Garetto, T.F.; Marchi, A.J. Steam-methane reforming at low temperature on nickel-based catalysts. Chem. Eng. J. 2014, 235, 158–166. [Google Scholar] [CrossRef]
- Christensen, K.O.; Chen, D.; Lødeng, R.; Holmen, A. Effect of supports and Ni crystal size on carbon formation and sintering during steam methane reforming. Appl. Catal. A Gen. 2006, 314, 9–22. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane steam reforming: II. Diffusional limitations and reactor simulation. AIChE J. 1989, 35, 97–103. [Google Scholar] [CrossRef]
- Baek, S.M.; Kang, J.H.; Lee, K.-J.; Nam, J.H. A numerical study of the effectiveness factors of nickel catalyst pellets used in steam methane reforming for residential fuel cell applications. Int. J. Hydrogen Energy 2014, 39, 9180–9192. [Google Scholar] [CrossRef]
- Cho, E.H.; Koo, K.Y.; Lee, H.W.; Park, Y.-K.; Yoon, W.L.; Ko, C.H. Preparation of egg-shell-type Ni/Ru bimetal alumina pellet catalysts: Steam methane reforming for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 18350–18357. [Google Scholar] [CrossRef]
- Roy, P.S.; Park, C.S.; Raju, A.S.; Kim, K. Steam-biogas reforming over a metal-foam-coated (Pd–Rh)/(CeZrO2–Al2O3) catalyst compared with pellet type alumina-supported Ru and Ni catalysts. J. CO2 Util. 2015, 12, 12–20. [Google Scholar] [CrossRef]
- Valizadeh, S.; Ko, C.H.; Lee, J.; Lee, S.H.; Yu, Y.J.; Show, P.L.; Rhee, G.H.; Park, Y.-K. Effect of eggshell-and homo-type Ni/Al2O3 catalysts on the pyrolysis of food waste under CO2 atmosphere. J. Environ. Manag. 2021, 294, 112959. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, E.; Ko, C.H. Preparation of Ni-based egg-shell-type catalyst on cylinder-shaped alumina pellets and its application for hydrogen production via steam methane reforming. Int. J. Hydrogen Energy 2019, 44, 5314–5323. [Google Scholar] [CrossRef]
- Cho, E.; Yu, Y.J.; Kim, Y.; Phan, T.N.; Park, D.; Ko, C.H. Egg-shell-type Ni supported on MgAl2O4 pellets as catalyst for steam methane reforming: Enhanced coke-resistance and pellet stability. Catal. Today 2020, 352, 157–165. [Google Scholar] [CrossRef]
- Vizcaíno, A.; Arena, P.; Baronetti, G.; Carrero, A.; Calles, J.; Laborde, M.; Amadeo, N. Ethanol steam reforming on Ni/Al2O3 catalysts: Effect of Mg addition. Int. J. Hydrogen Energy 2008, 33, 3489–3492. [Google Scholar] [CrossRef]
- Lisboa, J.d.S.; Santos, D.C.; Passos, F.B.; Noronha, F.B. Influence of the addition of promoters to steam reforming catalysts. Catal. Today 2005, 101, 15–21. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zheng, X. The deposition of coke from methane on a Ni/MgAl2O4 catalyst. Carbon 2007, 45, 1314–1321. [Google Scholar] [CrossRef]
- Takehira, K.; Shishido, T.; Wang, P.; Kosaka, T.; Takaki, K. Autothermal reforming of CH4 over supported Ni catalysts prepared from Mg–Al hydrotalcite-like anionic clay. J. Catal. 2004, 221, 43–54. [Google Scholar] [CrossRef]
- Lee, J.; Joo, C.; Cho, H.; Kim, Y.; Ga, S.; Kim, J. Design of multistage fixed bed reactors for SMR hydrogen production based on the intrinsic kinetics of Ru-based catalysts. Energy Convers. Manag. 2022, 268, 115981. [Google Scholar] [CrossRef]
- Lee, J.; Cho, H.; Kim, M.; Hall, S.; Moon, I. Double-tube reactor design and process optimization for on-site steam methane reforming processes. Ind. Eng. Chem. Res. 2020, 59, 18028–18038. [Google Scholar] [CrossRef]
- Hadian, N.; Rezaei, M.; Mosayebi, Z.; Meshkani, F. CO2 reforming of methane over nickel catalysts supported on nanocrystalline MgAl2O4 with high surface area. J. Nat. Gas Chem. 2012, 21, 200–206. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zhao, H.; Wang, X.; Zheng, X. Novel synthesis of high surface area MgAl2O4 spinel as catalyst support. Mater. Lett. 2004, 58, 1920–1923. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, K.; Rui, N.; Zhao, B.; Liu, C.-j. Improved activity of Ni/MgAl2O4 for CO2 methanation by the plasma decomposition. J. Energy Chem. 2015, 24, 655–659. [Google Scholar] [CrossRef]
- Tan, M.; Wang, X.; Wang, X.; Zou, X.; Ding, W.; Lu, X. Influence of calcination temperature on textural and structural properties, reducibility, and catalytic behavior of mesoporous γ-alumina-supported Ni–Mg oxides by one-pot template-free route. J. Catal. 2015, 329, 151–166. [Google Scholar] [CrossRef]
- Shen, J.; Reule, A.A.; Semagina, N. Ni/MgAl2O4 catalyst for low-temperature oxidative dry methane reforming with CO2. Int. J. Hydrogen Energy 2019, 44, 4616–4629. [Google Scholar] [CrossRef]
- Habibi, N.; Wang, Y.; Arandiyan, H.; Rezaei, M. Effect of substitution by Ni in MgAl2O4 spinel for biogas dry reforming. Int. J. Hydrogen Energy 2017, 42, 24159–24168. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.-W. Temperature-programmed-reduction studies of nickel oxide/alumina catalysts: Effects of the preparation method. Thermochim. Acta 1995, 256, 457–465. [Google Scholar] [CrossRef]
- Parmaliana, A.; Arena, F.; Frusteri, F.; Giordano, N. Temperature-programmed reduction study of NiO–MgO interactions in magnesia-supported Ni catalysts and NiO–MgO physical mixture. J. Chem. Soc. Faraday Trans. 1990, 86, 2663–2669. [Google Scholar] [CrossRef]
- Garbarino, G.; Valsamakis, I.; Riani, P.; Busca, G. On the consistency of results arising from different techniques concerning the nature of supported metal oxide (nano) particles. The case of NiO/Al2O3. Catal. Commun. 2014, 51, 37–41. [Google Scholar] [CrossRef]
- Matera, S.; Reuter, K. When atomic-scale resolution is not enough: Spatial effects on in situ model catalyst studies. J. Catal. 2012, 295, 261–268. [Google Scholar] [CrossRef]
- Bhat, S.A.; Sadhukhan, J. Process intensification aspects for steam methane reforming: An overview. AIChE J. 2009, 55, 408–422. [Google Scholar] [CrossRef]
- Ni, M. 2D heat and mass transfer modeling of methane steam reforming for hydrogen production in a compact reformer. Energy Convers. Manag. 2013, 65, 155–163. [Google Scholar] [CrossRef]
- Jeon, J.; Nam, S.; Ko, C.H. Rapid evaluation of coke resistance in catalysts for methane reforming using low steam-to-carbon ratio. Catal. Today 2018, 309, 140–146. [Google Scholar] [CrossRef]
- Arepalli, S.; Nikolaev, P.; Gorelik, O.; Hadjiev, V.G.; Holmes, W.; Files, B.; Yowell, L. Protocol for the characterization of single-wall carbon nanotube material quality. Carbon 2004, 42, 1783–1791. [Google Scholar] [CrossRef]
- Mansfield, E.; Kar, A.; Hooker, S.A. Applications of TGA in quality control of SWCNTs. Anal. Bioanal. Chem. 2010, 396, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhao, B.; Itkis, M.E.; Haddon, R.C. Nitric acid purification of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 13838–13842. [Google Scholar] [CrossRef]
- Neagu, D.; Oh, T.-S.; Miller, D.N.; Ménard, H.; Bukhari, S.M.; Gamble, S.R.; Gorte, R.J.; Vohs, J.M.; Irvine, J.T. Nano-socketed nickel particles with enhanced coking resistance grown in situ by redox exsolution. Nat. Commun. 2015, 6, 8120. [Google Scholar] [CrossRef]
- Jang, M.-S.; Cho, E.H.; Koo, K.Y.; Yoon, W.L.; Ko, C.H. Facile preparation of egg-shell-type pellet catalysts using immiscibility between hydrophobic solvent and hydrophilic solution: Enhancement of catalytic activity due to position control of metallic nickel inside alumina pellet. Appl. Catal. A Gen. 2017, 530, 211–216. [Google Scholar] [CrossRef]
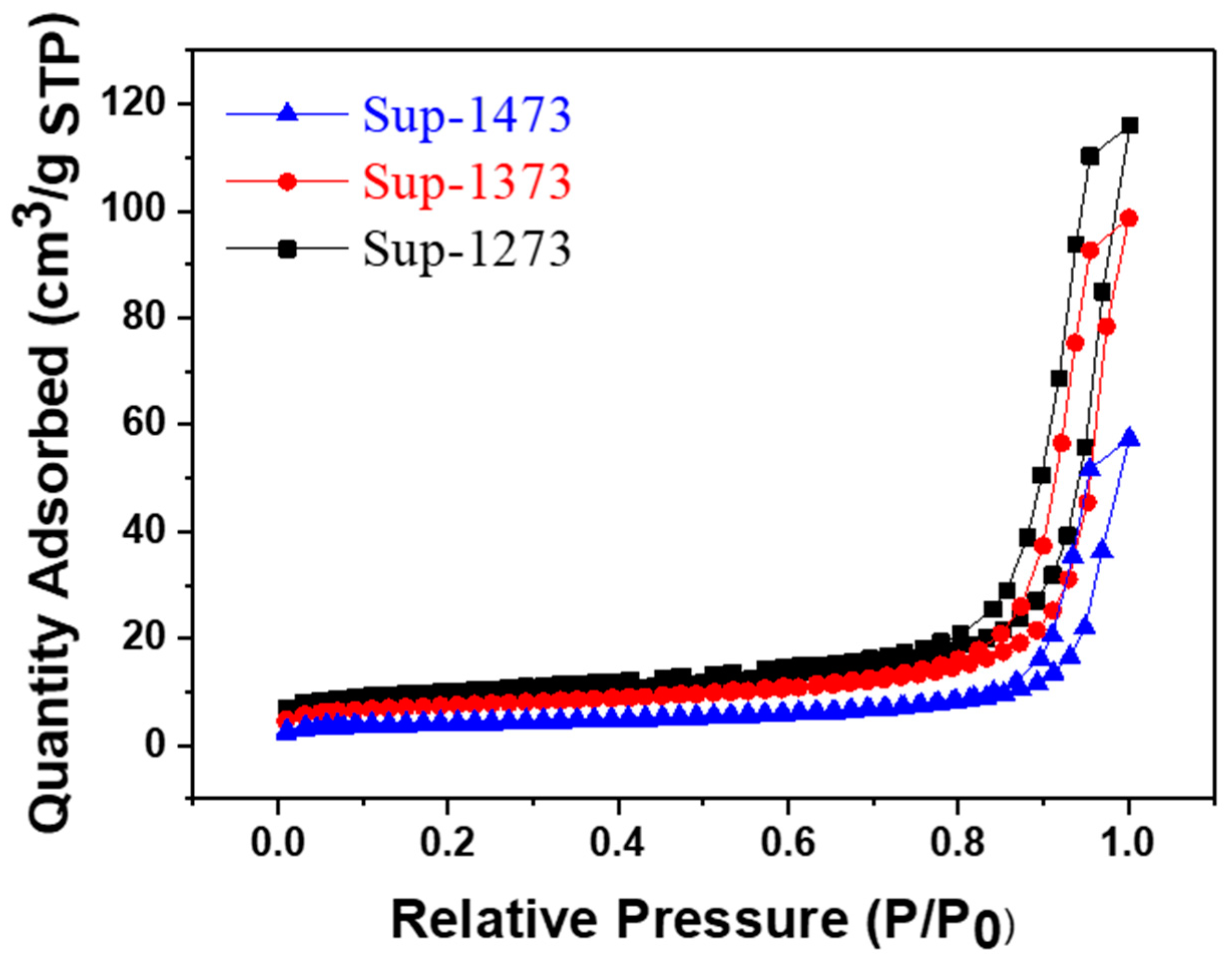
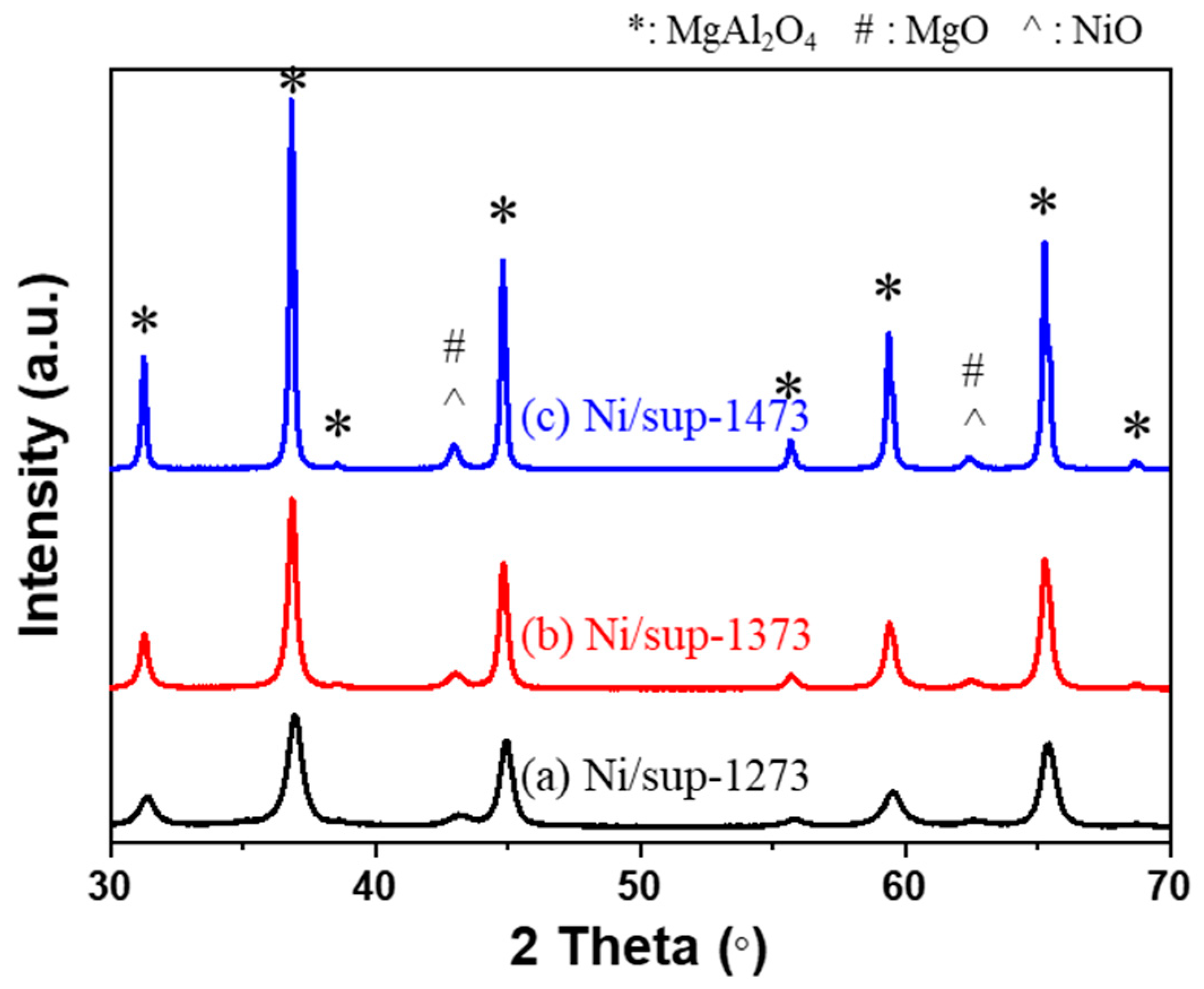
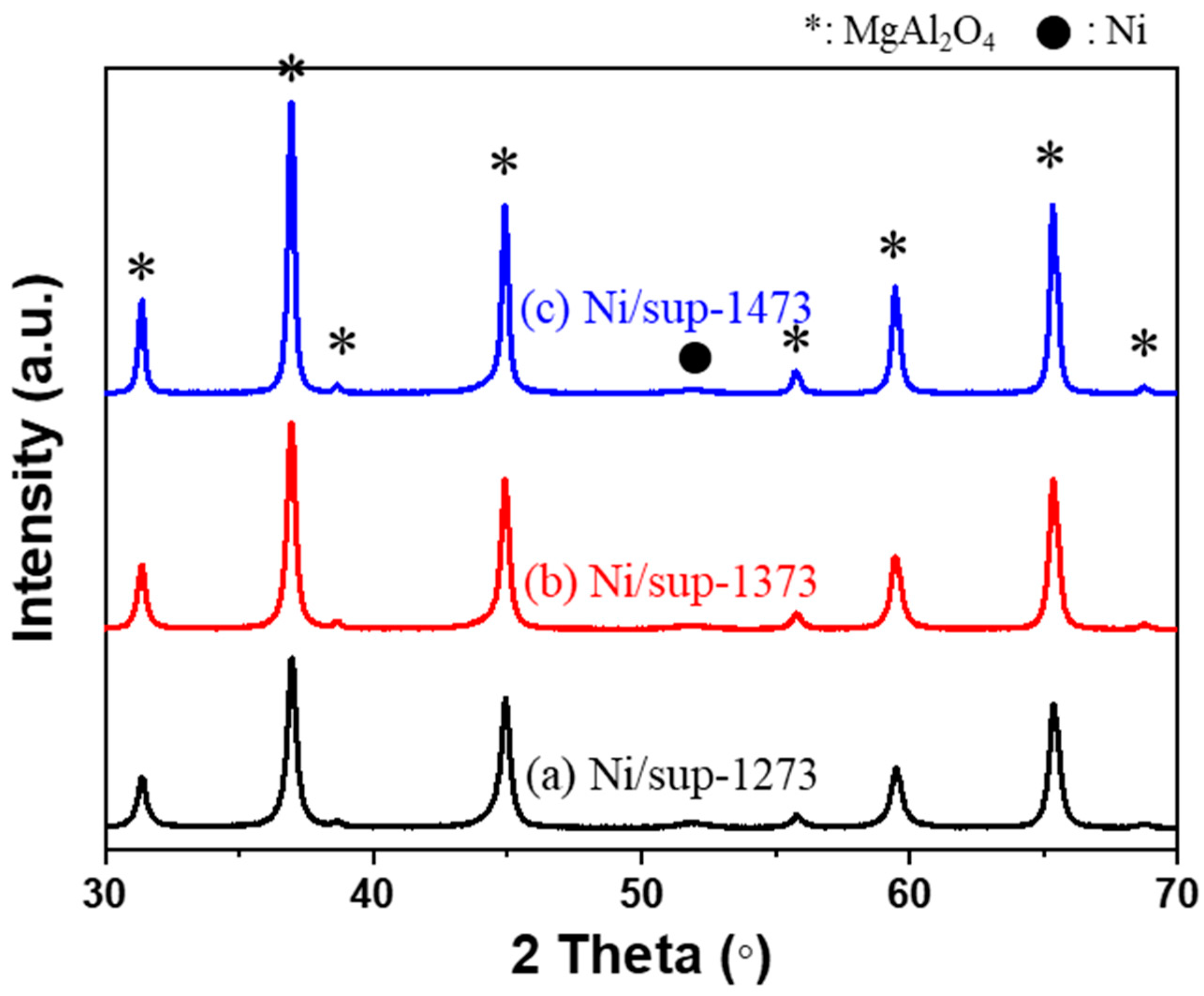
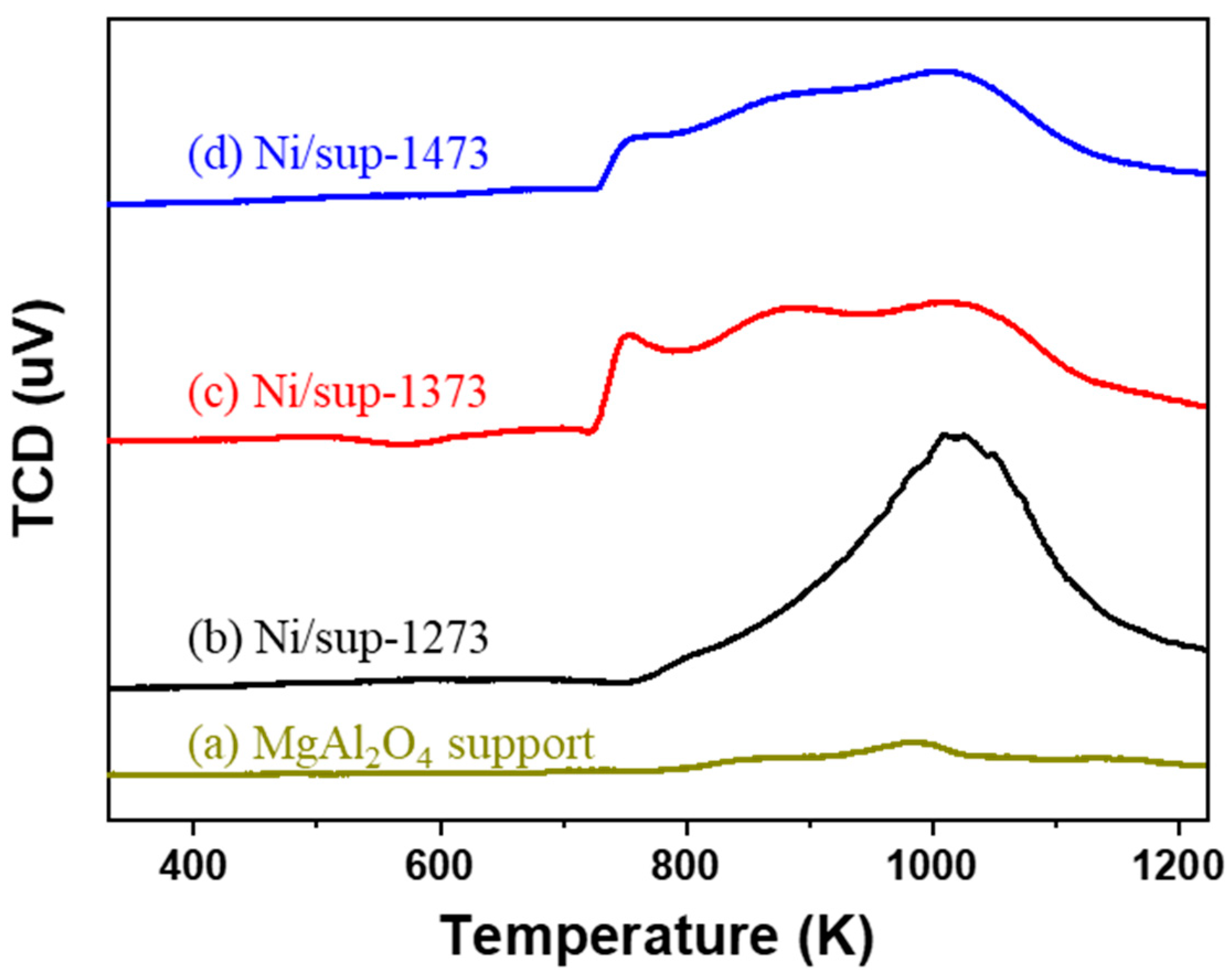

| Pellet Supports | Surface Area (m2/g) 1 | Pore Volume (cm3/g) 2 |
|---|---|---|
| Sup-1273 | 29.3 | 0.074 |
| Sup-1373 | 25.0 | 0.057 |
| Sup-1473 | 14.5 | 0.024 |
| Catalyst | Strength (N) |
|---|---|
| Ni/sup-1273 | 48 |
| Ni/sup-1373 | 123 |
| Ni/sup-1473 | 137 |
| Com-catalyst | Shattered |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.J.; Cho, E.; Ko, C.H. Egg-Shell-Type MgAl2O4 Pellet Catalyst for Steam Methane Reforming Reaction Activity: Effect of Pellet Preparation Temperature. Catalysts 2022, 12, 1500. https://doi.org/10.3390/catal12121500
Yu YJ, Cho E, Ko CH. Egg-Shell-Type MgAl2O4 Pellet Catalyst for Steam Methane Reforming Reaction Activity: Effect of Pellet Preparation Temperature. Catalysts. 2022; 12(12):1500. https://doi.org/10.3390/catal12121500
Chicago/Turabian StyleYu, Yeon Jeong, Eunkyung Cho, and Chang Hyun Ko. 2022. "Egg-Shell-Type MgAl2O4 Pellet Catalyst for Steam Methane Reforming Reaction Activity: Effect of Pellet Preparation Temperature" Catalysts 12, no. 12: 1500. https://doi.org/10.3390/catal12121500
APA StyleYu, Y. J., Cho, E., & Ko, C. H. (2022). Egg-Shell-Type MgAl2O4 Pellet Catalyst for Steam Methane Reforming Reaction Activity: Effect of Pellet Preparation Temperature. Catalysts, 12(12), 1500. https://doi.org/10.3390/catal12121500







