Improved Photoelectrochemical Performance of BiVO4 for Water Oxidation Enabled by the Integration of the Ni@NiO Core–Shell Structure
Abstract
1. Introduction
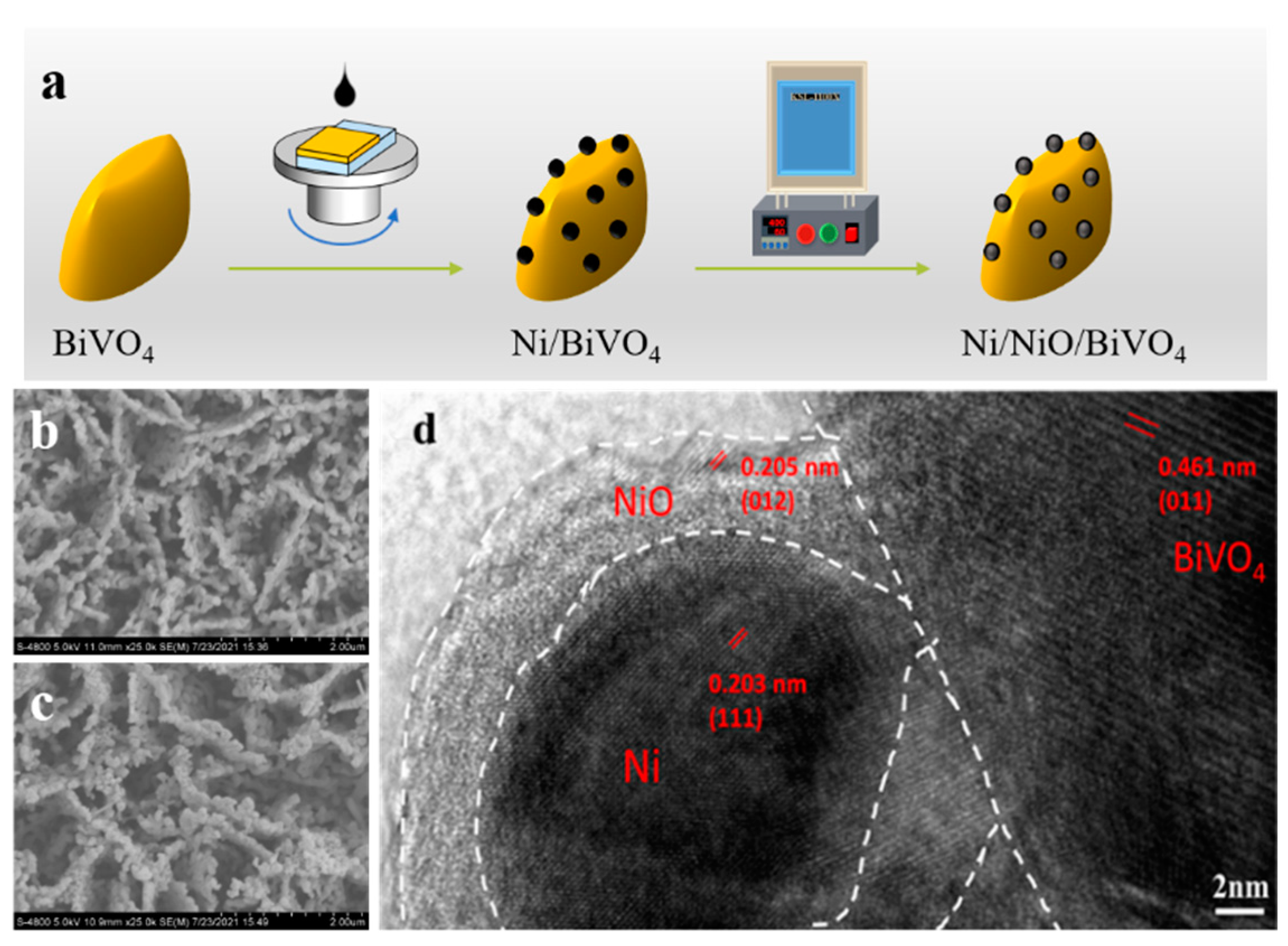
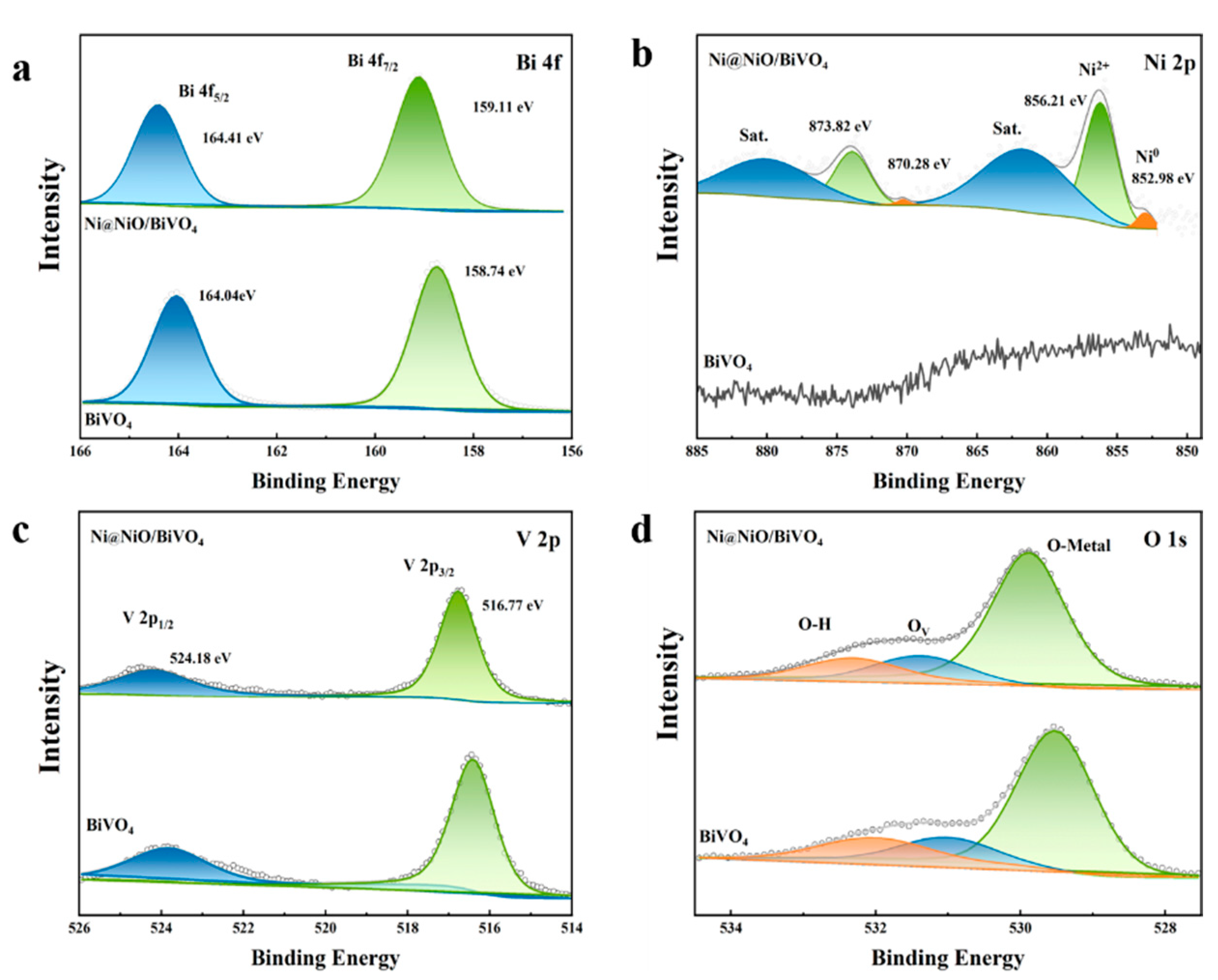
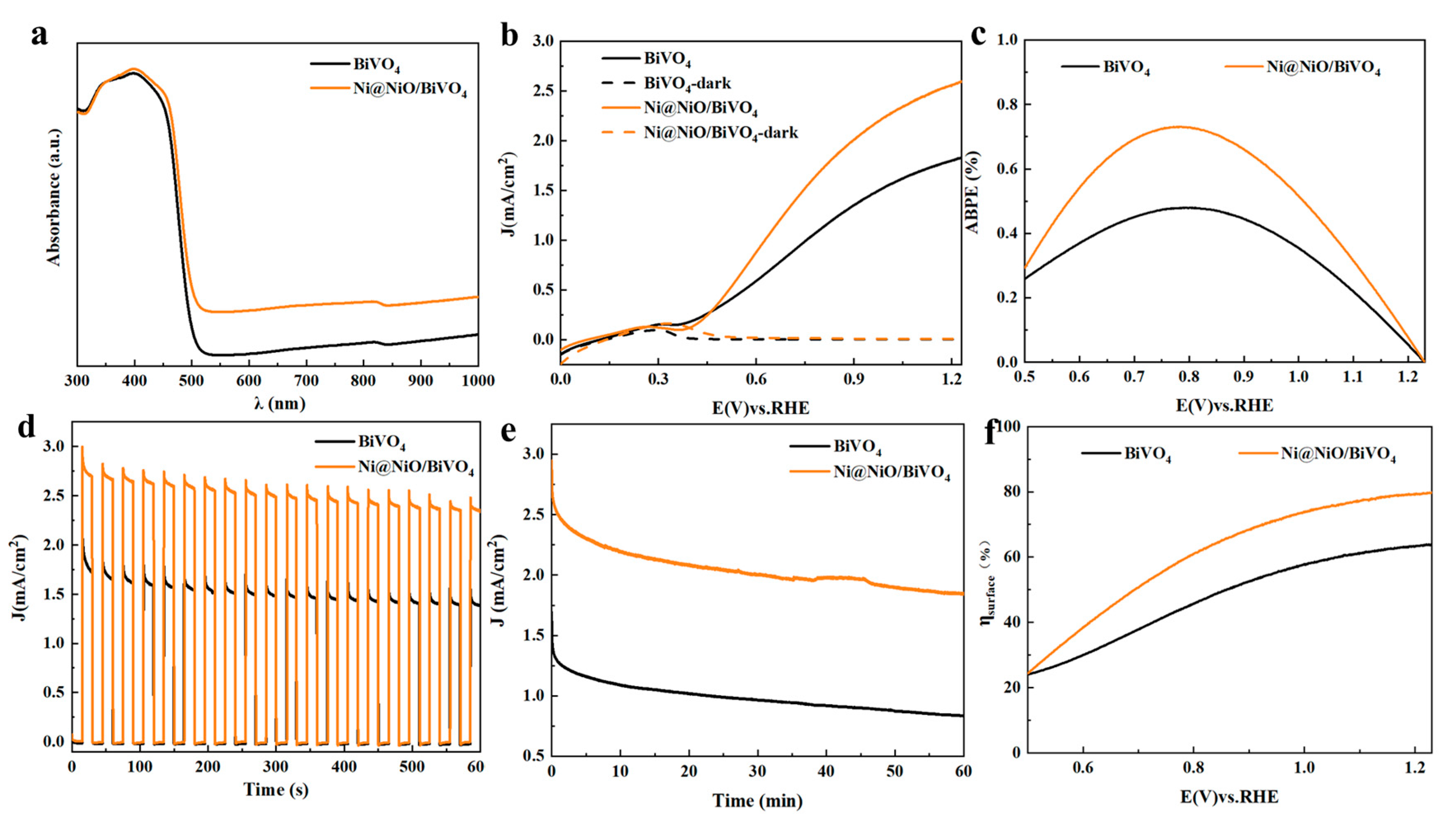
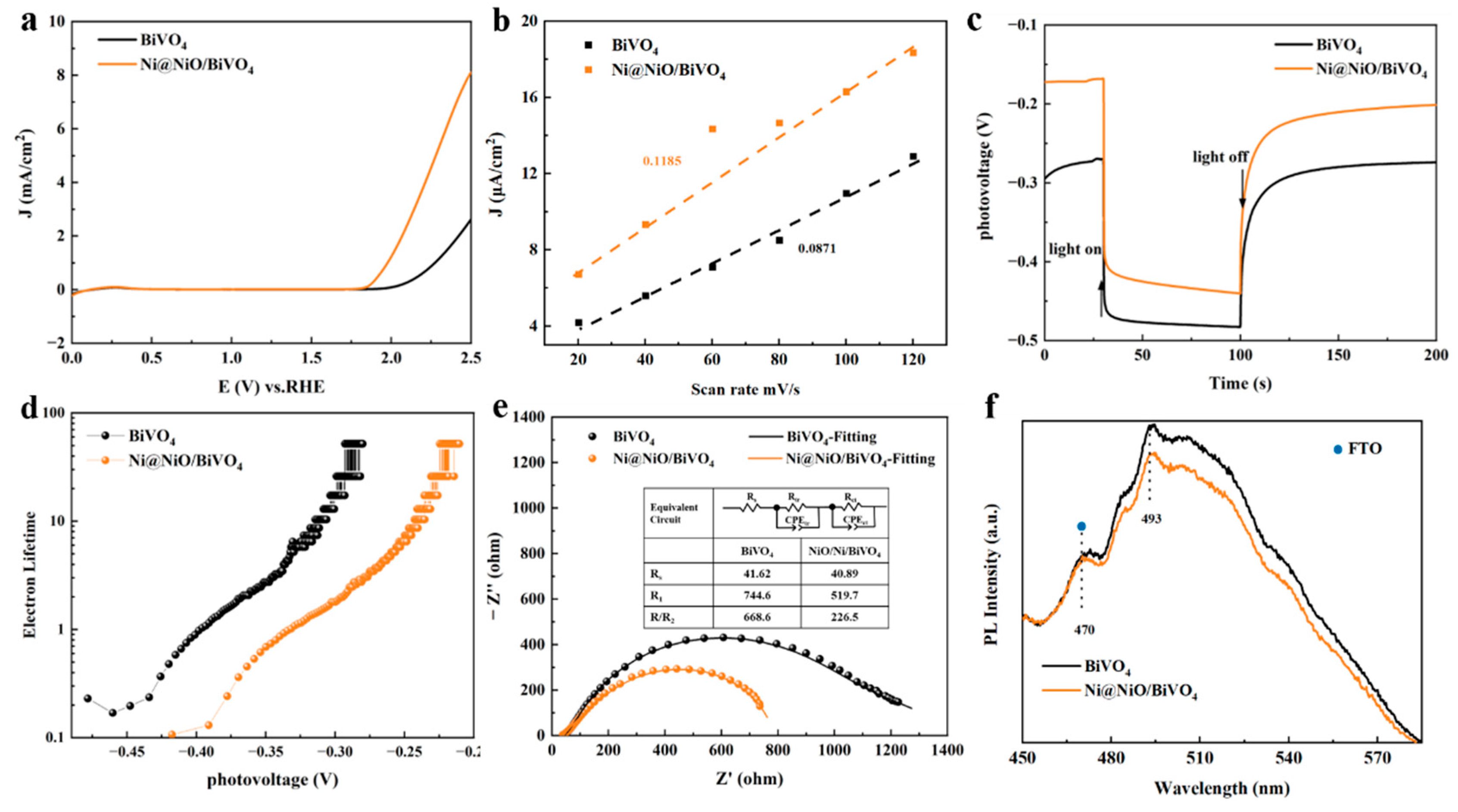
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Worm-Like BiVO4 Film
3.3. Preparation of Ni Nanoparticle
3.4. Preparation of Ni@NiO/BiVO4 Photoanode
3.5. Materials Characterization
3.6. Photoelectrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gratzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Sivula, K.; van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Xu, X.T.; Pan, L.; Zhang, X.W.; Wang, L.; Zou, J.J. Rational design and construction of cocatalysts for semiconductor-based photo-electrochemical oxygen evolution: A comprehensive review. Adv Sci. 2019, 6, 1801505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huang, X.; Zhang, Y.; Lu, G.; Chou, L.; Bi, Y. Unveiling the activity and stability origin of BiVO4 photoanodes with FeNi oxyhydroxides for oxygen evolution. Angew. Chem. Int. Ed. 2020, 59, 18990–18995. [Google Scholar] [CrossRef]
- Cao, X.; Xu, C.; Liang, X.; Ma, J.; Yue, M.; Ding, Y. Rationally designed/assembled hybrid BiVO4-based photoanode for enhanced photoelectrochemical performance. Appl. Catal. B Environ. 2020, 260, 118136. [Google Scholar] [CrossRef]
- Wang, S.C.; He, T.W.; Yun, J.H.; Hu, Y.X.; Xiao, M.; Du, A.J.; Wang, L.Z. New Iron-Cobalt Oxide Catalysts Promoting BiVO4 Films for Photoelectrochemical Water Splitting. Adv. Funct. Mater. 2018, 28, 1802685. [Google Scholar] [CrossRef]
- Pan, J.B.; Wang, B.H.; Wang, J.B.; Ding, H.Z.; Zhou, W.; Liu, X.; Zhang, J.R.; Shen, S.; Guo, J.K.; Chen, L.; et al. Activity and Stability Boosting of an Oxygen-Vacancy-Rich BiVO4 Photoanode by NiFe-MOFs Thin Layer for Water Oxidation. Angew. Chem. Int. Ed. 2021, 60, 1433–1440. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Chen, K.Y.; Huang, J.W.; Wang, L.; Zhang, M.Y.; Bai, B.; Liu, H.; Wang, Q.Z. Preparation of heterometallic CoNi-MOFs-modified BiVO4: A steady photoanode for improved performance in photoelectrochemical water splitting. Appl. Catal. B Environ. 2020, 266, 118513. [Google Scholar] [CrossRef]
- Zachaus, C.; Abdi, F.F.; Peter, L.M.; van de Krol, R. Photocurrent of BiVO4 is limited by surface recombination, not surface catalysis. Chem. Sci. 2017, 8, 3712–3719. [Google Scholar] [CrossRef]
- Wu, M.; Ding, T.; Wang, Y.; Zhao, W.; Xian, H.; Tian, Y.; Zhang, T.; Li, X. Rational construction of plasmon Au assisted ferroelectric-BaTiO3/Au/g-C3N4 Z-scheme system for efficient photocatalysis. Catal. Today 2020, 355, 311–318. [Google Scholar] [CrossRef]
- Isimjan, T.T.; Maity, P.; Llorca, J.; Ahmed, T.; Parida, M.R.; Mohammed, O.F.; Idriss, H. Comprehensive Study of All-Solid-State Z-Scheme Photocatalytic Systems of ZnO/Pt/CdZnS. ACS Omega 2017, 2, 4828–4837. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chou, P.H.; Yu, Y.H.; Kei, C.C. Deposition of Ni nanoparticles on black TiO2 nanowire arrays for photoelectrochemical water splitting by atomic layer deposition. Electrochim. Acta 2018, 284, 211–219. [Google Scholar] [CrossRef]
- Ding, C.M.; Shi, J.Y.; Wang, Z.L.; Li, C. Photoelectrocatalytic water splitting: Significance of cocatalysts, electrolyte, and interfaces. ACS Catal. 2017, 7, 675–688. [Google Scholar] [CrossRef]
- Han, T.T.; Shi, Y.Y.; Song, X.X.; Mio, A.; Valenti, L.; Hui, F.; Privitera, S.; Lombardo, S.; Lanza, M. Ageing mechanisms of highly active and stable nickel-coated silicon photoanodes for water splitting. J. Mater. Chem. A. 2016, 4, 8053–8060. [Google Scholar] [CrossRef]
- Lee, S.A.; Lee, T.H.; Kim, C.; Lee, M.G.; Choi, M.-J.; Park, H.; Choi, S.; Oh, J.; Jang, H.W. Tailored NiOx/Ni cocatalysts on silicon for highly efficient water splitting photoanodes via pulsed electrodeposition. ACS Catal. 2018, 8, 7261–7269. [Google Scholar] [CrossRef]
- Laskowski, F.A.L.; Nellist, M.R.; Venkatkarthick, R.; Boettcher, S.W. Junction behavior of n-Si photoanodes protected by thin Ni elucidated from dual working electrode photoelectrochemistry. Energy Environ. Sci. 2017, 10, 570–579. [Google Scholar] [CrossRef]
- Malara, F.; Fracchia, M.; Kmentová, H.; Psaro, R.; Vertova, A.; Oliveira de Souza, D.; Aquilanti, G.; Olivi, L.; Ghigna, P.; Minguzzi, A.; et al. Direct Observation of Photoinduced Higher Oxidation States at a Semiconductor/Electrocatalyst Junction. ACS Catal. 2020, 10, 10476–10487. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.-S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef]
- Zhang, X.; Zhai, P.; Zhang, Y.; Wu, Y.; Wang, C.; Ran, L.; Gao, J.; Li, Z.; Zhang, B.; Fan, Z.; et al. Engineering single-atomic Ni-N4-O sites on semiconductor photoanodes for high-performance photoelectrochemical water splitting. J. Am. Chem. Soc. 2021, 143, 20657–20669. [Google Scholar] [CrossRef]
- Zhou, B.X.; Ding, S.S.; Yang, K.X.; Zhang, J.; Huang, G.F.; Pan, A.L.; Hu, W.Y.; Li, K.; Huang, W.Q. Generalized Synthetic Strategy for Amorphous Transition Metal Oxides-Based 2D Heterojunctions with Superb Photocatalytic Hydrogen and Oxygen Evolution. Adv. Funct. Mater. 2021, 31, 2009230. [Google Scholar] [CrossRef]
- Muhmood, T.; Xia, M.Z.; Lei, W.; Wang, F.Y.; Khan, M.A. Design of Graphene Nanoplatelet/Graphitic Carbon Nitride Heterojunctions by Vacuum Tube with Enhanced Photocatalytic and Electrochemical Response. Eur. J. Inorg. Chem. 2018, 2018, 1726–1732. [Google Scholar] [CrossRef]
- Mahmood, A.; Muhmood, T.; Ahmad, F. Carbon nanotubes heterojunction with graphene like carbon nitride for the enhancement of electrochemical and photocatalytic activity. Mater. Chem. Phys. 2022, 278, 125640. [Google Scholar] [CrossRef]
- Muhmood, T.; Xia, M.Z.; Lei, W.; Wang, F.Y. Under vacuum synthesis of type-I heterojunction between red phosphorus and graphene like carbon nitride with enhanced catalytic, electrochemical and charge separation ability for photodegradation of an acute toxicity category-III compound. Appl. Catal. B Environ. 2018, 238, 568–575. [Google Scholar] [CrossRef]
- Ma, Z.; Song, K.; Wang, L.; Gao, F.; Tang, B.; Hou, H.; Yang, W. WO3/BiVO4 type-II heterojunction arrays decorated with oxygen-deficient ZnO passivation layer: A highly efficient and stable photoanode. ACS Appl. Mater. Interfaces 2019, 11, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, J.; Su, J.; Guo, L. Turning the unwanted surface bismuth enrichment to favourable BiVO4/BiOCl heterojunction for enhanced photoelectrochemical performance. Appl. Catal. B Environ. 2019, 241, 506–513. [Google Scholar] [CrossRef]
- Hongmanorom, P.; Ashok, J.; Chirawatkul, P.; Kawi, S. Interfacial synergistic catalysis over Ni nanoparticles encapsulated in mesoporous ceria for CO2 methanation. Appl. Catal. B. 2021, 297, 120454. [Google Scholar] [CrossRef]
- Malara, F.; Fabbri, F.; Marelli, M.; Naldoni, A. Controlling the surface energetics and kinetics of hematite photoanodes through few atomic layers of NiOx. ACS Catal. 2016, 6, 3619–3628. [Google Scholar] [CrossRef]
- Jian, J.; Shi, Y.; Ekeroth, S.; Keraudy, J.; Syväjärvi, M.; Yakimova, R.; Helmersson, U.; Sun, J. A nanostructured NiO/cubic SiC p–n heterojunction photoanode for enhanced solar water splitting. J. Mater. Chem. A. 2019, 7, 4721–4728. [Google Scholar] [CrossRef]
- Cai, Q.; Hong, W.; Jian, C.; Liu, W. Ultrafast hot ion-exchange triggered electrocatalyst modification and interface engineering on silicon photoanodes. Nano Energy 2020, 70, 104485. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, L.-W.; Liu, H.; Wang, J.-J. Electronic structure regulation and polysulfide bonding of Co-doped (Ni, Fe)1+xS enable highly efficient and stable electrocatalytic overall water splitting. Chem. Eng. J. 2022, 441, 136121. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.C.; Wu, J.Z.; Zhang, X.C.; Zhang, J.; Lyu, M.Q.; Luo, B.; Qian, G.R.; Wang, L.Z. Identifying dual functions of rGO in a BiVO4/rGO/NiFe-layered double hydroxide photoanode for efficient photoelectrochemical water splitting. J. Mater. Chem. A. 2020, 8, 13231–13240. [Google Scholar] [CrossRef]
- Srimurugan, V.; Jothiprakash, C.G.; Souparnika, V.; Prasanth, R. Photocorrosion-less stable heterojunction photoanode for efficient visible-light driven solar hydrogen generation. Int. J. Hydrogen Energy 2022, 47, 12515–12527. [Google Scholar] [CrossRef]
- Ren, S.J.; Sun, M.; Guo, X.T.; Liu, X.H.; Zhang, X.Y.; Wang, L. Interface-confined surface engineering via photoelectrochemical etching toward solar neutral water splitting. ACS Catal. 2022, 12, 1686–1696. [Google Scholar] [CrossRef]
- Zhu, S.S.; Zhang, Y.; Zou, Y.; Guo, S.Y.; Liu, H.; Wang, J.J.; Braun, A. Cu2S/BiVO4 Heterostructure Photoanode with Extended Wavelength Range for Efficient Water Splitting. J. Phys. Chem. C. 2021, 125, 15890–15898. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Zhu, S.S.; Liu, Y.Y.; Zhang, X.; Wang, J.J.; Braun, A. Covalent S-O Bonding Enables Enhanced Photoelectrochemical Performance of Cu2S/Fe2O3 Heterojunction for Water Splitting. Small 2021, 17, 2100320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Guo, Y.; Cai, L.L.; Li, H.; Wang, K.X.Z.; Cho, I.S.; Lee, C.H.; Fan, S.H.; Zheng, X.L. High-performance ultrathin BiVO4 photoanode on textured polydimethylsiloxane substrates for solar water splitting. ACS Energy Lett. 2016, 1, 68–75. [Google Scholar] [CrossRef]
- Tran-Phu, T.; Fusco, Z.; Di Bernardo, I.; Lipton-Duffin, J.; Toe, C.Y.; Daiyan, R.; Gengenbach, T.; Lin, C.H.; Bo, R.H.; Nguyen, H.T.; et al. Understanding the role of vanadium vacancies in BiVO4 for efficient photoelectrochemical water oxidation. Chem. Mater. 2021, 33, 3553–3565. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Zhao, Z.L.; Zhang, Q.H.; Huang, L.B.; Gu, L.; Lu, G.; Hu, J.S.; Wan, L.J. Steering elementary steps towards efficient alkaline hydrogen evolution via size-dependent Ni/NiO nanoscale heterosurfaces. Natl. Sci. Rev. 2020, 7, 27–36. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, L.W.; Liu, X.L.; Tan, T.; Liu, H.; Wang, J.J. Precisely engineering the electronic structure of active sites boosts the activity of iron-nickel selenide on nickel foam for highly efficient and stable overall water splitting. Appl. Catal. B Environ. 2021, 299, 120678. [Google Scholar] [CrossRef]
- Jiang, W.J.; Tang, T.; Zhang, Y.; Hu, J.S. Synergistic Modulation of Non-Precious-Metal Electrocatalysts for Advanced Water Splitting. Acc. Chem. Res. 2020, 53, 1111–1123. [Google Scholar] [CrossRef]
- Zhong, M.; Hisatomi, T.; Kuang, Y.; Zhao, J.; Liu, M.; Iwase, A.; Jia, Q.; Nishiyama, H.; Minegishi, T.; Nakabayashi, M.; et al. Surface modification of CoOx loaded BiVO4 photoanodes with ultrathin p-type NiO layers for improved solar water oxidation. J. Am. Chem. Soc. 2015, 137, 5053–5060. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, J.; Gao, L.L.; Hu, Y.P.; Long, X.F.; Wei, S.Q.; Wang, C.L.; Jin, J.; Ma, J.T. Construction of an efficient hole migration pathway on hematite for efficient photoelectrochemical water oxidation. J. Mater. Chem. A 2018, 6, 23478–23485. [Google Scholar] [CrossRef]
- Yu, F.; Li, F.; Yao, T.; Du, J.; Liang, Y.; Wang, Y.; Han, H.; Sun, L. Fabrication and kinetic study of a ferrihydrite-modified BiVO4 photoanode. ACS Catal. 2017, 7, 1868–1874. [Google Scholar] [CrossRef]
- Gu, S.N.; Li, W.J.; Wang, F.Z.; Wang, S.Y.; Zhou, H.L.; Li, H.D. Synthesis of buckhorn-like BiVO4 with a shell of CeOx nanodots: Effect of heterojunction structure on the enhancement of photocatalytic activity. Appl. Catal. B 2015, 170, 186–194. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, S.; Dai, Y.; Huang, X.; Chou, L.; Lu, G.; Dong, G.; Bi, Y. Nitrogen-incorporation activates NiFeOx catalysts for efficiently boosting oxygen evolution activity and stability of BiVO4 photoanodes. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.Y.; Huang, H.T.; Guo, W.X.; Xu, X.M.; Yao, Y.F.; Yu, Z.T.; Li, Z.S.; Zou, Z.G. Evaluating the promotional effects of WO3 underlayers in BiVO4 water splitting photoanodes. Chem. Eng. J. 2021, 417, 128095. [Google Scholar] [CrossRef]
- Huang, G.P.; Fan, R.L.; Zhou, X.X.; Xu, Z.H.; Zhou, W.Y.; Dong, W.; Shen, M.R. A porous Ni-O/Ni/Si photoanode for stable and efficient photoelectrochemical water splitting. ChemComm 2019, 55, 377–380. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.Y.; Zou, Y.; Li, T.T.; Liu, H.; Wang, J.J. Enhanced charge separation and conductivity of hematite enabled by versatile NiSe2 nanoparticles for improved photoelectrochemical water oxidation. Appl. Mater. Today 2022, 28, 101552. [Google Scholar] [CrossRef]
- Yang, F.; Chen, J.; Ye, Z.; Ding, D.; Myung, N.V.; Yin, Y. Ni-based Plasmonic/Magnetic Nanostructures as Efficient Light Absorbers for Steam Generation. Adv. Funct. Mater. 2020, 31, 2006294. [Google Scholar] [CrossRef]
- Fang, W.Q.; Lin, Y.M.; Xv, R.Z.; Fu, L. Boosting Photoelectrochemical Performance of BiVO4 Photoanode by Synergistic Effect of WO3/BiVO4 Heterojunction Construction and NiOOH Water Oxidation Modification. ACS Appl. Energy Mater. 2022, 5, 11402–11412. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y.; Ding, Q.; Yu, Y.; Huo, P.; Shi, W.; Xu, D. MOF derived NiO thin film formed p-n heterojunction with BiVO4 photoelectrode for enhancement of PEC performance. Colloids Surf. A 2022, 655, 130282. [Google Scholar] [CrossRef]
- Zeng, G.H.; Wang, X.J.; Yu, X.; Guo, J.; Zhu, Y.; Zhang, Y.M. Ultrathin g-C3N4/Mo:BiVO4 photoanode for enhanced photoelectrochemical water oxidation. J. Power Sources 2019, 444, 227300. [Google Scholar] [CrossRef]
- Liu, C.H.; Luo, H.; Xu, Y.; Wang, W.C.; Liang, Q.; Mitsuzaki, N.; Chen, Z.D. Cobalt-phosphate-modified Mo:BiVO4 mesoporous photoelectrodes for enhanced photoelectrochemical water splitting. J. Mater. Sci. 2019, 54, 10670–10683. [Google Scholar] [CrossRef]
- Sun, L.X.; Sun, J.H.; Yang, X.J.; Bai, S.L.; Feng, Y.J.; Luo, R.X.; Li, D.Q.; Chen, A.F. An integrating photoanode consisting of BiVO4, rGO and LDH for photoelectrochemical water splitting. Dalton Trans. 2019, 48, 16091–16098. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.-Y.; Zhu, S.-S.; Zou, Y.; Zhang, Y.; Yuan, S.-Y.; Li, T.-T.; Guo, S.-Y.; Liu, H.; Wang, J.-J. Improved Photoelectrochemical Performance of BiVO4 for Water Oxidation Enabled by the Integration of the Ni@NiO Core–Shell Structure. Catalysts 2022, 12, 1456. https://doi.org/10.3390/catal12111456
Cui J-Y, Zhu S-S, Zou Y, Zhang Y, Yuan S-Y, Li T-T, Guo S-Y, Liu H, Wang J-J. Improved Photoelectrochemical Performance of BiVO4 for Water Oxidation Enabled by the Integration of the Ni@NiO Core–Shell Structure. Catalysts. 2022; 12(11):1456. https://doi.org/10.3390/catal12111456
Chicago/Turabian StyleCui, Jun-Yuan, Shi-Shi Zhu, Yang Zou, Yan Zhang, Shao-Yu Yuan, Tian-Tian Li, Shi-Yi Guo, Hong Liu, and Jian-Jun Wang. 2022. "Improved Photoelectrochemical Performance of BiVO4 for Water Oxidation Enabled by the Integration of the Ni@NiO Core–Shell Structure" Catalysts 12, no. 11: 1456. https://doi.org/10.3390/catal12111456
APA StyleCui, J.-Y., Zhu, S.-S., Zou, Y., Zhang, Y., Yuan, S.-Y., Li, T.-T., Guo, S.-Y., Liu, H., & Wang, J.-J. (2022). Improved Photoelectrochemical Performance of BiVO4 for Water Oxidation Enabled by the Integration of the Ni@NiO Core–Shell Structure. Catalysts, 12(11), 1456. https://doi.org/10.3390/catal12111456






