Abstract
The oxygen reduction reaction (ORR) is one of the key processes for electrochemical energy storage, such as the cathode process in fuel cells and metal–air batteries. To date, the efficiency of the ORR half-reaction limits the overall performance of these energy storage devices. Traditional platinum-based materials are expensive and cannot provide the desired ORR efficiency. As an alternative, a new catalytic scheme for an ORR was proposed, which consisted of an electrode modified with a TEMPO-containing conductive polymer and a solution redox mediator system based on nitrogen oxides (NOx). NOx is perfect for oxygen reduction in solution, which, however, cannot be efficiently reduced onto a pristine electrode, while TEMPO is inactive in the ORR itself but catalyzes the electrochemical reduction of NO2 on the electrode surface. Together, these catalysts have a synergistic effect, enabling an efficient ORR in an acidic medium. In the present study, the synthesis of a novel TEMPO-containing conductive polymer and its application in the synergistic ORR system with a NOx mediator is described. The proposed mediator system may increase the performance of proton-exchange fuel cells and metal–air batteries.
1. Introduction
The electrochemical four-electron oxygen reduction reaction (ORR) comprises the cathodic process in fuel cells and metal–air batteries and is becoming one of the most important electrochemical reactions for energy storage and conversion [1]. The dioxygen molecule, in terms of thermodynamics, is one of the most powerful oxidants (Table 1), but the great stability of the double O=O bond results in a low reactivity of the oxygen [2]. In particular, due to its sluggish kinetics, the electrochemical reduction of the dioxygen molecule requires an efficient redox catalyst which transfers electrons from the electrode to the O2 molecule [3]. An ORR catalyst for energy storage applications should also possess high selectivity for the four-electron ORR over the adverse process of two-electron oxygen reduction to peroxides, which decreases the half-cell potential of the cathode and leads to the accumulation of hazardous peroxide products (Table 1, Equation (2)) [4,5,6].
Commercial ORR cathode materials are based on precious metals, mostly Pt, due to their catalytic activity and four-electron selectivity in the ORR reaction [7,8]. Platinum catalysts have high cost and low stability. Therefore, decreasing the use of platinum or replacing it in cathode catalysts is the main way for the development of new technologies for the commercial use of fuel cells [9,10,11,12]. The content of platinum in cathode materials can be reduced by using platinum alloys with other metals [13,14,15,16] or eliminated by using alternative catalytic systems, such as doped carbon nanomaterials [17,18,19,20,21,22]. The drawbacks of such catalysts are their tendency to agglomeration, uneven distribution of particles in polymer matrices, and washing out during operation, which deter the catalytic performance of the materials [23,24,25]. Therefore, the development of effective ORR catalysts is a key scientific problem to be solved for fuel cell commercialization.

Table 1.
Standard potentials of O2- and NOx- based half-reactions.
Table 1.
Standard potentials of O2- and NOx- based half-reactions.
| Nr. | Half-Reaction | E°, V | Ref. |
|---|---|---|---|
| 1 | O2 + 4 H+ + 4 ē ⇄ 2 H2O | 1.229 | [26] |
| 2 | O2 + 2 H+ + 2 ē ⇄ H2O2 | 0.695 | [26] |
| 3 | HNO2 + H+ + ē ⇄ NO•(g) + H2O | 0.984 | [26] |
| 4 | NO2•(g) + H+ + ē ⇄ HNO2 | 1.108 | [26] |
| 5 | NO2•(g) + 2 H+ + 2 ē ⇄ NO•(g) + H2O | 1.028 1 | [26] |
| 6 | TEMPO• + H+ + ē ⇄ TEMPOH | 0.65 | [27] |
| 7 | TEMPO+ + ē ⇄ TEMPO• | 0.745 | [27] |
| 8 | TEMPO+ + H+ + 2 ē ⇄ TEMPOH | 0.70 2 | [27] |
1 Calculated from Equations (3) and (4). 2 Calculated from Equations (6) and (7).
Nitrogen oxides (NOx) are considered promising mediators for the oxygen reduction reaction (ORR) [28,29,30,31]. The catalytic cycle of this mediator system in the electrochemical ORR is depicted in Figure 1a. NO, one of the components of the NOx system, efficiently reduces dioxygen, affording, through several intermediates, NO2, which can then be then reduced back to NO. This process provides fast kinetics of oxygen reduction and is thermodynamically favored, with NO ΔE° of the reduction of O2 by NO (Table 1, Equations (1) and (5)) of only 0.201 V, which means a high thermodynamic efficiency of the NOx mediator system in the ORR. The mediator cycle inherently produces H2O as a sole reduction product, which excludes the possibility of H2O2 formation [32].
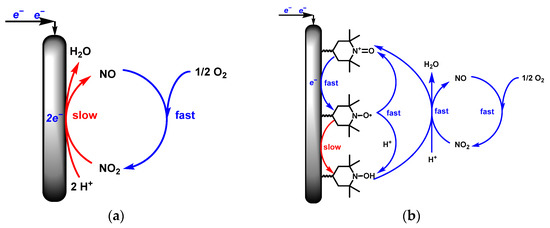
Figure 1.
(a) Simplified catalytic cycle of NOx-mediated ORR and (b) generalized reaction scheme for NOx-mediated ORR on a TEMPO-modified electrode.
However, the electroreduction of NO2 on the electrodes is limited by its poor kinetics and thus requires an additional catalytic system that will facilitate this process [32]. A possible solution to overcome this limitation is the use of an additional redox mediator, which can eliminate the kinetic limitations of electron transfer. An inorganic co-mediator system based on the VO2+/VO2+ couple was proposed for the NOx system; however, vanadyl itself showed poor reduction kinetics on the electrode [33,34]. Recently, aminoxyl derivatives, and, in particular, TEMPO, were proposed as dissolved co-mediators that facilitate the reduction of NO2 and overcome this issue (Figure 1b) [35]. The standard potential of the TEMPO•/TEMPO+ couple is 0.745 V (Table 1, Equation (7)), which is only 0.363 V lower than the NO2 reduction potential (Table 1, Equation (4)). This co-mediator minimizes energy losses in this step, and, at the same time, ensures fast kinetics for both NO2 reduction and electrode sites.
In our study, we propose an ORR catalytic system based on the NOx-aminoxyl mediator system with a TEMPO catalyst immobilized on the surface of the electrode (Figure 1b) as a potential catalytic system for fuel cells and metal–air batteries. TEMPO radicals undergo disproportionation in an acid medium, producing an active reducing agent—TEMPO− anions—which rapidly reduces NO2 species to NO, forming TEMPO+ cations, which can be easily electroreduced to neutral TEMPO species. The latter again produce TEMPO− species in an acid medium, and the mediator cycle continues. TEMPO fragments are bound on a conductive polymer backbone, which ensures a fast electron transport to the electrode surface, increasing the overall efficiency of the ORR. Such an architecture shifts one of the electron transfer processes from the solution to the electrode surface, which improves the overall electron transfer, reduces the amount of TEMPO used, and prevents the crossover of this mediator to the anode compartment. We prepared a new TEMPO-pyrrole monomer and deposited an acid-stable conductive polymer using this monomer. The catalytic system consisting of the electrode modified with this polymer and a solution of a NOx mediator system showed activity towards the ORR in an acidic medium.
2. Results and Discussion
2.1. Synthesis of the TEMPO-Containing Polymer
Existing TEMPO-pyrroles and polymers obtained thereof are based on an ester linker [36,37,38], which is acid-labile and cannot be utilized in acidic ORR systems. To overcome this issue, we prepared the new TEMPO-pyrrole PyC4NO with an acid-stable alkyl linker. The PyC4NO monomer was obtained in two steps (Figure 2). Briefly, TEMPOL was alkylated with an excess of 1,4-dibromobutane according to the literature to obtain product 1, which was then used for the N-alkylation of pyrrole. The alkylation with KOH under typical conditions failed, but the alkylation with NaH as the base yielded the desired monomer PyC4NO, with a 63% yield.
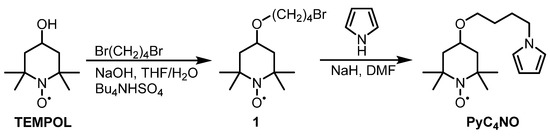
Figure 2.
Scheme for the synthesis of PyC4NO.
Films of poly-PyC4NO were deposited via an oxidative electrochemical polymerization of PyC4NO on the surface of the GC electrode in CV mode (Figure 3a). The irreversible current near an anodic boundary of the CV was attributed to pyrrole oxidation, and the cycle-to-cycle current increase of the peak pair indicated the polymerization of pyrrole PyC4NO and film growth. A feature worth mentioning is that polymerization reproducibility was scarcely achievable by repeating the vertex potentials, scan rate, and number of CV cycles. The PyC4NO polymerization process was strongly dependent on slight variations in the electrode potential and the previous history of the solution. To adjust the upper vertex potential accurately, the potential of the TEMPO oxidation of the monomer was used.
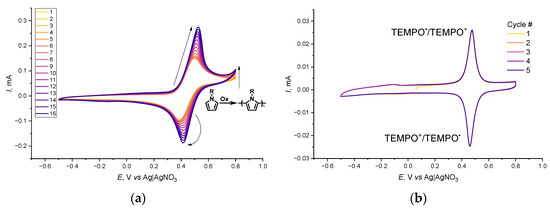
Figure 3.
CV curves for the (a) deposition of poly-PyC4NO from the electrolyte containing 1 mM PyC4NO and (b) cycling of poly-PyC4NO with IR compensation applied; 50 mV s−1, 0.1 M Et4NBF4 in CH3CN.
As seen in the SEM images of the obtained film (Figure 4), poly-PyC4NO appeared as a discontinuous coating consisting of globules with a diameter up to 200 nm. The globules formed a tuberous, but dense layer on the electrode surface.
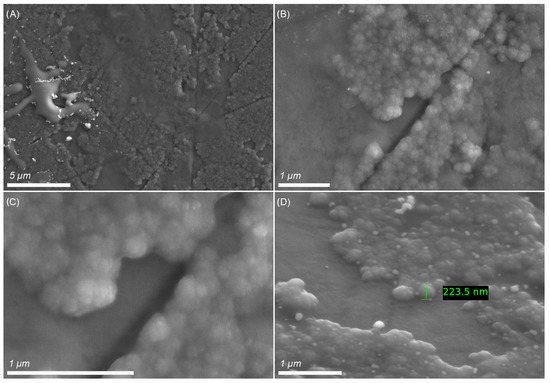
Figure 4.
SEM images of the poly-PyC4NO film at different magnifications.
The resulting polymer formed a visible film on the electrode, insoluble in a polymer-free electrolyte, which confirmed the deposition of the polymer. The electrodes obtained were used to study the electrochemical properties of poly-PyC4NO.
2.2. Electrochemical Properties of the Poly-PyC4NO-Modified Electrode
The CV spectrum of the freshly deposited film in a neutral electrolyte (Figure 3b) under anaerobic conditions contained a pronounced pair of peaks corresponding to the aminoxyl-oxoammonium one-electron process at 0.47 V. The low peak potential difference (16 mV) indicated the reversibility of this redox process and a fast electron transfer in the film, which acted as a thin layer system without internal diffusion limitations [39,40]. The electrochemical response of the film was stabilized in the second cycle, and no evolution or degradation occurred in the subsequent cycles.
Upon addition of trifluoroacetic acid (TFA) to the electrolyte, the electrochemical response of the poly-PyC4NO film under anaerobic conditions changed drastically (Figure 5a). In acidic conditions, the TEMPO• particles underwent a proton-coupled disproportionation, affording equal amounts of the corresponding hydroxylamine TEMPOH and oxoammonium cation TEMPO+ (Figure 5c). Accordingly, from the first CV cycle after the addition of TFA, the currents of the peak pair near 0.45 V dropped rapidly. The TEMPO• fragments underwent disproportionation to TEMPO+ and TEMPOH (despite the low reduction potential, the latter has a very sluggish oxidation kinetics), resulting in the decrease of the oxidation peak linked to the oxidation of TEMPO• to TEMPO+. The resulting TEMPO+, being reduced on the backward scan of the CV, disproportionated simultaneously, and the net result of this process was the complete reduction of the TEMPO fragments to TEMPOH. As a result, all TEMPO fragments were brought to the TEMPOH state, which resulted in the disappearance of the peaks of the TEMPO+/TEMPO• pair. This process was fully reversible and, when transferred back to the acid-free electrolyte, the electrode restored its electrochemical activity.
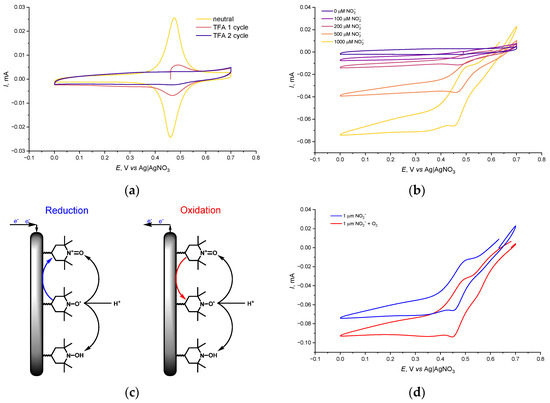
Figure 5.
CV curves of poly-PyC4NO (a) in a neutral electrolyte with 0.1 M TFA and (b) in 0.1 M TFA with Bu4NNO2 additives; (c) electrode processes of the TEMPO-containing polymer in an acidic electrolyte and (d) CV curves of poly-PyC4NO in anaerobic and aerobic conditions, 1 mM Bu4NNO2, 0.1 M TFA; 50 mV s−1, 0.1 M Et4NBF4 in CH3CN.
The gradual addition of Bu4NNO2 to the acidified electrolyte under anaerobic conditions resulted in an irreversible cathodic current (Figure 5b), which indicated a fast reduction of NO2 species on the TEMPO-modified electrode. The cathodic current increased proportionally to the concentration of NO2− up to 1 mM concentration.
In the presence of O2, an additional growth of the cathodic current was observed (Figure 5d), which means that some NO2 reduced on the TEMPO-modified electrode was instantly oxidized back to NO2 and then brought back to the reaction with a reduced form of TEMPO, according to the proposed double mediator scheme (Figure 1b). Thus, the proposed mediator/catalyst system facilitated the electrochemical reduction of O2. Encouraged by the performance of the developed system, we decided to study its performance in the ORR in bulk.
2.3. Bulk ORR Performance of the Catalytic System
The electrocatalytic properties of the poly-PyC4NO film were studied by potentiostatic electrolysis at the formal potential of the aminoxyl-oxoammonium process, 0.47 V, vs. Ag|AgNO3 (Figure 6a). As seen from the chronoamperograms, the reduction current in the presence of an oxygen flow became higher after about 10% of electrolysis time, which indicated the depletion of the solution containing NO2 in the absence of oxygen and its regeneration in the presence of oxygen.
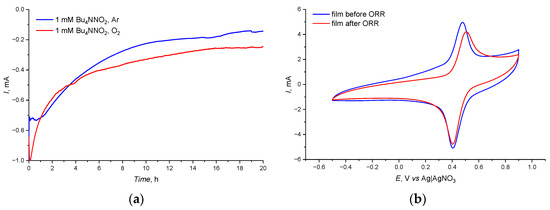
Figure 6.
(a) Potentiostatic chronoamperogram with and without O2 on a carbon paper electrode coated with the poly-PyC4NO film at 0.47 V vs. Ag|AgNO3, 1 mM Bu4NNO2 + 0.1 M TFA and (b) CV curve of poly-PyC4NO before and after the ORR experiments, 50 mV s−1; 0.1 M Et4NBF4 in CH3CN.
As seen from the CV curve of the poly-PyC4NO film recorded in an initial electrolyte after the ORR experiments (Figure 6b), the film recovered its electroactivity with only a minor decrease in current, which indicated a high stability of the film under ORR conditions.
3. Materials and Methods
General considerations. The 1H and 13C NMR spectra were recorded on a Brucker Avance 400 spectrometer at 400 and 101 MHz, respectively, in CDCl3. Pentafluorophenylhydrazine-d3 was used to quench the TEMPO radical in situ prior to acquiring the spectrum. ESI-HRMS was recorded using a Shimadzu LCMS-9030 spectrometer in positive mode. SEM was performed on a Zeiss Merlin microscope with a GEMINI II column at 5 kV accelerating voltage in secondary electron mode. The electrochemical experiments were performed on a BioLogic VMP-3e potentiostat.
Starting materials, reagents, and solvents of reagent grade were used as received. The compounds 4-bromobutoxyTEMPO (1) [41] and Bu4NNO2 [42] were prepared according to literature protocols. The dry DMF was prepared by fraction distillation from CaH2 in vacuo. Petroleum ether was dried by storage over activated silica. Electrochemical-grade CH3CN and Et4NBF4 were used for the electrochemical experiments. HPLC was performed on an ECOM TOY18DAD800 chromatograph equipped with a YMC 5 µm SiO2 column; TLC were performed on Merck 60 M F254 indicator silica gel plates.
Synthesis of PyC4NO. NaH (60% dispersion in mineral oil, 9.25 mmol, 370 mg) was placed in a 30 mL screw cap vial with septum under Ar, washed twice with dry petroleum ether, and dried in vacuo. Dry DMF (6 mL) was injected in the vial under Ar, the reaction mixture was cooled with ice, and then a solution of 1H-pyrrole (5 mmol, 335 mg, 346 µL) in dry DMF (1 mL) was added dropwise via a syringe. After the hydrogen evolution ceased, a solution of 1 (7.25 mmol, 2.27 g) in dry DMF (6 mL) was added dropwise via a syringe. The reaction mixture was stirred at RT for 18 h, quenched with 25 mL of H2O, and diluted with 30 mL of CH2Cl2. The organic layer was separated, thoroughly washed with brine 3 times, dried over Na2SO4, and the solvent was removed in vacuo. The crude product was purified by preparative HPLC (silica gel, hexane–acetone gradient), giving the desired product, an orange solid with a yield of 63% (922 mg).
PyC4NO: 1H NMR (CDCl3, 400 MHz) δ (ppm) 6.67 (t, J = 2 Hz, 2H), 6.15 (t, J = 2 Hz, 2H), 3.93 (t, J = 7.1 Hz, 2H), 3.61–3.49 (m, 1H), 3.44 (t, J = 6.3 Hz, 2H), 2.06–1.74 (m, 4H), 1.68–1.51 (m, 2H), 1.51–1.38 (m, 2H), 1.23 (s, 6H), 1.17 (s, 6H); 13C NMR (CDCl3, 101 MHz) δ (ppm) 120.5, 107.9, 70.5, 67.6, 59.2, 49.5, 44.8, 32.0, 28.6, 27.4, 20.7; ESI HRMS m/z [M]+ calcd for C17H29N2O2+ 293.2224, found 293.2226.
Electrochemical experiments. Poly-PyC4NO films were deposited onto the prepared glassy carbon (GC) electrodes (d = 3 mm) by oxidative electrochemical polymerization of PyC4NO from its 1 mM solution in CH3CN containing 0.1 M Et4NBF4 as a supporting electrolyte. The preparation of the GC electrode included subsequent polishing, rinsing with isopropyl alcohol and acetone, drying and electrochemical pretreatment procedures (application of constant potentials of 1.5, −1.0, 1.5, −1.0 V vs. an Ag|AgNO3 reference electrode for 2 min in 0.1 M Et4NBF4, CH3CN electrolyte). Polymerization was carried out in a standard three-electrode cell equipped with a Pt strip as an auxiliary electrode and a BASi MW-1085 Ag|AgNO3 non-aqueous reference electrode. Polymerization was carried out in the cyclic voltammetry (CV) mode in the range of −0.5–0.8 V with a scan rate of 50 mV for 16 cycles. After the deposition, the obtained films were washed with CH3CN and dried in air.
For the potentiostatic experiments, poly-PyC4NO films were deposited onto a carbon paper disc electrode (d = 16 mm) following the same procedure as described above, but with the electrode pre-treatment step omitted. Potentiostatic electrolysis was performed in a three-electrode cell with a glass frit dividing the working and the counter electrode compartments. The solution volume in the working electrode compartment was 25 mL. Before electrolysis, the solution was purged with argon or oxygen, and electrolysis was performed in a sealed cell under static pressure of the desired gas.
4. Conclusions
The immobilization of TEMPO-groups on the electrode surface by help of a conductive polymer backbone is an effective way to utilize the catalytic properties of the TEMPO radical in heterogeneous catalytic cycles. In this work, we demonstrated an efficient ORR cycle consisting of dissolved NOx and an electrode modified with a TEMPO-containing polymer. The former one provided a facile O2 reduction, while the latter one boosted the electron transfer from the electrode surface. The TEMPO-containing polymer was easily deposited directly on the electrode surface by an electrochemical polymerization. Immobilization of TEMPO on the electrode surface allowed us to reduce the amount of the TEMPO-containing catalyst with respect to that of the dissolved NOx mediator system and prevent the possible crossover of TEMPO species. Because of the high potential of both redox processes involved in the proposed cycle, this system preserved most of the energy of the ORR half-reaction. This system may be further employed in proton-exchange fuel cells. Since this system is able to operate under nonaqueous conditions, it can be exploited at temperatures below 0 °C, which is beneficial for transport and, in general, outdoor applications.
Author Contributions
Conceptualization, D.A.L. and O.V.L.; methodology, D.A.L., A.Y.K. and O.V.L.; validation, A.Y.K., O.Y.B., V.V.P. and L.G.R.; investigation, A.Y.K. and L.G.R.; resources, O.V.L.; data curation, A.Y.K.; writing—original draft preparation, D.A.L.; writing—review and editing, O.V.L.; supervision, O.V.L.; funding acquisition, O.V.L. All authors have read and agreed to the published version of the manuscript.
Funding
The reported study was funded by RFBR, Sirius University of Science and Technology, JSC Russian Railways and Educational Fund “Talent and success”, project number 20-33-51007.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the Research Center for Magnetic Resonance, the Center for Chemical Analysis and Materials Research, the Interdisciplinary Resource Centre for Nanotechnology, and the Centre for Extreme States of Materials and Constructions of Saint Petersburg State University Research Park for the measurements and fabrication processes.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed]
- Suermann, M.; Schmidt, T.J.; Büchi, F.N. Comparing the kinetic activation energy of the oxygen evolution and reduction reactions. Electrochim. Acta 2018, 281, 466–471. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Norskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
- Mase, K.; Ohkubo, K.; Fukuzumi, S. Efficient two-electron reduction of dioxygen to hydrogen peroxide with one-electron reductants with a small overpotential catalyzed by a cobalt chlorin complex. J. Am. Chem. Soc. 2013, 135, 2800–2808. [Google Scholar] [CrossRef] [PubMed]
- Baran, J.D.; Gronbeck, H.; Hellman, A. Analysis of porphyrines as catalysts for electrochemical reduction of O2 and oxidation of H2O. J. Am. Chem. Soc. 2014, 136, 1320–1326. [Google Scholar] [CrossRef]
- Inaba, M.; Yamada, H.; Tokunaga, J.; Matsuzawa, K.; Hatanaka, A.; Tasaka, A. Hydrogen Peroxide Formation as a Degradation Factor of Polymer Electrolyte Fuel Cells. ECS Trans. 2006, 1, 315–322. [Google Scholar] [CrossRef]
- Huang, L.; Zaman, S.; Tian, X.; Wang, Z.; Fang, W.; Xia, B.Y. Advanced Platinum-Based Oxygen Reduction Electrocatalysts for Fuel Cells. Acc. Chem. Res. 2021, 54, 311–322. [Google Scholar] [CrossRef]
- Sui, S.; Wang, X.; Zhou, X.; Su, Y.; Riffat, S.; Liu, C.-J. A comprehensive review of Pt electrocatalysts for the oxygen reduction reaction: Nanostructure, activity, mechanism and carbon support in PEM fuel cells. J. Mater. Chem. A 2017, 5, 1808–1825. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Gewirth, A.A.; Varnell, J.A.; DiAscro, A.M. Nonprecious Metal Catalysts for Oxygen Reduction in Heterogeneous Aqueous Systems. Chem. Rev. 2018, 118, 2313–2339. [Google Scholar] [CrossRef]
- Cui, J.; Chen, Q.; Li, X.; Zhang, S. Recent advances in non-precious metal electrocatalysts for oxygen reduction in acidic media and PEMFCs: An activity, stability and mechanism study. Green Chem. 2021, 23, 6898–6925. [Google Scholar] [CrossRef]
- Shahbaz, A.; Afaf, A.; Tahir, N.; Abid, U.; Saim, S. Non Precious Metal Catalysts: A Fuel Cell and ORR Study of Thermally Synthesized Nickel and Platinum Mixed Nickel Nanotubes for PEMFC. Key Eng. Mater. 2021, 875, 193–199. [Google Scholar] [CrossRef]
- Zhang, J.; Sasaki, K.; Sutter, E.; Adzic, R.R. Stabilization of platinum oxygen-reduction electrocatalysts using gold clusters. Science 2007, 315, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Iijima, Y.; Kondo, T.; Takahashi, Y.; Bando, Y.; Todoroki, N.; Wadayama, T. Oxygen Reduction Reaction Activities for Pt/Au(hkl) Bimetallic Surfaces Prepared by Molecular Beam Epitaxy. J. Electrochem. Soc. 2013, 160, F898–F904. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, Y.; Fu, G.; Duchesne, P.N.; Gu, L.; Zheng, Y.; Weng, X.; Chen, M.; Zhang, P.; Pao, C.-W.; et al. Interfacial Effects in Iron-Nickel Hydroxide–Platinum Nanoparticles Enhance Catalytic Oxidation. Science 2014, 344, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Markovic, N.M.; Stamenkovic, V.R. Advanced Platinum Alloy Electrocatalysts for the Oxygen Reduction Reaction. ACS Catal. 2012, 2, 891–898. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Cai, P.W.; Ci, S.Q.; Zhang, E.H.; Shao, P.; Cao, C.S.; Wen, Z.H. FeCo Alloy Nanoparticles Confined in Carbon Layers as High-activity and Robust Cathode Catalyst for Zn-Air Battery. Electrochim. Acta 2016, 220, 354–362. [Google Scholar] [CrossRef]
- Chai, G.-L.; Boero, M.; Hou, Z.; Terakura, K.; Cheng, W. Indirect Four-Electron Oxygen Reduction Reaction on Carbon Materials Catalysts in Acidic Solutions. ACS Catal. 2017, 7, 7908–7916. [Google Scholar] [CrossRef]
- Gong, X.; Liu, S.; Ouyang, C.; Strasser, P.; Yang, R. Nitrogen- and Phosphorus-Doped Biocarbon with Enhanced Electrocatalytic Activity for Oxygen Reduction. ACS Catal. 2015, 5, 920–927. [Google Scholar] [CrossRef]
- Shao, Y.; Jiang, Z.; Zhang, Q.; Guan, J. Progress in Nonmetal-Doped Graphene Electrocatalysts for the Oxygen Reduction Reaction. ChemSusChem 2019, 12, 2133–2146. [Google Scholar] [CrossRef]
- An, F.; Bao, X.-Q.; Deng, X.-Y.; Ma, Z.-Z.; Wang, X.-G. Carbon-based metal-free oxygen reduction reaction electrocatalysts: Past, present and future. New Carbon Mater. 2022, 37, 338–354. [Google Scholar] [CrossRef]
- Zheng, Q.; Cheng, X.; Jao, T.-C.; Weng, F.-B.; Su, A.; Chiang, Y.-C. Degradation analyses of Ru85Se15 catalyst layer in proton exchange membrane fuel cells. J. Power Sources 2012, 218, 79–87. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Merida, W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184, 104–119. [Google Scholar] [CrossRef]
- Elmas, S.; Beelders, W.; Pan, X.; Nann, T. Conducting copper(I/II)-metallopolymer for the electrocatalytic oxygen reduction reaction (ORR) with high kinetic current density. Polymers 2018, 10, 1002. [Google Scholar] [CrossRef]
- Bratsch, S.G. Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K. J. Phys. Chem. Ref. Data 1989, 18, 1–21. [Google Scholar] [CrossRef]
- Gerken, J.B.; Pang, Y.Q.; Lauber, M.B.; Stahl, S.S. Structural Effects on the pH-Dependent Redox Properties of Organic Nitroxyls: Pourbaix Diagrams for TEMPO, ABNO, and Three TEMPO Analogs. J. Org. Chem. 2018, 83, 7323–7330. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Sato, M.; Murase, T.; Tsukahara, K. Kinetic Studies of the Reaction of Nitrous Acid with Iodide Ion in the Presence of Molecular Oxygen in an Acid Solution. Bull. Chem. Soc. Jpn. 1993, 66, 2900–2906. [Google Scholar] [CrossRef]
- Anson, C.W.; Stahl, S.S. Mediated Fuel Cells: Soluble Redox Mediators and Their Applications to Electrochemical Reduction of O2 and Oxidation of H2, Alcohols, Biomass, and Complex Fuels. Chem. Rev. 2020, 120, 3749–3786. [Google Scholar] [CrossRef]
- Nutting, J.E.; Mao, K.; Stahl, S.S. Iron(III) Nitrate/TEMPO-Catalyzed Aerobic Alcohol Oxidation: Distinguishing between Serial versus Integrated Redox Cooperativity. J. Am. Chem. Soc. 2021, 143, 10565–10570. [Google Scholar] [CrossRef]
- Ryland, B.L.; Stahl, S.S. Practical aerobic oxidations of alcohols and amines with homogeneous copper/TEMPO and related catalyst systems. Angew. Chem. Int. Ed. Engl. 2014, 53, 8824–8838. [Google Scholar] [CrossRef] [PubMed]
- Ford, P.C.; Wink, D.A.; Stanbury, D.M. Autoxidation kinetics of aqueous nitric oxide. FEBS Lett. 1993, 326, 1–3. [Google Scholar] [CrossRef]
- Kummer, J.T.; Oei, D.G. A chemically regenerative redox fuel cell. II. J. Appl. Electrochem. 1985, 15, 619–629. [Google Scholar] [CrossRef]
- Bergens, S.H.; Gorman, C.B.; Palmore, G.T.; Whitesides, G.M. A Redox Fuel Cell That Operates with Methane as Fuel at 120 °C. Science 1994, 265, 1418–1420. [Google Scholar] [CrossRef] [PubMed]
- Gerken, J.B.; Stahl, S.S. High-Potential Electrocatalytic O2 Reduction with Nitroxyl/NOx Mediators: Implications for Fuel Cells and Aerobic Oxidation Catalysis. ACS Cent. Sci. 2015, 1, 234–243. [Google Scholar] [CrossRef]
- Xu, L.H.; Yang, F.; Su, C.; Zhang, C. Research of Properties on Li-Ion Batteries Based on a Polypyrrole Derivative Bearing TEMPO as a Cathode Material. Adv. Mater. Res. 2014, 936, 447–451. [Google Scholar] [CrossRef]
- Xu, L.; Guo, P.; He, H.; Zhou, N.; Ma, J.; Wang, G.; Zhang, C.; Su, C. Preparation of TEMPO-contained pyrrole copolymer by in situ electrochemical polymerization and its electrochemical performances as cathode of lithium ion batteries. Ionics 2017, 23, 1375–1382. [Google Scholar] [CrossRef]
- Xu, L.; Yang, F.; Su, C.; Ji, L.; Zhang, C. Synthesis and properties of novel TEMPO-contained polypyrrole derivatives as the cathode material of organic radical battery. Electrochim. Acta 2014, 130, 148–155. [Google Scholar] [CrossRef]
- Malev, V.V.; Levin, O.V. Criteria of the absence of short-range interactions within electroactive polymer films. Electrochim. Acta 2012, 80, 426–431. [Google Scholar] [CrossRef]
- Laviron, E. Adsorption, Autoinhibition and Autocatalysis in Polarography and in Linear Potential Sweep Voltammetry. J. Electroanal Chem. 1974, 52, 355–393. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, J.; Chen, H.; Feng, L.; Jing, R.; Lu, M.; Hu, B.; Ji, J. Magnetic Superhydrophobic Polymer Nanosphere Cage as a Framework for Miceller Catalysis in Biphasic Media. ChemCatChem 2014, 6, 1626–1634. [Google Scholar] [CrossRef]
- Bhatt, V.D.; Gohil, K. Ion exchange synthesis and thermal characteristics of some [N+4444] based ionic liquids. Thermochim. Acta 2013, 556, 23–29. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).